Abstract
In this article, we describe an incorrect use of logic which involves the careless application of the “necessary and sufficient” condition originally used in formal logic. This logical fallacy is causing frequent confusion in current biology, especially in neuroscience. In order to clarify this problem, we first dissect the structure of this incorrect logic (which we refer to as “misapplied-N&S”) to show how necessity and sufficiency in misapplied-N&S are not matching each other. Potential pitfalls of utilizing misapplied-N&S are exemplified by cases such as the discrediting of command neurons and other potentially key neurons, the distorting of truth in optogenetic studies, and the wrongful justification of studies with little meaning. In particular, the use of the word “sufficient” in optogenetics tends to generate misunderstandings by opening up multiple interpretations. To avoid the confusion caused by the misleading logic, we now recommend using “indispensable and inducing” instead of using “necessary and sufficient. “ However, we ultimately recommend fully articulating the limits of what our experiments suggest, not relying on such simple phrases. Only after this problem is fully understood and more rigorous language is demanded, can we finally interpret experimental results in an accurate way.
Keywords: logic, command neurons, necessary and sufficient, optogenetics, memory, Feeding neuron, indispensable and inducing, Drosophila
Introduction
Needless to say, life sciences, particularly those involving genetic techniques, have substantially increased in importance during the last few decades. Naturally, the number of publications in the field has also increased dramatically compared to most other scientific fields. However, history of science suggests that while such a surge of research promises significant advancements, it can also lead researchers to become careless: Seemingly trivial issues in the heat of rapid discovery may actually lead the entire research field towards invalid conclusions. A fundamental issue that can cause researchers to misinterpret their data is the incorrect use of logic, which often leads to outright erroneous conclusions.
One such problem involves the careless use of the “Necessary and Sufficient” (N&S) conditions used as a technical expression in formal logic, which is a field of study dealing with abstract reasoning. A property of true N&S is that it implies equivalence between the two statements, allowing one to be a definition of the other (for introductory reading, see White, 2008). However, many biologists use N&S in a manner that does not satisfy formal logic criteria; true equivalence is not present in their statements. For the purpose of this essay, we will define this biology-specific misuse of N&S as “misapplied-N&S.” It is commonly argued that N&S has a special meaning in biology that differs from that in formal logic, and they conclude that its deviation from the formal definition is just a trivial issue of wording. However, inappropriate use of such logic can misrepresent the truth, as it has in the past, and we will show with detailed examples. It is universally agreed upon that we must not inappropriately use math, the basis of our quantitative analysis, so why not logic, which lies at the basis of science, as well? Such misuse creates similar repercussions. For a quick and simple example, observe the claim “Gene X is necessary and sufficient for a biological phenomenon,” a format we often encounter. It literally means that no other gene is required for the biological phenomenon. This is simply untrue, as multiple molecules function together to execute the whole process in any biological phenomenon. While perhaps a simplified example, instances similar to this are rampant in current biology, and they are equally erroneous. Given that misapplied-N&S is distorting researchers’ way of thinking, as we will discuss below, this issue cannot be addressed by simply placing the blame on wording or semantics.
In this essay, we explain the structure of misapplied-N&S, its potential pitfalls, and the grave consequences of inappropriately utilizing N&S logic. First, we clarify what N&S exactly is in formal logic, where it is used as a method to define a concept. Then, we explain the misapplied-N&S frequently used in biology, followed by a typical example of it in neuroethology that is causing serious problems in the field. In this example, we will explain how a key concept, the “command neuron,” was inappropriately defined using misapplied-N&S, which led to long-lasting confusion that stagnated the research field. With additional analysis of other possible pitfalls frequently encountered in optogenetic studies, we will conclude that avoiding the use of necessary and sufficient in biology will allow researchers to circumvent many problems and confusions, thus enhancing our research community.
True “necessary and sufficient” in formal logic
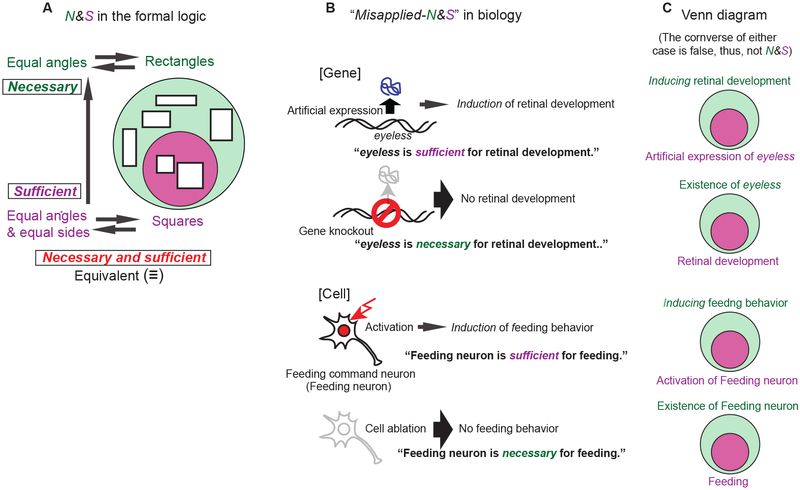
A simple example of the conditions that true N&S logic must satisfy can be given in the definition of a square (Fig. 1A). Because a square is also a rectangle, it must be a quadrilateral with four equal angles. In this case, we can say that being a quadrilateral with four equal angles (rectangle) is a necessary condition for being a square. Yet, it is not sufficient for defining a square, as there are countless rectangles that are not squares. The sufficiency is fulfilled, however, when the condition of having equal sides is added to this property of being a quadrilateral with four equal angles. Let us suppose a case where if statement A (being a square in Fig. 1A) is true, then statement B (being a rectangle) is also true (A ➔ B). In this case, we can say that A is a sufficient condition for B and that B is necessary condition for A. This relationship can be understood through the Venn diagram in Fig. 1A, where all the cases fulfilling statement A (squares) are included in the cases fulfilling statement B (rectangles). Only in special cases where both directions (A➔ B and B➔ A) are true, A and B are deemed to be necessary and sufficient for each other. In a Venn diagram of such a case, the set fulfilling condition A (a quadrilateral with equal angles and equal sides) should be exactly the same as the set fulfilling condition B (square). Because both sets are identical, A and B are logically equivalent. Therefore, A can be said to be a definition of B, and this is ultimately what true N&S must conclude. With this in mind, we will now explain how misapplied-N&S, frequently used in current biology, does not fit the framework of the real N&S illustrated above.
Figure 1.
Schematic diagrams to compare the real necessary and sufficient in formal logic and “misapplied-necessary and sufficient” commonly used in biology. (A) The relationship between squares and rectangles as a typical example of true necessary (being a rectangle; pale green) and sufficient condition (being a square; magenta) in formal logic. See text. (B) Two representative examples in biology to show misapplied-necessary and sufficient commonly used in biology experiments. Top two schemes; experiments of retinal formation in developmental biology. Bottom two schemes; experiments of command neurons for feeding behavior (Flood et al., 2013b) in neuroethology. See text. (C) Interpretations of each experiment in B according to formal logic. Consistent with the example of the rectangle/square, sets corresponding to the necessary conditions are represented as the circle in pale green while sets corresponding to the sufficient conditions are represented as the smaller circle in magenta. Note that converses to each example are false. Thus, there is no real necessary and sufficient condition in any of the cases.
“Misapplied-necessary and sufficient” in biology
One of the more common sources of misapplied-N&S is genetic studies. As shown in the upper half of Fig. 1B, two representative methods of genetic manipulation are (1) gene knockout (mutation) and (2) artificial expression of a gene. Some journal articles and scientific talks first propose that “Gene X is necessary for a phenomenon” when a knockout of Gene X leads to a disruption of the normally occurring phenomenon. Then, they propose that “Gene X is sufficient for the biological phenomenon” when artificial expression of Gene X artificially “induces” the phenomenon. From these observations, the claim is ultimately made that Gene X is necessary and sufficient for the phenomenon. The gene eyeless in a fruit fly, Drosophila, is a prime example of the Gene X in such statements, with a clear phenotype of retinal development, and will be used to illustrate our point. One paper made a statement that “Pax6/eyeless genes are necessary and sufficient for retinal development.” (Davis et al., 2003)*, as shown in the upper half of Fig. 1B.
Understanding true N&S allows one to easily recognize that this framework of thinking is nothing more than misapplied-N&S. To claim that eyeless is necessary for retinal development (Davis et al., 2003), they first show disruption of naturally occurring retinal development through the knockout of eyeless (for example, by deleting the eyeless gene), thus concluding that:
If there is no eyeless, then there is no retinal development ** (i)
Formal logic guarantees that the contrapositive (non B ➔ non A) is always true when the original proposition (A➔ B) is true. Therefore, one can conclude:
If retinal development occurs, then eyeless exists (ii)
Thus, we can at least say that the existence of eyeless is necessary for retinal development, in a similar way that being a rectangle was necessary for being a square in Fig. 1A. In the Venn diagram, the set of cases where a retina is developed is included in the other set, where eyeless is present (2nd Venn diagram in Fig. 1C).
A problem is encountered when the sufficient condition is then added. Following the format of the square example above, a proposition to claim that eyeless is sufficient for retinal development (Davis et al., 2003) should be the opposite direction (converse) to (ii), which is:
If eyeless exists, then a retina is developed (iii)
However, proposition (iii) is clearly false as other genes and factors are required for complete retinal development: eyeless’s existence alone is obviously not enough to form a retina. It is evident that the set of cases where “eyeless exists” is larger than the set with cases of “retinal development,” which requires more factors such as genes at the downstream of eyeless and genes cooperatively functioning with it (Jusiak et al., 2014). This is analogous to how squares have more requirements and form a smaller set, which is then included in the larger set of rectangles that has less requirements. The statement is represented pictorially by the 2nd Venn diagram in Fig. 1C. Thus, “eyeless exists” cannot be a sufficient condition for natural retinal development.
This problem arises because the sufficient condition used in the misapplied-N&S experiment (artificial expression of eyeless causing artificial retinal development) is different from the logically appropriate sufficiency statement (iii) and does not match its respective necessary condition. To match the true N&S conditions in formal logic, the sufficient condition must include all of the requirements for retinal development (White, 2008). However, the sufficient condition in misapplied-N&S deals with only one such factor– eyeless in this case. The statement “artificial expression of eyeless is capable of promoting a series of events that culminates in retinal development,” suggested by the above misapplied-N&S experiment, is not identical to the statement “eyeless alone is ‘sufficient’ for retinal development.”
Alternatively, one could also analyze a misapplied-N&S statement from the other way around. Instead of starting with a valid necessary condition, it could start with a legitimate sufficient condition and attempt to construct an appropriate necessary condition. This is actually how another publication stated, “Eyeless/Pax6 is necessary and in some circumstances sufficient to induce eye development” (Kumar and Moses, 2001). While adding the word “induce” may make it sound more plausible than the previous case this statement still fails to match true N&S conditions.
The second half of the statement is actually true, in that activation of eyeless signaling by its artificial expression is indeed sufficient for manipulating retinal development. No other method of manipulation is required for retinal development.
If eyeless is artificially expressed, then retinal development is induced*** (iv)
As the opposite direction (converse) to (iv), a proposition to claim the necessity of eyeless expression for promotion of retinal development should logically be:
If retinal development is being induced, then eyeless should have been artificially expressed (v)
However, we cannot test (v) because it would require the unfeasible task of trying every possible method of manipulation to check for any induction of retinal development. For example, since it is not realistic to express each gene in the whole genome one by one, concluding the absolute necessity of eyeless expression for the induction of retinal development is nearly impossible. In the case of eyeless, it is reported that artificial expression of other genes also promotes retinal development (Jusiak et al., 2014), and thus (v) is indeed false. As represented pictorially by the 1st Venn diagram in Fig. 1C, the set fulfilling “retinal development is induced” is larger than the set fulfilling “eyeless is artificially expressed.” Thus, the condition, “eyeless is artificially expressed,” is sufficient, but is not necessary for the condition, “retinal development is induced.” And so, the statement that eyeless is necessary and sufficient for inducing retinal development is also faulty. People are tempted to claim necessity by simply demonstrating failed retinal development when eyeless is knocked out (Davis et al., 2003; Kumar and Moses, 2001). However, it does not actually match the sufficiency statement (iv), as (v) is the logically legitimate necessity statement that should accompany (iv).
The sufficient condition in this misapplied-N&S is based on experiments where we suggest causality by spatiotemporal manipulation of gene expression to actively correlate it to a biological phenomenon. On the other hand, the necessary condition is based on experiments where gene expression is shut down to check if it is definitely an essential requirement for the whole biological phenomenon. This means that there is little evidence to suggest causality, as the necessary condition can also be fulfilled in cases where, for example, it is a prerequisite such as a housekeeping molecule or even a supporting structure. Therefore, the two parts (necessary and sufficient) of misapplied-N&S are dealing with completely different issues based on completely different kinds of experiments: There is no logical rationale to suggest that they should be associated together like this. However, they tend to be paired in such a way simply because they are two representative kinds of manipulations in current biology (artificial expression and shutting down expression). While people may be tempted to place them together, the fact remains that these statements are not actually matching logically. We can see the same logical structure in neuroscience, where people tend to claim that a certain neuron is N&S for a certain pattern of behavior based on two representative kinds of manipulations in current neurobiology, activation of the neuron and shutting down activity of the neuron. This is further explained in A “Witch Hunt” of Command Neurons section (the lower half of Fig. 1B, C).
In conclusion, we should avoid the careless use of the phrase “necessary and sufficient.” Some may try to rationalize its use by suggesting that there is perhaps a “biological” interpretation of misapplied-N&S, a simple figure of speech that does not have to follow the standards of formal logic. This may seem attractive to use because it seemingly imparts an air of formal logic to incorrectly enhance the importance of a gene/neuron****. In the following sections, we introduce specific examples where such careless use of misapplied-N&S has indeed led to serious misunderstanding of experimental results. The grave repercussions of its use will make clear that one should refrain from using the phrase, no matter what the intent.
A “witch-hunt” of command neurons
Wewould like to pick a particularly troubling example where such misapplied-logic has led to great confusion on the key concept of “command neurons” in the neuroethology field, so much so that it nearly resulted in the mistaken loss of the concept entirely. This is a serious issue because neuronal network analysis through optogenetics* (see Pitfalls II section for details) has been explosively popular in recent years, and thus the problem will only exacerbate if left unchecked. The concept of the command neuron was originally conceived through neuroethological experiments by K. Ikeda on crayfish behavior in the lab of C.A.G. Wiersma at CalTech (Ikeda and Wiersma, 1964; Wiersma and Ikeda, 1964). Ikeda recorded repeated bursts of neural activity (Ikeda and Wiersma, 1964), which associated with oscillatory movement of crayfish swimmerets. He also found neurons that could turn this activity on or off. Consequently, Wiersma and Ikeda (1964) established the concept of “command neurons” to vividly characterize the role of these “switch” neurons. Fourteen years after the above report, a “formal” definition of command neurons was proposed not by Wiersma and Ikeda themselves, but by Kupfermann and Weise (1978): “We suggest that a command neuron be defined as a neuron that is both necessary and sufficient for the initiation of a given behavior. Three criteria can be tested by: (1) establishing the response pattern of the putative command neuron during presentation of a given stimulus and execution of a well specified behavior; (2) removing the neuron and showing that the response is no longer elicited by the stimulus (necessary condition); and (3) firing the neuron in its normal pattern and showing that the complete behavioral response occurs (sufficient condition).” After understanding our above discussion, one could see that this new definition is based on the same, typical misapplied-N&S (the lower half of Fig. 1B). Since the “necessary and sufficient” used here does not correspond to each other (bottom two Venn diagrams in Fig. 1C), their “definition” does not follow any formal logic. But because they arrogated the terminology from formal logic which sounded very careful, strict, and thoughtful, their “rigid” definition (Marder and Calabrese, 1996) has unfortunately become widely accepted in the neuroethology field.
Understanding the flaw with their reasoning requires some further examination. The first demonstration of a command neuron by Wiersma and Ikeda (1964) was done by triggering a certain pattern of behavior by activating neurons with a certain pattern of electrical activity, which fulfilled the sufficient condition of Kupfermann and Weise. However, the true necessary condition to meet this sufficient condition is not based on the removal of neurons as Kupfermann and Weise suggested. Rather, the appropriate necessary condition should be that induction of the pattern of behavior occurs only through the electrical activation of the neuron (initiating the behavior ➔ the electrical activation of the neuron). As explained with the example of induction of retinal development, this condition is simply impossible to test because it is rather unrealistic to test every kind of manipulation, for example, by stimulating every neuron in the brain one by one. Thus, it is unfeasible to fulfill the true N&S condition in any way. Kupfermann and Weise’s necessary condition through the removal of some neurons is not matching the sufficient condition, and thus does not have any logical reason to be tied together (see Pitfalls I section to see how their necessary condition is unnecessary).
The unnecessarily “rigid” requirement incurred by Kupfermann and Weise’s misapplied-N&S logic has led to an awkward situation resembling a “witch-hunt” in the neuroethology field. For example, the necessary condition in misapplied-N&S has mistakenly led to the exclusion of the Mauthner cell from being classified as a command neuron (Eaton et al., 2001) although its unique and insightful properties make it an exemplary model system of the sort (Korn and Faber, 2005). Indeed, failure to fulfill the necessary condition of misapplied-N&S has excluded many appropriate command neurons in a similar fashion. Even among animals with simple nervous systems, only two pairs of command neurons (one in a lobster, the other in a sea slug) had been able to fulfill Kupfermann and Weise’s unnecessarily strict criteria (Frost and Katz, 1996; Meyrand et al., 1991). The feeding command neuron, which we have recently discovered in the brain of fruit flies (Flood et al., 2013b) (see What is the “merit” section) has become only the third one in the world to meet Kupfermann and Weise’s criteria. While a few pairs of neurons may have passed these unnecessarily rigid criteria, this situation has placed the command neuron concept itself in danger, as the term and its strictness was often avoided altogether. Marder and Calabrese (1996) commented, “these criteria were so rigid that the concept has largely been abandoned, and the term command neuron has virtually disappeared from the literature on control of rhythmic motor systems.” People have started to use other phrases such as “higher-order neurons” (Kupfermann and Weiss, 2001; Marder et al., 2005) or “command-like neurons” (Eaton et al., 2001) in order to circumvent the strict definition, but they lack the intuitive diction of the original term. In reality, it was the wrong logic of misapplied-N&S, introduced by Kupfermann and Weise, that polluted the command neuron concept, which does not have any fault in and of itself.
What is the “merit” of misapplied-N&S in neuroscience? –suggesting a natural function and no redundancy through misapplied-N
Before we discuss the complications of using the term specifically, let us start by laying out one possible positive side of misapplied-N&S, taking an example from the feeding command neuron in fruit flies (Flood et al., 2013b).
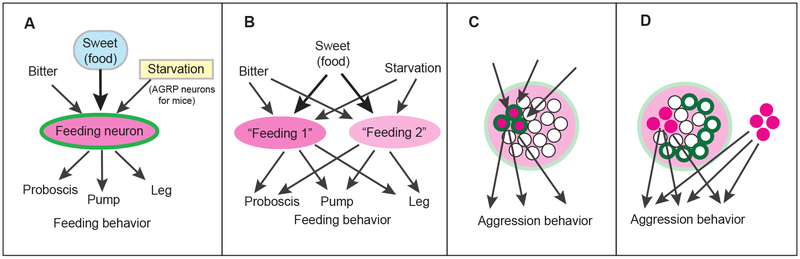
As mentioned above, we have discovered a pair of command neurons that direct the whole feeding behavior, and it happens to meet Kupfermann and Weise’s misapplied-logic criteria to qualify as the third “certified” command neuron. The feeding command neuron (“Feeding neuron”) is located at the pivot of the feeding circuit in the downstream of gustatory signals and upstream of motor neurons, thus controlling feeding behavior. Genetic activation of the Feeding neuron by opening heat-activated channels induces the feeding behavior, fulfilling the sufficient condition in misapplied-N&S (condition (2) of Kupfermann & Weise’s definition). The Feeding neuron also satisfies Kupfermann and Weise’s necessary condition in misapplied-N&S, in that its inactivation or ablation can suppress the natural feeding behavior of a fly (condition (3) of Kupfermann & Weise’s definition). This necessary condition in misapplied-N&S reflects two facts: it supports the assumption that (1) the Feeding neuron functions in natural feeding behavior (it is a natural function; the misapplied-sufficiency result was not a phenomenon, which occurs only by artificial activation) and (2) there are no other cells that can substitute the Feeding neuron in triggering feeding behavior (there is no redundancy). Therefore, as shown in Fig. 2A, we can gather that the Feeding neuron is at the “pivot” of the signaling pathway that controls fly feeding behavior. This incidental case of a pivotal role without redundancy enables the Feeding neuron to coincidentally fulfill the misapplied-N&S condition, since a loss of a pivotal neuron would lead to a loss of the whole behavior and activation of the pivotal neuron would induce it. This is how, through a similar consideration, the gene eyeless (the upper half of Fig. 1B) is believed to function as a master regulator on the only signaling pathway for natural retinal development. The experimental results which fulfill misapplied-N&S help us identify possible key neurons in this way, and thus we guess this is why some researchers feel some merit of misapplied-N&S*. If someone wants to use some term to specify misapplied-N&S condition because of this kind of merit, we recommend using another phrase such as “indispensable and inducing” (see So, what should we do instead? section for detailed reasoning).
Figure 2.
Schematic diagrams of neuroscience experiments that address commanding or “misapplied-sufficient” function of neurons. (A) An example of a feeding command neuron in a fly brain (Flood et al., 2013b). Various signals such as food or starvation flow into a pair of feeding command neurons (Feeding neuron), and the Feeding neuron makes the decision to trigger the whole feeding behavior. The Feeding neuron fulfills the “rigid” requirements of misapplied-N&S. See text. (B) A hypothetical case with redundancy, where a second imaginary feeding command neuron (Feeding 2) can substitute the first command neuron (Feeding 1). Feeding 1 fulfills the sufficient condition in misapplied-N&S, but it does not fulfill the necessary condition in misapplied-N&S. (C) A group of neurons are triggering a whole pattern of behavior such as aggression (Lin et al., 2011). The group of neurons fulfills requirements of misapplied-N&S (pale magenta represents the area tested for “sufficiency” and pale green represents “necessity”). However, it is possible that only a fraction of the cells (circles with deep colors) are responsible for the behavior. The deep magenta represents “sufficiency” and deep green for “necessity”. (D) Another possible scenario; the group of neurons fulfills requirements of misapplied-N&S, but the cells responsible for “necessity” might be different from the cells responsible for “sufficiency”. Color code is the same as that of C. This situation allows a group of redundant cells (right) to have a potential to be “sufficient” for triggering the behavior as well. The “necessity” experiment does not check if it is a natural function or not either. It is far from the true N&S shown in Fig.1A and the words “necessary and sufficient” are misleading here. See text.
Pitfalls of N&S in neuroscience I. --Failing the necessary condition in misapplied-N&S does not mean “unimportant” (A better definition of the command neuron)
Some researchers may want to claim the practical advantage of utilizing misapplied-N&S from this aforementioned “merit.” Thus, they attempt to justify the use of “necessary and sufficient” by claiming it is a biology-specific figure of speech. By using it in such a way supposedly unrelated to formal logic, they may claim that “necessary and sufficient” points to a neuron/gene having a pivotal role, or even that it is simply important for whatever other reason. While seemingly innocuous upon first inspection, this kind of thinking is still quite troublesome.
First of all, the misapplied-N&S test is not a reliable test for indicating the importance of a neuron: Despite Kupfermann and Weise’s criteria (Kupfermann and Weise, 1978) on command neurons, failure in fulfilling the necessary condition of misapplied-N&S does not automatically mean that the neuron is unimportant. As a simple metaphor to illustrate the nature of this misunderstanding, let us imagine a soccer game. Suppose that one great soccer player A is leading the team as a playmaker. If the team becomes dysfunctional whenever player A is gone, perhaps due to a red card, it would be clear that player A is a real playmaker. However, there could also be a case where another player B is good enough that he can essentially substitute that role of a playmaker. If the team can still operate without him, could we automatically say that player A is neither important nor functional? Does this suggest that player A is not playing the playmaker role in the games he does indeed play? Of course not: he is still playing that essential role regardless of whether another player can substitute him or not. Like this soccer metaphor, failing the necessary condition of misapplied-N&S by retaining behavior even after a neuron is ablated or inactivated should not necessarily discourage people who are working on the neuron, as it may just indicate redundancy such as the instance depicted in Fig. 2B. Such redundancy can be expected in many important mammalian functions, especially in the nervous system, in the same way that a loss of one kidney still allows for waste filtration*. The nervous system also has incredible flexibility, where loss of important neurons can be substituted by other circuits originally for another function, as widely observed in rehabilitation. It is still entirely possible that the neuron is playing a pivotal role even after failing the necessity test as shown in Fig. 2B. Despite this fact, some researchers are misled into believing that their neurons are unimportant for a given function if inactivation or ablation of their neuron does not lose the function because the misapplied-N&S is so wide spread and accepted as an authentic “logic” in current biology.
As we stated earlier, the necessary condition in misapplied-N&S may suggest the possibility of a natural role of the neuron, but since it does not guarantee this**, the natural role is more clearly identified by directly observing neuronal activity. The best method for confirming command neuron we can conduct in current biology is (1) recording an electrophysiological response to a key stimulus from a neuron and then (2) mimicking the firing pattern of the same neuron to induce the behavior pattern***. Once electrophysiological recording/stimulation has been conducted, the necessary condition in misapplied-N&S experiments is only a means of checking redundancy; since the command function has already been established, the necessary condition in misapplied-N&S is not required to prove it. Thus, the necessary condition from misapplied-N&S should never have been included in Kupfermann and Weise’s “definition” of the command neuron. However, many people in current neuroscience are confused by this misapplied-logic into believing that the test for the necessary condition in misapplied-N&S is a decisive experiment for confirming the importance of the neuron(s). This belief forced them to discredit potentially important neurons such as the Mauthner cell, as we explained in the “witch-hunt” section. The Drosophila P1 neuron that triggers courtship behavior (Kimura et al., 2008) also does not fulfill Kupfermann and Weise’s criteria (Yamamoto and Koganezawa, 2013), but it does fit our two criteria stated above (Kohatsu et al., 2011; von Philipsborn et al., 2011). Thus, both the Mauthner cell and the P1 neuron should be credited as command neurons. We have to stray away from Kupfermann and Weise’s misleading definition, rooted in misapplied logic. Instead, we propose the definition of a command neuron to be “an interneuron(s), the natural activity of which triggers a specific motor program (Flood et al., 2013b),” modified from Edwards et al. (1999).
Pitfalls of N&S in neuroscience II. -- Misapplied-N&S simplifies the truth too much, skewing our understanding.
Optogenetic techniques first demonstrated in Drosophila (Lima and Miesenbock, 2005) sparked a surge of studies in modern neuroscience where animal behavior is controlled by acute activation of neurons through photo-manipulation. Photo-activatable ion channels were exogenously introduced into animals such as mice. Then, they were illuminated to induce behavior. Their acute induction of behavior helped revive the command neuron concept, and optogenetic studies often use the term “sufficient” to express their exciting observations of behavior induced by photo-activating only a small group of neurons. But because the word “sufficient” is often used in the likes of misapplied-N&S by Kupfermann and Weise, we frequently see confusion and misunderstanding surrounding these studies. We will illustrate this by taking examples of feeding circuits. As shown in Fig 2A, the starvation signal is incorporated into the Feeding neuron in the fly brain (Flood et al., 2013b) (see What is the “merit” section). Detailed optogenetic studies on starvation have been conducted in the brain of mice, and some groups of neurons (AGRP neurons) were found to be involved in a motivating process for feeding (Aponte et al., 2011). In those studies, they observed a motivated state of feeding through the activation of the neurons and stated “AGRP neurons are sufficient to orchestrate feeding behavior.” This sounds as if AGRP neurons function like the Feeding neurons by commanding the behavior. In fact, the AGRP neurons are playing another important but completely different role as a motivator for the feeding behavior. The Feeding neuron in the Drosophila brain is naturally activated by the presence of food, and its artificial activation can trigger feeding behavior even in the absence of food. Meanwhile, it is the absence of food that activates AGRP neurons (Yang et al., 2011), and the artificial activation of AGRP neurons sets the motivated state, but the starting of feeding behavior still requires the existence of food. Thus, these neurons behave quite differently, but the roles of these completely different types of neurons have been blurred from the meaning behind the word “sufficient.” Optogenetic induction of behavior after the stimulation of AGRP neurons simply suggests that other forms of manipulation are not needed for inducing the behavior. It does not imply that activity of other neurons, nor other signals such as food, are not needed. However, the statement about the AGRP neuron being “sufficient to orchestrate” sounds as if AGRP neurons are commanding the feeding behavior because the word “sufficient” implies that the induction of the behavior does not need other factors, such as the very food that is directly triggering the behavior. Thus, a statement with “sufficient” is confusing because it tends to generate overstatements. The title of the AGRP neuron paper is obviously an overstatement because other neurons are required for orchestrating the feeding behavior as recently shown in mice by another group (Han et al., 2017). Instead of conducting optogenetic experiments, monitoring the activation of neurons would be more helpful in finding the exact function of the cells because it can differentiate between these two types of functions, “commanding” and “motivating.” Command neurons such as the Feeding neuron are activated by key stimuli such as the food signal (Flood et al., 2013b) while motivating neurons such as the AGRP neurons are activated by metabolic states such as the starvation signal (Yang et al., 2011). Since the food signal triggers feeding behavior without passing through the AGRP neurons, AGRP neurons cannot be said to “orchestrate” the feeding behavior: It only monitors the metabolic state to set the probability of the behavior occurring. Such sufficiency statements produce overstatements akin to an important supporting actor being made to look like the lead actor.
Let’s see another example; “Previous studies have demonstrated the important role of DA (dopamine) in the processing of salient signals and goal-directed behaviors. However, it has been unclear whether other circuits and neurotransmitter systems are required at the same time and whether specific patterns of activity in distinct neuron types are sufficient to effect place preference” (Tsai et al., 2009). Optogenetic experiments are not enough to address this question since spontaneous activity of neurons (unrelated to the stimulus) in other circuits may be contributing to affecting place preference. However, people are often confused between the two possibilities of (1) the activity of the neuron is truly sufficient (other factors, such as coupled spontaneous activity of other neurons, are not required at all for specifying the place preference) and (2) the optogenetic activation of the neuron by itself is only sufficient as manipulation when observing the behavior (other forms of manipulation are not required for specifying the place preference). As the authors’ statement conveys, most readers would assume it means that other neuronal activity is not needed (1). This is not addressed by the optogenetic experiment, which only confirms (2). Thus, we recommend avoiding the use of “sufficient” to explain results from any optogenetic experiment at all. Otherwise, demonstrating the sufficient condition in misapplied-N&S may inaccurately suggest there is absolutely no need of other factors, such as other neuronal circuits which are often needed. It seems that some researchers may be unaware that the “logic” at the basis of their study is ambiguous. Instead of carelessly borrowing such strong and determining words from formal logic, which actually make things vaguer rather than precise, we must maintain the discipline to actually describe what physiological truth we can read out from each experiment.
Pitfalls of N&S in neuroscience III. -- In studies of a group of neurons, misapplied-N&S can falsely make insignificant neurons look important as well
In the vertebrate brain, multiple neurons often synergistically cooperate to perform one function. Therefore, activation of a single neuron may not bring about a readily-apparent function, and alternatively a loss of a single neuron may not cause an obvious loss of that function. This can be problematic for experimental purposes, as it would be hard to clearly identify a cause-and-effect relationship when synergistic/redundant characteristics of brains mitigate or negate the effects of the control. To counteract this issue, some experiments activate and ablate/inactivate an entire group of neurons. The explosive popularity of optogenetics is making misapplied-N&S analysis regarding population of cells quite common. Some neurons were concluded to be “necessary and sufficient” for aggression behavior in this way, as they connected the activation of VMHvl neurons in a mouse to its increased aggression towards another mouse (Lin et al., 2011). The potential “merit” of misapplied-N&S, exemplified by the case of the Feeding neuron (see What is the “merit” section), allowed this experiment to give an intriguing insight for brain function (Lin et al., 2011). It supported the possibility that this group of neurons is the only one that triggers aggression behavior in the natural condition. This suggests the possibility that the group of neurons may be located at the pivot of the aggression circuit. Despite such promising prospects, we have to be even more careful in cases involving multiple neurons such as this because the strong nuance of misapplied-N&S opens up additional issues that were absent in single neuron analysis.
As depicted in Fig. 2C, misapplied-N&S can be fulfilled even in a case where only a small number of neurons are actually responsible for executing the behavior. However, the strong nuance of the word necessary in misapplied-N&S gives the impression that every single cell in the whole group is important and essential. We have to bear in mind that misapplied-N&S experiments only confirm that the group of cells includes the essential cells, although “necessary and sufficient” makes it sound as if this entire group of cells is the minimum requirement for the function. Moreover, it is even possible that the cells in the tested group responsible for the necessary condition of misapplied-N&S and those responsible for the sufficient condition are completely different cells altogether (Fig. 2D). In the aggression example, some cells in the group may induce the aggression behavior (sufficient), while another part of the cells may be a prerequisite for the natural aggression behavior (necessary) as we explained in the Footnote** in the Pitfalls I section. It is possible that a loss of the latter cells, such as those regulating leg movements, might make the animal less energetic, leading to loss of aggression behavior. The possibility of different subgroups playing different roles in the given group would undermine the two possible insights of misapplied-N&S, checking if it is a natural function and that there is no redundancy (see What is the “merit” section). This problem is depicted in Fig. 2D. Thus, we have to bear in mind that the use of misapplied-N&S for a group of cells has the substantial possibility of misleading us away from the truth. This confusion results from the fact that misapplied-N&S sounds “logical,” despite the lack of basis in any proper logic, and thus nobody has questioned its resulting conclusion. The necessary condition in misapplied-N&S gives the impression that all of those cells are really necessary, when it is entirely possible that some in the group do not serve any purpose in the function.
Pitfalls of N&S in neuroscience IV. --The most extreme and concerning example: an inappropriate framework of thinking based on misapplied-N&S generating studies lacking scientific insight
The strong sound of misapplied-N&S is not only leading to the distortion of understanding or definitions, but it also tempts some researchers into conducting unproductive or even misleading studies. For example, one group published a paper claiming that Calcium is N&S for inducing long-term memory formation, measuring an association between an olfactory stimulus and sucrose stimulus in honeybees (Perisse et al., 2009). The original question of the study, “whether Ca2+ is necessary and sufficient for inducing long-term memory formation,” is based on misapplied-N&S; because the question itself does not have a logical foundation, the design of the experiments (injection of calcium chelator to reduce free calcium for the necessary condition and artificial increase of calcium for the sufficient condition) as a whole unfortunately does not offer scientific insight, and the final conclusion itself is another typical instance of misapplied-N&S. In the animal brain, the calcium ions play an essential or “pivotal” role in chemical synaptic transmission, which is a major synaptic transmission in the animal brain (Katz and Miledi, 1967). Thus, it is quite obvious that the calcium ion is necessary for memory formation. Then, we also know that there are other factors in memory formation, and so we know that the calcium ion is definitely not sufficient for memory formation. Therefore, we do not even need to ask the question of whether calcium is N&S for long-term memory formation, and we definitely do not need any experiment to find this out. Their necessity experiment suppresses synaptic transmission at many synapses, so it is quite obvious that it will compromise proper memory formation. Then, their sufficiency experiments abnormally increase synaptic transmission at many synapses, which should change the behavioral output in an unpredictable manner. If neuronal activities induced from the olfactory and sucrose stimulus, which are supposed to be associated in the experiment, change due to an increase of the calcium ion at previous steps in memory formation, it would result in a change of the behavioral output. However, this does not imply that there was a change in the fundamental property or potential of memory. Here we should note a serious pitfall underlying memory studies, where some researchers are tempted to claim that there is a change in the property of memory when only the neuronal activities to be associated for remembering had changed, leading to change in behavioral output*. However, we cannot address property of memory based on such experiments showing changes in behavioral output alone. As a representative example of the pitfall, we cannot receive any insight on the memory mechanism from experiments that manipulate the calcium ion, as it changes neuronal activity in general, and not just the memory formation in question. While it is possible that the property of memory may also be changed by the level of Ca2+, we cannot address it thorough manipulation of overall Ca2+ level as everything in neurophysiology is calcium-dependent.
A serious problem arose as a misleading study sounded meaningful due to misapplied-N&S. A case that is as clearly faulty as this calcium/memory example may be somewhat rare, but so many other reported experiments have the same structure. Some deal with the effect of expression/knockout (Xu et al., 2014) or pharmacological activation/inactivation (Pagnussat et al., 2015) of various molecules on memory. If a biological phenomenon is based on a complex system, it is difficult to pinpoint where certain molecular and cellular processes constitute the phenomenon, such as memory formation. The common problem underlining these experiments is that misapplied-N&S can be fulfilled even if the molecule is not functioning for memory per se, and so the strong nuance of misapplied-N&S makes it sound as if the molecule is critical for memory. This problem derives from the fact that molecules such as Ca2+ function at so many aspects for biological phenomena based on complex systems (at all synapses in the case of Ca2+, for example). In a similar manner, we should be careful in cases where a group of neuromodulatory neurons such as dopamine neuron, serotonin neurons, etc. may influence on multiple sites in the neuronal network. We may be misled by experiments fulfilling the misapplied-N&S through manipulation of such neurons because behavioral change may be caused from the outside of the focused point such as parts carrying memory.
The take home message from this example is that we should truly understand the physiological processes underlying each experiment, rather than giving a sloppy summary that utilizes misapplied-N&S. But because misapplied-N&S evokes an implication of importance, reviewers and editors of some journals usually do not notice the lack of meaning in such studies. As a result, such studies are appearing in prestigious journals, while most readers do not even notice how unproductive and misleading those studies are. Our scientific community must cease this spread of misapplied-N&S!
So, what should we do instead?
More often than in published papers, we encounter misapplied-N&S in academic talks. One principal investigator repeated more than ten times in a talk that a certain molecule is N&S for Long Term Potentiation (LTP), a representative phenomenon (Bliss and Lomo, 1973) for an experimental model of memory formation. As is obvious from our discussion above, a molecule can never be truly N&S for the whole phenomenon. Such careless statements from established, professional scientists are having quite a bad influence on young researchers, and we frequently see students “proudly” claiming something is “necessary and sufficient” in their thesis defense or qualifying exam. We must stop this foolish and worrying situation before many young scientists start misleading experiments like the study of whether calcium is N&S for memory formation (Perisse et al., 2009) or fasting-dependent long-term memory (Hirano et al., 2013). Thus, let us propose the following solution.
First of all, our overall discussion reveals that we must be careful not to misuse the N&S conditions, so that we can correct past confusions while preventing future ones. The main problem comes from the word “sufficient,” which is often used to emphasize that artificial expression of only a single gene or activation of only a single neuron can cause a substantial and presumably relevant effect on the whole process of interest. Although it may be sufficient as an experimental manipulation for triggering the effect, it is not actually sufficient for executing the whole effect itself (Fig. 1C). Therefore, this term is only erroneous when it is coupled with the word “necessary,” and it could be said that its use would not be wrong if the context is restricted to artificial manipulation. However, it often leads into mixing up the three following possibilities (taking the command neuron/optogenetic study for simplicity):
(1) the activity of the neurons is truly sufficient for a pattern of behavior (activity of any other neuron is not required for executing the whole behavior)
(2) the activity of the neurons is sufficient for triggering a pattern of behavior (spontaneous activity of any other neuron or any other extrinsic factors are not required for triggering the behavior)
(3) the artificial activation of neurons is sufficient for triggering a pattern of behavior (any other form of manipulation is not required for triggering the behavior)
The first sufficiency matches the necessary condition of misapplied-N&S, although it is rather unrealistic as we explained in the Misapplied-N&S in biology section. Its downstream neurons should also be activated to realize any pattern of behavior. The second one is what people often believe to be the case, due to the confusion behind the use of the word “sufficient,” although it is not supported by neuronal stimulation/optogenetic experiments. The nuance of “sufficient” causes people to ignore not only spontaneous activity, but also extrinsic key stimuli such as food as explained in Pitfall II. But within these three options, optogenetic analysis only addresses and supports the third possibility. Thus, we should be careful when using the term in our statements, but it would certainly be better to simply avoid using the word “sufficient” entirely for studies on the artificial activation of neurons.
In the past, the word “sufficient” was not used in the misleading way as it often is nowadays because the word “sufficient” by itself does not evoke the meaning that it is single-handedly causing the whole process. Only fairly recently has sufficient become common in biology literature, even more frequently in talks. We guess that the main impetus behind this verbal change is to tie it with the word “necessary” for claiming misapplied-N&S, although it does not make sense to use the word “sufficient” if they are not examining factors that are the minimum requirements for that function. Rather, the importance of “sufficiency” experiments lies in demonstrating a causal link through optogenetic activation of neurons (or misexpression of a gene). Thus, words such as triggers, promotes, induces, switches, or initiates may better reflect or express the desired nuance without creating such confusion.
The term “necessary” may be appropriate in terms of the formal logic if it is not tied with the word “sufficient”. However, we recommend against using “necessary” because its contrary, “not necessary,” gives the nuance of unimportance in ordinary conversation as discussed in Pitfall I. Thus, we propose use of a word, “indispensable” instead of “necessary” because its antonym, “dispensable,” sounds not only unimportant, but also substitutable.
Let us give an example that describes a pair of experiments which fits misapplied-N&S. Suppose:
Artificial activation of a Feeding neuron pair induced feeding behavior.
When a Feeding neuron pair was inactivated or ablated, natural feeding behavior ceased.
To interpret these experiments, an appropriate statement for a conclusion may be “activity of a Feeding neuron induces feeding behavior at probably only one pivot of the natural feeding circuit (but, it is recommended to monitor activity of Feeding neuron to confirm natural activity).”
If you prefer to use a simple sentence like N&S, we recommend you to say something akin to, “the Feeding neuron is indispensable and inducing for feeding behavior.” However, rather than simply stating the conclusion using a short sentence, we must carefully consider what is going on in our experiments and what our results suggest in each experiment: fulfilling the indispensable and inducing criteria does not necessarily suggest the pivotal role of the neuron*.
Conclusions
Even if there happened to be a few instances where real necessity and sufficiency can be validated, we have never seen a single case where the use of N&S has helped our understanding of biology because its use would require too many premises to offer any actual additional insight. However, we have encountered far too many cases where misapplied-N&S has misled the interpretation of experimental results. It is more worthwhile to carefully examine what we can learn from each experiment, rather than superficially borrowing terminology from formal logic without adhering to the actual definitions of the terms. To avoid subsequent confusions, it may be more sensible to simply abolish the use of N&S entirely. Alternatively, indispensable and inducing would work better to make it clear that the two manipulations are not logically converse in order to avoid overstatements. Fulfilling the indispensable and inducing condition should not be the end goal of our research: that alone suggests very little for our understanding. We need more experiments such as activity monitoring to truly understand brain function.
The words “necessary and sufficient” are not only misleading, but the way of thinking by researchers when they use misapplied-N&S is often incorrect. We must stress that the problem we have addressed here is far deeper than simply an issue of semantics. The incorrect use of logic undermines perfectly executed research with misleading conclusions that misunderstand nature. Thus, we conclude that “necessary and sufficient” in biology is not necessarily necessary.
ACKNOWLEDGEMENTS
We thank Prof. Ryushi Goto at Department of Mathematics, Osaka University and Prof. Yoshinori Namikawa at Department of Mathematics, Kyoto University for advice on logic, Ms. Nobuko Yoshihara and Christina Kingman for their kind support and advice, Dr. Takeo Watanabe, Mr. Louis Watanabe, Drs. Masahiro J. Go, Nao Uchida, Benjamin White, Daisuke Yamamoto, Soh Kohatsu and members of Yoshihara lab such as Akira Sakurai for their comments, Kazuhiro Oiwa, Hiroaki Kojima and Iwao Hosako for generous supports at Kobe. This work was supported by National Institute of Health Grant, U.S.A. (MH85958 to Motojiro Y.) and KAKENHI (26891030 to MotojiroY.) from JSPS, Japan. This article is dedicated to Motojiro Y’s mentor, Dr. Kazuo Ikeda, who is the founder of the command neuron concept and passed away on May 3rd, 2016 at the age of 89.
Footnotes
Pax6 is a mammalian homolog (ortholog) of eyeless.
Strictly speaking, we cannot even state (i) just through the evidence from the knockout of eyeless alone. There is still the possibility that some unknown experimental manipulation may be able to cause retinal development without eyeless, and it would be impossible to test all hypothetical cases in order to disprove it. However, since such a possibility is rather unusual in biology, let us assume that proposition (i) is true for the sake of simplicity in our discussion.
We cannot even state (iv) in a strict manner as well, because we again cannot test all hypothetical cases. But like the case of (i), we can claim (iv) if restricted to the experimental condition, where other factors for inducing retinal development already exist. Thus, we can assume that proposition (iv) is true for the sake of simplicity as well.
In some cases, the term “necessary and sufficient” is used in an even less meaningful manner. Such cases include interpreting the loss of a function in a mutant of a gene as suggesting its “necessity” and the rescue of the function through expression of the gene as “sufficiency.” Rescuing a mutant of a gene with its transgene is a critical confirmation of the requirement of the gene for the biological function (necessity), but is not related to sufficiency at all. Restoring expression of a gene to that of its natural state would obviously return the phenotype if the gene is really responsible for it (confirming this is the purpose of rescue experiments). Thus, it is unable to suggest anything about its sufficiency. Because it is just restoring a lost function caused by a lost gene and not inducing an ectopic biological function, we may refer to such a statement as “further-misapplied-N&S.” Taking this further-misapplied-N&S even further, some researchers designed an experiment stating that an expression of an effector transgene to suppress synaptic transmission in a certain group of neurons indicates “necessity” of the neurons. Then, they suppressed the expression of the very same transgene to restore synaptic transmission and supposedly justify “sufficiency” of the neurons (Liu et al., 2012). We shall not discuss these cases any further because such “further-misapplied-N&S” are just selling words; they are pure rhetoric without any positive biological insight or logical meaning.
Optogenetics is a method to activate or inactivate neurons through expression of ion channels or transporters, which can be activated by illuminating the neurons as explained in the Pitfall II section.
Experimentally speaking, this special state fulfilling misapplied-N&S is exactly what may make the system quite easy to analyze. We deliberately sought the feeding command neuron in the Drosophila brain (Flood et al., 2013a) for simple circuit analyses of Pavlovian conditioning with this in mind (Yoshihara, 2012; Yoshihara and Ito, 2012) to connect synaptic plasticity (Yoshihara et al., 2005) to memory. As Pavlov’s dogs associated the stimulus of sound and food in order to induce automatic salivation through the sound by itself, we are trying a similar conditioning paradigm to associate a sensory stimulus like sound to observable feeding behavior such as proboscis extension (Sakurai and Yoshihara, in preparation). If the stimulus were integrated into the Feeding neuron, we could perform a single cell analysis of the association in such a simple system. We hope we can connect synaptic plasticity on the Feeding neuron to memory as behavioral change. If there were any redundancy, which breaks misapplied-N&S, such analysis would be much more complicated and encounter difficulty in making clear-cut conclusions.
Since relatively simple animals such as C. elegans and Drosophila have limited redundancy in their genomes, genes in worms (Chalfie et al., 1981; Horvitz and Sulston, 1980) and flies (Nusslein-Volhard and Wieschaus, 1980) are often irreplaceable for their given function. Such limited redundancy is not present between neurons in the mammalian brain, perhaps not even in many cases in nervous systems of simple model animals, where many neurons function together for one purpose. The Drosophila Feeding neuron, which is indispensable and inducing for feeding behavior as a pair of neurons, might be fairly exceptional.
Fulfilling the necessary condition in misapplied-N&S by itself cannot guarantee the natural role. Suppose glial cells surround a command neuron, which trigger a pattern of behavior. Since glial cells are playing a supporting role to maintain excitability of neurons, ablation of the glial cells could inhibit excitability of the command neuron, resulting in the loss of the behavior and “indicating” its necessity. Like this putative example from glial cells, the necessary condition in misapplied-N&S may even be fulfilled by cells that have a supporting role as the prerequisite for the behavior. Thus, we cannot automatically conclude a natural triggering function with only the necessary condition in misapplied-N&S. To make a decisive statement, we have to monitor how the signals actually flow. We have to be careful on this issue especially in studies on a group of manipulated neurons, where necessary cells such as glial cells and sufficient neurons such as one of groups of neurons with commanding function are different within an optically-manipulated group of neurons (see Pitfall III section and Fig. 2D).
To substitute electrophysiology, which is technically intricate, we have visually recorded the activity through calcium imaging and optically induced the firing of the Feeding neuron in a fruit fly. We developed this experimental system to record brain activity in a fly performing feeding behavior (Yoshihara, 2012). Even though the time resolution of such imaging/optical stimulation is not as good as electrophysiological recording/stimulation so far, it still provided fairly convincing evidence for the commanding function of the Feeding neuron (Flood et al., 2013b). We still have to make it completely certain through electrophysiological recording/stimulation with an identical firing pattern.
For example, flies change behavior patterns when they are starved, reflecting that neuronal activity in a fly’s brain changes in the starved state. One group associated an odor to escape behavior to an electric shock before and after starvation (Hirano et al., 2013). They reported somewhat of a difference between the starved and satiated flies in their odor-induced escaping behavior, and they concluded that “fasting facilitated long-term memory.” However, it does not imply that the property of associative memory itself was changed in the brain due to starvation. It is well known that general locomotive activity (Linford et al., 2015) and response to olfactory signals change when flies are starved (Farhan et al., 2013; Root et al., 2011). It is quite likely that both the neuronal activity for the escaping locomotion and neuronal activity in response to odors changed before association when starved. Therefore, mechanism of memory at the association cannot be addressed by simply measuring the whole escaping locomotion.
This kind of starvation-induced increase in the whole escaping locomotion from the odor has given rise to an opportunity for another misapplied-N&S. Expressing a constitutive active form of a protein, CRTC, in mushroom bodies (MBs) increased the escaping locomotion from the odor. This apparent “memory alteration” in a similar manner to the effect of starvation was recognized as sufficiency. As necessity, they showed that RNAi knockdown of the protein ceased the starvation-induced increase of the apparent “memory.” Then, they concluded that “CRTC activity in MBs is necessary and sufficient to form the fasting-dependent long-term memory” (Hirano et al., 2013). These experiments are like a “house built on sand,” where their very foundation itself is rather vacant. Thus, it is difficult for them to ever be meaningful in expanding our understanding of memory mechanism. It is very likely that manipulating CRTC activity just resulted in altered escaping locomotion in general, not particularly memory mechanism. But the misapplied-N&S makes CRTC activity sound “equivalent to,” or very important for, the “fasting-dependent long-term memory”. Both the mushroom body study (Hirano et al., 2013) and the calcium study (Perisse et al., 2009) doubly violated proper logic both in the misapplied-N&S pitfall and in the memory pitfall, leading to a lack of scientific insight for memory mechanism.
“Indispensable and inducing condition → only one pivot” is not true whereas “only one pivot → indispensable and inducing condition” is true. Thus, fulfilling the indispensable and inducing condition does suggest the possibility of a pivotal role, but does not guarantee it, while failing the indispensable and inducing condition does not prove against a pivotal role either, as explained in the Pitfall I section. We should be especially careful in neuroscience, where so many neurons are forming complex networks, making experimental results hard to interpret. In developmental genetics, however, fulfilling the indispensable and inducing criteria tends to lead to the pivotal role of a gene in many cases such as eyeless. This is due to hierarchical gene cascade being fairly common during development. Thus, we rarely see this logic issue being a serious problem in developmental genetics as much as in neuroscience.
References
- Aponte Y, Atasoy D, and Sternson SM (2011). AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, and Lømo T (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Horvitz HR, and Sulston JE (1981). Mutations that lead to reiterations in the cell lineages of C. elegans. Cell 24, 59–69. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Tavsanli BC, Dittrich C, Walldorf U, and Mardon G (2003). Drosophila retinal homeobox (drx) is not required for establishment of the visual system, but is required for brain and clypeus development. Dev Biol 259, 272–287. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Lee RK, and Foreman MB (2001). The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol 63, 467–485. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, and Krasne FB (1999). Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci 22, 153–161. [DOI] [PubMed] [Google Scholar]
- Farhan A, Gulati J, Grobetae-Wilde E, Vogel H, Hansson BS, and Knaden M (2013). The CCHamide 1 receptor modulates sensory perception and olfactory behavior in starved Drosophila. Scientific reports 3, 2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood TF, Gorczyca M, White BH, Ito K, and Yoshihara M (2013a). A large-scale behavioral screen to identify neurons controlling motor programs in the Drosophila brain. G3 3, 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood TF, Iguchi S, Gorczyca M, White B, Ito K, and Yoshihara M (2013b). A single pair of interneurons commands the Drosophila feeding motor program. Nature 499, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, and Katz PS (1996). Single neuron control over a complex motor program. Proc Natl Acad Sci U S A 93, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Rangel MJ Jr., Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, and de Araujo IE (2017). Integrated Control of Predatory Hunting by the Central Nucleus of the Amygdala. Cell 168, 311–324 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Masuda T, Naganos S, Matsuno M, Ueno K, Miyashita T, Horiuchi J, and Saitoe M (2013). Fasting launches CRTC to facilitate long-term memory formation in Drosophila. Science 339, 443–446. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, and Sulston JE (1980). Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics 96, 435–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, and Wiersma CA (1964). Autogenic Rhythmicity in the Abdominal Ganglia of the Crayfish: The Control of Swimmeret Movements. Comp Biochem Physiol 12, 107–115. [DOI] [PubMed] [Google Scholar]
- Jusiak B, Karandikar UC, Kwak SJ, Wang F, Wang H, Chen R, and Mardon G (2014). Regulation of Drosophila eye development by the transcription factor Sine oculis. PLoS One 9, e89695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, and Miledi R (1967). The timing of calcium action during neuromuscular transmission. J Physiol 189, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Hachiya T, Koganezawa M, Tazawa T, and Yamamoto D (2008). Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759–769. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, and Yamamoto D (2011). Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508. [DOI] [PubMed] [Google Scholar]
- Korn H, and Faber DS (2005). The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47, 13–28. [DOI] [PubMed] [Google Scholar]
- Kumar JP, and Moses K (2001). Eye specification in Drosophila: perspectives and implications. Semin Cell Dev Biol 12, 469–474. [DOI] [PubMed] [Google Scholar]
- Kupfermann I & Weiss KR (1978). The command neuron concept. Behav. Brain. Sci. 1, 3–39. [Google Scholar]
- Kupfermann I, and Weiss KR (2001). Motor program selection in simple model systems. Curr Opin Neurobiol 11, 673–677. [DOI] [PubMed] [Google Scholar]
- Lima SQ, and Miesenbock G (2005). Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, and Anderson DJ (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Ro J, Chung BY, and Pletcher SD (2015). Gustatory and metabolic perception of nutrient stress in Drosophila. Proc Natl Acad Sci U S A 112, 2587–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, and Tanimoto H (2012). A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488, 512–516. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, and Taylor AL (2005). Invertebrate central pattern generation moves along. Curr Biol 15, R685–699. [DOI] [PubMed] [Google Scholar]
- Marder E, and Calabrese RL (1996). Principles of rhythmic motor pattern generation. Physiol Rev 76, 687–717. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Simmers J, and Moulins M (1991). Construction of a pattern-generating circuit with neurons of different networks. Nature 351, 60–63. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, and Wieschaus E (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801. [DOI] [PubMed] [Google Scholar]
- Pagnussat N, Almeida AS, Marques DM, Nunes F, Chenet GC, Botton PH, Mioranzza S, Loss CM, Cunha RA, and Porciuncula LO (2015). Adenosine A receptors are necessary and sufficient to trigger memory impairment in adult mice. British journal of pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisse E, Raymond-Delpech V, Neant I, Matsumoto Y, Leclerc C, Moreau M, and Sandoz JC (2009). Early calcium increase triggers the formation of olfactory long-term memory in honeybees. BMC Biol 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, and Wang JW (2011). Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, and Deisseroth K (2009). Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, and Dickson BJ (2011). Neuronal control of Drosophila courtship song. Neuron 69, 509–522. [DOI] [PubMed] [Google Scholar]
- White HI, Discovering philosophy, 2nd edition, (Prentice Hall, 2008) [Google Scholar]
- Wiersma CA, and Ikeda K (1964). Interneurons Commanding Swimmeret Movements in the Crayfish, Procambarus Clarki (Girard). Comp Biochem Physiol 12, 509–525. [DOI] [PubMed] [Google Scholar]
- Xu N, Zhou WJ, Wang Y, Huang SH, Li X, and Chen ZY (2014). Hippocampal Wnt3a is Necessary and Sufficient for Contextual Fear Memory Acquisition and Consolidation. Cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D, and Koganezawa M (2013). Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci 14, 681–692. [DOI] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, and Sternson SM (2011). Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146, 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M (2012). Simultaneous recording of calcium signals from identified neurons and feeding behavior of Drosophila melanogaster. J Vis Exp 62, e3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, and Littleton JT (2005). Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science 310, 858–863. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, and Ito K (2012). Acute genetic manipulation of neuronal activity for the functional dissection of neural circuits-a dream come true for the pioneers of behavioral genetics. J Neurogenet 26, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]