Abstract
Articular cartilage is critical for painless and low-friction range of motion; however, disruption of articular cartilage, particularly in the knee joint, is common. Treatment options are based on the size and depth of the chondral defect, as well as involvement of subchondral bone. The gold standard for evaluation of articular cartilage is with arthroscopy, but it is limited by its ability to objectively judge the depth and severity of chondral damage. Optical reflection spectroscopy has been introduced to objectively assess the thickness of cartilage. We present a technique to systematically evaluate the articular cartilage of the knee using BioOptico optical reflection spectroscopy (Arthrex) to better evaluate those with visible chondral and subchondral defects.
The role of articular cartilage is to permit joint motion that is near frictionless, with damage to this surface leading to the development of arthritis. Articular lesions are common, with an estimated 1 million patients affected annually in the United States alone.1 Furthermore, a retrospective review of knee arthroscopy procedures found a 63% prevalence of chondral lesions with an average of 2.7 lesions per knee.2 Patients with these lesions can present with complaints of knee pain, swelling, and disability. These lesions are difficult to evaluate with plain radiography and magnetic resonance imaging (MRI) is commonly used for diagnosis, but the ability of MRI to diagnose these lesions has been called into question.1, 3 Thus, the gold standard for diagnosis remains visual inspection and probing of the articular surface during arthroscopy.4
These chondral lesions have a limited potential for healing because of the lack of blood supply, lymphatic system, and neurology connection to the rest of the body in adults.5 When cartilage injury occurs, the involvement of the subchondral bone dictates the type of healing that takes place. Without penetration of the subchondral bone, there is a brief induction of cell replication and matrix production by adjacent chondrocytes, whereas penetration of the subchondral bone leads to fibrocartilage healing.5 Because of the limited ability to heal, treatment of these injuries remains a challenge. Current treatment options remain limited to chondroplasty versus chondral resurfacing techniques such as microfracture, osteochondral autograft transfer, osteochondral allograft transplant, or matrix autologous chondrocyte implantation.6, 7
The choice of treatment is often dictated by subchondral bone involvement and characteristics of the chondral defect such as the size, depth, and location of the lesion. The size and depth can be quantified by measuring cartilage thickness, and several methods have been proposed for this. The most frequently used techniques to achieve this include needle probe methods (in which a sharp needle is place into cartilage with force and displacement is measured), high-resolution ultrasound, MRI, and optical coherence tomography based on interferometry.1 More recently, specially designed arthroscopes have been used to accomplish this goal.1 As new treatments are introduced, the development of improved methods for quantitatively grading lesions and cartilage quality are important.
One such method is the introduction of optical reflection spectroscopy to determine the thickness of cartilage.1, 8, 9 The optical absorption of cartilage is different than that of subchondral bone, and optical reflection spectroscopy can use this to estimate cartilage thickness through the reflectance spectrum taken from the joint surface.1, 8, 9 By relating the reflected intensities at specific wavelengths to the reflected intensity of a reference wavelength, cartilage thickness can be estimated.1, 8, 9 Consequently, this technology has been shown to be able to evaluate cartilage thickness and visualize the thickness distribution over a knee joint using wavelengths.9 By incorporating this technology into an arthroscope, measurement of cartilage depth can be made in vivo during an arthroscopy. A previous study determined that when using an arthroscope implemented with this technology, the thickness of cartilage can be estimated to within 0.28 to 0.30 mm.1
By using an arthroscope implemented with this technology, cartilage depth will be quantified in vivo, providing the surgeon with better information to guide treatment selection. This technique article describes our technique for assessing cartilage depth during arthroscopy of the knee using spectral and texture enhancement provided by BioOptico (Arthrex, Naples, FL).
Technique
Using BioOptico
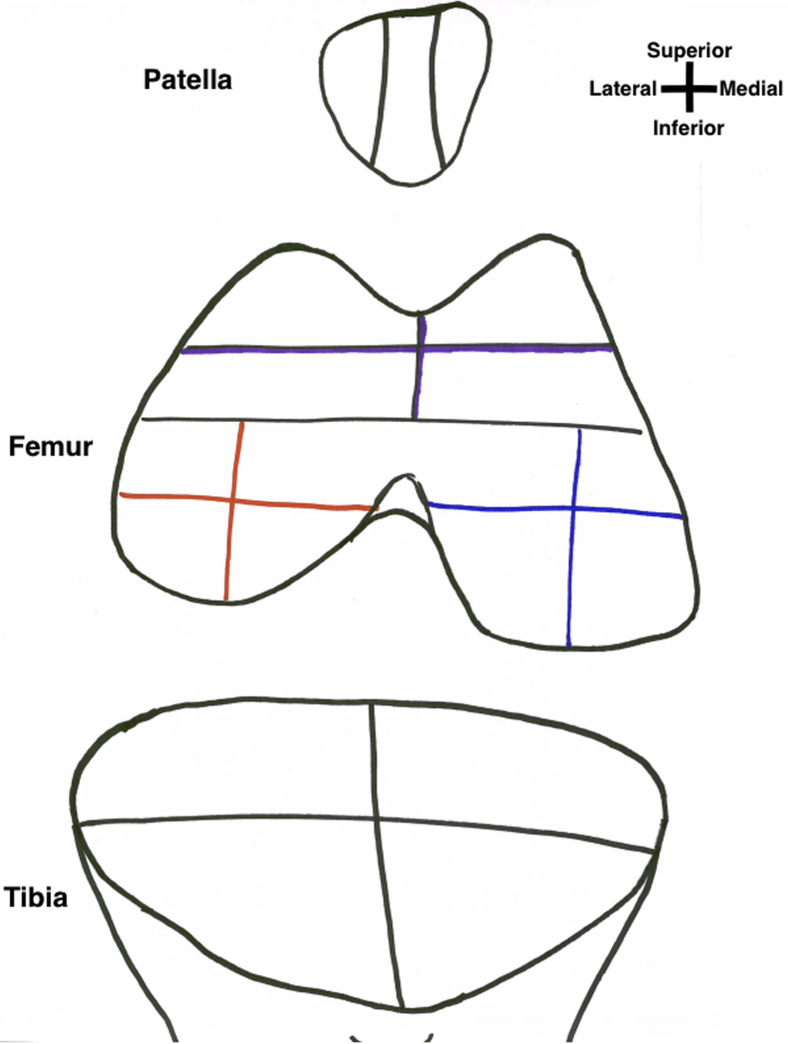
To systematically evaluate the cartilage of the knee using BioOptico technology, the articular surface of the knee is divided into 22 sections to allow for systematic evaluation (Fig 1, Fig 2, Fig 3, Fig 4, Video 1). We present a technique to visualize each section. Once the section is adequately visualized, the BioOptico imaging system will assess the depth of the cartilage based on the color-coded schematic shown on the respective screen. No previous grading scale exists to compare reflectance spectroscopy readings to chondral thickness; therefore, a classification system mirroring the Outerbridge system is proposed with corresponding color readouts (types 0-3, Video 1). Type 0 has no color on reflectance spectroscopy and represents near-normal cartilage. Type 1 is colored pink and represents minor cartilage loss. Type 2 is colored red and represents moderate cartilage loss. Type 3 is colored dark red and represents severe cartilage loss (Table 1). Once the operating surgeon is satisfied with the visualization, he or she may manually capture the arthroscopic image along with the BioOptico evaluation for future reference before moving onto the next section. In addition to capturing the arthroscopic BioOptico image, we recommend manually recording the cartilage grade for each section on a 2-dimensional diagram (Fig 5). This can be recorded during the actual procedure by an assistant and used for further documentation.
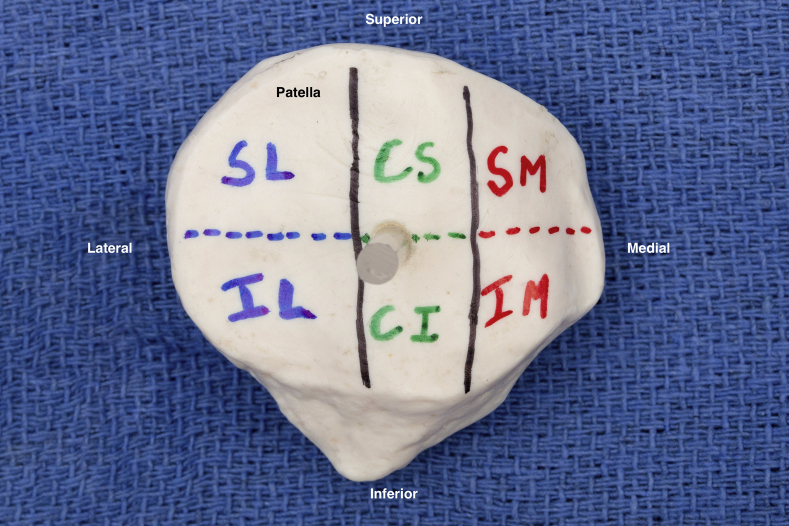
Fig 1.
Illustration of the 6 patella sections. (CI, inferior central facet; CS, superior central facet; IL, inferior lateral facet; IM, inferior medial facet; SL, superior lateral facet; SM, superior medial facet.)
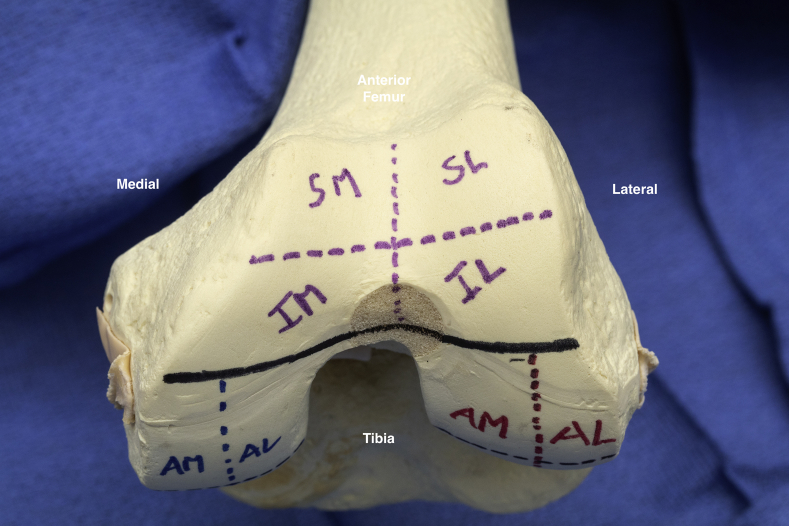
Fig 2.
Illustration of the 4 trochlear sections. (AL, anterior lateral; AM, anterior medial; IL, inferior lateral facet; IM, inferior medial facet; SL, superior lateral facet; SM, superior medial facet.)
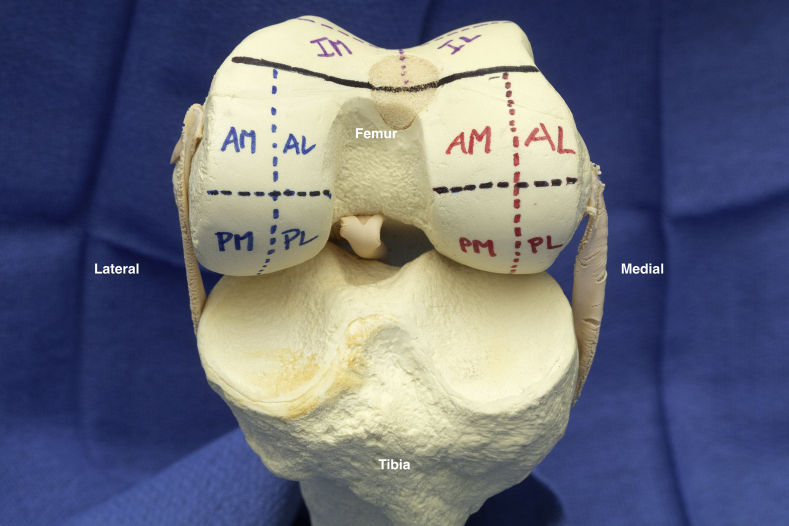
Fig 3.
Illustration of the 4 medial femoral condyle sections (red) and the 4 lateral femoral condyle sections (blue). (AL, anterior lateral; AM, anterior medial; IL, inferior lateral facet; IM, inferior medial facet; SL, superior lateral facet; SM, superior medial facet.)
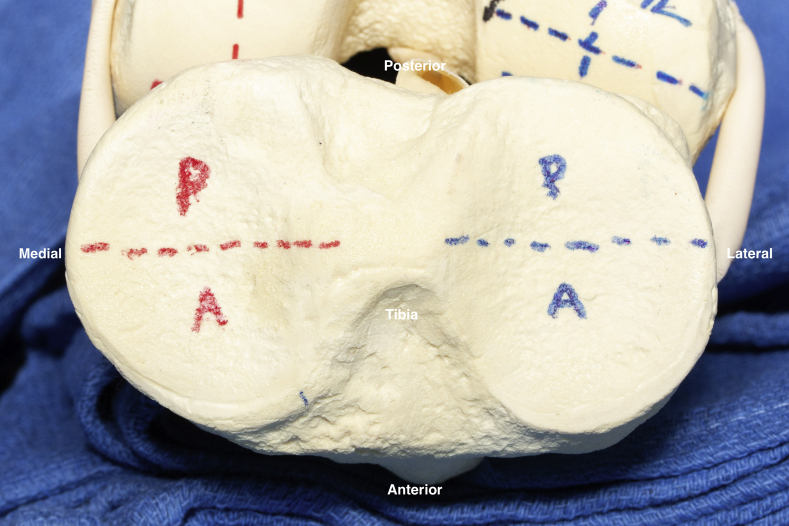
Fig 4.
Illustration of the 2 medial tibial plateau sections (red) and the 2 lateral tibial plateau sections (blue). A, anterior; P, posterior.
Table 1.
Cartilage Grading Scale Based on Optical Reflection Spectroscopy
| Type | BioOptico Color | Cartilage Damage |
|---|---|---|
| 0 | No color | Normal cartilage |
| 1 | Pink | Mild degeneration/thinning |
| 2 | Red | Moderate degeneration/thinning |
| 3 | Dark red | Severe degeneration/thinning |
Fig 5.
Proposed diagnostic arthroscopy cartilage grading sheet that can be used during the procedure to record the grade of each cartilage section.
Position and Preparation
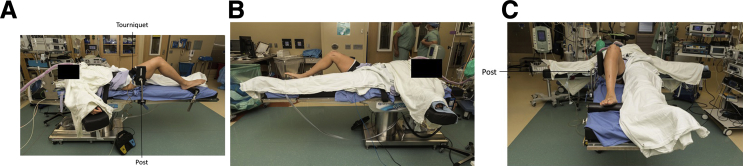
After induction of anesthesia, the patient is positioned in the supine position on the operating table. A post of the surgeon's preference should be placed on the operative side to position the leg and assist in producing a valgus force for diagnostic arthroscopy (Fig 6, Video 1). A nonsterile pneumatic tourniquet is placed on the patient's operative thigh and the lower extremity is prepped and draped in a normal sterile fashion. A surgical marker is used to outline standard anterolateral and anteromedial portals (Video 1). The portals are then established using an 11-blade scalpel and a hemostat is used to widen them.
Fig 6.
Left knee, patient in supine position. (A) Lateral, (B) medial, and (C) end-table view of operating room setup in preparation for BioOptico evaluation. Note the patient is supine with knee at 90°. Post and sterile draping can be set up per the surgeon's preference.
Diagnostic Technique
After establishing the anterolateral and anteromedial arthroscopic portals, a 30° arthroscope is placed in the anterolateral portal. Evaluation of the articular cartilage using BioOptico technology can now be carried out by following the sequence of a standard diagnostic scope. To better aid in systematically evaluating and documenting the cartilage, the articular surface of the knee is divided into 22 sections.
Beginning with the knee in full extension, the patellofemoral joint is visualized first. With the scope camera pointed superiorly, advance the scope through the anterolateral portal until the superior and inferior medial facet, as well as the superior and inferior central facet, of the patella is visualized (Fig 7, Video 1). Next, a probe is inserted through the anteromedial portal and used to subjectively grade the cartilage on this surface using a grading system such as Outerbridge. Once this surface has been subjectively graded, the mode on the arthroscope can be switched to begin using BioOptico.
Fig 7.
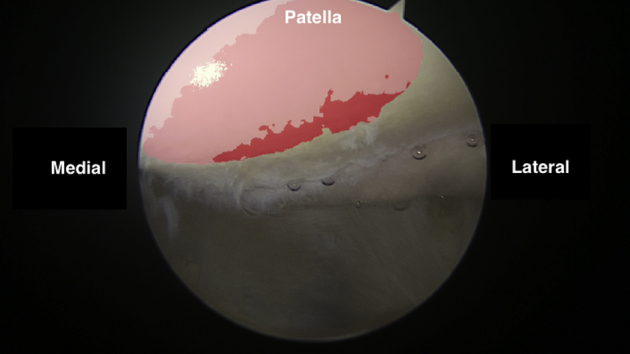
Arthroscopic view of the medial facet of the patella as viewed from the anterolateral portal.
Once activated, the articular surface of interest is visualized and the BioOptico imaging system will assess the depth of remaining cartilage and display these depths as corresponding articular cartilage color on the video screen. When the operating surgeon is satisfied with the visualization seen with BioOptico in the section and cartilage depth is adequately assessed, he or she may manually capture the arthroscopic image with BioOptico evaluation for future reference. The BioOptico mode is then deactivated and the arthroscope is rotated 180° downward to evaluate the medial trochlea, divided into a superior and inferior section (Video 1). Again, a probe through the anteromedial portal is used to first evaluate these sections of articular cartilage subjectively in a similar manner to that of the patellar surface. Once subjectively accessed, BioOptico mode is activated and the cartilage surface depth is objectively evaluated and the findings can be recorded as demonstrated above. After evaluation of the central and medial patellofemoral sections is complete from the anterolateral portal, the arthroscope is removed and placed into the anteromedial portal to best visualize the remaining superior and inferior lateral facets of the patella and the corresponding superior lateral and inferior lateral trochlear zones (Fig 8).
Fig 8.
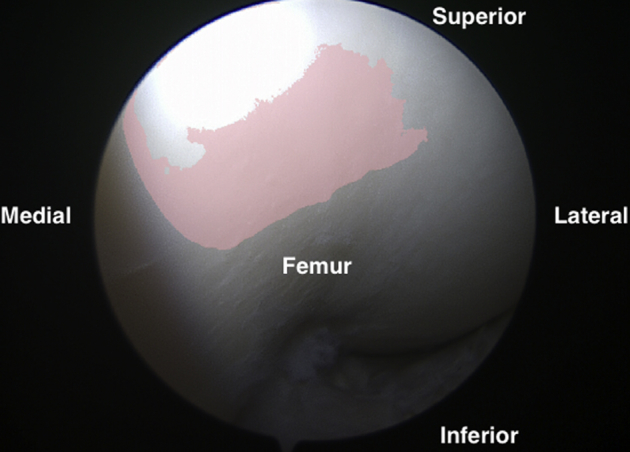
Arthroscopic view of the entire trochlea as viewed from the anteromedial portal.
The arthroscope is advanced through the anteromedial portal until the superior and inferior lateral patellar facets come into view. Using a probe through the anterolateral portal, these surfaces can be subjectively graded. Next, the BioOptico mode is activated and the lateral patellar facets are objectively graded. The BioOptico mode is then deactivated and the arthroscope is rotated 180° inferiorly to bring the superior lateral and inferior lateral trochlear zones into view. With the BioOptico mode deactivated, the probe, through the anteromedial portal, is used to first subjectively evaluate the surface and then BioOptico mode is reactivated to objectively grade the surface. To use the BioOptico mode with the best accuracy, ensure that the arthroscope is as perpendicular as possible to the cartilage surface being assessed because increasing obliqueness can lead to decreased accuracy of articular cartilage thickness.
With arthroscopic examination of the patellar surface complete, the arthroscope is removed and placed back into the anterolateral portal; the knee is then flexed to approximately 30° and a valgus force is applied to allow visualization of the medial compartment (Video 1). With this degree of flexion, the anterior lateral and anterior medial sections of the medial femoral condyle can be evaluated (Fig 9). A probe is placed through the anteromedial portal and is used to subjectively grade the anterior lateral and anterior medial sections of the medial femoral condyle. Once subjectively graded, the BioOptico mode is then activated; these surfaces are objectively graded and results are recorded. From this position, the anterior and posterior sections of the medial tibial plateau can be visualized by rotating the arthroscope 180° downward (Fig 10). After rotating the arthroscope, a probe is used to subjectively evaluate the cartilage with the BioOptico mode deactivated and then the mode is reactivated to objectively evaluate these surfaces. Next, the arthroscope is retracted slightly and rotated back upward 180°. The valgus force is removed and the knee is then hyperflexed to approximately 110°. This hyperflexion brings the posterolateral and posteromedial sections of the medial femoral condyle into visualization. From this position, the arthroscopic probe is used to subjectively evaluate the posterior sections with the BioOptico mode switched off. Once subjectively evaluated, the mode is reactivated and the posterior sections can be objectively evaluated.
Fig 9.
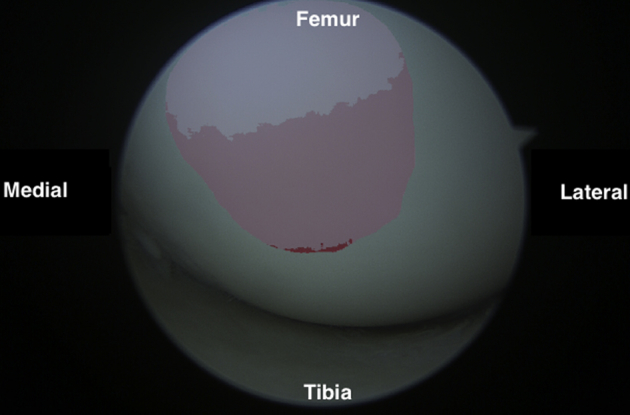
Arthroscopic view of the superior medial femoral condyle as viewed from the anterolateral portal.
Fig 10.
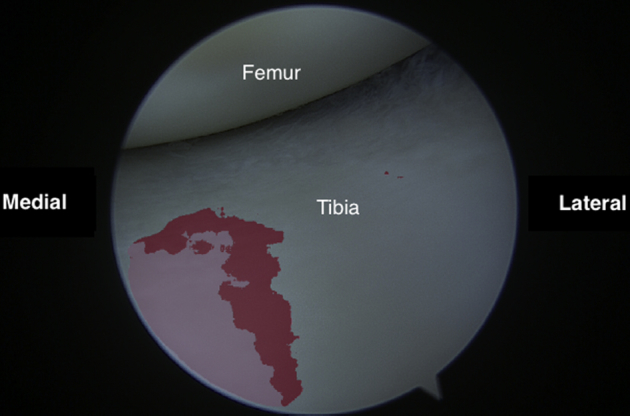
Arthroscopic view of the anteromedial tibial plateau as viewed from the anterolateral portal.
The arthroscope is then retracted from the anterolateral portal and placed back into the anteromedial portal with the knee in slight flexion because attention is now turned to the lateral surfaces of the knee. The knee is brought into a “figure 4” position, with the knee flexed to approximately 90° and the hip in external rotation. This allows for better visualization of the lateral compartment. In this position, the arthroscope is advanced until the lateral femoral condyle comes into view, in particular the anterior medial and lateral sections (Fig 11). Next, the arthroscopic probe is inserted through the anterolateral portal and the surfaces are probed to subjectively evaluate them. Once subjectively evaluated, the BioOptico mode on the arthroscope is activated and the anterior medial and lateral sections are objectively evaluated (Video 1). Next, the arthroscope is rotated 180° downward to facilitate evaluation of the anterior and posterior sections of the lateral tibial plateau (Fig 12). With the BioOptico mode first deactivated, the arthroscopic probe is used to evaluate these surfaces, followed by objective evaluation with the BioOptico mode activated. To evaluate the posterolateral and posteromedial sections of the lateral femoral condyle, the arthroscope is retracted slightly and rotated 180° upward as the knee is brought into hyperflexion >110°. Once hyperflexed, the BioOptico mode is deactivated and the probe is used to provide subjective evaluation of the cartilage. The BioOptico mode is then reactivated, providing for the posterolateral and posteromedial sections of the condyle to be objectively evaluated.
Fig 11.
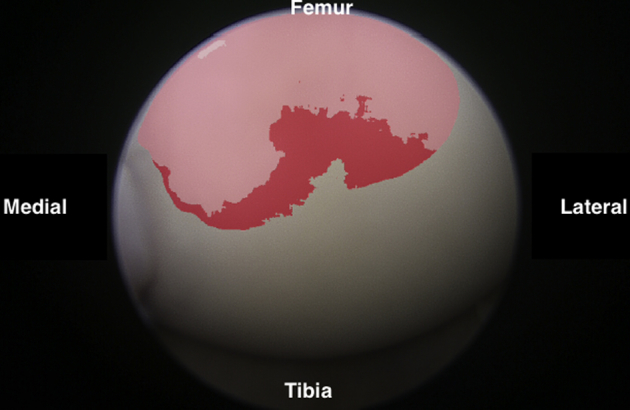
Arthroscopic view of the anterolateral femoral condyle as viewed from the anteromedial portal.
Fig 12.
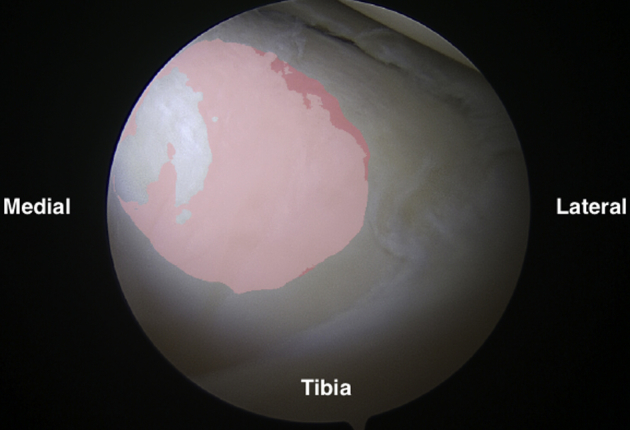
Arthroscopic view of the anterolateral tibial plateau as viewed from the anteromedial portal.
Through our experience, adaptions have been made to better allow for evaluation of the cartilage surface. First, if the surgeon does not feel he or she achieved adequate visualization of the patellofemoral compartment through the standard portals, an accessory superolateral or superomedial portal may be created (Video 1). These portals allow for visualization of the contralateral side of the patellofemoral joint and can be evaluated as previously described. Also, to examine the cartilage surface of interest at an optimal perpendicular angle, it may be useful to use a 70° arthroscopic camera during the procedure. In addition, adjusting the overall brightness of the light source can allow for a clearer examination. The combination of additional portals, alternative arthroscopic cameras, and brightness adjustment allows for optimal visualization of all surfaces in the majority of cases (Table 2).
Table 2.
Technical Considerations for Optimizing the Evaluation of Cartilage Surface Using Optical Reflection Spectroscopy
| Optical Reflection Spectroscopy Technical Considerations |
|---|
| Use contralateral portal from side of interest to better visualize articular surface (e.g., anterolateral portal to view medial-sided structures). |
| Examine articular surface of interest at perpendicular angle to increase accuracy of reflectance spectroscopy. |
| Adjust light source brightness to obtain a clearer picture. |
| Use a 70° scope for visualization when otherwise difficult to obtain perpendicular visualization of articular surface. |
| For patellofemoral visualization, it may be necessary to view through an accessory superior medial or superior lateral portal. |
| Use multiple knee flexion angles to examine chondral surface of interest to aid in reflectance spectroscopy accuracy. |
After evaluation is complete, other planned procedures can be carried out, such as meniscus, chondral, or ligamentous surgery. Once completed, the knee is copiously flushed with irrigation fluid and arthroscopic equipment is removed. Arthroscopic portals may be closed with No. 2-0 nylon interrupted sutures and a sterile dressing placed over the incisions per the operating surgeon's preference.
Discussion
Traumatic and degenerative injuries to the cartilage are 1 of the leading causes of disability worldwide, with chondral lesions commonly found in patients older than age 40.1 These injuries are commonly to the result of acute trauma or the repetitive overload of the knee joint found in chronic cases. Impaction of the joint surface leads to softening or fissuring of the cartilage, tearing, or delamination. Because of various biologic factors, chondral injuries have a limited ability for healing, leading to the potential to worsen over time. With the limited ability to heal, treatment is limited to conservative measures, debridement and chondroplasty, chondral resurfacing techniques, microfracture, osteochondral autograft transfer, osteochondral allograft transplant, or matrix autologous chondrocyte implantation.6, 7
The diagnosis of chondral injuries can be a difficult task. Using patient signs and symptoms, such as pain, effusion, crepitus, or decreased motion, has been shown to have a low specificity and predictive value.4 Also, the joint space narrowing, subchondral sclerosis, and loose bodies seen on plain radiography with chondral injuries do not appear until late in the disease process.10 This leaves MRI or arthroscopic evaluation as the main methods by which cartilage injuries are diagnosed, with MRI being the only noninvasive technique. The ability of MRI to diagnose these lesions, however, depends on both the technique of the MRI and radiologist experience, with its validity being questioned.3 When MRI is used to evaluate cartilage lesions, 1 study found only a moderate interobserver validity of 0.80.11 In addition, artifacts (such as the “magic angle” effect) can complicate the evaluation of these lesions and oftentimes small lesions are overlooked.4, 12 Only newer techniques, such as Delayed gadolinium-enhanced MRI of cartilage, make it possible to evaluate initial lesions, but they are not routinely used secondary to prohibitive cost and availability.4, 13
Arthroscopic evaluation is the most valid method to evaluate the cartilage and is considered the gold standard because it allows for the direct viewing of the lesion.4 Arthroscopy is invasive, but the direct viewing allows for the subjective grading of the cartilage by the surgeon, which dictates treatment. Under direct visualization, a hook is used to palpate the cartilage and assign a grade to the lesion. This palpation has been found to be highly subjective, depending on the manual pressure applied by the surgeon and on the geometry of the distal end of the hook.14 In addition, there is still no consensus regarding the true validity of arthroscopy to diagnose chondral lesions.15 A previous study determined that using arthroscopy to diagnose cartilage lesions only had an interobserver agreement of .67 between arthroscopists.16 Studies have found that there is more agreement when intact cartilage is present or with lesions at the ends of the spectrum, such as Outerbridge I or IV. Brismar et al.17 determined a mean interobserver agreement of >80% was found with grade I or IV lesions, but agreement dropped to 65% for grade II or III lesions. In a survey of highly trained arthroscopists, the majority of surgeons thought differentiation of high- or low-grade lesions was valid, but almost 50% believed there was a “need for improvement” in differentiation between grade I and II lesions and grade II and III lesions.4 In addition, 13.3% and 61.9% responded that the incorporation of objective measurements for these intermediate lesions would be “very useful” and “somewhat useful,” respectively.4 By replacing the subjective grade assigned by surgeons with technology that can objectively evaluate cartilage, the validity of arthroscopy to diagnose chondral lesions will be improved, especially those of intermediate grades.
The advent of optical reflection spectroscopy, as found in BioOptico, provides for an objective way to measure cartilage thickness. Cartilage is connective tissue comprising proteoglycans, collagen, and water. Because it lacks blood perfusion, the absorption and scattering properties of light are mainly determined by these components.9 The subchondral bone, in contrast, has blood perfusion and thus its absorption and scattering properties are mainly determined by hemoglobin and other pigments in the blood.9 By measuring the difference between the wavelengths that are absorbed and scattered between the 2 tissue types, the cartilage thickness can ultimately be measured.9 By applying this technology to the video stream provided during arthroscopy, an objective measurement of cartilage thickness in vivo can be accomplished. This method has been shown to objectively measure cartilage thickness with an error of 0.28 to 0.30 mm and the error was lowest when cartilage thickness was <1.5 mm.1 This roughly correlates to grade II and III cartilage lesions, providing a reliable quantitative assessment of cartilage grades found to have the greatest interobserver variability.
Cartilage lesions are typically graded based on the subjective descriptions of surgeons made during arthroscopy and according to either the Outerbridge or the more recent International Cartilage Repair Society grading systems.9 In both of these systems, grading of the lesion relies on the intact surrounding cartilage thickness.9 With the quantitative assessment provided by the optical reflection spectroscopy technology, a new grading scheme could be developed that was only dependent on cartilage that remains in the lesion, with the potential to better correlate with symptoms or the ability to heal.9 An additional possible avenue for this technology is to incorporate it into in-office arthroscopy. This would improve the ability to diagnose early articular degeneration found in osteoarthritis and allow for some treatment modalities that might not be possible with later disease.9
The limitations of the outlined technique are many. Although reflectance spectroscopy better allows for objective assessment of cartilage lesions, minimal data exist correlating the proposed grading system and progression/symptomatology of said lesions. Additionally, the ability to accurately assess the 22 sections previously outlined requires a learning curve as well as increased operative time. Outside of operative time, minimal additional risk is placed upon the patient with this technique.
The use of BioOptico during arthroscopy allows for the objective measurement of cartilage thickness, allowing for better assessment of lesions. Its use can help guide surgeons in treatment choice, especially in the indeterminate grade lesions that have proven difficult in the past.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: A.C. reports personal fees as a consultant for Arthrex and Trice Medical, outside the submitted work. D.E.H. reports personal fees as a consultant for Arthrex, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Video demonstrating a technique for arthroscopic evaluation of knee cartilage using optical reflection spectroscopy.
References
- 1.Johansson A., Kuiper J.-H., Sundqvist T. Spectroscopic measurement of cartilage thickness in arthroscopy: Ex vivo validation in human knee condyles. Arthroscopy. 2012;28:1513–1523. doi: 10.1016/j.arthro.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Curl W.W., Krome J., Gordon E.S., Rushing J., Smith B.P., Poehling G.G. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 3.Figueroa D., Calvo R., Vaisman A., Carrasco M.A., Moraga C., Delgado I. Knee chondral lesions: Incidence and correlation between arthroscopic and magnetic resonance findings. Arthroscopy. 2007;23:312–315. doi: 10.1016/j.arthro.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Spahn G., Klinger H.M., Hofmann G.O. How valid is the arthroscopic diagnosis of cartilage lesions? Results of an opinion survey among highly experienced arthroscopic surgeons. Arch Orthop Trauma Surg. 2009;129:1117–1121. doi: 10.1007/s00402-009-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OʼDriscoll S.W. The healing and regeneration of articular cartilage. J Bone Joint Sur Am. 1998;80:1795–1812. [PubMed] [Google Scholar]
- 6.Knutsen G., Engebretsen L., Ludvigsen T.C. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86:455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Dervin G.F., Stiell I.G., Rody K., Grabowski J. Effect of arthroscopic débridement for osteoarthritis of the knee on health-related quality of life. J Bone Joint Surg Am. 2003;85:10–19. doi: 10.2106/00004623-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Oberg P.A., Sundqvist T., Johansson A. Assessment of cartilage thickness utilising reflectance spectroscopy. Med Biol Eng Comput. 2004;42:3–8. doi: 10.1007/BF02351004. [DOI] [PubMed] [Google Scholar]
- 9.Johansson A., Sundqvist T., Kuiper J.-H., Öberg P.Å. A spectroscopic approach to imaging and quantification of cartilage lesions in human knee joints. Phys Med Biol. 2011;56:1865–1878. doi: 10.1088/0031-9155/56/6/021. [DOI] [PubMed] [Google Scholar]
- 10.Spahn G., Wittig R., Kahl E., Klinger H.M., Mückley T., Hofmann G.O. [Evaluation of cartilage defects in the knee: validity of clinical, magnetic-resonance-imaging and radiological findings compared with arthroscopy] Unfallchirurg. 2007;110:414–424. doi: 10.1007/s00113-006-1225-z. [in German] [DOI] [PubMed] [Google Scholar]
- 11.Drapé J.L., Pessis E., Auleley G.R., Chevrot A., Dougados M., Ayral X. Quantitative MR imaging evaluation of chondropathy in osteoarthritic knees. Radiology. 1998;208:49–55. doi: 10.1148/radiology.208.1.9646792. [DOI] [PubMed] [Google Scholar]
- 12.Glaser C. [Imaging of cartilage] Radiologe. 2006;46:16–25. doi: 10.1007/s00117-005-1287-x. [in German] [DOI] [PubMed] [Google Scholar]
- 13.Spahn G., Plettenberg H., Kahl E., Klinger H.M., Mückley T., Hofmann G.O. Near-infrared (NIR) spectroscopy. A new method for arthroscopic evaluation of low grade degenerated cartilage lesions. Results of a pilot study. BMC Musculoskelet Disord. 2007;8:47. doi: 10.1186/1471-2474-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L.P., Herzog W. Arthroscopic evaluation of cartilage degeneration using indentation testing--influence of indenter geometry. Clin Biomech (Bristol, Avon) 2006;21:420–426. doi: 10.1016/j.clinbiomech.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Friemert B., Oberländer Y., Schwarz W. Diagnosis of chondral lesions of the knee joint: Can MRI replace arthroscopy? A prospective study. Knee Surg Sports Traumatol Arthrosc. 2004;12:58–64. doi: 10.1007/s00167-003-0393-4. [DOI] [PubMed] [Google Scholar]
- 16.Jerosch J., Castro W.H., de Waal Malefijt M.C., Busch M., van Kampen A. [Interobserver variation in diagnostic arthroscopy of the knee joint. “How really objective are arthroscopic findings?”] Unfallchirurg. 1997;100:782–786. [in German] [PubMed] [Google Scholar]
- 17.Brismar B.H., Wredmark T., Movin T., Leandersson J., Svensson O. Observer reliability in the arthroscopic classification of osteoarthritis of the knee. J Bone Joint Surg Br. 2002;84:42–47. doi: 10.1302/0301-620x.84b1.11660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video demonstrating a technique for arthroscopic evaluation of knee cartilage using optical reflection spectroscopy.