Abstract
Study Objectives:
Disturbed sleep is a hallmark feature of posttraumatic stress disorder (PTSD). However, few studies have examined sleep objectively in individuals with PTSD compared to trauma-exposed controls. This study used wrist actigraphy to measure and compare sleep patterns in trauma-exposed Australian Vietnam veterans (VV) with and without PTSD.
Methods:
Trauma-exposed Australian VV with and without PTSD were recruited from the PTSD Initiative. VV wore wrist accelerometers over 14 days and completed daily sleep diaries. Sleep parameters were compared between groups including sleep latency (SL), time in bed (TIB), total sleep time (TST), wake after sleep onset (WASO), and movement index (MI). Night-to-night and overall within-individual variability were assessed by root mean squared successive differences and comparison of individual standard deviations. Correlations between sleep diary (self-reported) and wrist actigraphy (objective) variables were also assessed.
Results:
A total of 40 male VV (20 with PTSD) participated in the study. We found no difference in sleep patterns determined by wrist actigraphy between groups with the exception of reduced SL in VV with PTSD (3.9 ± 0.9 versus 4.9 ± 1.4 minutes, P < .05). Overall within-individual variability was significantly greater in VV with PTSD for TIB, TST, WASO, and MI. Self-reported and objective TST and WASO were more strongly correlated in VV without PTSD than those with PTSD.
Conclusions:
Although there were no significant differences in sleep parameters, VV with PTSD had increased within-individual overall sleep variability and reduced correlation between self-reported and objective sleep parameters compared to trauma-exposed controls. Further evaluation of extended sleep patterns by actigraphy in VV with PTSD is warranted.
Citation:
Theal R, McLeay S, Gleeson S, Lowrie F, O'Sullivan R; PTSD Initiative. Comparison of sleep patterns in Vietnam veterans with and without posttraumatic stress disorder using wrist actigraphy. J Clin Sleep Med. 2019;15(5):725–732.
Keywords: objective sleep, posttraumatic stress disorder, sleep, sleep diaries, sleep patterns, sleep variability, veterans, wrist actigraphy
BRIEF SUMMARY
Current Knowledge/Study Rationale: Limited studies have used actigraphy to examine objective sleep patterns in veterans with posttraumatic stress disorder (PTSD). This study aimed to compare sleep patterns in Australian Vietnam veterans with and without PTSD using wrist actigraphy and sleep diaries to better understand the relationship between actual sleep and PTSD.
Study Impact: Compared to trauma-exposed controls, veterans with PTSD had reduced sleep latency, significantly increased overall within-individual variability in several sleep parameters, and increased sleep perception discrepancies. Further evaluation of extended sleep patterns by actigraphy in Vietnam veterans with PTSD is warranted.
INTRODUCTION
For many veterans, posttraumatic stress disorder (PTSD) is a major health issue associated with significant morbidity and mortality. Because of the nature of combat, military veterans are at particular risk of PTSD. In Vietnam veterans (VV), the lifetime prevalence of PTSD is estimated to be 20% to 30% of individuals.1,2 In addition to psychological distress, sleep disturbances and disorders including nightmares and insomnia are characteristic of PTSD. Untreated sleep issues may exacerbate PTSD and underlying psychiatric conditions in addition to adversely affecting health outcomes.3,4
The relationship between sleep and PTSD is evident, with almost 90% of individuals reporting some type of sleep disturbance.5 Pharmacological and psychological interventions including cognitive behavioral therapy for insomnia have shown promise in improving both sleep disturbances and reducing PTSD symptomology.6 In VV, PTSD severity has been positively associated with increased self-reported sleep difficulties,7,8 reduced sleep quality, and increased nightmares.9
Recently, the Gallipoli Medical Research Institute (GMRI) conducted the PTSD Initiative, a cross-sectional cohort study in 214 trauma-exposed Australian VV with and without PTSD.10 Despite reporting significantly increased sleep disturbances,10,11 objective sleep parameters measured by polysomnography (PSG) in a subset of participants were no different between VV with PTSD compared to trauma-exposed controls.12 This was consistent with a study in elderly veterans with and without PTSD who were exposed to trauma, where PSG measures were clinically similar between groups despite those with PTSD reporting reduced sleep quality.13 Other PSG studies comparing individuals with PTSD to trauma-exposed controls have been variable, with some reporting limited or no differences between groups in both veteran and civilian populations.6,14–16
Although PSG is used for clinical diagnosis of certain sleep disorders such as obstructive sleep apnea (OSA) and periodic limb movement disorder, it is limited to short-term measurement of sleep and is not generally indicated for routine investigation of insomnia or disturbances in circadian rhythm or sleep phase. As such, PSG may not be appropriate for the evaluation of certain PTSD-related sleep disturbances. Actigraphy, in combination with a sleep diary, has been shown to be useful in documentation of extended sleep patterns including the diagnosis of circadian rhythm disorders.17,18
Evaluating sleep patterns by actigraphy may be important in understanding PTSD-related sleep disturbances. This is highlighted by a recent study in contemporary veterans in which those with PTSD had significantly greater sleep pattern variability compared to individuals with insomnia and healthy controls.19 Currently, few studies have used actigraphy to assess extended sleep patterns in VV with PTSD. Therefore, the primary aim of this exploratory study was to compare objective sleep patterns determined by wrist actigraphy monitoring over 14 successive days in VV with and without PTSD from the GMRI PTSD Initiative. We also aimed to determine whether there were differences in perception of sleep, that is, the correlation between self-reported and objective sleep parameters, between groups.
METHODS
Participants
Participants were recruited from the GMRI PTSD Initiative, a large cross-sectional cohort study that investigated physical and psychological health outcomes in Australian VV exposed to trauma with and without PTSD.10 A total of 40 male VV participated in the current study between March and October 2016 (PTSD n = 20; trauma-exposed controls n = 20). PTSD status and trauma exposure were determined by psychiatric evaluation and the Clinician-Administered PTSD Scale (CAPS) for Diagnostic Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as part of the PTSD Initiative.10 Participants were selected for recruitment based on highest and lowest available CAPS-5 scores. Ethics approval was obtained from the Greenslopes Research Ethics Committee (Protocol 15/66) and Department of Veteran Affairs Human Research Ethics Committee (E016/003). Informed consent was obtained prior to study initiation.
Measures
Wrist actigraphy data were collected and analyzed in accordance with the SBSM Guide to Actigraphy Monitoring.20 Participants wore wrist accelerometers (wGT3X-BT activity monitors, ActiGraph, Pensacola, Florida, United States) day and night for 14 consecutive days on their nondominant wrist. Daily paper sleep diaries were completed concurrently to collect self-reported data including sleep latency (SL), wake after sleep onset (WASO), total sleep time (TST), caffeine, alcohol, food, and medication intake and timing, nap frequency and duration, time in and out of bed, and abnormal events affecting sleep or daily routine. Participants also completed a standardized self-report sleep questionnaire to assess overall sleep patterns, nightmares, nighttime movement, sleep medications, and reported history of OSA.10 OSA diagnosis was determined by previous diagnostic sleep study if available. Comorbid major depressive disorder (MDD) was determined using the Mini-International Neuropsychiatric Interview.21 Participants were recruited and data collected equally from each group from March to October 2016, in Australia.
Wrist actigraphy data were downloaded from wrist accelerometers and analyzed using ActiLife 6 (ActiGraph). Actigraphy parameters were set as standard with a sample rate of 30 Hz. Downloaded data were validated by a blinded skilled sleep scientist and were manually adjusted using the self-reported data, such as time in and out of bed, as a guide. The self-reported information was interpreted in view of the wrist actigraphy data, including reduced movement index (MI) and luxe monitor (light detection) to establish sleep duration and patterns. MI settings and luxe monitor settings were programmed to default. After wrist actigraphy data and sleep diary data had been rationalized, automated algorithms generated values for total time in bed (TIB), sleep efficiency (SE), SL, TST, WASO, number of awakenings, average duration of awakening, and MI.
Statistical Analysis
Group demographics were compared by unpaired t test for continuous variables and Fisher exact test for binary variables.
Sleep parameters (TST, SL, SE, TIB, WASO, number of awakenings, awakening length, bed time, wake time and MI) were compared between groups in terms of group means and variability. Night-to-night variability within individuals (herein referred to as night-to-night variability) was assessed by the root mean squared successive difference (RMSSD) calculated as:
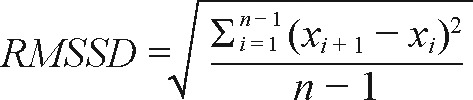 |
where x is the parameter and i is the night. Overall variability within individuals (overall variability) was assessed by computing individual standard deviations (SDs) for parameters across the 14 nights. Assessment of night-to-night sleep variability by comparison of RMSSDs has been used by Straus et al.22 whereas overall sleep variability by comparison of SDs has been used previously by Kay et al.23
Due to the non-normal distribution of most sleep parameters, group comparisons were performed using non-parametric Mann-Whitney U tests. Cohen effect sizes were calculated for each significantly different variable as the test statistic Z divided by √N, with values corresponding to parametric estimates of small (0.1), medium (0.3), and large (0.5) group effects.24 Where confounding demographics of sleep medication use, alcohol and caffeine intake, and OSA were found to differ between groups, these were included as variables in an additive multiple regression model to explore independence of PTSD diagnosis as an explanatory variable.
To assess the relationship between PTSD severity and sleep parameters, Spearman correlations were performed using CAPS-5 scores and sleep parameter averages or individual SDs (overall variability).
To assess sleep phase, participants were considered as having an advanced sleep phase if their average bedtime was before 8:00 pm or delayed sleep phase if their average out of bed time was after 8:00 am. Differences between groups were determined using Fisher exact tests.
Sleep perception differences in SL, TST, and WASO were assessed by determining the correlation between mean self-reported (sleep diary) and objectively measured (wrist actigraphy) values for each group.
Statistical analyses were performed using R (Version 3.2 The R Foundation for Statistical Computing, Vienna, Austria). All P values were two-tailed, with significance level set at P < .05.
RESULTS
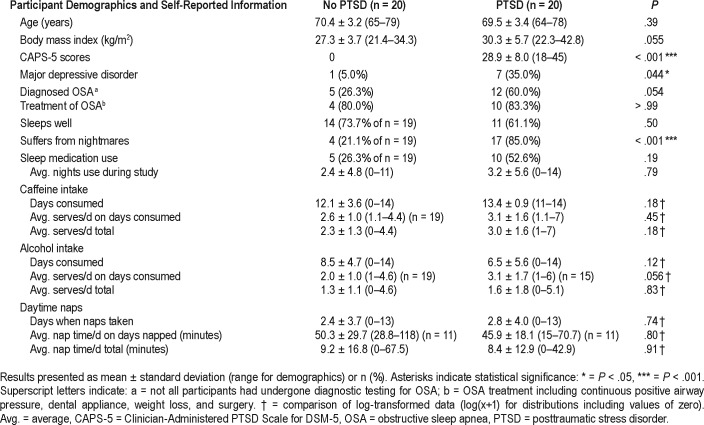
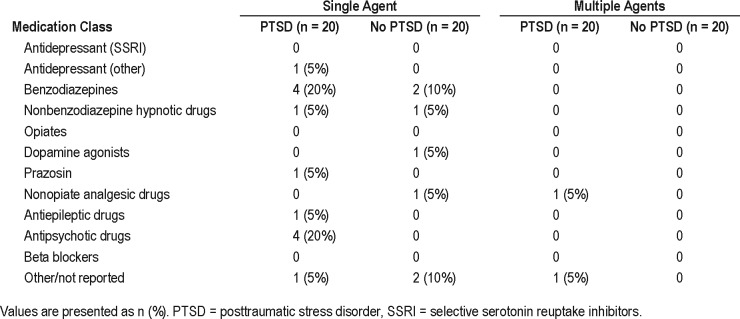
Table 1 shows group demographics and self-reported information. A full description of medication use is listed in Table 2. The PTSD group had significantly increased CAPS-5 scores and increased prevalence of MDD compared to that of the control group. There were no significant differences between groups for age, body mass index (BMI), diagnosis of OSA, or risk of sleep-disordered breathing; however, BMI and OSA diagnosis were trending toward significance (P = .051 and .054, respectively), with higher mean BMI and increased prevalence of OSA in the PTSD group. Although there was higher use of sleep medication in the PTSD group (52.6% versus 26.3%), this was not significant (P = .19), nor was there a significant difference in the number of nights medications were used during the course of the study (P = .79). Daily caffeine and alcohol intake was comparable between groups, as was daytime napping (Table 1). As expected, 85% of the PTSD group reported experiencing nightmares compared to only 20% of the control group (P < .001).
Table 1.
Participant demographics and self-reported information.
Table 2.
Medication usage.
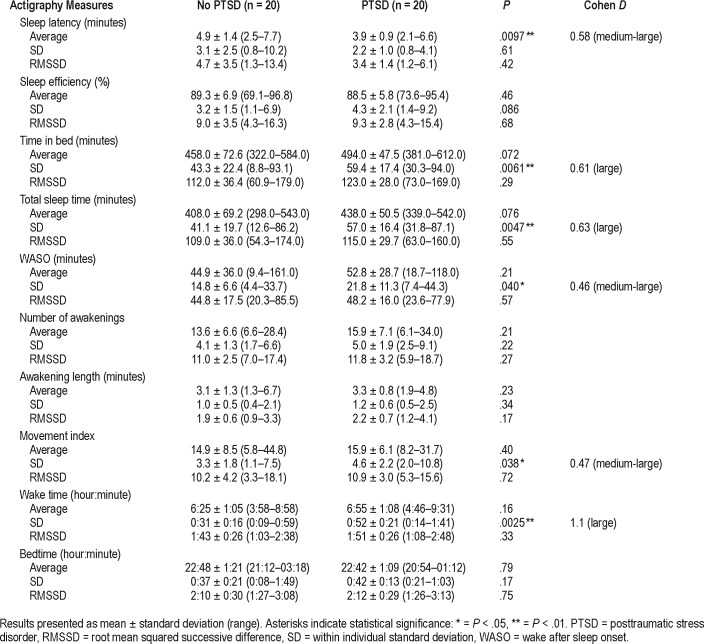
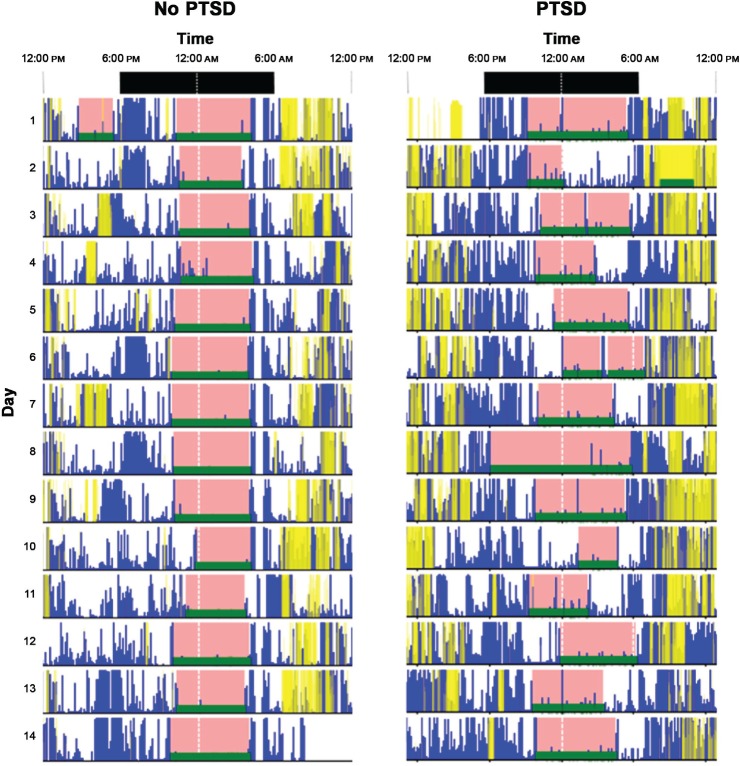
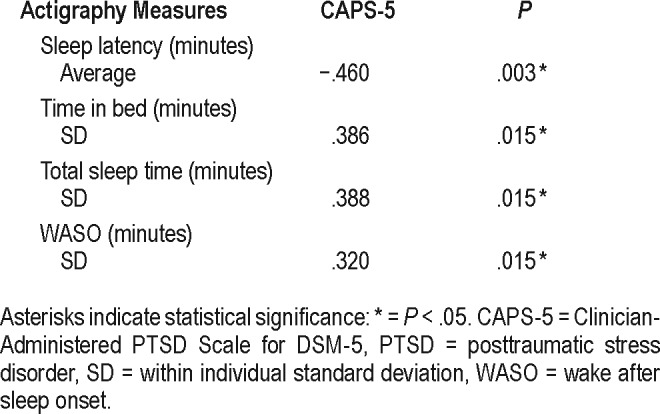
Average SL was the only significantly different average measure between groups, being lower in the PTSD group of 3.9 minutes compared to 4.9 minutes in the control group (Table 3). No differences were observed in night-to-night variability between groups (as assessed by comparison of individual RMSSD); however, overall sleep pattern variability within individuals (as assessed by comparison of individual SD) was significantly higher in the PTSD group for several parameters including TIB, TST, WASO, wake time, and MI, all of which showed medium-large differences between groups (Table 3). An example of the difference in variability in TIB/ TST observed between two participants (one from each group) is shown in Figure 1. PTSD severity determined by CAPS-5 scores was moderately and positively correlated with overall variability in TIB, TST, and WASO (Table 4). In addition, PTSD severity was negatively correlated with SL.
Table 3.
Comparison of actigraphy measures.
Figure 1. Example 14-night actigraphy graphs for participants in each group.
PTSD = posttraumatic stress disorder.
Table 4.
Correlation with sleep patterns measured by actigraphy and PTSD severity (CAPS-5).
None of the 40 participants had an advanced sleep phase, and there was no significant difference between groups in the proportion of participants with a delayed sleep phase, with only 4 participants (1 in the control group and 3 in the PTSD group, P = .60) having an average out of bed time after 8:00 am.
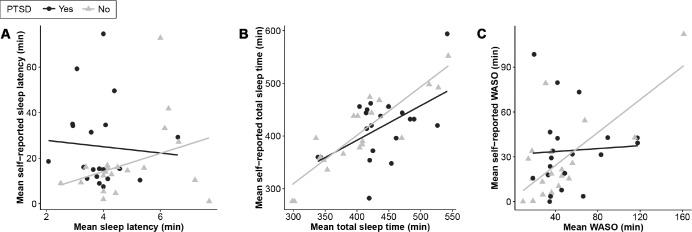
There was a positive correlation between mean self-reported SL for the control group (r = .34) and a weakly negative correlation for the PTSD group (r = −.07) (Figure 2A), although neither association was significant (P > .05). Self-reported estimation of TST was more strongly correlated with actigraphy for the control group (r = .88, P < .001) compared to the PTSD group (r = .52, P = .018) (Figure 2B), and also for WASO, with a highly significant correlation between self-report and objective WASO for the control group (r = .71, P < .001) but no correlation for the PTSD group (Figure 2C).
Figure 2. Correlation between self-reported and objective parameters.
PTSD = posttraumatic stress disorder, WASO = wake after sleep onset.
DISCUSSION
This study was the first to objectively measure extended sleep patterns using wrist actigraphy in trauma-exposed Australian VV. Although limited mean objective differences were observed, VV with PTSD showed significantly greater overall variability in sleep parameters compared to trauma-exposed controls. Additionally, VV with PTSD demonstrated reduced correlation between self-reported sleep diary and objective wrist actigraphy sleep parameters. Understanding the relationship between objective sleep patterns and PTSD is essential in establishing interventions that not only improve sleep, but also PTSD symptomology. This may be particularly relevant in an older veteran population when age and comorbid disease increase the risk for disrupted sleep.25
With the exception of SL, we found no differences in objective sleep parameters including TST, TIB, WASO, and SE between trauma-exposed VV with and without PTSD. This corresponds with the PSG results from a similar cohort12 and with previous studies demonstrating limited objective sleep differences between those with PTSD and trauma-exposed controls.6,14–16 In contrast to previous studies,26,27 however, SL was significantly lower in VV with PTSD compared to that in controls, and this was not because of the use of sleep medications or respiratory depressants/sedatives. A possible explanation may be that reduced SL is a result of increased daytime sleepiness, which although was not assessed at the time of this study, was significantly higher in VV with PTSD from the larger cohort.11 It should be noted, however, that these results must be interpreted cautiously because actigraphy devices may be inaccurate in estimating SL.
Perception of sleep was variable between VV with and without PTSD. Specifically, self-reported and actigraphy-measured TST and WASO were more strongly correlated in VV without PTSD compared to VV with PTSD. This could suggest that self-reported sleep patterns estimated by VV with PTSD were less accurate than those without PTSD. Correspondingly, increased discrepancy between self-reported and objective sleep has been associated with reduced mood upon waking and higher levels of presleep cognitive activity.28 Misperception of sleep is associated with insomnia as well as other psychological disorders and can contribute to sleep-related anxiety.29 This highlights the need to objectively measure sleep in populations of PTSD. VV with PTSD also tended to report increased sleep disturbances including nightmares, OSA, and restless legs syndrome. Of note, there was no difference in overall perception of sleeping well between groups. However, a more detailed assessment of self-reported sleep quality was lacking in this study. Furthermore, night-to-night sleep quality was not reported.
Night-to-night and overall within-individual variability were also compared between groups. Overall variability was significantly increased in VV with PTSD compared to controls for several sleep parameters including TST, TIB, WASO, wake time, and MI. However, despite differences in overall variability, night-to-night variability was not significantly different between veterans with and without PTSD, suggesting there were gradual shifts in sleep parameters over the 2 weeks rather than substantial night-to-night changes. This is important when addressing sleep hygiene issues and regular rise times to strengthen circadian rhythms.
Increased night-to-night variability has been associated with other sleep and mental disorders including insomnia, depressive symptoms, and bipolar disorder.30,31 Additionally, in a study by Straus et al., younger veterans (age 35 ± 9 years) with PTSD were found to have greater night-to-night variability compared to both veterans with insomnia and healthy controls. However, unlike the current study, controls from the Straus study were not trauma-exposed.23 Trauma exposure may significantly mediate sleep,6 and may influence night-tonight variability and other sleep patterns independent from PTSD status. Use of trauma-exposed controls in this study may have resulted in limited differences in night-to-night variability and other sleep patterns in this cohort. Future studies would benefit from the addition of a control group not exposed to trauma to better understand the relationship between trauma and disturbed sleep.
In addition to trauma exposure, other factors including age, PTSD severity, nightmares, depression, substance abuse, and comorbid disease may significantly influence sleep patterns and variability.6,22,25,32 These factors may contribute to the variable relationship between objective sleep and PTSD demonstrated in the literature, particularly when compared to trauma-exposed controls. Increased PTSD severity, comorbid MDD, and nightmare incidence in VV with PSTD may have significantly contributed to increased overall within-individual variability compared to that in controls. Correspondingly, this study demonstrated a significant positive correlation between CAPS-5 scores and overall variability for TIB, TST, and WASO (Table 4). Age and alcohol intake were similar between VV with and without PTSD. Unfortunately, data on daily nightmare incidence were unavailable for this analysis and would be worthwhile to explore in future studies.
Specifically in an older VV population, age is an important consideration. Normal aging has been associated with reduced sleep quality and increased sleep disorders.25 Additionally, older individuals may have increased comorbid diseases and associated medications, which could also negatively affect sleep.25,33 Age-related sleep disturbances may conceal differences between VV with and without PTSD. Additionally, younger age has been previously associated with increased sleep variability,30 and may explain why increased night-tonight variability was reported in younger veterans with PTSD19 and not seen in our older cohort. Age, trauma, and other possible factors contributing to PTSD-related sleep disturbances and sleep variability should be further investigated.
Limitations
We recognize that this exploratory study is limited by small sample size, which may have insufficient power to detect small differences between groups; however, any clinically significant differences in parameter means would have been identified in trends toward significance (P < .1 or .2). Furthermore, the large difference in CAPS-5 scores between veterans with PTSD and controls exposed to trauma also increased the likelihood of detecting differences attributable to PTSD status.
We also note that wrist actigraphy has certain limitations including underestimation of SL and overestimation of WASO compared to PSG.18 Nevertheless, in combination with a sleep diary, wrist actigraphy is still a useful and cost-effective research tool to measure objective sleep patterns over extended periods, and is particularly useful to measure individual sleep variability, which is not possible with only 1 or 2 nights of PSG.
Our results are limited to older Caucasian male veterans and may not be applicable to other demographic groups, including younger veterans of recent conflicts. Similarly, participants of this study were experiencing relatively mild to moderate PTSD symptomology, which may influence sleep differently compared to more severe cases of PTSD. Furthermore, the addition of more variables including daily nightmare incidence and sleep quality indices would be beneficial to better understand potential factors influencing sleep. As previously mentioned, this study did not specifically include daily reporting of nightmare incidence and therefore could not assess the relationship between nightmares and sleep patterns. Nonetheless, this study provides an interesting insight into objective sleep patterns of an aging Australian veteran population, 40 or more years after conflict.
CONCLUSIONS
In conclusion, limited objective sleep pattern differences were observed in trauma-exposed Australian VV with and without PTSD. However, VV with PTSD demonstrated greater overall within-individual variability and reduced correlation between self-reported and objective outcomes compared to controls. Overall sleep variability and perceptions of sleep may be important features of PTSD-related sleep disturbances and should be further investigated. This study highlights a future role for wrist actigraphy in monitoring extended sleep patterns in individuals with PTSD. Interventions to improve sleep, targeting reduced sleep variability and improved sleep perception, with the use of actigraphy to provide biofeedback, may be beneficial.
DISCLOSURE STATEMENT
Work for this study was performed at Gallipoli Medical Research Institute and Greenslopes Private Hospital, Brisbane, QLD Australia. All authors have seen and approved the manuscript. Funding was provided by Returned & Services League (RSL) of Australia (Queensland Branch). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
PTSD Initiative Members
Sarah McLeay, BSc(Hons), PhD1
Wendy Harvey, BSc(Hons), MBBS, MPH1
Madeline Romaniuk, BA, GradDipPsych, BBehSc(Hons), DPsych(Clinical)1,2
Darrell Crawford, MBBS, FRACP, MD1,3,4
David Colquhoun, MBBS, FRACP1,3,4
Ross McD Young, PhD1,5
Miriam Dwyer, BSc, HDipEd1
John Gibson, MBBS, FRANZCP1,4
Robyn O'Sullivan, MBBS, FRACP1,3,4
Graham Cooksley, MBBS, MD, FRACP1,3
Christopher Strakosch, MD, FRACP1,3,4
Rachel Thomson, MBBS, GradDipClinEpi, PhD, FRACP1,3,4
Joanne Voisey, BSc(Hons), PhD1,2
Bruce Lawford, MBBS, FRANZCP, FAChAM (RACP)1,2,3,4
1Gallipoli Medical Research Foundation, Greenslopes Private Hospital, Greenslopes, Queensland, Australia
2School of Biomedical Sciences, Faculty of Health and Institute of Health and Biomedical Innovation, Queensland University of Technology, Kelvin Grove, Queensland, Australia
3School of Medicine, The University of Queensland, Herston, Queensland, Australia
4Greenslopes Private Hospital, Newdegate St, Greenslopes, Queensland, Australia
5Faculty of Health, Queensland University of Technology, Kelvin Grove, Queensland, Australia
The authors gratefully acknowledge the dedicated efforts of the participants and their families, and the clinical and support staff involved in data collection.
ABBREVIATIONS
- BMI
body mass index
- CAPS-5
Clinician-Administered Posttraumatic Stress Disorder Scale for DSM-5
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- GMRI
Gallipoli Medical Research Institute
- MDD
major depressive disorder
- MI
movement Index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- PTSD
posttraumatic stress disorder
- RMSSD
root mean squared successive difference
- SD
standard deviation
- SE
sleep efficiency
- SL
sleep latency
- TIB
time in bed
- TST
total sleep time
- VV
Vietnam veterans
- WASO
wake after sleep onset
Contributor Information
Collaborators: Sarah McLeay, Wendy Harvey, Madeline Romaniuk, Darrell Crawford, David Colquhoun, Ross McD Young, Miriam Dwyer, John Gibson, Robyn O'Sullivan, Graham Cooksley, Christopher Strakosch, Rachel Thomson, Joanne Voisey, and Bruce Lawford
REFERENCES
- 1.Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related PTSD: a critical review. Aust N Z J Psychiatry. 2010;44(1):4–19. doi: 10.3109/00048670903393597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam veterans health study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331–340. doi: 10.1093/ije/25.2.331. [DOI] [PubMed] [Google Scholar]
- 3.Leggett A, Burgard S, Zivin K. The impact of sleep disturbance on the association between stressful life events and depressive symptoms. J Gerontol B Psychol Sci Soc Sci. 2015;71(1):118–128. doi: 10.1093/geronb/gbv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang JH, Huang PT, Lin YK, et al. Association between sleep duration and sleep quality, and metabolic syndrome in Taiwanese police officers. Int J Occup Med Environ Health. 2015;28(6):1011–1023. doi: 10.13075/ijomeh.1896.00359. [DOI] [PubMed] [Google Scholar]
- 5.Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567–590. doi: 10.2165/00023210-200620070-00003. [DOI] [PubMed] [Google Scholar]
- 6.Cox RC, Tuck BM, Olatunji BO. Sleep disturbance in posttraumatic stress disorder: epiphenomenon or causal factor? Curr Psychiatry Rep. 2017;19(4):22. doi: 10.1007/s11920-017-0773-y. [DOI] [PubMed] [Google Scholar]
- 7.Bourn LE, Sexton MB, Raggio GA, Porter KE, Rauch SAM. Posttraumatic stress disorder and somatic complaints: contrasting Vietnam and OIF/OEF veterans' experiences. J Psychosom Res. 2016;82(Supplement C):35–40. doi: 10.1016/j.jpsychores.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Gehrman PR, Harb GC, Cook JM, Barilla H, Ross RJ. Sleep diaries of Vietnam War veterans with chronic ptsd: the relationships among insomnia symptoms, psychosocial stress, and nightmares. Behav Sleep Med. 2015;13(3):255–264. doi: 10.1080/15402002.2014.880344. [DOI] [PubMed] [Google Scholar]
- 9.Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929–933. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 10.McLeay SC, Harvey WM, Romaniuk MN, et al. Physical comorbidities of post-traumatic stress disorder in Australian Vietnam War veterans. Med J Aust. 2017;206(6):251–257. doi: 10.5694/mja16.00935. [DOI] [PubMed] [Google Scholar]
- 11.Baird T, McLeay S, Harvey W, Theal R, Law D, O'Sullivan R. Sleep disturbances in Australian Vietnam veterans with and without posttraumatic stress disorder. J Clin Sleep Med. 2018;14(5):745–752. doi: 10.5664/jcsm.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baird T, Theal R, Gleeson S, McLeay S, O'Sullivan R. Detailed polysomnography in Australian Vietnam veterans with and without posttraumatic stress disorder. J Clin Sleep Med. 2018;14(9):1577–1586. doi: 10.5664/jcsm.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engdahl BE, Eberly RE, Hurwitz TD, Mahowald MW, Blake J. Sleep in a community sample of elderly war veterans with and without posttraumatic stress disorder. Biol Psychiatry. 2000;47(6):520–525. doi: 10.1016/s0006-3223(99)00201-2. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi I, Lavela J, Bell K, Mellman TA. The impact of posttraumatic stress disorder versus resilience on nocturnal autonomic nervous system activity as functions of sleep stage and time of sleep. Physiol Behav. 2016;164(Pt A):11–18. doi: 10.1016/j.physbeh.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi I, Lavela J, Mellman TA. Nocturnal autonomic balance and sleep in PTSD and resilience. J Trauma Stress. 2014;27(6):712–716. doi: 10.1002/jts.21973. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Li Y, Zhu H, et al. Characteristics of objective daytime sleep among individuals with earthquake-related posttraumatic stress disorder: a pilot community-based polysomnographic and multiple sleep latency test study. Psychiatry Res. 2017;247:43–50. doi: 10.1016/j.psychres.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30(11):1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straus LD, Drummond SP, Nappi CM, Jenkins MM, Norman SB. Sleep variability in military-related PTSD: a comparison to primary insomnia and healthy controls. J Trauma Stress. 2015;28(1):8–16. doi: 10.1002/jts.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(sup1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 22.Straus LD, Drummond SP, Nappi CM, Jenkins MM, Norman SB. Sleep variability in military-related PTSD: a comparison to primary insomnia and healthy controls. J Trauma Stress. 2015;28(1):8–16. doi: 10.1002/jts.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay DB, Buysse DJ, Germain A, Hall M, Monk TH. Subjective-objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment. J Sleep Res. 2015;24(1):32–39. doi: 10.1111/jsr.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Lawrence Erlbaum Associates; 1977. [Google Scholar]
- 25.Espiritu JRD. Aging-related sleep changes. Clin Geriatr Med. 2008;24(1):1–14. doi: 10.1016/j.cger.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Calhoun PS, Wiley M, Dennis MF, Means MK, Edinger JD, Beckham JC. Objective evidence of sleep disturbance in women with posttraumatic stress disorder. J Trauma Stress. 2007;20(6):1009–1018. doi: 10.1002/jts.20255. [DOI] [PubMed] [Google Scholar]
- 27.Werner KB, Griffin MG, Galovski TE. Objective and subjective measurement of sleep disturbance in female trauma survivors with posttraumatic stress disorder. Psychiatry Res. 2016;240:234–240. doi: 10.1016/j.psychres.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbert V, Pratt D, Emsley R, Kyle SD. Predictors of nightly subjective-objective sleep discrepancy in poor sleepers over a seven-day period. Brain Sci. 2017;7(3):E29. doi: 10.3390/brainsci7030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey AG, Tang N. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138(1):77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bei B, Wiley JF, Trinder J, Manber R. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:108–124. doi: 10.1016/j.smrv.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11(1):56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 33.Doghramji K, Jangro WC. Adverse effects of psychotropic medications on sleep. Sleep Med Clin. 2016;11(4):503–514. doi: 10.1016/j.jsmc.2016.08.001. [DOI] [PubMed] [Google Scholar]