Abstract
Study Objectives:
Many patients struggle with adherence to positive airway pressure (PAP) therapy for sleep apnea. In this systematic review we examined the effect that patient-facing applications (PFA)—web-based applications that interact directly with the patient—have on PAP adherence.
Methods:
A comprehensive search of PubMed, CINAHL, MEDLINE, and SCOPUS databases was performed. We looked for studies where: (1) patients were adults with sleep apnea initiating PAP therapy for the first time; (2) the intervention was a PFA that incorporated individual PAP use data; (3) the comparison was usual and/or telemedicine care, and (4) outcomes of objective PAP adherence data were recorded.
Results:
Seven studies were identified (two randomized trials, one prospective cohort trial, four retrospective cohort studies). Cumulatively the studies enrolled 304,328 patients, with individual enrollment ranging between 61 and 172,678 patients. Six studies showed that PFA use was associated with using PAP for significantly more hours per night (range 0.7–1.3 hours more). PFA cohorts used PAP a greater proportion of nights and had a lower rate of mask leak. There was no difference in apnea-hypopnea index and self-reported measures of symptoms between study groups.
Conclusions:
PFA use was associated with improved adherence to PAP therapy. Although this conclusion is based on only two small trials and predominantly observational studies and therefore should be tested in large prospective trials, the PAFs are inexpensive, do not draw on health care resources, and show promise in improving PAP therapy for OSA.
Citation:
Shaughnessy GF, Morgenthaler TI. The effect of patient-facing applications on positive airway pressure therapy adherence: a systematic review. J Clin Sleep Med. 2019;15(5):769–777.
Keywords: internet, obstructive sleep apnea, patient compliance, self-care, self-management, telemedicine
BACKGROUND
Positive airway pressure (PAP) therapy is the most commonly prescribed treatment for obstructive sleep apnea (OSA), but even when adherence is defined as ≥ 4 hours of nightly use, 46% to 83% of patients are nonadherent.1 Importance of adherence is underscored by the dose-dependent relationship between PAP adherence and outcomes.2,3 Therefore, there is a need for successful methods to improve PAP adherence.
Because technology allows objective measurement of PAP usage, challenges with PAP nonadherence have been known for more than 25 years.4 However, nonadherence is a ubiquitous challenge in the management of chronic diseases. A meta-analysis of 569 studies of nonpsychiatric prescription therapy found an average adherence rate of only 40% for chronic diseases.5,6 In addition to reducing costs of therapy, simplifying regimens, and creating effective reminder packaging, several studies have emphasized multiple patient-level educational interventions with behavioral support as effective.7 The major goal of educational and behavioral support is to improve the ability of patients to manage their own illness.8 Successful self-management requires self-monitoring of symptoms or physiologic processes, decision making to make changes in response to observations from self-monitoring, and problem solving.9
Educational and behavioral support to enhance self-management may be provided during in-person visits, or via tele-medicine.1 An intensive in-person program improved PAP adherence by an average of 1.5 h/night compared to usual care, but required very frequent interactions with the patient.10 Tele-medicine is increasingly seen as a tool to deliver cost-effective care while increasing accessibility. Telemonitoring for management of OSA has been shown to improve patient adherence.11–13 However, these interventions can be labor and resource intensive, and may fail to engage the patient in self-care.
With growing internet access, there is a developing role for web-based patient-facing applications (PFA) to assist patients in self-management of OSA. PFAs do not require interaction with health care providers and give patients direct access to their PAP data in real time. These platforms are commonly equipped with educational material and troubleshooting tips.14,15 In addition to researcher-developed applications, two examples of PAP manufacturer-developed PFAs are Dream-Mapper (previously SleepMapper) (Philips Respironics, Murrysville, Pennsylvania, United States) and myAir (ResMed Corp, San Diego, California, United States).14,16 Because of their content and design, PFAs could potentially help the patient self-manage OSA and improve adherence while decreasing health care resource utilization. The aim of this systematic review was to assess whether patient use of PFAs for PAP therapy affects treatment adherence.
METHODS
Literature Review
A comprehensive search of PubMed, CINAHL, MEDLINE, and SCOPUS was performed from inception until February 2, 2018. No restrictions on language, time, or study design were applied. The search phrase formula, composed of free-text and Medical Subject Headings terms, was: (sleep disordered breathing OR sleep apnea syndromes OR obstructive sleep apnea) AND (patient engagement OR self-monitoring OR mobile applications OR telemedicine OR real-time feedback OR web-based OR access to information OR patient participation) NOT (remote monitoring). Bibliographies of all included studies and the publications citing the included studies were reviewed. Phillips Respironics and ResMed were contacted to inquire about any additional studies not yet indexed.
Study Selection
Studies were eligible if they met the following “PICO” (population studied, intervention, comparison group, outcomes of interest) criteria: (1) population studied was adult (age 18 years or older); (2) population studied was initiating or using PAP therapy for sleep apnea; (3) intervention was using a PFA with access to real-time individual PAP data; (4) comparison groups were either usual care and/or telemonitoring; (5) outcomes included average PAP use (time/night). Exclusion criteria were: (1) intervention did not provide patients with access to PAP data; (2) PAP prescription was not new; (3) publication type was case report, meta-analysis, editorial, review, note, or letter. The title and abstract of publications meeting criteria were screened by two authors, and responsive manuscripts were then reviewed in their entirety, consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist.17
Data Collection
We extracted the country, study design, study period, study population and demographic characteristics, interventions, follow-up period, and outcome measures from each publication. Meta-analysis was not performed because of heterogeneity and the small number of studies.
Quality Assessment
Methodologic quality of the studies was assessed independently by two reviewers (G.F.S. and T.I.M; Table 1). Study design was assessed based on the Oxford Centre for Evidence-Based Medicine Levels of Evidence.18 Prospective and retrospective cohort studies were evaluated using the Newcastle-Ottawa Scale.19 The methodologic quality of the randomized trials was assessed with the Cochrane risk of bias tool.20
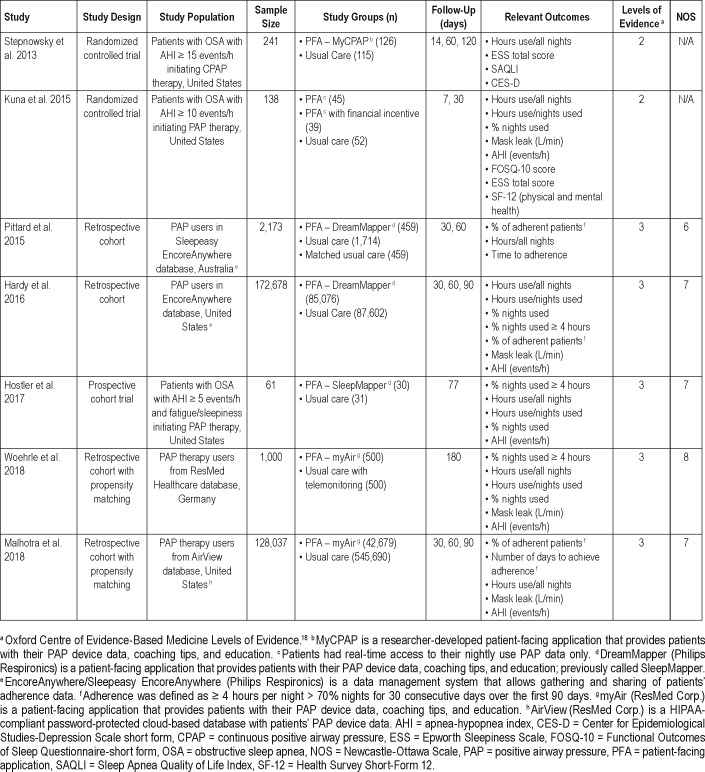
Table 1.
Study characteristics.
RESULTS
Study Selection
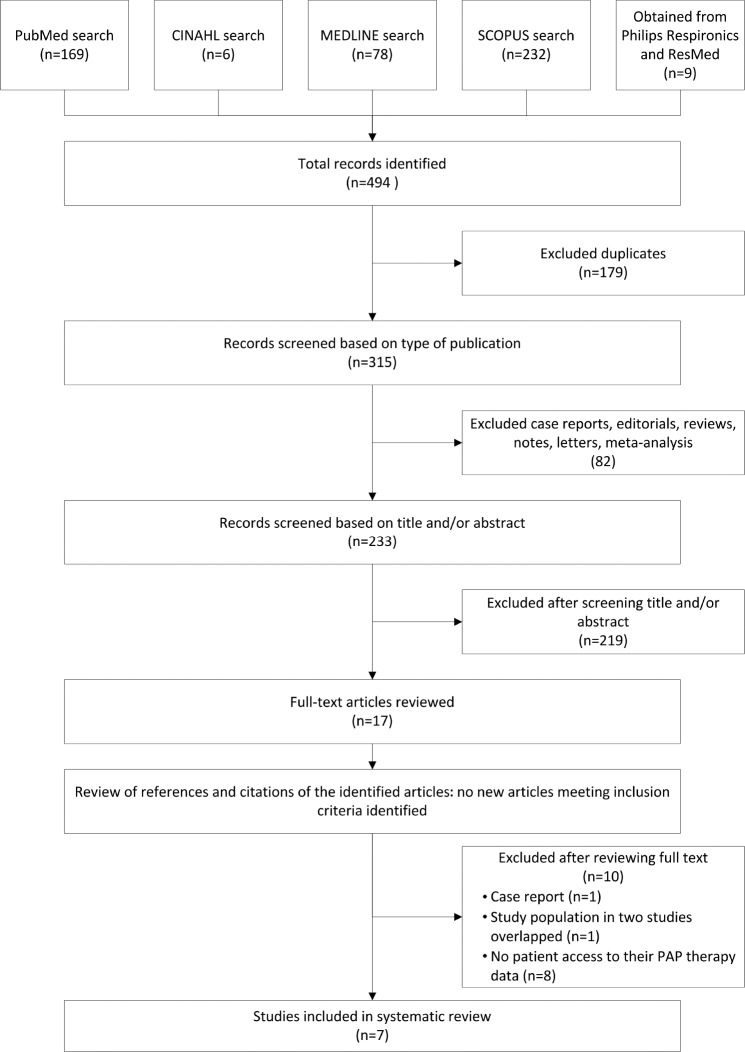
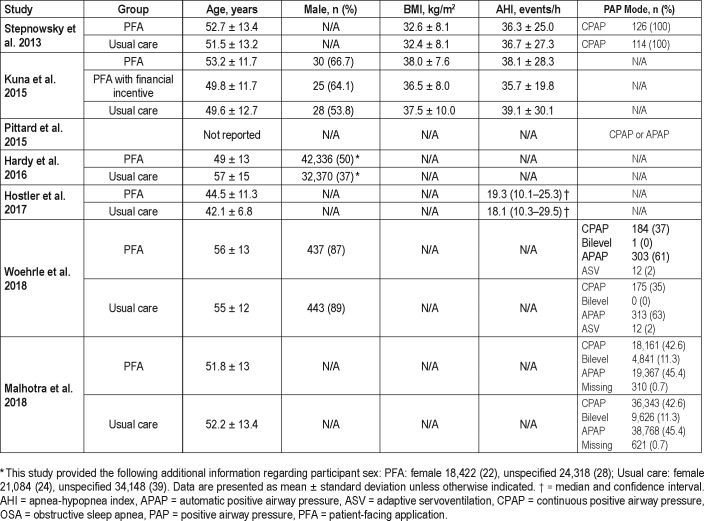
The literature search identified 315 unique publications, of which 7 met review criteria (Figure 1). Two studies were controlled clinical trials,21,22 one was a prospective cohort study,23 and four were retrospective cohort studies.14,15,24,25 The cumulative number of participants was 304,328. Study information and participant characteristics are summarized in Table 1 and Table 2.
Figure 1. Flow diagram of study selection.
Table 2.
Demographic and baseline characteristics.
The first described PFA for PAP therapy used in a randomized trial was MyCPAP, a tool that enabled patients to review nightly adherence, apnea-hypopnea index (AHI), and mask leak.21
The second randomized trial had three study groups: usual care, usual care with access to PAP data, and usual care with access to PAP data and financial incentive.22 The website only provided the patients with the hours of PAP use. Patients in the financial incentive group received a reward during the first week for each login day and for each night PAP was used for a minimum of 4 hours.22
One prospective cohort study recruited participants sequentially by offering Monday clinic patients access to PFA and recruiting Wednesday clinic patients as control participants. Patients who agreed to use the tool were given access to Sleep-Mapper (now DreamMapper).23
The four retrospective cohort studies were similar in concept. Hardy et al. retrieved patient data from Philips Respironics' PAP database. The aim was to assess DreamMapper's effect on adherence.15 Pittard et al. explored the adherence of patients with DreamMapper utilizing the Australian Philips Respironics' database.25 Woehrle et al.24 published a retrospective analysis of data from ResMed Healthcare Germany, evaluating the effect of myAir, ResMed's PFA. Uniquely, usual care in this study involved telemonitoring with feedback.24 A study by Malhotra et al. explored the adherence of patients using myAir from ResMed's United States database.14
Average Hours of Use Per Night
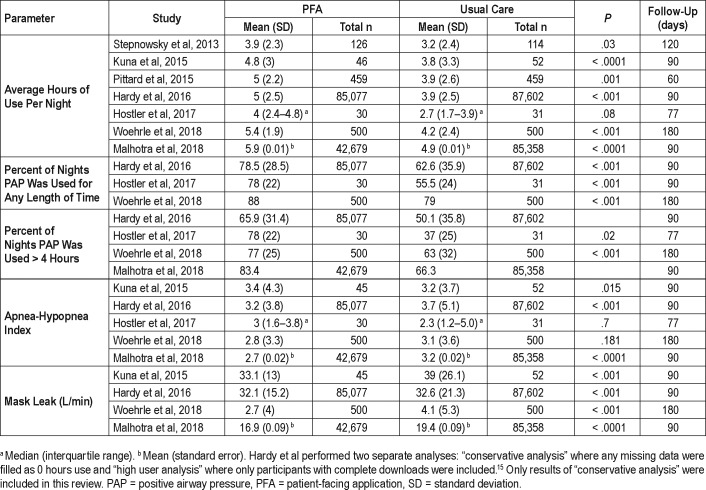
All studies showed an increase in nightly PAP use in patients using PFA versus the control groups, with significance (P < .05) in six out of seven studies. The difference in average nightly use between the study groups varied between 0.721 and 1.3 hours23 (Table 3). According to the study by Kuna et al., financial incentive had no additional benefit on average hours of use at 7 or 90 days.22
Table 3.
Results.
Percentage of Nights PAP Was Used for Any Amount of Time
All studies that assessed the number of nights patients used PAP found a significant difference.15,22–24 According to the study by Hardy et al., at 90 days the PFA cohort used PAP 78.5% of nights versus 62.6% nights in the usual care cohort.15 An even greater difference was found by Hostler et al., with 78% nights in the PFA group versus 55.5% in usual care.23 Even at 180 days the PFA group was significantly more adherent24 (Table 3).
Kuna et al. reported a mean number of days per week that PAP device was used. Patients using the PFA with and without financial incentive outperformed usual care group with 5.6 nights per week for both intervention arms as compared to 4.7 nights for usual care (P < .0001 and P < .0001).22
Apnea-Hypopnea Index
Five studies evaluated the effect of PFA on residual AHI measured by the PAP device. Although technically three out of five studies found a lower AHI in the PFA groups and two showed lower AHI in usual care groups, the AHI was within normal limits (less than 5 events/h) for all groups in all studies after treatment initiation.14,15,22–24
Mask Leak
Mask leak was significantly lower in PFA cohorts both at 90 and 180 days, as demonstrated by four studies.14,15,22,24 Interestingly, the randomized clinical trial by Kuna et al. showed 5.9 L/ min less mask leak in the PFA cohort even though patients did not have access to mask leak information or any troubleshooting recommendations22 (P < .001, Table 3).
Other Outcomes
Woehrle et al. assessed persistence of PAP therapy by recording the rate of PAP device return to the durable medical equipment provider. The PFA group terminated treatment by 180 days less often (0.6% versus 3.4% in the usual care group, P < .001).24
Two studies looked at the quality-of-life questionnaires. In the trial performed by Stepnowsky et al., the Epworth Sleepiness Scale (ESS) mean score decreased in both trial arms after 4 months of PAP therapy (usual care from 10.5 ± 5.4 to 6.5 ± 4.2; intervention group from 10.7 ± 5.2 to 7.1 ± 4.5). The scores were not statistically significantly different between the two groups.21 Similarly, both groups in the trial performed by Kuna et al. had a decrease in the mean ESS score without any significant difference between the groups. Mean change in usual care was −3.6 ± 4.8 and in the PFA group −3.0 ± 5.4 (P = .604).22 The Sleep Apnea Quality of Life scores were not statistically significantly different between the trial groups after 4 months of therapy in the trial by Stepnowsky et al. (usual care 4.6 ± 2.6; PFA group 5.1 ± 2.0, P not significant).21 Neither was there any difference in the results of the Center for Epidemiological Studies - Depression score (usual care 7.1 ± 4.9; PFA group 8.6 ± 5.5, P not significant).21 The Functional Outcomes of Sleep Questionnaire – short form score improved significantly with PAP therapy in both study groups in the trial by Kuna et al., but the degree of change was not significantly different between the groups (mean change of 1.4 ± 2.5 in usual care and 1.3 ± 3.0 in PFA group).22 The Health Survey Short Form 12 - mental health component scores improved after 3 months of treatment in both groups, though the change was only significant in usual care (usual care mean change 0.3 ± 0.4; PFA group mean change 0.2 ± 0.5).22 Health Survey Short Form 12 – physical component did not improve in either of the groups.22
Kuna et al. evaluated frequency of PFA access over time. During the first week 53.0 ± 9.9% of the PAP data access group logged in versus 72.9 ± 6.6% in the financial incentive group. After financial incentive ceased there was no difference between the groups. The proportion of patients who logged in to the PFA steadily decreased over time to approximately 10% at 90 days.22
Risk of Bias
The randomized trial by Stepnowsky et al. had a low risk of bias for blinding of outcome assessment, high risk of bias for participant blinding and selective reporting, and unclear risk for other components.21 The trial by Kuna et al. had low risk of bias in all components except for a high risk of participant blinding.22 See Table 4 for details. All cohort studies involved registries where patients self-selected to use or not use PFAs, providing a high risk of bias in cohort construction.14,15,24,25
Table 4.
Risk of bias assessment for randomized trials using the Cochrane risk of bias tool.20
DISCUSSION
The studies indicate that PFAs are associated with improved PAP therapy adherence and reduced mask leaks when compared with usual care models.14,15,22–26 One study also found a higher therapy persistence rate.24 These improvements in PAP therapy adherence were similar to those observed in telemonitoring interventions, which have shown 0.4 to 2 hours of increased PAP use per night.12,13,27,28 Telemonitoring systems require health care provider–initiated patient contact and deployment of health care resources, whereas PFAs engage patients directly without placing additional burden on health care. One study compared outcomes in patients using a telemonitoring system with those using the telemonitoring system plus a PFA.24 PFA addition resulted in significant improvement in nightly PAP usage and in treatment persistence.24 PFAs seem to uniquely contribute to improved PAP adherence.
The PFAs included in this review all provided the patients with feedback regarding the pattern and amount of individual PAP usage. In addition, several PFAs incorporated features to enhance self-efficacy skills informed by theories of behavior change. Self-efficacy is confidence in one's ability to change.29,30 This concept is a part of social cognitive theory, which regards self-efficacy as a major determinant of behavior change.29 Strong self-efficacy beliefs are associated with the ability to withstand failure and persist in efforts.30,31 Empirical evidence supports the association between self-efficacy and improved treatment adherence in sleep medicine.12,32 MyCPAP, DreamMapper, and myAir enhance self-monitoring by providing the patient with easy-to-view reports of trends in usage, and AHI. All three PFAs also supply feedback about mask leak. myAir additionally monitors mask off/on events and gives a summary score based on proprietary weighting of the aforementioned factors. Although MyCPAP provides educational materials and troubleshooting tips, they must be selected by the patient. In contrast, the commercial products serve up educational suggestions based on the monitoring as well as suggestions for selecting intermediate goals of care, possibly better equipping patients to problem solve and to set goals. Perhaps future research could evaluate which features are most important in helping patients achieve their goals of care.
Only two studies explored the effect of PFAs on OSA symptoms.21,22 The study groups in the trial by Stepnowsky et al. did not differ at baseline in any of these measures. Both groups in this study received identical instruction and education on OSA and PAP. Usual care consisted of follow-up with clinic staff at predetermined intervals. Beyond this, participants were encouraged to call should any issues or concerns arise. The clinical interaction in intervention groups after PAP initiation was dependent on patient's needs, symptoms, and objectively measured nightly data.21 In the trial by Kuna et al., all participants received the same usual care with one clinic visit with the sleep specialist after treatment initiation. In this study all patients with any other sleep disorder diagnosis were excluded.22 Two studies that assessed functional outcomes were small in sample size and perhaps not able to detect differences. As the benefit in using PFA resulted in 0.7 to 1.3 hours more PAP use, it could be that this difference has a minimal effect on symptom improvement. Furthermore, PAP adherence does not always translate into symptom improvement, and the scores could be affected by coexisting conditions.33 Insomnia, for example, is a known risk factor for PAP nonadherence, though the study by Kuna et al. did try to eliminate confounding due to comorbidities.34 These findings highlight that even optimally delivered PAP therapy leaves many patients with residual symptoms that require alternative management strategies. The reported slow decline in PAP adherence despite PFAs suggests a need for additional interventions.22 Perhaps helping patients establish peer support via a forum or discussion board incorporated in the PFA could aid in long-term adherence. Additional studies are needed to determine whether PFAs have an effect on symptoms, quality of life, and clinical outcomes of OSA. No studies have addressed adherence 1 year or longer after PAP treatment was initiated. The study by Woehrle et al. was the only one that looked at adherence as far as 180 days after therapy initation.24 It will be important to evaluate long-term adherence in future research.
Not all patients with access to PFA will actually use it. In fact, most of the studies enrolled patients if they signed up for access to the PFA, but were not able to assess the actual use of the application. The study by Kuna et al. was the only one that tracked website use and only about half of participants logged in during the first week. The proportion of patients using the website declined with time.22 It is not unusual for patients to lose interest in internet based interventions.35 A study assessed long-term use of a patient online portal and found that long-term use could only be predicted by having broadband access at home, high self-rated ability for internet use, and overall online behavior, none of which are easily modifiable.36 One study indicated that self-selected users of PFA were younger than those who elected not to use a PFA, perhaps reflecting comfort levels with internet and smart-phone technologies.15
There are potential non-patient–related barriers to PFA adoption. PFAs require that PAP data be transmitted to a central database wirelessly, enabling patient, provider, and commercial access to usage data. PAP manufacturers use proprietary cloud-based data platforms and utilize different algorithms for respiratory event detection. Therefore, health care professionals may require access to several different databases and because not all patients use the same brand of PAP devices, technical standards are needed to enable aggregation into one's health care record.37,38 Privacy may also be an issue. While most users in the trial by Stepnowsky et al. stated that they were “not at all” concerned about their information being sent over the internet, telemedicine does present privacy and security concerns.21,38 Finally, there are practice standard and business model barriers. There are no guidelines regarding who and how frequently patient data should be reviewed, or regarding what ought to be done when adherence is suboptimal.38 Billing and reimbursement for electronic data review and tele-medicine services are not well developed either.37
A major limitation of this systematic review is the small number of studies, with only two randomized trials.21,22 Retrospective studies were subjected to selection bias because it was the patients' choice to use the PFA. Therefore, the results may be confounded because patients who chose to use the PFA may be more invested in their therapy to begin with. The large retrospective studies that primarily relied on PAP cloud data also could not control for multiple baseline factors that can have significant effect on adherence, such as disease severity, comorbidity, or cultural and socioeconomic influences. There was no adjustment for age, severity of sleep apnea, socioeconomic status, education, access to internet, or internet and electronic device comfort level. Another potential confounder in registry studies such as that by Hardy et al. is the possibility that some patients may have been experienced PAP users prior to joining the registry rather than new PAP users.14,15,25 Such patients might react differently to PFAs than PAP-naïve patients. However, prior studies show that PAP usage patterns are established soon after PAP initiation, and it seems unlikely that providing feedback data and recommendations to experienced users would result in greater improvement in PAP adherence than what was observed in PAP-naïve patients.22 In addition, improvement in PAP adherence in experienced PAP patients, a successful rescue intervention, would be an even more favorable outcome than anticipated. If PAP-experienced patients made up a significant part of database study populations, the results would likely be biased against improvement in PAP utilization rather than for it. In the study by Hardy et al., the investigators thought this situation was, however, rare (personal communication).15 Additionally, the “usual care” varies between medical facilities and makes generalization of results difficult. The results of studies that used PAP manufacturer databases are in this regard closest to real life because patients come from many different health care providers.14,15,24,25
CONCLUSIONS
The use of PFAs is associated with improved PAP therapy adherence and possibly with improved therapy persistence. Although PFAs are likely to become routinely used, further efforts are needed to identify key features, their effect on outcomes of interest, and which patients would benefit from them most.
DISCLOSURE STATEMENT
Work for this study was performed at Mayo Clinic, Rochester, MN. All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Mr. Emerson Kerr from Philips Healthcare and Dr. Adam Benjafield, PhD from ResMed, who kindly provided additional material and studies about DreamMapper (Philips Respironics) and myAir (ResMed Corp), respectively.
Author contributions: GFS takes full responsibility for the content of this systematic review. The systematic review protocol was designed primarily by GFS with the assistance of TIM. The literature search was performed by GFS. Both authors independently screened literature search results and independently assessed the quality of included studies. The first draft of the manuscript was prepared by GFS. The manuscript was reviewed and edited by TIM. Both authors made the decision to submit the manuscript for publication and assume responsibility for accuracy and completeness of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ESS
Epworth Sleepiness Scale
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PFA
patient-facing application
REFERENCES
- 1.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128(2):624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 4.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149(1):149–154. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- 5.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 6.Toy EL, Beaulieu NU, Mchale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. doi: 10.1016/j.rmed.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 8.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 9.Shekelle PG, Maglione M, Chodosh J, et al. Chronic Disease Self Management for Diabetes, Osteoarthritis, Post-Myocardial Infarction Care, and Hypertension. U.S. Department of Health and Human Services. 2003. [Accessed June 10, 2018]. https://www.rand.org/pubs/reprints/RP1258.html.
- 10.Hoy CJ, Vennelle M, Kingshott RN, Engelman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med. 1999;159(4 Pt 1):1096–1100. doi: 10.1164/ajrccm.159.4.9808008. [DOI] [PubMed] [Google Scholar]
- 11.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomized controlled trial. Thorax. 2010;65(12):1061–1066. doi: 10.1136/thx.2009.133215. [DOI] [PubMed] [Google Scholar]
- 13.Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2012;35(4):477–481. doi: 10.5665/sleep.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy. Chest. 2018;153(4):843–850. doi: 10.1016/j.chest.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy W, Powers J, Jasko JG, Stitt C, Lotz G, Aloia MS. DreamMapper. A mobile application and website to engage sleep apnea patients in PAP therapy and improve adherence to treatment. Philips Respironics. 2016. [Accessed January 20, 2018]. http://alliednlassets.s3.amazonaws.com/rt-mrkt/phlprp-dm-1611/PR_DreamMapperWhitePaperV2-Final.pdf.
- 16.Hardy W, Powers J, Jasko JG, Stitt C, Lotz G, Aloia MS. SleepMapper. A mobile application and website to engage sleep apnea patients in PAP therapy and improve adherence to treatment. Philips Respironics. 2014. [Accessed January 20, 2018]. http://cdn.sleepreviewmag.com/sleeprev/2014/06/SleepMapper-Adherence-White-Paper.pdf.
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. [Accessed April 6, 2018]. https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf.
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011. [Accessed April 4, 2018]. http://handbook-5-1.cochrane.org/
- 21.Stepnowsky C, Edwards C, Zamora T, Barker R, Agha Z. Patient perspective on use of an interactive website for sleep apnea. Int J Telemed Appl. 2013;2013:239382. doi: 10.1155/2013/239382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuna ST, Shuttleworth D, Chi L, et al. Web-based access to positive airway pressure usage with or without an initial financial incentive improves treatment use in patients with obstructive sleep apnea. Sleep. 2015;38(8):1229–1236. doi: 10.5665/sleep.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hostler JM, Sheikh KL, Andrada TF, Khramtsov A, Holley PR, Holley AB. A mobile, web-based system can improve positive airway pressure adherence. J Sleep Res. 2017;26(2):139–146. doi: 10.1111/jsr.12476. [DOI] [PubMed] [Google Scholar]
- 24.Woehrle H, Arzt M, Graml A, et al. Effect of a patient engagement tool on positive airway pressure adherence: analysis of a German healthcare provider database. Sleep Med. 2018;41:20–26. doi: 10.1016/j.sleep.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Pittard J, Yarascavitch J, Jasko J, Stitt C, Aloia M. The use of SleepMapper (a patient self-management application) improves CPAP adherence in Australian patients. Koninklijke Philips N.V. 2015 Available on request from Philips Respironics. [Google Scholar]
- 26.Stepnowsky CJ, Palau JJ, Gifford AL, Ancoli-Israel S. A self-management approach to improving continuous positive airway pressure adherence and outcomes. Behav Sleep Med. 2007;5(2):131–146. doi: 10.1080/15402000701190622. [DOI] [PubMed] [Google Scholar]
- 27.Stepnowsky CJ, Palau JJ, Marler MR, Gifford AL. Pilot randomized trial of the effect of wireless telemonitoring on compliance and treatment efficacy in obstructive sleep apnea. J Med Internet Res. 2007;9(2):e14. doi: 10.2196/jmir.9.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munafo D, Hevener W, Crocker M, Willes L, Sridasome S, Muhsin M. A telehealth program for CPAP adherence reduces labor and yields similar adherence and efficacy when compared to standard of care. Sleep Breath. 2016;20(2):777–785. doi: 10.1007/s11325-015-1298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloia MS, Arnedt JT, Riggs RL, Hecht J, Borrelli B. Clinical management of poor adherence to CPAP: motivational enhancement. Behav Sleep Med. 2004;2(4):205–222. doi: 10.1207/s15402010bsm0204_3. [DOI] [PubMed] [Google Scholar]
- 30.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 31.Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271–277. doi: 10.1016/S0738-3991(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 32.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007;30(5):635–640. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 33.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: A clinical case series. Sleep Med. 2010;11(8):772–776. doi: 10.1016/j.sleep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Glasgow RE, Boles SM, McKay HG, Feil EG, Barrera M., Jr The D-Net diabetes self-management program: long-term implementation, outcomes, and generalization results. Prev Med. 2003;36(4):410–419. doi: 10.1016/s0091-7435(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 36.Woods SS, Forsberg CW, Schwartz EC, et al. The association of patient factors, digital access, and online behavior on sustained patient portal use: a prospective cohort of enrolled users. J Med Internet Res. 2017;19(10):e345. doi: 10.2196/jmir.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh J, Badr MS, Diebert W, et al. American Academy of Sleep Medicine (AASM) position paper for the use of telemedicine for the diagnosis and treatment of sleep disorders. J Clin Sleep Med. 2015;11(10):1187–1198. doi: 10.5664/jcsm.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swieca J, Hamilton GS, Meaklim H. The management, privacy and medico-legal issues of electronic CPAP data in Australia and New Zealand: Electronic CPAP data management in Australia and New Zealand. Sleep Med. 2017;(Suppl 1):S48–S55. doi: 10.1016/j.sleep.2017.03.018. [DOI] [PubMed] [Google Scholar]