Abstract
Study Objectives:
To examine the efficacy of imagery rehearsal (IR) combined with cognitive behavioral therapy for insomnia (CBT-I) compared to CBT-I alone for treating recurrent nightmares in military veterans with posttraumatic stress disorder (PTSD).
Methods:
In this randomized controlled study, 108 male and female United States veterans of the Iraq and Afghanistan conflicts with current, severe PTSD and recurrent, deployment-related nightmares were randomized to six sessions of IR + CBT-I (n = 55) or CBT-I (n = 53). Primary outcomes were measured with the Nightmare Frequency Questionnaire (NFQ) and Nightmare Distress Questionnaire (NDQ).
Results:
Improvement with treatment was significant (29% with reduction in nightmare frequency and 22% with remission). Overall, IR + CBT-I was not superior to CBT-I (NFQ: −0.12; 95% confidence interval = −0.87 to 0.63; likelihood ratio chi square = 4.7(3), P = .2); NDQ: 1.5, 95% confidence interval = −1.4 to 4.4; likelihood ratio chi square = 7.3, P = .06).
Conclusions:
Combining IR with CBT-I conferred no advantage overall. Further research is essential to examine the possibly greater benefit of adding IR to CBT-I for some subgroups of veterans with PTSD.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: Cognitive Behavioral Therapy (CBT) for Nightmares in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans; Identifier: NCT00691626; URL: https://clinicaltrials.gov/ct2/show/NCT00691626
Citation:
Harb GC, Cook JM, Phelps AJ, Gehrman PR, Forbes D, Localio R, Harpaz-Rotem I, Gur RC, Ross RJ. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757–767.
Keywords: cognitive behavioral therapy, insomnia, nightmares, posttraumatic stress disorder
BRIEF SUMMARY
Current Knowledge/Study Rationale: Imagery rehearsal (IR), the American Academy of Sleep Medicine-recommended therapy for posttraumatic stress disorder (PTSD)-associated nightmares, has been combined with components of cognitive behavioral therapy for insomnia (CBT-I) in many treatment protocols. The main aim of this dismantling study was to determine whether IR was essential to the efficacy of a treatment combining IR and CBT-I in reducing nightmare frequency and distress in military veterans with combat-related PTSD.
Study Impact: In male and female military veterans with PTSD and recurrent nightmares, the addition of IR to CBT-I did not, overall, result in greater treatment gains compared to CBT-I alone. However, adding IR may benefit veterans with lower nightmare severity and female veterans in particular.
INTRODUCTION
Recurrent nightmares are an integral feature of posttraumatic stress disorder (PTSD) and often an impetus for treatment seeking in combat veterans and others with PTSD.1 Frequent nightmares are commonly associated with poor sleep quality, impaired daytime functioning, depression, and suicidality.2–4 Although existing evidence-based psychotherapies for PTSD may have positive effects on posttraumatic sleep disturbances, recurrent nightmares remain clinically signifi-cant for many treatment completers5–7 and require targeted adjunctive treatment.8
Imagery rehearsal (IR), a form of cognitive behavioral treatment (CBT), is recommended by the American Academy of Sleep Medicine for the treatment of recurrent posttraumatic nightmares.9 It involves assisting the patient in revising the storyline of a nightmare during waking and encouraging rehearsal of a new, nondistressing dream script prior to bedtime. In recent meta-analyses of IR, average reductions in nightmare frequency and improvements in sleep quality and overall PTSD symptomatology were moderate to large, with effects maintained at 6 and 12 months after the completion of treatment.10–13 However, existing studies of IR are predominantly uncontrolled, and the two that included an active treatment control condition found reductions in the nightmare disturbance, as well as insomnia severity, that were smaller than those reported in uncontrolled or placebo-controlled studies.14,15
IR treatment protocols have varied widely with regard to the addition of various components of CBT for insomnia (CBT-I).16 There is some evidence that CBT-I alone can be effective for treating the posttraumatic nightmare disturbance.17 In a small uncontrolled trial in Iraq War veterans with PTSD and recurrent nightmares, we demonstrated that IR combined with components of CBT-I reduced nightmare frequency and sleep disturbance.18 Our aim in the current dismantling study was to test whether IR is essential to the efficacy of a combined treatment, IR plus CBT-I (IR + CBT-I), in reducing the nightmare disturbance in United States military veterans with combat-related PTSD. We predicted that IR + CBT-I would outperform CBT-I.
Evidence for the efficacy of IR in reducing the nightmare disturbance in PTSD is strongest in civilian samples, which have included 80% to 100% female participants with a history of sexual assault.12 Therefore, we investigated sex as a potential treatment modifier in the current randomized controlled trial (RCT). Other potential modifiers we considered were baseline nightmare severity and traumatic brain injury (TBI), which can lead to verbal memory deficits such as those associated with poor response to CBT.19
Aims of the Study
The aims of the study were to determine: (1) whether IR combined with CBT-I could alleviate the nightmare disturbance and improve sleep quality in United States military veterans with PTSD and (2) whether IR added to any efficacy of CBT-I.
METHODS
Participants
Of the 150 veterans of Operations Enduring Freedom (OEF), Iraqi Freedom (OIF), and New Dawn (OND) assessed for eligibility, 108 were enrolled. Participants were current patients receiving mental health care at the Corporal Michael J. Crescenz Department of Veterans Affairs Medical Center (CMCVAMC) in Philadelphia, Pennsylvania or its community-based outpatient clinics (n = 102) or at the Department of Veterans Affairs Connecticut Healthcare System (VACHS) in West Haven, Connecticut (n = 6). Recruitment at the Connecticut site was stopped because of staffing changes, and participants did not differ between sites. Inclusion criteria were current deployment-related PTSD (ie, resulting from combat and other deployment-related events) according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),20 assessed with the Clinician-Administered PTSD Scale (CAPS)21; recurrent deployment-related nightmares (at least one every 2 weeks for at least 6 months); and a global sleep disturbance, as indicated by a score of five or greater on the Pittsburgh Sleep Quality Index (PSQI).22 A comorbid anxiety or depressive disorder diagnosis, alcohol and cannabis abuse, as well as dementia and amnestic disorder related to mild to moderate head injury were allowed. Concurrent psychoactive medications, including sedative-hypnotic medications and medications sometimes used for the treatment of nightmares (eg, prazosin), were also allowed if they were first prescribed at least 2 weeks prior to the prospective participant's assessment for inclusion in the study. Medication changes over the course of the study were discouraged, but permitted if considered clinically indicated by the treating psychiatrist. Enrolled veterans were also allowed to continue mental health treatment as usual; however, veterans currently receiving prolonged exposure23 or cognitive processing therapy24 were not eligible.
Exclusion criteria for the study were: nightmares and PTSD primarily related to military sexual trauma (to avoid a heterogeneous participant sample), bipolar disorder, delirium, dementia and amnestic disorder not related to mild to moderate head injury, and schizophrenia and other psychotic disorders. In addition, individuals with substance dependence during the preceding 12 months and those with “at risk” drinking behavior over the past month (for men: more than 4 drinks in a day, more than 3 days a week, or more than 14 drinks total in a week; for women: more than 3 drinks in a day, more than 3 days a week, or more than 7 drinks total in a week)25 were excluded. Veterans who reported severe TBI (loss of consciousness or alteration of mental status greater than 24 hours; or peritraumatic memory loss or any posttraumatic amnesia greater than 7 days) also were excluded.26 Although sleep disorders including narcolepsy, circadian rhythm sleep disorders, and periodic limb movement disorder were cause for exclusion, veterans in treatment for sleep apnea or who had declined apnea treatment or not benefited from it were not excluded.
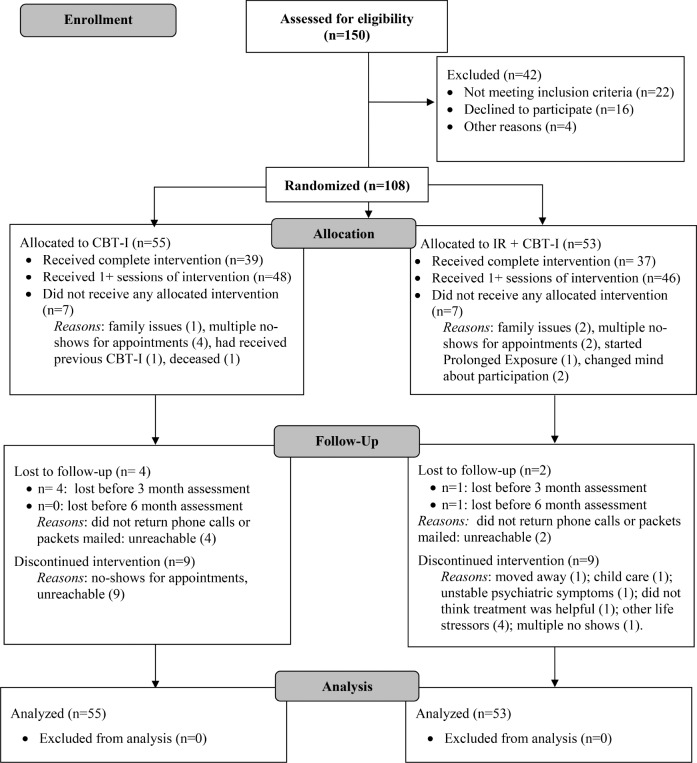
The flow of participants through the trial is shown in the CONSORT diagram in Figure 1. Forty-two of the 150 veterans screened were not enrolled: 22 did not meet eligibility criteria, and 20 withdrew before enrollment. The 108 veterans who met criteria and agreed to participate were randomized to CBT-I (n = 55) or IR + CBT-I (n = 53) by computer code that stratified by site and participant sex, with balance by blocking (three block sizes randomly permuted). Allocation concealment created by project statisticians was implemented with sealed envelopes by the research coordinator. Dropout from treatment was comparable between groups: 20% and 19% of those who initiated treatment with IR + CBT-I and CBT-I, respectively, did not complete all sessions. Every effort was made to obtain both baseline and follow-up data for every individual, and all 108 who were randomized were included in the intention to treat (ITT), ie, “as randomized,” analyses. There were no study-related adverse events.
Figure 1. Consort flowchart.
CBT-I = cognitive behavioral therapy for insomnia, IR = imagery rehearsal.
Measures
The CAPS,21 the 30-item gold standard clinician-administered structured interview with sound psychometrics,27 was used to ascertain a current diagnosis of PTSD. The four master's level CAPS raters across sites exhibited excellent reliability during training (intraclass correlations of 1.0 for presence of PTSD diagnosis and 0.95 for CAPS total score).
The Structured Clinical Interview for DSM-IV-Patient Version28 is a semi-structured interview widely used to ascertain current Axis I diagnoses according to DSM-IV criteria, and to screen for psychotic symptoms.
Veterans completed a brief trauma exposure screen to assess lifetime exposure to 12 types of potentially traumatic experiences. Deployment experiences were assessed using the Combat Experiences Scale29 and six subscales (15 to 20 items each) of the Deployment Risk and Resiliency Inventory (DRRI).30 Also, we devised an interview to elicit veterans' self-report of deployment-related injuries, blast exposure, and TBI.
Primary Outcomes
The Nightmare Frequency Questionnaire (NFQ)31 is a self-report measure of number of nights with nightmares per week and number of nightmares per week. It has demonstrated high test-retest reliability, validity with retrospective and prospective reports of nightmare frequency, and good discriminant validity.32
The Nightmare Distress Questionnaire (NDQ)33 is a self-report measure of the distress associated with nightmares. It contains 13 questions assessing anxiety, avoidance, realism, and importance associated with nightmares, summed for a total distress score. The NDQ has been shown to be reliable and valid.33
Secondary Outcomes
Additional self-report measures were administered: (1) PSQI,22 (2) the PSQI Addendum for PTSD (PSQI-A)34 to assess PTSD-related sleep disturbances, (3) the Nightmare Effects Survey31 to assess psychosocial impairment attributed to nightmares, (4) the Beck Depression Inventory,35 (5) the 12-Item Short Form Health Survey36 to assess functional health status, and (6) the PTSD Checklist-Military (PCL-M)37 to measure self-reported PTSD symptoms.
Procedure
The study was approved by the CMCVAMC and VACHS Institutional Review Boards. Recruitment and enrollment occurred from 2009 to 2014 at the CMCVAMC and from 2009 to 2010 at the VACHS. Because of budgetary constraints, recruitment was stopped in January 2015.
Potential participants were referred by treatment providers at the CMCVAMC, VACHS, and four CMCVAMC community-based outpatient clinics. Referred veterans were screened for eligibility, and gave written informed consent prior to participation in the assessment. Pretreatment and posttreatment assessments were conducted by master's level independent assessors unaware of treatment assignment (and without access to study files). The two-session baseline assessment and the posttreatment assessment (within 1 week of completing the final treatment session) included structured clinical interviews and self-report questionnaires. Two additional self-report follow-up assessments followed 3 and 6 months later.
Treatment, Supervision, and Fidelity
CBT-I and IR + CBT-I were administered in six weekly individual sessions lasting approximately 1 hour each, using detailed therapist manuals (available from the first author on request). Participants completed standard daily sleep diaries. The protocols equalized therapist contact in the two treatments by ensuring an equal number of sessions and by increasing time spent on the discussion of daily stressors in CBT-I to balance extra time spent on IR elements in IR + CBT-I. The active comparison treatment (CBT-I) controlled for both nonspecific effects of treatment (eg, instillation of hope, expectation of improvement) and non-IR therapy elements that can ameliorate sleep disturbances.8 We chose not to use a less active comparison condition, such as psychoeducation only, in order to provide all participants with some form of sleep-focused treatment given their high degree of sleep disturbance and level of distress.
Cognitive Behavioral Therapy for Insomnia
This treatment included psychoeducation about sleep and post-traumatic sleep problems and the following elements of standard CBT-I16: grounding, aimed at reducing arousal and/or dissociation after waking from nightmares (session 1), progressive muscle relaxation and discussion of the relationship between daily stressors, sleep, and nightmares (session 2), sleep hygiene and setting a regular sleep schedule (session 3), stimulus control (session 4), reduction of cognitive hyperarousal (session 5), and relapse prevention (session 6). Although regulating sleep schedules sometimes resulted in a reduction in time in bed, intentional “sleep restriction,” typically a core component of CBT-I, was not included; a pilot study18 and clinical experience had shown that most OEF/OIF veterans with PTSD have a sleep duration of less than 5 hours, usually the minimum time in bed used for sleep restriction, leaving little room to implement this strategy. We chose instead to focus on the other components of CBT-I that were more applicable to this population. Discussion of nightmare content was discouraged, and no imagery rescripting techniques were taught.
IR + CBT-I
In this condition, IR was combined with CBT-I, as described previously. In session 2, veterans were asked to select any recurrent deployment-related nightmare to target in treatment and write it out in detail. After brainstorming potential changes to the nightmare storyline with the therapist in session 3, participants wrote a new dream script in session 4. The new script was anchored in the original nightmare by using the same beginning as that of the nightmare, with subsequent departure into new, more emotionally neutral or positive imagery. Participants were instructed to practice imagining the new script “in their mind's eye” nightly before bed.
The developer of the manuals (GH) trained eight doctoral-level psychologists to deliver both treatments. All sessions were videotaped and reviewed by experts in CBT-I or IR + CBT-I (PG and AP, respectively), who provided weekly supervision, including feedback regarding treatment delivery and adherence to manuals. At study completion, a random sample of 10% of videotapes for each treatment was rated by a doctoral-level psychologist who was not a member of the study team. The treatment fidelity measure, which was adapted from previous PTSD/nightmare RCTs,14,38 rated common treatment elements and condition-specific elements on a scale from −2 to +2 (“not enough” to “too much”) and therapist competence from 0 to 4 (“poor” to “highly skilled”). In addition, global adherence, interpersonal effectiveness, and overall session quality were rated (0 = poor to 4 = excellent). Fidelity was similar between treatments. Overall, 88% of the sessions were rated as “excellent” for global adherence to the protocol, and none less than “good.” Similarly, the interpersonal effectiveness, pacing of sessions, and overall session quality were excellent in 79% to 88% of sessions. For specific treatment elements, the mean adherence score (−0.02, standard deviation [SD] = 0.06) was “just right” (score = 0). Therapist competence was high (mean = 3.6, SD = 0 .76).
Sample Size Estimation
The results from our previous RCT of IR for Vietnam veterans14 were not yet available at study inception. Therefore, for planning purposes, power calculations were conducted using the best available example of an IR trial with PSQI global score data. Krakow and colleagues39 found that, with n = 53, the mean (SD) PSQI score decreased from 10.9 (3.7) to 8.2 (4.0), whereas it was unchanged among control patients. Using simulation, the gold standard for power calculations, datasets were created with baseline and follow-up for 75 persons per group. To test power using the proposed mixed-effects model for the analysis of actual data, we assumed a random intercept with 4.0 SD (corresponding to that found by Krakow et al.),39 and a random slope (SD = 0.25), corresponding to a substantial degree of individual variation over time. Then, using a within-person-time variation of 1.0 SD, we found 84% power to detect a significant change in the treatment group versus the control group if the true relative improvement in the treatment group is as low as 1.0. For budgetary reasons, data collection was stopped at n = 108. To reflect actual power for the primary outcomes of interest, and given the actual sample of recruited patients (n = 108) and some patient dropout, we report 95% confidence intervals (CIs) to reflect poststudy power.40
Statistical Analysis
For descriptive characteristics, the convention mean (SD) was used. The primary analyses were ITT, using data from all 108 patients randomized to treatment. Outcomes were analyzed using linear mixed-effects models with a random intercept for each patient. Time was coded as a categorical factor representing baseline and three follow-up times. To allow for variation across participants over time, the model also included a random slope for time, coded as a linear term of 0, 2, 5, and 8 to correspond to the approximate number of months across visits. This approach allowed for nonlinear trajectories of outcome over time, but limited the number of terms for estimation in the model. In keeping with current statistical recommendations, the baseline value was treated as an outcome rather than as a covariate.41 The model included baseline covariates to adjust for differences in initial PTSD severity (PSQI score and CAPS score) for the two treatment arms. This approach also supports ITT analysis, which assumes that dropouts (missing outcomes) and loss to follow-up are at random, that is, not associated with group assignment, and permits simpler estimates of change within treatment group over time.
To assess whether the two groups differed by the degree of variability of outcomes over time, we fit mixed- effects models that allowed for the variance of random slopes (for individual participants) to differ by treatment group, an addition of one degree of freedom (df) to the model. Then, we used likelihood ratio tests to assess whether this larger model differed in fit from the smaller model that assumed the same variation of individual random slopes across treatment groups.
For estimates of effect modification, we modeled three-way interactions of treatment (CBT-I; IR + CBT-I) × time (baseline; follow-up) × modifier (sex; race; baseline nightmare severity; TBI). For the key contrasts of interest, for example, the change over time from baseline to last follow-up, we estimated the standardized expected value for each treatment at each time point, and then evaluated the difference in change over time between groups and estimated 95% confidence bounds for this difference. The figures in the Results section depict these expected values over time and their changes, emphasizing the key contrasts of interest as estimated on the original outcome scales. Where possible we opted to use likelihood ratio tests to estimate statistical significance of models and differences in models, as contrasted with Wald-based tests, because of their superior statistical properties.41 Analyses were performed using Stata Versions 13 and 14 (StataCorp LLC, College Station, Texas, United States). No interim analyses were planned or conducted.
RESULTS
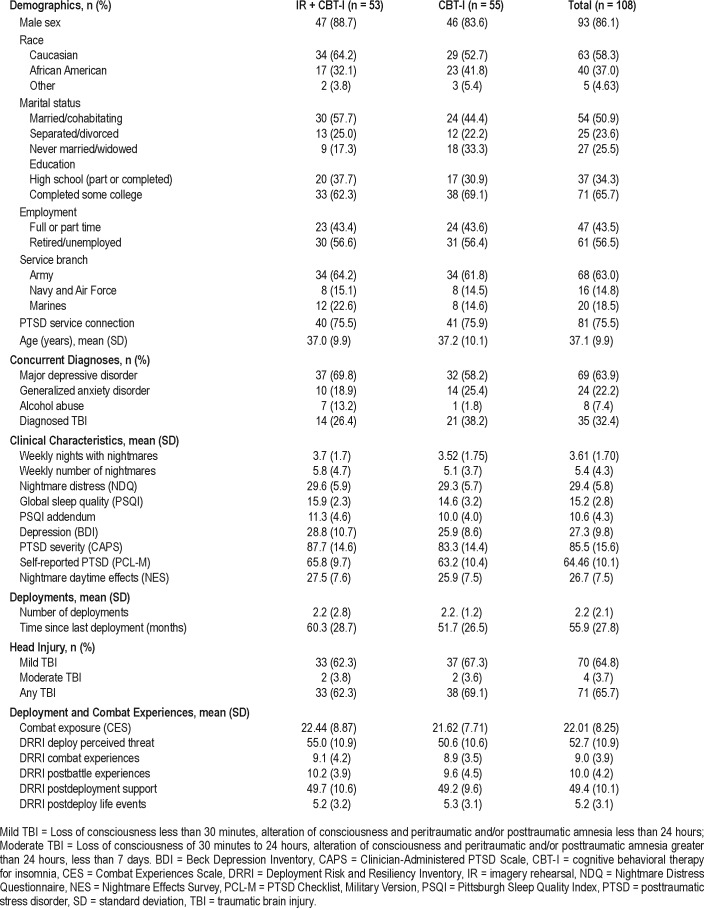
There were no substantial baseline differences between the two treatment groups on any sociodemographic characteristic (Table 1). Most participants (86%) were male and Caucasian (58%). Almost all had completed high school (98%), and approximately two-thirds had completed some college (66%); 44% were employed full-time or part-time. The average CAPS score indicated severe PTSD. The most common comorbid diagnoses were depressive disorders, and 32% of participants had a diagnosed TBI, for which 40% were in ongoing treatment at the Polytrauma Clinic of the CMCVAMC. Per retrospective self-report, 65% of participants had suffered a mild TBI, and 4% a moderate TBI, usually from blast exposure (mostly improvised explosive devices and mortar attacks); the percentage did not differ between the two conditions. The number of statistically significant differences between groups (n = 3: alcohol abuse, PSQI, DRRI Deployment Concerns) was what would be expected across more than 60 baseline comparisons, suggesting randomization performed as expected. Residual, possibly important baseline differences in initial symptoms (eg, IR + CBT-I group demonstrated poorer global sleep quality and more severe PTSD) were controlled in analyses with the PSQI and the CAPS as baseline covariates.
Table 1.
Baseline sociodemographic and clinical characteristics by group.
Prior and Concurrent Treatment
Prior and concurrent treatment data were available for 102 participants (94%). Of these, three participants had completed a prior course of prolonged exposure before enrollment, and none had undergone cognitive processing therapy. At the time of enrollment, 69% were in concurrent psychotherapy, 78% were being seen by a psychiatrist, and 75% were being prescribed psychotropic medication (27% were prescribed one, 32%: two, 14%: three, 3%: four, and 1%: five). The most commonly prescribed medications were selective serotonin reup-take inhibitors (47%), the alpha-1 adrenoceptor antagonist prazosin (30%), and hypnotic drugs (25%). There were no differences between groups in treatment variables including psychotropic prescriptions (P = .17). In an exploratory analysis, the use of prazosin during the course of the study (n = 89) did not improve outcome significantly: NDQ (likelihood ratio [LR] test = 2.0 [df = 3], P = .57); NFQ (LR test = 0.66 [df = 3], P = .66).
ITT Analyses
Differences Between Treatments Over Time
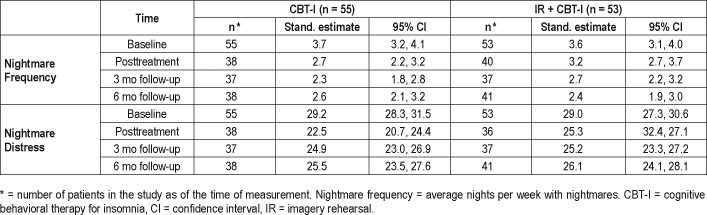
Improvements in each treatment group were statistically significant, but the two groups did not differ in improvement for primary outcomes (Table 2 and Table 3). The IR + CBT-I treatment did not lead to significantly larger improvements than the CBT-I treatment (Table 2) over time (Figure 2A and Figure 2B). For nightmare frequency (NFQ), the IR + CBTI group improved from baseline to the last follow-up by 1.14 nights compared to the CBT-I group (1.02 nights), for a difference of −0.12 (95% CI = −0.87 to 0.63; LR chi square = 4.7(3), P = .2). For nightmare distress (NDQ), the CBT-I group improved slightly more than the IR + CBT-I group, but the difference was not significant (1.5, 95% CI = −1.4 to 4.4; LR chi square = 7.3, P = .06). Among participants who completed all therapy sessions, improvements also did not differ between the treatment groups.
Table 2.
Mixed effects model results: nightmare frequency and distress.
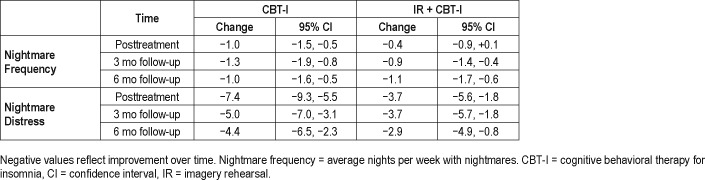
Table 3.
Differences in outcomes within treatment groups (CBT-I and IR + CBT-I): nightmare frequency and distress.
Figure 2. ITT outcomes for CBT-I and IR + CBTI.
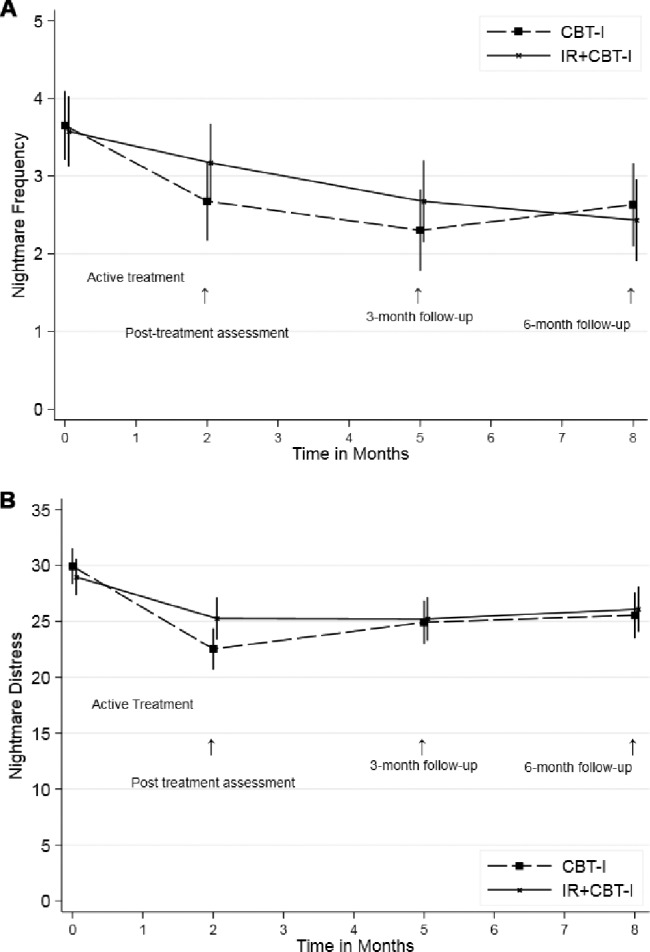
(A) Changes in nightmare frequency over time, by treatment group. (B) Changes in nightmare distress over time, by treatment group. CBT-I = cognitive behavioral therapy for insomnia, IR = imagery rehearsal, ITT = intention to treat.
Overall, nightmare frequency and distress declined significantly from baseline to 6 months posttreatment for both the CBT-I and IR + CBT-I groups (Table 3). For the number of nights with nightmares, both treatments showed a reduction in frequency of approximately 1 night per week at 6 months, decreasing from 3.6 to 2.6. For nightmare distress, the NDQ decreased about 4 points from baseline. As an additional descriptor of changes in nightmare frequency, 29% of participants showed a “clinically significant” reduction in nightmare frequency as defined by Cook et al.14 (reduction of two or more nightmares/week), and 22% achieved remission of nightmares to below the inclusion criterion frequency of one nightmare every 2 weeks.
Treatments did not produce significantly different effects for the secondary outcomes, sleep quality and PTSD symptoms (PSQI: 0.0, CI = −1.5 to 1.5; LR test = 0.94, df = 3, P = .82; PCLM: −2.2, 95% CI = −6.2 to + 1.8; LR test = 2.4, df = 3, P = .57). However, sleep quality improved in both groups, by 2.9 PSQI points for the CBT-I group and 2.8 points for the IR + CBT-I group (95% CI = −3.9 to −1.8 and −3.8 to −1.7, respectively).
Modification of Treatment Effects
To explore the possibility that a subgroup demonstrated better or worse treatment response, we compared treatments in subgroups defined by sex, race (Caucasian versus all other), severity of baseline nightmares (severe versus extreme scores on the nightmare frequency and intensity items on the CAPS), and self-reported TBI (presence versus absence). These analyses used the same baseline covariates of PSQI and CAPS scores.
No treatment modification effects were found for race and TBI, as seen in nonsignificant three-way interactions (race: NDQ: LR test [df = 3] = 2.9, P = .41; NFQ: LR test [df = 3] = 3.0, P = .40; TBI: NDQ: LR test [df = 3] = 2.6, P = .46; NFQ: LR test [ = 3] = 1.8, P = .62). Although absolute differences were small, confidence bounds were large (race: NDQ: −0.69, 95% CI = −6.8, 5.4; NFQ: 0.025, 95% CI = −1.3, 1.8; TBI: NDQ: 1.5, 95% CI = −4.9, 8.0; NFQ: −0.59, 95% CI = −2.3, 1.1).
We found no significant treatment modification effect for sex for either outcome (distress: NDQ: LR test [df = 3] = 3.4, P = .33; frequency: NFQ: LR test [df = 3] = 3.6, P = .32). Although differences were not significant, confidence bounds were large (NFQ: 1.3, 95% CI = −0.7, 3.3; NDQ: 6.8, 95% CI = −0.8, 14.3). In an exploratory analysis (Figure 3A), considering the IR + CBT-I group only, differences between men and women became more pronounced over time, although these did not attain statistical significance (NDQ: LR test [df = 3] = 7.5, P = .06; NFQ: LR test [df = 3] = 5.3, P = .15). Women's scores tended to improve more than men's and maintained improvement throughout the follow-up. With regard to the baseline severity of nightmare symptoms, the LR for the three-way interaction of treatment × time × baseline nightmare distress was significant (LR test [df = 3] = 9.3, P = .025). As shown in Figure 3B, those participants with more severe baseline nightmare symptoms (above the median level) benefitted less from IR + CBT-I than from CBT-I and, compared to the less severe subgroup, benefitted less from treatment. For nightmare frequency, we found no modification effect for baseline severity of nightmare symptoms (LR test = 3.35, df = 3, P = .34).
Figure 3. Modification of treatment effects: sex and nightmare severity.
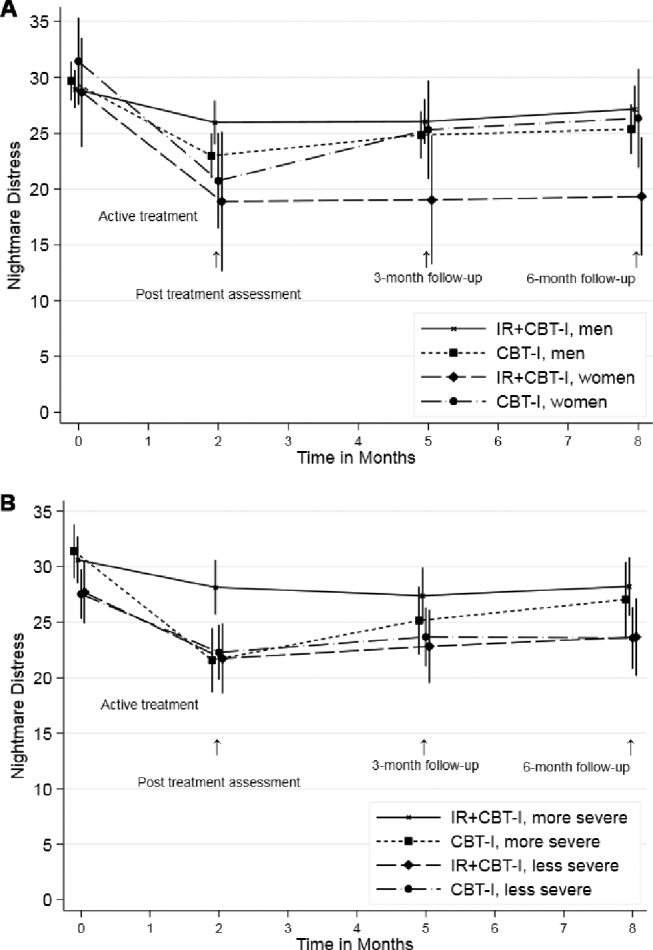
(A) Treatment modification by sex and group. (B) Treatment modification by baseline nightmare symptom severity and group. CBT-I = cognitive behavioral therapy for insomnia, IR = imagery rehearsal.
DISCUSSION
This RCT is the first that aimed to identify the therapeutic elements essential to the efficacy of IR combined with CBT-I in treating the nightmare disturbance in PTSD. Contrary to our prediction, we found that, in male and female OEF/OIF/OND veterans with PTSD and recurrent nightmares, the addition of IR to CBT-I did not, overall, result in greater treatment gains compared to CBT-I alone. Over time, both CBT-I, a treatment focused only on insomnia, and IR + CBT-I, a treatment focused on recurrent nightmares as well as insomnia, reduced nightmare frequency and nightmare distress, and improved sleep quality. This finding is consistent with reports by others17,42 that CBT-I alone could reduce the nightmare disturbance, as well as the insomnia, in PTSD. The mechanism(s) by which CBT-I, a non-nightmare–focused treatment, could improve nightmare symptoms is unclear and requires further investigation. We suggest that affective distress, which has been linked to nightmare production,4 is reduced by a treatment predicated on diminishing the conditioned association between environmental stimuli and anxious arousal.
A strength of the current study is the use of an active control group, which allows for examination of the effects of IR, specifically, when it is combined with CBT-I. Earlier meta-analyses of IR found moderate to large effects on nightmare frequency.10–13 However, the strongest evidence for the efficacy of IR derived largely from uncontrolled or waitlist controlled studies, from which it is not possible to distinguish a specific effect of IR from a nonspecific effect of psychotherapy.
An additional key difference between this trial and most of those in prior meta-analyses is our study population, with a large percentage of men, whereas greatest support for the benefits of IR has been obtained from studies with a majority of female participants.12 Significantly, a meta-analysis of response to trauma-focused psychological interventions for PTSD found that women obtained greater benefit than men.43 Indeed, exploratory analyses from the current study suggest that adding IR to CBT-I could improve the performance of insomnia-focused psychotherapy in female veterans specifically. Consistent with the known increased prevalence of nightmares in women compared to men,4 it is reasonable to hypothesize that brain mechanisms underlying nightmare production, and presumably response to nightmare treatment, differ between the sexes. The role of sex and the factors underlying a likely greater responsiveness to IR in women warrant further investigation.
Another possible explanation for the absence of a large effect of IR on nightmare symptoms in this trial relates to Criterion A for a PTSD diagnosis, the type of traumatic stressor.20 All the participants were military veterans who had experienced combat trauma, unlike the traumatic stressors reported by the civilian subjects in most earlier trials. A meta-analysis of PTSD psychotherapies found the smallest effect sizes for studies of combat compared to other types of trauma.44
Severity of the nightmare disturbance is another factor that could have diminished the overall efficacy of IR + CBT-I in this trial. As mentioned previously, most prior trials of IR involved populations with generally less severe symptomatology and failed to take account of baseline PTSD and nightmare severity. Interestingly, in a recent meta-analysis of psychotherapy treatment studies in veterans and active duty military personnel, symptom severity was shown to affect treatment response; both low and high PTSD severity levels, compared with a moderate severity level, were associated with poor outcome.45 In the current trial, veterans with more severe baseline nightmare symptomatology showed a somewhat smaller reduction in nightmare distress with IR + CBT-I than with CBT-I. It is possible that individuals with severe nightmare symptoms avoided exposure to nightmare content, thereby failing to engage in, and benefit from, IR. The suggestion of Haagen and colleagues45 that emotional activation may need to be titrated during exposure-based PTSD treatment could be relevant to IR, which entails some exposure to nightmare content. How severity of the nightmare disturbance should enter into decisions regarding individualized treatment is a topic for further investigation.
Strengths of the current study's methodology include a high ratio of enrolled to assessed participants, a comparatively large sample size, and excellent therapist adherence to therapy protocol. Rates of dropout from the two treatments were in the range of other psychotherapy trials in PTSD.46 The broad eligibility criteria, the enrollment of veterans receiving treatments standard in the United States Department of Veterans Affairs health care system, and the utilization of clinically relevant outcome measures add to the significance of these findings and their applicability to clinical care.
There are several limitations to consider when interpreting the results. First, because of the difficulty in recruiting OEF/ OIF/OND veterans with PTSD, we could not enroll and retain the full number of subjects originally planned, nor did we anticipate the degree of variability of responses to treatment across participants; therefore, power to detect group effects may have been attenuated. However, CIs were overlapping, and the magnitudes of differences between groups at the endpoints were quite small and unlikely to have been affected by greater power. Therefore, results would not likely have been shifted by data from additional participants.
Second, because of the participants' involvement in prior and concurrent PTSD therapies, a ceiling effect may have interfered with our ability to detect a difference between the two treatments. However, this is unlikely given that participants reported stable and chronic nightmare symptoms prior to enrollment and that most participants had not received evidence-based PTSD treatments. Furthermore, there is little evidence that even evidence-based psychotherapies for PTSD mitigate the sleep and nightmare disturbances.5–7
Third, this trial did not include a control group that received only treatment as usual. Therefore, only the relative efficacies of IR + CBT-I and CBT-I could be determined. Nevertheless, as reviewed previously, many prior studies have demonstrated the superiority of IR to a no-treatment waitlist condition, and therefore it is unlikely that the treatment effects we did observe were nonspecific. Finally, we did not investigate the efficacy of IR without CBT-I. However, the findings in two meta-analyses that the addition of CBT-I elements to IR improved sleep quality suggests that an IR-alone condition would not have outperformed IR + CBT-I.13
Fourth, participants in the current study were allowed to have diagnosed obstructive sleep apnea (OSA) as long as it was being treated or treatment had been ineffective or declined, but we did not assess all participants for it. OSA is recognized as a prevalent comorbidity in PTSD populations.47 There is evidence from one retrospective study in veterans with PTSD and comorbid OSA that treatment with positive airway pressure can reduce nightmare frequency.48 We have no reason to believe that individuals with OSA would have been assigned disproportionately to the experimental groups, confounding our results. Future research on nightmare treatment should provide greater detail on OSA comorbidity.
Although both IR and IR + CBT-I reduced nightmare frequency and distress, the nightmare disturbance did not remit with either intervention. Therefore, future research should consider modifications or extensions of both treatment protocols that could reduce nightmare symptoms to nonclinical levels. For CBT-I, the manualized treatment for veterans that has been adopted by the United States Veterans Health Administration can serve as a reference.16 In the case of IR + CBT-I, there has been no consensus for any single protocol, an issue for future research efforts.49
In conclusion, our results suggest that adding IR to CBT-I for the treatment of recurrent nightmares does not improve outcomes overall in veterans with chronic deployment-related PTSD. Exploratory analyses suggest that the addition of IR may be of benefit for certain groups of veterans, females and individuals with a less severe nightmare disturbance in particular. Other factors that merit investigation as moderators of treatment outcome are engagement in treatment, including compliance with homework assignments, symptom chronicity, cognitive functioning,50 and, with IR + CBT-I, characteristics of the nightmare targeted for rescripting. Treatment duration should be optimized, and consideration given to adding booster sessions. Because the current trial enrolled only veterans with combat-related PTSD, it will be essential to seek to generalize our findings to other traumatized populations. Finally, continuing research into the pathophysiology of trauma-related nightmares, with translation of findings to the clinic, is essential.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This project was supported by the Congressionally Directed Medical Research Program (CDMRP) Department of Defense, Award W81XWH-08-2-0104. The views expressed in this article do not represent those of the U.S. Government or its Department of Veterans Affairs. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of all study therapists and independent assessors. We further thank Jennifer Greene, MS, for providing invaluable, expert study coordination; Mark S. Cary, PhD, for his knowledgeable execution of statistical analyses, and J. Cobb Scott, PhD, for his thoughtful comments on the manuscript.
ABBREVIATIONS
- CAPS
Clinician-Administered PTSD Scale
- CBT
cognitive behavioral therapy
- CBT-I
cognitive behavioral therapy for insomnia
- CDMRP
Congressionally Directed Medical Research Programs
- CI
confidence interval
- CMCVAMC
Corporal Michael J. Crescenz VA Medical Center
- DRRI
Deployment Risk and Resiliency Inventory
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- IR
imagery rehearsal
- ITT
intention-to-treat
- LR
likelihood ratio
- NDQ
Nightmare Distress Questionnaire
- NFQ
Nightmare Frequency Questionnaire
- OEF
Operation Enduring Freedom
- OIF
Operation Iraqi Freedom
- OND
Operation New Dawn
- OSA
obstructive sleep apnea
- PCL-M
PTSD Checklist, Military Version
- PSQI
Pittsburgh Sleep Quality Index
- PSQI-A
Pittsburgh Sleep Quality Index Addendum
- PTSD
posttraumatic stress disorder
- RCT
randomized controlled trial
- SD
standard deviation
- TBI
traumatic brain injury
- VA
Veterans Affairs
- VACHS
Department of Veterans Affairs Connecticut Healthcare System
REFERENCES
- 1.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. [Google Scholar]
- 2.Levin R, Fireman G. Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep. 2002;25(2):205–212. [PubMed] [Google Scholar]
- 3.Bernert RA, Joiner TE, Cukrowicz KC. Suicidality and sleep disturbances. Sleep. 2005;28(9):1135–1141. doi: 10.1093/sleep/28.9.1135. [DOI] [PubMed] [Google Scholar]
- 4.Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull. 2007;133(3):482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- 5.Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. J Psychosom Res. 2011;70(4):318–327. doi: 10.1016/j.jpsychores.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Galovski TE, Monson C, Bruce SE, Resick PA. Does cognitive-behavioral therapy for PTSD improve perceived health and sleep impairment? J Trauma Stress. 2009;22(3):197–204. doi: 10.1002/jts.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutner CA, Casement MD, Gilbert KS. Change in sleep symptoms across cognitive processing therapy and prolonged exposure: a longitudinal perspective. Behav Res Ther. 2013;51(12):817–822. doi: 10.1016/j.brat.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownlow JA, Harb GC, Ross RJ. Treatment of sleep disturbances in post-traumatic stress disorder: A review of the literature. Curr Psychiatry Rep. 2015;17(6):41. doi: 10.1007/s11920-015-0587-8. [DOI] [PubMed] [Google Scholar]
- 9.Morgenthaler TI, Auerbach S, Casey KR, et al. Position paper for the treatment of nightmare disorder in adults: an American Academy of Sleep Medicine position paper. J Clin Sleep Med. 2018;14(6):1041–1055. doi: 10.5664/jcsm.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augedal AW, Hansen KS, Kronhaug CR, Harvey AG, Pallesen S. Randomized controlled trials of psychological and pharmacological treatments for nightmares: a meta-analysis. Sleep Med Rev. 2013;17(2):143–152. doi: 10.1016/j.smrv.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Hansen K, Höfling V, Kröner-Borowik T, Stangier U, Steil R. Efficacy of psychological interventions aiming to reduce chronic nightmares: a meta-analysis. Clin Psychol Rev. 2013;33(1):146–155. doi: 10.1016/j.cpr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Casement MD, Swanson LM. A meta-analysis of imagery rehearsal for post-trauma nightmares: effects on nightmare frequency, sleep quality, and posttraumatic stress. Clin Psychol Rev. 2012;32(6):1–9. doi: 10.1016/j.cpr.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seda G, Sanchez-Ortuno MM, Welsh CH, Halbower AC, Edinger JD. Comparative meta-analysis of prazosin and imagery rehearsal therapy for nightmare frequency, sleep quality, and posttraumatic stress. J Clin Sleep Med. 2015;11(1):11–22. doi: 10.5664/jcsm.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook JM, Harb GC, Gehrman PR, et al. Imagery rehearsal for posttraumatic nightmares: a randomized controlled trial. J Trauma Stress. 2010;23(5):553–563. doi: 10.1002/jts.20569. [DOI] [PubMed] [Google Scholar]
- 15.Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89–96. doi: 10.1016/j.jpsychores.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manber R, Friedman L, Siebern AT, et al. Cognitive Behavioral Therapy for Insomnia in Veterans: Therapist Manual. Washington, DC: US Department of Veteran Affairs; 2014. [Google Scholar]
- 17.Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harb GC, Cook JM, Gehrman PR, Gamble GM, et al. Post-traumatic stress disorder nightmares and sleep disturbance in Iraq War veterans: a feasible and promising treatment combination. J Aggress Maltreat Trauma. 2009;18(5):516–531. [Google Scholar]
- 19.Wild J, Gur RC. Verbal memory and treatment response in post-traumatic stress disorder. Br J Psychiatry. 2008;193(3):254–255. doi: 10.1192/bjp.bp.107.045922. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2005. [Google Scholar]
- 21.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Foa E, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- 24.Resick PA, Monson CM, Chard K. Cognitive Processing Therapy: Veteran/ Military Version: Therapist and Patient Materials Manual. Washington, DC: Department of Veterans Affairs; 2014. [Google Scholar]
- 25.National Institute on Alcohol Abuse and Alcoholism. Rethinking Drinking: Alcohol and Your Health. Rockville, MD: NIAAA; 2010. [Google Scholar]
- 26.The Management of Concussion/mTBI Working Group MOCMW. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev. 2009;46(6):CP1–CP68. [PubMed] [Google Scholar]
- 27.Weathers FW, Keane TM, Davidson J. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 29.Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychol Assess. 1989;1(1):53–55. [Google Scholar]
- 30.King DW, King LA, Vogt DS. Manual for the Deployment Risk and Resilience Inventory (DRRI): A Collection of Measures for Studying Deployment-Related Experiences of Military Veterans. Boston, MA: National Center for PTSD; 2003. [Google Scholar]
- 31.Krakow B, Hollifield M, Schrader R, et al. A controlled study of imagery rehearsal for chronic nightmares in sexual assault survivors with PTSD: a preliminary report. J Trauma Stress. 2000;13(4):589–609. doi: 10.1023/A:1007854015481. [DOI] [PubMed] [Google Scholar]
- 32.Krakow BJ, Melendrez DC, Johnston LG, et al. Sleep dynamic therapy for Cerro Grande fire evacuees with posttraumatic stress symptoms. J Clin Psychiatry. 2002;63(8):673–684. doi: 10.4088/jcp.v63n0804. [DOI] [PubMed] [Google Scholar]
- 33.Belicki K. Nightmare frequency versus nightmare distress: relations to psychopathology and cognitive style. J Abnorm Psychol. 1992;101(3):592. doi: 10.1037//0021-843x.101.3.592. [DOI] [PubMed] [Google Scholar]
- 34.Germain A, Hall M, Krakow B, Katherine Shear M, Buysse DJ. A brief Sleep Scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Dis. 2005;19(2):233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT. Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 36.Ware JH, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Weathers FW, Litz BT, Herman D, Huska J, Keane TM. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Paper presented at: Annual Convention of the International Society for Traumatic Stress Studies; July 1993; San Antonio, TX. [Google Scholar]
- 38.Schnurr PP, Friedman MJ, Foy DW, et al. Randomized trial of trauma-focused group therapy for posttraumatic stress disorder: results from a Department of Veterans Affairs cooperative study. Arch Gen Psychiatry. 2003;60(5):481–489. doi: 10.1001/archpsyc.60.5.481. [DOI] [PubMed] [Google Scholar]
- 39.Krakow B, Hollifield M, Johnston L, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. 2001;286(5):537–545. doi: 10.1001/jama.286.5.537. [DOI] [PubMed] [Google Scholar]
- 40.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121(3):200–206. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- 41.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 42.Pruiksma KE, Cranston CC, Rhudy JL, Micol RL, Davis JL. Randomized controlled trial to dismantle exposure, relaxation, and rescripting therapy (ERRT) for trauma-related nightmares. Psychol Trauma. 2018;10(1):67–75. doi: 10.1037/tra0000238. [DOI] [PubMed] [Google Scholar]
- 43.Wade D, Varker T, Kartal D, Hetrick S. Gender difference in outcomes following trauma-focused interventions for posttraumatic stress disorder: systematic review and meta-analysis. Psychol Trauma. 2016;8(3):356–364. doi: 10.1037/tra0000110. [DOI] [PubMed] [Google Scholar]
- 44.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162(2):214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 45.Haagen JFG, Smid GE, Knipscheer JW, Kleber RJ. The efficacy of recommended treatments for veterans with PTSD: a metaregression analysis. Clin Psychol Rev. 2015;40:184–194. doi: 10.1016/j.cpr.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Imel ZE, Laska K, Jakupcak M, Simpson TL. Meta-analysis of dropout in treatments for posttraumatic stress disorder. J Consult Clin Psychol. 2013;81(3):394–404. doi: 10.1037/a0031474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krakow BJ, Ulibarri VA, Moore BA, McIver ND. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev. 2015;24:37–45. doi: 10.1016/j.smrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA) J Clin Sleep Med. 2014;10(6):631–636. doi: 10.5664/jcsm.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harb GC, Phelps AJ, Forbes D, Ross RJ. A critical review of the evidence base of imagery rehearsal for posttraumatic nightmares: pointing the way for future research. J Trauma Stress. 2013;26(5):570–579. doi: 10.1002/jts.21854. [DOI] [PubMed] [Google Scholar]
- 50.Scott JC, Harb GC, Brownlow JA, Greene J, Gur RC, Ross RJ. Verbal memory functioning moderates psychotherapy treatment response for PTSD-related nightmares. Behav Res Ther. 2017;91:24–32. doi: 10.1016/j.brat.2017.01.004. [DOI] [PubMed] [Google Scholar]