Abstract
Study Objectives:
The purpose of this study was to determine sleep quality and presence of sleep disorders in participants with spinal cord injury (SCI).
Methods:
A web-based survey, available online from February 2011 to July 2013, using validated sleep questionnaires, advertised via the internet and locally through SCI consumer organizations in the United States, Australia, New Zealand, and Canada, was designed to evaluate sleep in adults with self-reported SCI. Demographic characteristics and medical history were obtained from participant self-report.
Results:
In our study population, 70% of the 304 participants were male with a mean age of 45 ± 13 years. The mean duration of injury was 16 ± 12 years. Cervical injuries were reported by 49% and thoracic injuries noted in 40% of participants. Increased sleep apnea risk was noted in 31% of participants, with 66% reporting snoring. Insomnia symptoms were reported by 54% of the respondents. Almost 40% of participants ranked their sleep quality as “fairly bad” to “very bad” in the previous month, 29% reported “often” or “almost always” waking up because of pain, and 22% had difficulty falling asleep because of leg cramps. In the past year, 27% of the respondents reported daily uncomfortable leg sensations and 28% found these leg symptoms to be “moderately to extremely distressing.”
Conclusions:
This study increases the awareness that insomnia, sleep apnea, and poor sleep quality are common in individuals with chronic SCI; often coexisting. There is a need for increased screening for sleep problems by healthcare providers taking care of individuals living with SCI.
Citation:
Shafazand S, Anderson KD, Nash MS. Sleep complaints and sleep quality in spinal cord injury: a web-based survey. J Clin Sleep Med. 2019;15(5):719–724.
Keywords: excessive daytime sleepiness, insomnia, sleep apnea, sleep quality, spinal cord injury
BRIEF SUMMARY
Current Knowledge/Study Rationale: Spinal cord injury (SCI) is universally recognized as a catastrophic disability affecting every aspect of life. Sleep disturbances have an important effect on quality of life. The aim of this study was to determine sleep quality, prevalence of excessive daytime sleepiness, insomnia symptoms, symptoms of restless legs, and sleep apnea risk in participants with SCI.
Study Impact: This study increases awareness that insomnia symptoms, sleep apnea, poor sleep quality, and excessive daytime sleepiness are common in individuals with chronic SCI and need to be diagnosed and addressed accordingly.
INTRODUCTION
Spinal cord injury (SCI) is universally recognized as a catastrophic disability affecting every aspect of life. The prevalence of traumatic SCI is estimated at 906 per million patients with an annual incidence of 40 new patients per million in the United States.1 Disability from SCI imposes significant emotional, personal, economic, and social burdens.2,3 Most people with SCI sustain their injuries in their third or fourth decade of life, and life span after SCI remains foreshortened, depending on injury level and extent of impairment. Medical comorbidi-ties including cardiovascular disease (CVD) have emerged as leading causes of mortality in chronic SCI with a prevalence exceeding nondisabled individuals, and an accelerated trajectory in early life.4 Prevalence rates of asymptomatic and symptomatic CVD in SCI populations range from approximately 25% to more than 50%. In contrast, among age-matched nondisabled populations, the prevalence of CVD is typically reported to be in the range of 5% to 10%.5 In addition to medical complications, 10% to 60% of individuals with SCI experience comorbid cognitive impairments in the areas of processing speed, attention, memory, and cognitive flexibility.6–9 Cognitive impairments may prolong near-term and chronic adjustment to disability, and delay learning of new skills. An emerging clinical challenge is the management of these medical, psychological, and cognitive long-term sequelae of SCI.
An area that is gaining increased attention in SCI is the effect of sleep disorders on psychological, cognitive, and cardiovascular health. People with SCI report higher rates of self-reported sleep disturbances than the general population, particularly restless sleep, early morning awakenings, muscle spasms, daytime sleepiness, and snoring.10–12 In the general population, sleep disorders and poor sleep quality adversely affect cardiovascular health, mood, emotional well-being, cognition, and health-related quality of life.13,14 In particular, obstructive sleep apnea (OSA), characterized by periods of complete cessation of breathing (apnea) or marked reductions in airflow (hypopnea), sleep fragmentation, and frequent oxygen desaturations, is associated with impaired cognition, depression, greater susceptibility to pain, increased risk of stroke, hypertension, atrial fibrillation, diabetes, and perhaps even mortality in the general population.15–18 Sleep disorders in SCI (in particular OSA and insomnia) could serve as an important risk factor for CVD and cognitive impairment in a population that already has higher than average prevalence of both conditions. It is therefore important, as a first step, to determine the prevalence and symptomatic characteristics of sleep disorders in this population.
Most studies reported in the literature, to date, report sleep problems in small clinical samples of SCI. To better understand the extent of sleep complaints and sleep quality from a stake-holder perspective, we conducted a web-based survey directed toward individuals with SCI across several countries. Stake-holder perceptions of risk and effects may differ from those individuals tested in clinical settings in both prevalence and quality, and may identify a need for more diligent surveillance of sleep disorders. The aim of the survey was to determine sleep quality, sleep habits, prevalence of excessive daytime sleepiness (EDS), insomnia symptoms, symptoms of restless legs, and risk for OSA in participants with SCI. Secondary aims of the study were to explore the demographic, medical, and sleep-related characteristics of individuals who report insomnia or OSA symptoms.
METHODS
In this cross-sectional study, we designed and implemented a web-based survey using validated sleep questionnaires and advertised via the internet through SCI consumer organizations in the United States, Australia, New Zealand, and Canada as well as to the local “Miami Project to Cure Paralysis” research volunteer registry. The survey was available online from February 2011 to July 2013 and was administered using the SurveyMonkey platform (SurveyMonkey, San Mateo, California, United States).
To qualify for the study, individuals had to have a spinal injury and be age 18 years or older (obtained via self-report). A waiver of signed consent was granted by the University of Miami Institutional Review Board. All of the elements of informed consent were described on the study information page, which was displayed on the first page of the survey. Consent and qualification were inferred by agreement and further participation in the survey. Those individuals who did not agree to the information provided on the study information page elected to not complete the survey. To prevent multiple survey completions by the same individual, eligible individuals contacted the research team to receive a randomly generated passcode for entry into the secure website. Upon completion of the survey, the passcode used by an individual to enter the website was unlinked from his/her answers, thereby preserving anonymity.
To further explore demographic, medical, and sleep characteristics of individuals with SCI, we divided participants into four groups: insomnia versus no insomnia, and high risk for OSA versus low risk for OSA. These two sleep disorders were chosen a priori because of their known effect on physical, cognitive, and emotional well-being in the general population13–18 and therefore their potential importance for the functional well-being of individuals with SCI.
Study Variables
Demographic characteristics and medical history including comorbidities, medication use, smoking history, height, weight, level of injury, and duration of injury were obtained from participant self-report. Participants completed questionnaires to determine risk for OSA, insomnia symptoms, sleep habits, daytime somnolence, and sleep quality.
Questionnaires
The Berlin questionnaire,19 a validated questionnaire with good performance characteristics in the general population, was used to determine increased risk of OSA. Self-reported daytime sleepiness was measured using the Epworth Sleepiness Scale (ESS).20 Eight items are rated on a scale of 0–3, and total scores range from 0–24, with higher scores indicating a greater propensity to fall asleep in different situations. Five questions were included to determine presence of symptoms suggestive of restless legs syndrome (RLS). These questions incorporate the most recent clinical diagnostic criteria established by the International RLS Study Group.21
The Sleep Heart Health Study22 Sleep Habits questionnaire was used to determine insomnia symptoms, sleep habits, and factors that disturb nocturnal sleep. Additionally, participants were asked to rate their sleep quality in the prior month on a four-point scale from very bad to very good.
Data Analysis
We report mean, and standard deviation (SD), and frequency. Participants were divided into two groups (No insomnia and Insomnia) with insomnia defined as those participants who reported “often or almost always” difficulties with sleep onset, sleep maintenance, or waking up too early in the morning. Additionally, demographic, medical, and sleep characteristics were compared between participants with high risk for OSA and those with low risk for OSA. Differences in study variables were compared using the chi-square test statistic, Fisher exact test, or t test. High risk for OSA was calculated as the proportion of individuals with a Berlin score ≥ 2.19 EDS was defined as proportion of participants with ESS score ≥ 10.23
We accepted a two-tailed value of P < .05 as statistically significant for all analyses and analyzed data using SPSS for Windows, version 21.0 (SPSS; Chicago, Illinois, United States).
RESULTS
Characteristics of Study Participants
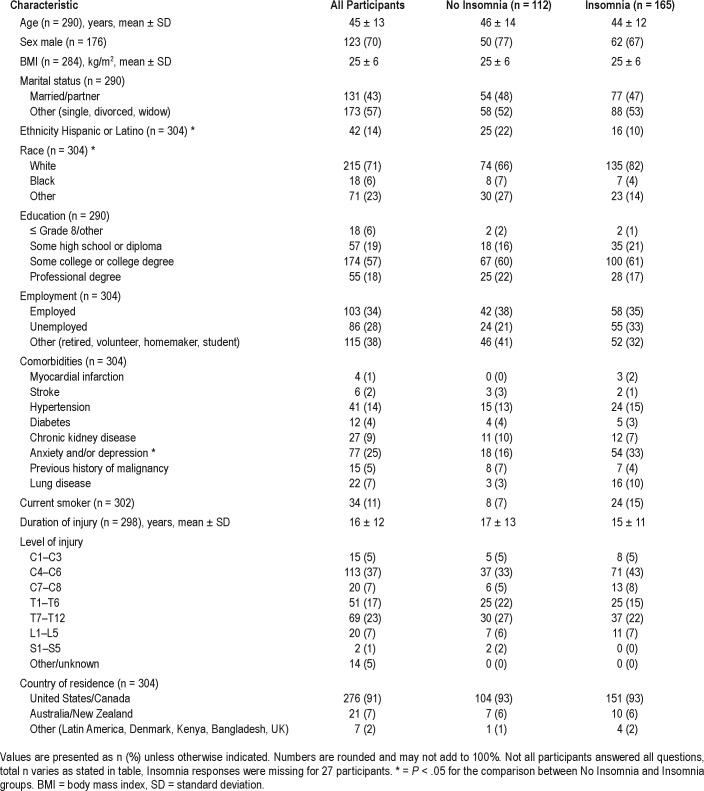
The study sample consisted of 304 participants with SCI, 70% male, with a mean age of 45 ± 13 years. The mean body mass index was 25 ± 6 kg/m2 (Table 1), 43% were married at the time of survey completion, 22% were employed full time, 14% of participants identified themselves as Hispanic, and 71% as white. The mean duration of injury was 16 ± 12 years. Cervical injuries were reported by 49%, thoracic level injuries noted in 40%, and lumbar injuries reported in 7% of participants. There were no statistically significant differences in most demographic characteristics, comorbidities, level of injury, duration of injury, and country of residence between those with and without insomnia symptoms. Race and ethnicity differed between the two groups; whites were more likely than Blacks to report insomnia symptoms, and Hispanics were less likely than non-Hispanics to report insomnia. Participants with insomnia symptoms were more likely to report depression and anxiety.
Table 1.
Characteristics of study participants.
Risk of OSA
Only 13% of all participants reported that they received an OSA diagnosis from a doctor. However, an increased risk for OSA, using responses to the Berlin questionnaire, was noted in 31% of participants. Snoring was reported by 66% of participants and self-reported apneas were noted in 4% of participants. Compared with those with no insomnia symptoms, participants with insomnia symptoms were more likely to be at high risk for OSA (39% versus 27%, P = .04).
Individuals living with SCI who were high risk for OSA, compared with those with low OSA risk, were more likely to be male (84% versus 16%, P < .05), report EDS (52% versus 26%, P < .001), and have poor sleep quality (57% versus 36%). Those with high risk for OSA compared with the low risk for OSA group had statistically significant higher body mass index (28 versus 24 kg/m2. P < .001) and were older (49 versus 43 years, P < .001). There was no statistically significant difference between the two OSA risk groups in duration of and level of SCI. Only 8% of the study population reported being prescribed OSA treatment with positive airway pressure (PAP) therapy.
Insomnia Symptomatology and Sleep Duration
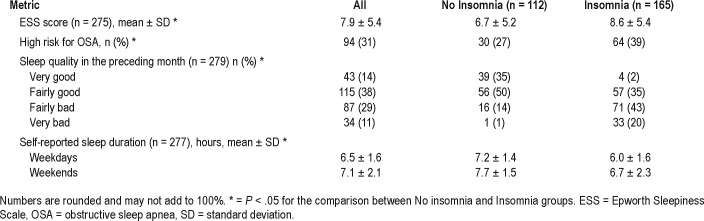
Symptoms suggestive of insomnia were reported by 54% of the respondents. Difficulties with sleep induction were reported by 36%, 39% had problems with sleep maintenance “often” or “almost always,” 32% reported waking up too early in the morning, and 42% thought that their sleep was not restful, “often” or “almost always”. Not surprisingly, participants with insomnia symptoms were more likely to report poor sleep quality compared with those with no insomnia symptoms (38% versus 6%, P < .001).
Participants reported on average 6.5 ± 1.6 hours of sleep during weekdays and 7.1 ± 2.1 hours of sleep during weekends. There was a statistically significant difference in sleep duration between those with and without insomnia symptoms. Participants with insomnia symptoms had shorter self-reported sleep duration on weekdays and weekends (Table 2).
Table 2.
Sleep questionnaires.
Sleep Quality, RLS, and EDS
Almost 40% of participants ranked their sleep quality as “fairly bad” to “very bad” in the previous month. When queried about their sleep, 29% reported “often” or “almost always” waking up due to pain, and 22% had difficulty falling asleep because of leg cramps.
In the past year, 27% of the respondents reported daily uncomfortable leg sensations and 28% found these leg symptoms to be “moderately to extremely distressing.” An increased urge to move their legs while sitting or lying down was reported by 51%; 63% thought these sensations were worse at rest, and 23% experienced the symptoms mostly at night. Compared with those without insomnia, participants with insomnia were more likely to report nighttime unpleasant leg sensations and “urge to move legs” (31% versus 18%, P < .05).
EDS was reported by 31% of all participants. A statistically significant difference in EDS was noted, with 40% of participants with insomnia symptoms and only 26% of the no insomnia participants reporting EDS (P = .01). The mean ESS score for the entire study population was 7.9 ± 5.4, with a statistically significant difference noted between groups (Table 2).
DISCUSSION
This study describes responses from a large sample of individuals with chronic SCI. Validated questionnaires were used to better describe sleep symptoms in this population. In agreement with studies in Denmark and Australia that report sleep disturbances in 35% to 49% of their surveyed SCI population, our study also demonstrates that people living with SCI have a high prevalence of sleep complaints that adversely affects their sleep quality.11,24
Symptoms suggestive of sleep apnea were reported by one-third of our study participants and yet only 13% of the participants reported a physician-based diagnosis of OSA and only 8% were prescribed PAP therapy. This suggests that sleep apnea may be underrecognized and undertreated in the SCI population.
The most commonly reported objectively diagnosed sleep disorder in patients with SCI, particularly in those with cervical lesions, but also present in thoracic-level injuries, is OSA with an estimated prevalence of between 50% to 80%.10,24–28 Reported estimates vary depending on the level of injury and the methodology used to measure OSA. Regardless, the prevalence of OSA in individuals with SCI far exceeds what is reported in the general population.29,30 In the general population, sleep apnea has been associated with cardiovascular morbidity,31 and individuals with chronic SCI are at increased risk for accelerated onset of CVD.5 It is plausible that the combination of SCI and OSA may indeed increase cardiovascular morbidity and mortality.32
The wealth of evidence in the non-SCI population combined with findings from our study highlight the importance of considering and diagnosing OSA in the SCI population. In our study duration of SCI was not a good predictor of sleep apnea symptoms. Using the Berlin questionnaire, we report that 31% of the study participants are high risk for OSA based on symptoms. This is lower than estimates reported in the literature that use objective polysomnography to diagnose OSA. Traditional screening questionnaires such as the Berlin questionnaire are useful in predicting risk, but are weakly correlated with objective polysomnography and may underestimate the true prevalence of OSA in SCI.27 Until better screening tools are identified, a high index of clinical suspicion followed by definitive testing using polysomnography may be warranted to diagnose OSA in SCI. The gold standard of sleep apnea therapy, PAP therapy, has been used with success in individuals with SCI. 33 Studies are needed to determine whether PAP use improves quality of life, CVD, and cognitive morbidity in individuals with chronic SCI. As in the general population, adherence to PAP therapy in SCI may be low and the limiting factor in any potential clinical improvement.33
EDS was reported by slightly more than one-third of our study participants with many factors associated with its occurrence. In addition to symptoms suggestive of sleep apnea, individuals with insomnia symptoms, and those with pain and uncomfortable leg sensations while sitting or lying down, were more likely to report EDS. EDS was significantly correlated (r = −.27, P < .001) with sleep quality, with those participants reporting daytime sleepiness being more likely to report poor sleep quality. Traditionally EDS has been defined by a score of 10 or higher on the ESS23; however, it is unclear whether this is an appropriate threshold in individuals with SCI, and a lower score may be more representative of the daytime sleepiness in vulnerable populations.34,35 Additionally, some people with SCI cannot or do not drive and elimination of that particular question on the ESS may alter sensitivity thresholds and overall validity. There is need for the development of questionnaires that better represent the “daytime sleepiness” construct in SCI.
Insomnia symptoms were reported by more than half of our study participants, with most complaints being restless sleep or difficulty maintaining sleep. There was no difference in insomnia symptoms based on level or duration of injury. Importantly in this study, insomnia symptoms coexisted with other sleep disorders including sleep apnea symptoms and symptoms of restless legs. This highlights the need for a comprehensive approach to the management of insomnia complaints in individuals with SCI, including evaluation for other sleep disorders (sleep apnea, restless legs) and conditions that interfere with sleep quality. Additional determinants of sleep disturbance in SCI include presence of pain, paresthesias, spasms, bladder distension/bladder management routines, anxiety, depression, and medication side effects.36 Melatonin, a hormone secreted by the pineal gland, plays an important role in the regulation of circadian rhythm and sleep induction. Melatonin levels are noted to be low in individuals with cervical injuries and may play a role in sleep disturbances noted in these individuals, including shorter sleep duration, poor sleep quality, longer time to achieving rapid eye movement sleep, and longer wakefulness after sleep onset.36
The strength of this large cross-sectional study is its use of validated questionnaires to determine the presence of various sleep complaints in a large sample of participants with chronic SCI across North America and Australia with varying levels and duration of injury.
Limitations
Our study has several limitations. We cannot provide true population prevalence estimates for sleep disorders because we did not use randomized sampling techniques. SCI diagnosis was by self-report and not medically verified by our study team. This was a convenience sample that may overrepresent those who have more sleep complaints. However, individuals were recruited from representative populations with chronic SCI, using a research registry restricted to patients with SCI and consumer SCI organizations. Participants had varying levels and durations of injuries, which strengthens the generalizability of our findings. We did not collect data from the general population and therefore do not provide direct, controlled comparisons of those with and without SCI. However, in comparisons with prior published general populations norms, individuals with SCI do have more sleep complaints and a high prevalence of symptoms suggestive of sleep apnea and insomnia. Recall bias is inherent to all questionnaire-based studies and we were not able to verify the medical history reported by study participants.
CONCLUSIONS
In summary, this study increases awareness that insomnia symptoms, sleep apnea, poor sleep quality, and EDS are common in individuals with chronic SCI. Our study highlights the need for increased attentiveness to screening for sleep problems by health care providers taking care of individuals living with SCI. Much work remains to be done to determine whether a combination of pharmacological and nonpharmacological therapies aimed at improving sleep quality and sleep disorders will improve quality of life and cognitive and motor morbidity in individuals with SCI.
DISCLOSURE STATEMENT
Work for this study was completed at the University of Miami. Drs. Nash and Shafazand were supported by a grant from the National Institute for Disability, Independent Living, and Rehabilitation Research, U.S. Department of Health and Human Services #H133G100217 [Nash, PI]. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: All authors had access to the data and contributed substantially to the design, acquisition, and analysis of data, and writing of the manuscript.
ABBREVIATIONS
- CVD
cardiovascular disease
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- RLS
restless legs syndrome
- SCI
spinal cord injury
REFERENCES
- 1.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French DD, Campbell RR, Sabharwal S, Nelson AL, Palacios PA, Gavin-Dreschnack D. Health care costs for patients with chronic spinal cord injury in the Veterans Health Administration. J Spinal Cord Med. 2007;30(5):477–481. doi: 10.1080/10790268.2007.11754581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz R, Czaja SJ, Lustig A, Zdaniuk B, Martire LM, Perdomo D. Improving the quality of life of caregivers of persons with spinal cord injury: a randomized controlled trial. Rehabil Psychol. 2009;54(1):1–15. doi: 10.1037/a0014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg ME, Castellote JM, de Pedro-Cuesta J, Mahillo-Fernandez I. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27(8):1517–1528. doi: 10.1089/neu.2009.1138. [DOI] [PubMed] [Google Scholar]
- 5.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–152. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 6.Davidoff GN, Roth EJ, Richards JS. Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch Phys Med Rehabil. 1992;73(3):275–284. [PubMed] [Google Scholar]
- 7.Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc. 1997;3(5):464–472. [PubMed] [Google Scholar]
- 8.Dowler RN, O'Brien SA, Haaland KY, Harrington DL, Feel F, Fiedler K. Neuropsychological functioning following a spinal cord injury. Appl Neuropsychol. 1995;2(3–4):124–129. doi: 10.1080/09084282.1995.9645349. [DOI] [PubMed] [Google Scholar]
- 9.Jegede AB, Rosado-Rivera D, Bauman WA, et al. Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res. 20(1):3–9. doi: 10.1007/s10286-009-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlowitz DJ, Spong J, Gordon I, Howard ME, Brown DJ. Relationships between objective sleep indices and symptoms in a community sample of people with tetraplegia. Arch Phys Med Rehabil. 2012;93(7):1246–1252. doi: 10.1016/j.apmr.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Biering-Sorensen F, Biering-Sorensen M. Sleep disturbances in the spinal cord injured: an epidemiological questionnaire investigation, including a normal population. Spinal Cord. 2001;39(10):505–513. doi: 10.1038/sj.sc.3101197. [DOI] [PubMed] [Google Scholar]
- 12.Spong J, Graco M, Brown DJ, Schembri R, Berlowitz DJ. Subjective sleep disturbances and quality of life in chronic tetraplegia. Spinal Cord. 2015;53(8):636–640. doi: 10.1038/sc.2015.68. [DOI] [PubMed] [Google Scholar]
- 13.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundermann B, Krieg JC, Schreiber W, Lautenbacher S. The effect of sleep deprivation on pain. Pain Res Manag. 2004;9(1):25–32. doi: 10.1155/2004/949187. [DOI] [PubMed] [Google Scholar]
- 15.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 16.Naegele B, Thouvard V, Pepin JL, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18(1):43–52. [PubMed] [Google Scholar]
- 17.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 18.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Allen RP, Burchell BJ, MacDonald B, Hening WA, Earley CJ. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10(10):1097–1100. doi: 10.1016/j.sleep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 23.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103(1):30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 24.Spong J, Graco M, Brown DJ, Schembri R, Berlowitz DJ. Subjective sleep disturbances and quality of life in chronic tetraplegia. Spinal Cord. 2015;53(8):636–640. doi: 10.1038/sc.2015.68. [DOI] [PubMed] [Google Scholar]
- 25.Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil. 2005;86(6):1193–1199. doi: 10.1016/j.apmr.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Leduc BE, Dagher JH, Mayer P, Bellemare F, Lepage Y. Estimated prevalence of obstructive sleep apnea-hypopnea syndrome after cervical cord injury. Arch Phys Med Rehabil. 2007;88(3):333–337. doi: 10.1016/j.apmr.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med. 2014;10(1):65–72. doi: 10.5664/jcsm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sankari A, Martin JL, Bascom AT, Mitchell MN, Badr MS. Identification and treatment of sleep-disordered breathing in chronic spinal cord injury. Spinal Cord. 2015;53(2):145–149. doi: 10.1038/sc.2014.216. [DOI] [PubMed] [Google Scholar]
- 29.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 30.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 31.Lavie P, Lavie L. Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharml Des. 2008;14(32):3466–3473. doi: 10.2174/138161208786549317. [DOI] [PubMed] [Google Scholar]
- 32.Sankari A, Martin JL, Badr M. A retrospective review of sleep-disordered breathing, hypertension and cardiovascular diseases in spinal cord injury patients. Spinal Cord. 2015;53(6):496–497. doi: 10.1038/sc.2015.16. [DOI] [PubMed] [Google Scholar]
- 33.Berlowitz DJ, Spong J, Pierce RJ, Ross J, Barnes M, Brown DJ. The feasibility of using auto-titrating continuous positive airway pressure to treat obstructive sleep apnoea after acute tetraplegia. Spinal Cord. 2009;47(12):868–873. doi: 10.1038/sc.2009.56. [DOI] [PubMed] [Google Scholar]
- 34.Cluydts R, De Valck E, Verstraeten E, Theys P. Daytime sleepiness and its evaluation. Sleep Med Rev. 2002;6(2):83–96. doi: 10.1053/smrv.2002.0191. [DOI] [PubMed] [Google Scholar]
- 35.Rosenthal LD, Dolan DC. The Epworth sleepiness scale in the identification of obstructive sleep apnea. J Nerv Ment Dis. 2008;196(5):429–431. doi: 10.1097/NMD.0b013e31816ff3bf. [DOI] [PubMed] [Google Scholar]
- 36.Giannoccaro MP, Moghadam KK, Pizza F, et al. Sleep disorders in patients with spinal cord injury. Sleep Med Rev. 2013;17(6):399–409. doi: 10.1016/j.smrv.2012.12.005. [DOI] [PubMed] [Google Scholar]