Abstract
Study Objectives:
Previous studies have shown that non-rapid eye movement (NREM) sleep parasomnias commonly coexist with restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) in children, leading to speculation that RLS/PLMD may precipitate or worsen parasomnias. However, there are limited data about the effect of the treatment of RLS/PLMD on parasomnias in children. Hence, we performed this study to determine whether the treatment of RLS/PLMD with oral iron therapy is associated with improvement of parasomnias in children.
Methods:
A retrospective database was created for children with RLS/PLMD who were treated with iron therapy. These participants were followed for at least 1 year at Cincinnati Children's Hospital Medical Center. All participants had ferritin level testing and were treated with iron therapy. In addition, all participants underwent polysomnography before starting iron therapy for RLS/PLMD except for one participant who was already on iron but required a higher dose. Most participants underwent polysomnography after iron therapy.
Results:
A total of 226 participants were identified with the diagnosis of RLS/PLMD. Of these, 50 had parasomnias and 30 of them were treated with iron therapy. Of the 30 participants, RLS symptoms improved in 15 participants (50%) and resolution of parasomnias was noted in 12 participants (40%) participants after iron therapy. Repeat polysomnography after iron therapy was performed in 21 participants (70%). After iron therapy, there was a significant decrease in periodic limb movement index (17.2 ± 8.8 [before] versus 6.7 ± 7.3 [after] events/h, P < .001). In addition, there were significant decreases in PLMS (24.52 ± 9.42 [before] versus 7.50 ± 7.18 [after] events/h, P < .0001), PLMS-related arousals (4.71 ± 1.81 [before] versus 1.35 ± 1.43 [after] events/h, P < .0001), and total arousals (11.65 ± 5.49 [before] versus 8.94 ± 3.65 [after] events/h, P < .01) after iron therapy.
Conclusions:
Parasomnias are common in our cohort of children with RLS/PLMD. Iron therapy was associated with a significant improvement in periodic limb movement index, RLS symptoms, and resolution of a significant proportion of NREM sleep parasomnias, suggesting that RLS/PLMD may precipitate NREM sleep parasomnia.
Citation:
Gurbani N, Dye TJ, Dougherty K, Jain S, Horn PS, Simakajornboon N. Improvement of parasomnias after treatment of restless leg syndrome/ periodic limb movement disorder in children. J Clin Sleep Med. 2019;15(5):743–748.
Keywords: parasomnias, periodic limb movement disorder, restless leg syndrome
BRIEF SUMMARY
Current Knowledge/Study Rationale: Non-rapid eye movement (NREM) sleep parasomnias have been reported to occur in children with restless leg syndrome (RLS)/periodic limb movement disorder (PLMD). Our literature review confirms that there are limited published data on the effect of treatment of RLS/PLMD with oral iron therapy on parasomnias in children.
Study Impact: This study confirms results of previous studies that have suggested the RLS/PLMD may precipitate NREM sleep parasomnias in children. In addition, the results of this study suggest that treatment of RLS/PLMD with oral iron therapy may help to improve parasomnias.
INTRODUCTION
Parasomnias are abnormal sleep-related events including complex movements, behaviors, emotions, dreams, and autonomic nervous activity that can result in sleep disruption, injuries, and psychosocial effects. Parasomnias can occur during non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, or during sleep-wake transitions. Parasomnias are classified as NREM-related, REM-related, other parasomnias, or isolated symptoms and normal variants.1 NREM-related parasomnias including confusional arousals, sleep terrors, and sleepwalking are commonly seen in children.1–3 These disorders arise from partial arousals from slow wave sleep, typically during the first half of the night and resolve spontaneously with children returning to deep sleep.1,3
There are several predisposing and precipitating factors that can trigger NREM-related parasomnias in children. Sleep deprivation, stress, and certain medications are common triggers of parasomnias.3 There is a genetic predisposition that has been suggested by various studies, particularly for sleepwalking.1 Obstructive sleep apnea and other sleep-related respiratory events have been recognized as triggers of disorders of arousal in children.4 Previous studies suggest that parasomnias occur commonly in children with restless legs syndrome (RLS) and can be precipitated by periodic limb movements in sleep (PLMS).5 Dye et al. also studied 105 participants with RLS/periodic limb movement disorder (PLMD) and confirmed increased prevalence of parasomnias in children with RLS/ PLMD.6 Guilleminault et al. studied 84 children with sleep walking or sleep terrors and documented that sleep-disordered breathing and RLS/PLMD may be comorbidities that trigger parasomnias and management of sleep-disordered breathing and RLS/PLMD led to resolution of the parasomnias.2
There are limited data in the literature regarding the effects of the treatment of RLS/PLMD on parasomnias in children. Thus, the purpose of our study is to examine whether the oral iron therapy used for the treatment of RLS/PLMD in children is associated with resolution of parasomnias.
METHODS
This was a retrospective study approved by the institutional board review at Cincinnati Children's Hospital Medical Center (CCHMC). At CCHMC, iron therapy is typically used as the first line of treatment for children with RLS/PLMD when the ferritin level is less than 50 ng/mL. The dosing for iron therapy typically used for management of RLS/PMD is 3 mg elemental iron per kg per day in the form of iron sulfate. Iron studies are conducted at 3- to 6-month intervals after starting iron therapy while adjusting iron therapy dose to keep serum ferritin levels ≥ 50 ng/mL.
A retrospective database was created for children with RLS/PLMD. A review of this database was performed to identify children who also had parasomnias. Children with RLS/PLMD and parasomnias who were treated with iron therapy and followed for at least 1 year at CCHMC were included in our study. As part of our electronic medical records in our sleep center, sleep history is obtained using a standardized list of questions that capture symptoms of all sleep disorders including parasomnias. These standardized questions are used to interview patients and their family at the initial and all follow-up visits. The patients were seen in the clinic by a sleep physician who obtained history based on the standard questions and may go into further detail if there are concerns. All participants underwent iron studies including serum iron and ferritin levels and were treated with iron therapy. All participants underwent polysomnography (PSG) before starting iron therapy for RLS/ PLMD, with the exception of one participant who was already on iron but required a higher dose. Most participants underwent PSG after iron therapy.
RLS and PLMD were diagnosed using International Classification of Sleep Disorders (ICSD)-2 criteria.1 Only those meeting definite RLS diagnosis were included. The essential RLS criteria for RLS include (1) an urge to move accompanied by or caused by an unpleasant sensation in legs, (2) symptoms that begin or worsen during periods of rest or inactivity, (3) partial or total relief of symptoms by movements, and (4) symptoms get worse or only occur during evenings or at night. For definite RLS, these essential criteria have to be met along with either a description in the child's own words that is consistent with leg discomfort, or the child meets all these essential criteria but does not relate a description in the child's own words that is consistent with leg discomfort and the child has two of three supportive criteria (including sleep disturbances for age, positive family history in parents or siblings with definite RLS, or periodic limb movement index [PLMI] ≥ 5 events/h on PSG). PLMD was diagnosed if PLMI > 5 events/h on the overnight diagnostic sleep study and the patient had clinically significant sleep disturbance or daytime fatigue that was not explained by any other disorders. Parasomnias were diagnosed using ICSD-2 criteria.1
PSG was performed at CCHMC using the Grass system (Grass Telefactor, West Warwick, Rhode Island, United States). The standard pediatric montage was used and the following parameters were recorded simultaneously: bilateral electro-oculogram (EOG), electroenphalography (EEG; F3A2, F4A1, C3A2, C4A1, O1A2, O2A1), chin electromyogram (EMG), anterior tibialis EMG, tracheal microphone, electrocardiography (EKG), pulse oximetry and pulse waveform, thoracic and abdominal inductance plethysmography, nasal thermistor, nasal pressure transducer, and end-tidal CO2 (ETCO2). PSG was performed in accordance with the American Academy of Sleep Medicine (AASM) guidelines7; results were scored by registered sleep technologists and reviewed by board certified or board eligible pediatric sleep specialists.
Arousals, K complexes, sleep spindles, sleep staging, and PLMS were scored based on The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. A limb movement was defined as having a minimum amplitude of 8 μV above baseline EMG and lasting a minimum duration of 0.5 seconds and a maximum duration of 10 seconds. PLMS were scored when there were four or more consecutive limb movements that were separated by at least 5 seconds and a maximum for 90 seconds. Micro-arousals were scored using a modified version of the American Sleep Disorders Association (ASDA) criteria8,9; a definition based on ASDA criteria10 using a range of 1.5 to 3.0 seconds. This definition has been implemented in similar studies,9,11 including those studying adolescents.4,12 Total arousals reported are periodic limb movement arousals, isolated limb movement arousals, and spontaneous arousals. All respiratory-related arousals were excluded.
Of the 30 participants included in the study, 21 had PSG performed both before and after the initiation of iron therapy. The original PSG test files were available for review for 14 of these participants, with the remainder of the participants having only reports available. These 28 studies (from 14 participants) were reviewed in a blinded fashion to calculate the frequency of various EEG correlates to PLMS. We specifically examined PLMS and isolated limb movements along with both spontaneous and movement associated arousals, microarousals, and slow wave firings (K complexes and K spindle complexes). Changes to sleep microarchitecture have been associated with PLMS.13,14
Data were obtained from initial and subsequent clinical encounters and included demographics, symptoms, laboratory studies, medications, and PSG results. Descriptive statistics were calculated for key demographic and PSG variables. Means and standard deviations were reported for continuous variables and frequencies and percentages were reported for categorical variables. To compare continuous variable outcomes, the t test was used. A value of P < .05 was considered statistically significant. Changes to sleep stages were calculated using paired t test with the exception of data that were not normally distributed, for which the Wilcoxon signed-rank test was used.
RESULTS
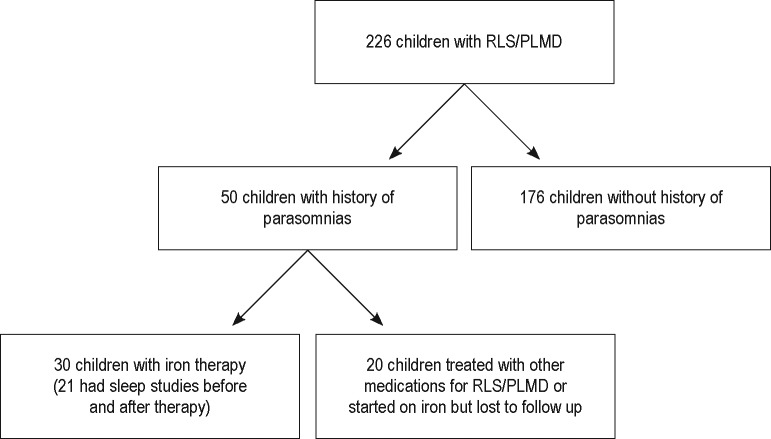
Figure 1 shows the flow diagram for participants who were included and excluded from this study. We started with 226 children with RLS/PLMD. Of these, 50 children had history of parasomnias and 30 of 50 children underwent iron therapy and were included in our study. The demographic features and presenting symptoms of the participants in this study are outlined in Table 1.
Figure 1. Flow diagram for participant inclusion.
PLMD = periodic limb movement disorder, RLS = restless legs syndrome.
Table 1.
Demographics and clinical presentation.
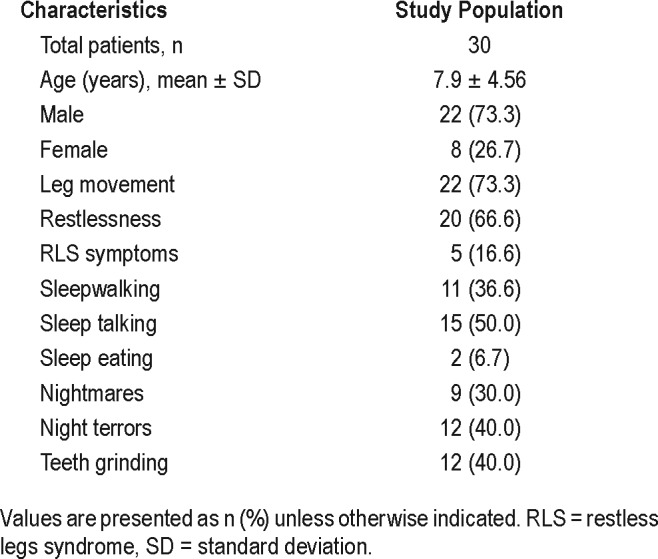
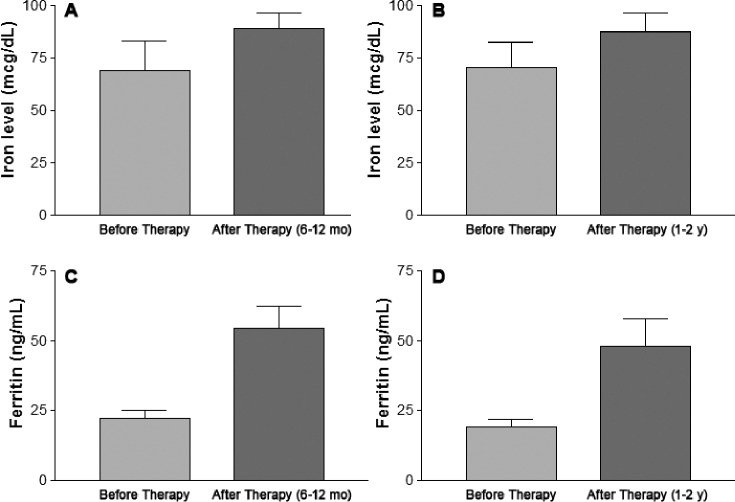
Figure 2 represents changes in iron and ferritin levels 6 to 12 months and 1 to 2 years after initiation of iron therapy. For those participants with repeated changes in ferritin level at 6 to 12 months, the mean ferritin level before oral iron sulfate therapy was 22.32 ± 10.6 ng/mL (n = 13) and it increased to 54.5 ± 28.6 ng/mL (n = 13, P < .001) after oral iron sulfate therapy. For those participants with repeated ferritin level changes at 1 to 2 years, the mean ferritin level before oral iron sulfate therapy was 19.2 ± 9.4 ng/mL (n = 12, P < .05) and it increased to 48.2 ± 33.6 ng/mL after oral iron sulfate therapy. Serum iron levels were not significantly different after oral iron therapy. The mean serum iron level was 69.2 ± 45.9 μg/mL (n = 11, P = NS) before iron therapy and was 89 ± 25.02 μg/mL (n = 11, P = NS) 6 to 12 months after iron therapy. The mean serum iron level was 70.75 ± 33.5 μg/mL (n = 8, P = NS) before iron therapy and was 87.4 ± 25.6 μg/mL (n = 8, P = NS) 1 to 2 years after iron therapy.
Figure 2. Iron and ferritin levels before and after iron therapy.
(A) P = NS, n = 11; (B) P = NS, n = 8; (C) P < .001, n = 13; (D) P < .05, n = 12.
The change in PLMI before and after iron therapy is shown in Figure 3. There was a significant decrease in PLMI (17.2 ± 8.8 [before] versus 6.7 ± 7.3 [after] events/h, P < .001) after iron therapy. Of the 30 participants who underwent iron therapy, RLS symptoms improved in 15 participants (50%) and resolution of parasomnias was noted in 12 participants (40%) after iron therapy. Of these 12 participants, 7 had improvement within 3 to 6 months, 3 had improvement within 6 to 12 months, and 2 had improvement within 1 to 2 years after starting iron therapy.
Figure 3. PLMI before and after iron therapy.
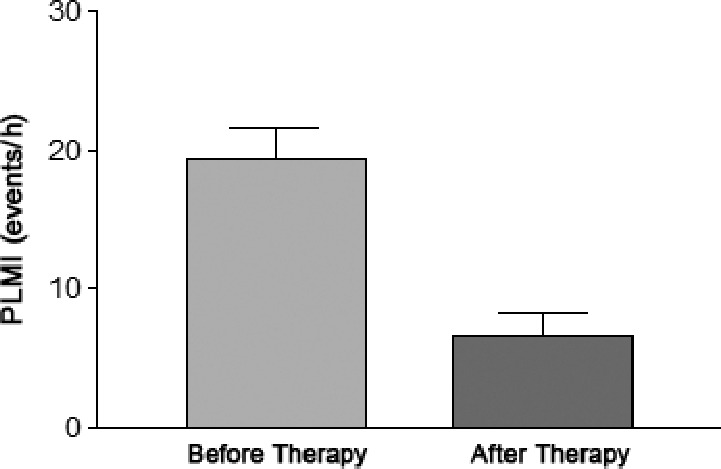
P < .001, n = 21. PLMI = periodic limb movement index.
Of the 30 participants included in this study, 1 participant was on iron therapy before the first sleep study, which showed persistent PLMS, so the dosage of the iron therapy was increased before the second sleep study. Also, another participant tried pramipexole and ropinirole before trying iron therapy for RLS/PLMD. PSG indicated the presence of obstructive sleep apnea in 9 of 30 participants with a mean apnea-hypopnea index (AHI) of 2.83 ± 1.61 events/h and mean obstructive AHI of 2.59 ± 1.33 events/h. Of the nine participants with mild obstructive sleep apnea, five were treated with nasal steroids or montelukast, two with noninvasive positive pressure support, and two with no intervention.
When comparing EEG correlates to PLMS in sleep studies before and after iron supplementation in 14 participants, significant differences were found for the following categories: PLMS (24.52 ± 9.42 [before] versus 7.50 ± 7.18 [after] events/h, P < .0001), PLMS-related arousals (4.71 ± 1.81 [before] versus 1.35 ± 1.43 [after] events/h, P < .0001), and total arousals (11.65 ± 5.49 [before] versus 8.94 ± 3.65 [after] events/h, P < .01). In addition, reductions of PLMS-related microarousals (0.74 ± 0.43 [before] versus 0.27 ± 0.26 [after] events/h, P < .01) and PLMS-related slow wave firings (1.34 ± 1.23 [before] versus 0.31 ± 0.38 [after] events/h, P < .01) were also seen. There were no statistical differences in the percentage of sleep stages and WASO in sleep studies before and after iron supplementation: N1 (2.67% ± 2.36% [before] versus 3.85% ± 3.43% [after] P > .1), N2 (51.47% ± 11.30% [before] versus 51.24% ± 11.65% [after], P > .5), N3 (26.72% ± 6.77% [before] versus 26.17% ± 9.24% [after], P > .5, REM (19.14% ± 7.67% [before] versus 18.74% ± 5.46% [after], P > 5, and WASO (12.03% ± 11.45% [before] versus 14.77% ± 16.39% [after], P > 0.2).
DISCUSSION
Our study sought to evaluate the effects of the treatment of RLS/PLMD with iron therapy on parasomnias in children. Our results indicate that iron therapy leads to significant improvement in PLM index as well as RLS symptoms. Our findings confirm previous studies on short-term responses to iron therapy in children with RLS and PLMD.15–17 In addition, Dye et al. showed a sustained long-term response to iron therapy for more than 2 years following initiation of iron therapy.6
Parasomnias have also been shown to be more common in children with RLS/PLMD and it has been suggested that they are possibly triggered by PLMS and sleep deprivation.2,4,18 Our study is the largest study evaluating the effect of iron therapy for RLS/PLMS on parasomnias. Resolution of significant proportion of parasomnias was noted in our cohort 3 to 6 months and 1 to 2 years after initiation of iron therapy, suggesting that RLS/PLMD may precipitate parasomnias.
Previous studies have shown that parasomnias can be associated with primary sleep disorders including sleep-disordered breathing and RLS/PLMD. Guilleminault et al. reported a series of 84 prepubertal children with parasomnias, of which 49 children had sleep-disordered breathing and 2 children had RLS/PLMS based on PSG findings.2 The 2 children with RLS/ PLMS were treated with pramipexole and follow-up 5 to 12 weeks later showed resolution of report of parasomnias. In this study, the mean respiratory disturbance index of the 49 children with sleep-disordered breathing was 5.4 ± 1.5. In our cohort, we also found that parasomnias were fairly common in our participants with RLS/PLMD, with 22% who had a history of parasomnias. RLS symptoms including leg movement and restlessness improved in 15 participants (50%) and complete cessation of parasomnias was noted in 12 participants (40%) after iron therapy. Interestingly, 30% of our cohort with parasomnias and RLS/PLMD had coexisting mild sleep-disordered breathing with a mean AHI of 2.83 ± 1.61 events/h. Most of them were treated conservatively and only two were treated with noninvasive positive pressure support.
It has been speculated that sleep disorders that lead to sleep disruption or trigger arousals could precipitate parasomnias in children. Guilleminault et al. reported improvement in PLMI from 11 to 0.2 events/h and from 16 to 0.2 events/h in the 2 participants treated with pramipexole.2 In our cohort, there were several interesting findings regarding PLMS and the EEG features associated with PLMS. There was a 71.3% reduction in PLMS-related arousals and a 63.5% reduction in the PLMS-related microarousals following iron supplementation, which is supportive of an overall improvement in sleep continuity. In addition to arousals, there was a 76.9% reduction in the number of PLMS related slow wave firings (K complexes and K-spindle complexes). This further supports an improvement in sleep continuity following iron supplementation given the likely role of K complexes in preserving sleep continuity.19 Finally, although there was a significant decrease in the total number of arousals as well as PLMS-related arousals following iron therapy, there was no change in the frequency of spontaneous arousals. This finding could suggest that in the setting of relative iron insufficiency PLMS-related arousals are secondary to the PLMS themselves (as opposed to the possibility that relative iron insufficiency results in a greater tendency for arousals events, which in turn trigger PLMS), although further studies would be needed to confirm this.
Although the EEG tracing was not available to review for every participant included in the cohort, the 69.4% reduction in the number of PLMS after iron therapy for the 14 participants included in the detailed PSG review was similar to the 61.0% reduction seen in the overall study population. The ratio of PLMS-related arousals to total PLMS seen in this cohort was similar to previous studies.20,21 There was no reduction in the amount of isolated limb movements following iron supplementation. This finding is of interest because it reinforces the importance of periodicity in the phenomenology of PLMD.
There are certain limitations in our study. First, this is a retrospective study so the time interval between each clinical visit and the duration from initiation of iron therapy to follow-up sleep study varies. Second, we assume that these participants were compliant with iron therapy as prescribed. However, increase in ferritin levels after therapy suggests that most patients were undergoing iron therapy. One of our participants had previously tried pramipexole and ropinirole before trying iron therapy, and this may contribute to improvement of parasomnias in this particular participant. Third, the timing of parasomnias improvement in our sleep study is variable from 3 to 6 months to 1 to 2 years. Although, parasomnias can improve over time as slow wave sleep decreases with age, it is unlikely that slow wave sleep percentage declines significantly within a 1- to 2-year period. Finally, 30% of the participants with RLS/PLMD and parasomnias treated with iron therapy also had sleep-disordered breathing. Although the sleep-disordered breathing was mild in our cohort, it may lead to sleep fragmentation and precipitation of parasomnias. However, it is unlikely that sleep-disordered breathing will respond to iron therapy
In conclusion, our study has demonstrated that parasomnias frequently occur concurrently with RLS/PLMD. Oral iron therapy was associated with a reduction of PLMS and PLMS-related arousal indices, and improvement in RLS symptoms, and resolution of a significant proportion of parasomnias in this cohort suggests that RLS/PLMD may be a causative factor in the development of parasomnia. Larger-scale prospective studies are needed to confirm the association between RLS/ PLMD and parasomnias in children.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This research was previously presented at SLEEP 2015, the annual meeting of the APSS, Seattle, Washington. This study was supported by the Cincinnati Children's Hospital Research Fund. The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- CCHMC
Cincinnati Children's Hospital Medical Center
- ICSD
International Classification of Sleep Disorders
- PLMD
periodic limb movement disorder
- PLMI
periodic limb movement index
- PLMS
periodic limb movements in sleep
- PSG
polysomnography
- REM
rapid eye movement
- RLS
restless legs syndrome
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Guilleminault C, Palombini L, Pelayo R, Chervin RD. Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics. 2003;111(1):e17–e25. doi: 10.1542/peds.111.1.e17. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer LJ, Mindell JA. Sleep and sleep disorders in children and adolescents. Psychiatr Clin North Am. 2006;29(4):1059–1076. doi: 10.1016/j.psc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 4.De Gennaro L, Ferrara M, Bertini M. EEG arousals in normal sleep: variations induced by total and selective slow-wave sleep deprivation. Sleep. 2001;24(6):673–679. doi: 10.1093/sleep/24.6.673. [DOI] [PubMed] [Google Scholar]
- 5.Picchietti DL, Bruni O, de Weerd A, et al. Pediatric restless legs syndrome diagnostic criteria: an update by the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(12):1253–1259. doi: 10.1016/j.sleep.2013.08.778. [DOI] [PubMed] [Google Scholar]
- 6.Dye TJ, Jain SV, Simakajornboon N. Outcomes of long-term iron supplementation in pediatric restless legs syndrome/periodic limb movement disorder (RLS/PLMD) Sleep Med. 2017;32:213–219. doi: 10.1016/j.sleep.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 8.Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med. 2007;3(2):133–145. [PubMed] [Google Scholar]
- 9.Martin SE, Engleman HM, Kingshott RN, Douglas NJ. Microarousals in patients with sleep apnoea/hypopnoea syndrome. J Sleep Res. 1997;6(4):276–280. doi: 10.1111/j.1365-2869.1997.00276.x. [DOI] [PubMed] [Google Scholar]
- 10.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 11.Poyares D, Guilleminault C, Rosa A, Ohayon M, Koester U. Arousal, EEG spectral power and pulse transit time in UARS and mild OSAS subjects. Clin Neurophysiol. 113(10):1598–1606. doi: 10.1016/s1388-2457(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 12.Mathur R, Douglas NJ. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep. 1995;18(5):330–333. doi: 10.1093/sleep/18.5.330. [DOI] [PubMed] [Google Scholar]
- 13.Ferri R, Manconi M, Arico D, et al. Acute dopamine-agonist treatment in restless legs syndrome: effects on sleep architecture and NREM sleep instability. Sleep. 2010;33(6):793–800. doi: 10.1093/sleep/33.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrino L, Boselli M, Buccino GP, Spaggiari MC, Di Giovanni G, Terzano MG. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol. 1996;13(4):314–323. doi: 10.1097/00004691-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26(6):735–738. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- 16.Starn AL, Udall JN., Jr Iron deficiency anemia, pica, and restless legs syndrome in a teenage girl. Clin Pediatr (Phila) 2008;47(1):83–85. doi: 10.1177/0009922807303930. [DOI] [PubMed] [Google Scholar]
- 17.Grim K, Lee B, Sung AY, Kotagal S. Treatment of childhood-onset restless legs syndrome and periodic limb movement disorder using intravenous iron sucrose. Sleep Med. 2013;14(11):1100–1104. doi: 10.1016/j.sleep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz K, Kilincaslan A, Aydin N, Kor D. Prevalence and correlates of restless legs syndrome in adolescents. Dev Med Child Neurol. 2011;53(1):40–47. doi: 10.1111/j.1469-8749.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 19.Cash SS, Halgren E, Dehghani N, et al. The human K-complex represents an isolated cortical down-state. Science (New York, NY) 2009;324(5930):1084–1087. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005;6(3):259–267. doi: 10.1016/j.sleep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51(3):103–107. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]