ABSTRACT
Cable bacteria belonging to the family Desulfobulbaceae couple sulfide oxidation and oxygen reduction by long-distance electron transfer over centimeter distances in marine and freshwater sediments. In such habitats, aquatic plants can release oxygen into the rhizosphere. Hence, the rhizosphere constitutes an ideal habitat for cable bacteria, which have been reported on seagrass roots recently. Here, we employ experimental approaches to investigate activity, abundance, and spatial orientation of cable bacteria next to the roots of the freshwater plant Littorella uniflora. Fluorescence in situ hybridization (FISH), in combination with oxygen-sensitive planar optodes, demonstrated that cable bacteria densities are enriched at the oxic–anoxic transition zone next to roots compared to the bulk sediment in the same depth. Scanning electron microscopy showed cable bacteria along root hairs. Electric potential measurements showed a lateral electric field over centimeters from the roots, indicating cable bacteria activity. In addition, FISH revealed that cable bacteria were present in the rhizosphere of Oryza sativa (rice), Lobelia cardinalis and Salicornia europaea. Hence, the interaction of cable bacteria with aquatic plants of different growth forms and habitats indicates that the plant root–cable bacteria interaction might be a common property of aquatic plant rhizospheres.
Keywords: cable bacteria, filamentous bacteria, aquatic plants, rhizosphere, oxygen distribution, electric field
Cable bacteria are ubiquitous and active in the rhizosphere of aquatic plants.
INTRODUCTION
In water-saturated, organic-rich sediments, oxygen from the water column does not diffuse beyond the uppermost millimeters, whereas deeper sediments often contain millimolar concentrations of sulfide. Aquatic plants adapt to survive in such anoxic sediments by an extensive system of gas spaces supplying oxygen from photosynthesis to the roots (Armstrong et al. 1996). Moreover, aquatic plants can release oxygen into the rhizosphere—the zone surrounding the roots—producing geochemical gradients that are subject to daily fluctuations as photosynthesis ceases during night and the oxygenated zone diminishes (Lenzewski et al. 2018). In the oxygenated zone, abiotic and biotic sulfide oxidation play an essential role in protecting the roots against phytotoxic sulfide (Sand-Jensen, Prahl and Stokholm 1982; Jensen, Kühl and Priemé 2007; Lamers et al. 2013; Brodersen et al. 2018).
Sulfide-oxidizing cable bacteria are filamentous, multicellular members of the family Desulfobulbaceae (Pfeffer et al. 2012), which live in marine and freshwater sediments and may extend several centimeters from the oxic surface into the sulfidic sediment. By means of internal electron conduction, they couple the oxidation of sulfide at their deep, anodic end to the reduction of molecular oxygen or nitrate at the cathodic end on the sediment surface (Pfeffer et al. 2012; Marzocchi et al. 2014). The unique morphology and metabolism of cable bacteria can be used to determine their identity and activity in sediments. Strings underneath the outer membrane of cable bacteria run along the length of the filaments, which give rise to their resilient structure and characteristic ridges (Jiang et al. 2018). Moreover, the transport of charges by cable bacteria causes an electric field, which can be measured as an increase of electric potential with depth (Risgaard-Petersen et al. 2014). The electric field drives an ion current in the pore water, completing the electric circuit and maintaining a charge balance. The activity of cable bacteria strongly impacts the geochemistry of the sediment (Risgaard-Petersen et al. 2012), which leads to characteristic geochemical fingerprints: a pH maximum in the oxic zone due to the cathodic oxygen reduction and a decreased pH in the sulfidic zone due to the anodic sulfide oxidation. The lower pH in deeper layers likely promotes iron-sulfide dissolution and, hence, replenishes the electron donor sulfide. An additional source of sulfide replenishment to sustain cable bacteria metabolism can be sulfide diffusion from deeper sediment layers or local sulfate reduction (Nielsen and Risgaard-Petersen 2015). The spatial separation of the two redox reactions causes a suboxic zone of several centimeters that is devoid of measurable sulfide and oxygen (van de Velde et al. 2016). Consequently, cable bacteria have a competitive advantage over unicellular sulfide oxidizers, which can thrive only where the gradients of sulfide and oxygen meet. Furthermore, the large span of the cable bacteria makes them tolerant to fluctuating gradients of sulfide and oxygen, and, if needed, they can move to new locations by gliding motility (Bjerg et al. 2016). Cable bacteria have a global distribution in marine and freshwater sediments (Trojan et al. 2016; Burdorf et al. 2017), including the surface of oxygen-releasing seagrass roots, where they have been proposed to protect the plant against toxic sulfide (Martin et al. 2018).
This study aimed at testing not only the presence but also the activity and spatial arrangement of cable bacteria around oxygen-releasing roots. We hypothesize that cable bacteria actively perform sulfide oxidation in the rhizosphere of marine and freshwater aquatic plants extending to centimeters away from the oxygen-releasing roots. Thus, these filamentous bacteria conserve energy from oxidation of the electron donor sulfide, produced by sulfate-reducing bacteria in bulk sediments, and reduction of the electron acceptor oxygen, released from the plant roots. Employing microscopic techniques, we investigated the activity and localization of cable bacteria in the rhizosphere of Littorella uniflora, which is a small, evergreen, freshwater plant known to release oxygen from the roots (Christensen, Revsbech and Sand-Jensen 1994). Furthermore, we studied the presence of cable bacteria in the rhizosphere of rice, Lobelia cardinalis and Salicornia europaea, to assess whether the relationship between cable bacteria and aquatic plants could be a common phenomenon.
MATERIALS AND METHODS
Plants and growth conditions
The freshwater plants Li . uniflora and Lo. cardinalis (Zoo Zajac, Duisburg, Germany) were grown in a 20-L tank filled with tap water and a layer of quartz sand. Light was supplied in 11 h photoperiods by LED lamps (Classic LED Aquarium Light; Nicrew, Shenzhen City, China).
The halophyte S. europaea was sampled from Aggersund, Denmark (57° 0'1.08‘N, 9°17'19.10’E) using a sediment corer. Plants were rinsed in seawater before measurements.
Rice seeds (Oryza sativa) were soaked in water for 24 h and grown in drained soil pots before planting in bowls with a sieved, nutrient-rich and reduced sediment from the banks of river Giber Å, Denmark. The sediment was covered with 1-cm tap water. Plants received daylight, were kept at room temperature and grown for more than 3 months. Subsequently, the rice plants were transferred into a sediment that had been kept anoxic for more than 2 months, and were illuminated by LED lamps (Reflector PAR38; Megaman, Hong Kong, China) for 4 weeks until sampling.
Rhizo-sandwich
The rhizo-sandwich set-up was used to visualize oxygen dynamics by planar optode measurements, determine electric fields by electric potential profiling, and sample in precise spots for scanning electron microscopy (SEM) and quantification of cable bacteria with fluorescence in situ hybridization (FISH). The sediment was retrieved from an exposed mud flat in the Giber Å stream system at Vilhemsborg, Denmark (56° 4'3.78‘N, 10°11'9.04’E) at a temperature of 5°C and conductivity of the pore water of 651 µS/cm. There was no odor of H2S, but the blackish color indicated the presence of iron sulfides. Geochemical characteristics of comparable sediments from stream banks 500 m upstream were described previously (Risgaard-Petersen et al. 2015). The sediment was sieved with a mesh size of 0.5 mm and stored in an anaerobic chamber at room temperature for a minimum of 2 weeks to minimize bioturbation during the experiment.
To construct the rhizo-sandwich (Fig. S1A, Supporting Information), microscope slides were glued with silicone sealant (734 Clear; Dow Corning, Midland, Michigan, USA) to the edges of a glass plate (24.5 × 14 cm2) leaving one side open. A planar optode foil was attached to the inside of the glass plate (Brodersen et al. 2017). The sediment was spread out and roots of Li. uniflora were placed on the sediment before closing with a second glass plate, clamping and tightening with modeling clay. The sides were covered with an opaque plastic foil and incubated at room temperature in a continuously mixed tap water tank (58 × 29 × 30 cm3). The rhizo-sandwiches were illuminated on a 10:14 h light–dark cycle by an LED lamp (Reflector PAR38; Megaman, Hong Kong, China) and 300 µmol photons/m2 reached the canopy of the plants.
Root-sandwich
In contrast to the rhizo-sandwich, which includes the entire below-ground biomass and sediment, the root-sandwich contains only single plant roots. The root-sandwich maintained the rhizosphere spatially intact while sampling and preparing for FISH analysis.
Roots of Li. uniflora and Lo. cardinalis were aligned on a microscope slide (75 × 26 mm) with cover slips as spacers (Figs S1B and S2A, Supporting Information). A suspension of 10 mM iron sulfide (Müller et al. 2016) and the iron-reducing culture 1MN, containing groundwater cable bacteria belonging to the family Desulfobulbaceae up to 95% of the microbial community (Marozava et al. 2018), was added before closing with a second microscope slide and clamping with hair clips. Plants were placed on top of the sediment and sand was filled up to the leaf sheaths, covering both roots and microscope slides. The jar was filled with a 6-cm sediment overlaid with 4 cm of water phase fully submerging the plants. To further stimulate the microbial community, the sediment was inoculated with the culture 1MN by using a syringe. The plants were illuminated on a 10:14 h light–dark cycle by a fluorescent tube (Flora Sun; Zoo Med, USA).
After 13 days, the root-sandwiches containing a single root were removed, wrapped in an aluminum foil, and shock-frozen in liquid nitrogen. A frozen sandwich was placed with the flat side on a laboratory bench so that the lower slide melted while the upper remained frozen. The frozen side containing the root was pulled away, frozen again, covered with pre-cooled glutaraldehyde (final concentration ∼ 2.5%) avoiding rapid thawing and dried at 50°C for 3 h to prepare for FISH analysis. A root-sandwich containing a dissected root (no contact through plant tissue to the water column) was taken as a control.
The Desulfobulbaceae-enriched culture 1MN was cultivated in 50 mL freshwater mineral medium (Widdel and Bak 1992) in 125-mL serum bottles sealed with butyl rubber stoppers under anoxic conditions. The culture was grown with initial concentrations of 30 mM S0, 30 mM Fe(OH)3 and 0.7 mM HS−, with 30 mM bicarbonate buffer at pH 6.5. Quartz sand (400 mL, grain size 0.0036–1 mm, porosity 0.2) was washed thrice with tap water, amended with 3 µmol/cm3 FeS and filled into a 2-L glass jar.
Planar optode measurements
A fluorescence imaging-based planar optode system (Larsen et al. 2011; Brodersen et al. 2017) was used to visualize the oxygen dynamics in the rhizosphere of Li. uniflora and S. europaea. An amount of 1.5 mg of platinum(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin (PtTFPP; Frontier Scientific, Logan, Utah, USA), 1.5 mg of Macrolex fluorescence yellow (Lanxess, Köln, Germany) as well as 100 mg of polystyrene (Sigma-Aldrich, Taufkirchen, Germany) were dissolved in 1 g of chloroform and knife-coated (∼12 µm thickness) on a dust-free PET foil (Puetz Folien, Taunusstein, Germany). On top of that an optical isolation layer, consisting of 100 mg of carbon black (Kremer Pigmente, Aichstetten, Germany) in silicone (ELASTOSIL E4, Wacker, München, Germany), was coated (Larsen et al. 2011). The foil was attached with tap water to the rhizo-sandwich slide (Li. uniflora) or a transparent acrylic chamber (10 × 10 × 29 cm3) (S. europaea) and a vinyl electrical tape (Super 33+; Scotch, Minnesota, USA) was used to seal the edges. Roots of S. europaea were held close to the planar optode with 5-mm-wide plankton net stripes while the seawater and sediment from the sampling site was carefully added. The water column above the sediment was approximately 2 cm, submersing most of the biomass. The set-up was equilibrated for 10 h in the dark and 1 h in the light before optode measurements.
The planar optode was excited with an LED lamp (i-led, ILH-GD01-DEBL-SC201; r-s components, Copenhagen, Denmark) powered by a USB-controlled LED driver unit (http://imaging.fish-n-chips.de). Images were taken with an SLR camera (EOS 1300D; Canon, Tokio, Japan) equipped with a macro objective (100mm f/2.8 AT-X M100 AF Pro D; Tokina, Tokio, Japan) and a 530-nm-long pass filter (52 mm x 2 mm). LED excitation and image acquisition were controlled and synchronized with the software look@RGB (http://imaging.fish-n-chips.de). Pieces of the used sensor foils were calibrated in similar tanks with tap water and seawater from the sampling site by bubbling with air and N2 (Na2SO3 was added to obtain fully anoxic conditions). O2 concentrations within the tank were simultaneously monitored with a calibrated oxygen meter (FireStingGO2 equipped with a robust sensor probe; Pyroscience, Aachen, Germany) and expressed as % air saturation.
Image processing was performed with ImageJ (https://imagej.nih.gov/ij/) using the plugin Ratio Plus (https://imagej.nih.gov/ij/plugins/ratio-plus.html). The image resolution was 40 pixels/mm. Due to drifts throughout the prolonged incubation of the sample (>2 weeks), it was necessary to correct the images. This was achieved by assuming that the bulk sediment was anoxic at all times. Hence, the baseline of the concentration profiles was corrected by subtracting the lowest value in the reduced zone.
Optode imaging of the rhizosphere of Li. uniflora was conducted continuously over one light–dark shift in 20 min intervals and over one dark–light shift in 10 min intervals.
FISH analysis
The entire rhizo-sandwich, including one intact plant of Li. uniflora, was shock-frozen in liquid nitrogen. Upon thawing, one glass plate could be removed while the sediment remained frozen to the other before it thawed completely. Oxidation of the sediment led to the formation of visible bright shaded zones millimetres in width and thus allowing for sampling in defined areas. Samples were taken from the oxidized rhizosphere and top sediment layer (bright) and the reduced rhizosphere and top sediment layer (dark) 0–5 mm distant from the oxidized zone. Furthermore, samples of bulk sediment were taken, defined as >4 cm away from the oxidized rhizosphere and >2 cm from the water–sediment interface. Sediment samples (15–80 µL) were fixed in 96% ethanol and stored at 20°C.
The rhizosphere of S. europaea was sampled as a sediment core containing an entire plant, drained and cut into slices. The bowl containing rice plants and the sediment was drained. Rice plants and the sediment were carefully lifted out and separated with gloved hands, leading to the emergence of roots partly covered with the sediment. The adhering sediment on the roots was taken off from the roots with a spatula tip, fixed in 96% ethanol and stored at −20°C.
Sediment samples were homogenized by mild ultrasound 3 × 20 s at intervals of 10 s (SonopulsHD 2070; Bandelin, Berlin, Germany). Aliquots were diluted 10-fold in 0.1% sodium pyrophosphate and 1% agarose was added in a ratio of 1:50 [v/v]. FISH was performed as described by Pernthaler et al. (2001) with the specific oligonucleotide probes DSB706 (Loy et al. 2002) and FliDSB194 (Müller et al. 2016) (Table S1, Supplementary Information) at a formamide concentration of 35% for the detection of filamentous Desulfobulbaceae. Samples were covered with Citifluor (Citifluor, Hatfield, Pennsylvania, USA), Vecta Shield (VWR, Søborg, Denmark) and counterstained with 1 mg/mL 4′,6-diamidin-2-phenylindol (DAPI). Rhizo-sandwiches, S. europaea, and rice samples were additionally hybridized with the oligonucleotide probes EUB-MIX (Amann et al. 1990; Daims et al. 1999). The negative control probe NON-EUB (Manz et al. 1992) was run along with each analysis. For the determination of the density of cable bacteria (m/cm3), the length of all positively stained filaments consisting of at least four cells was measured using the imaging software NIS-Elements (Version 4.50; Nikon Instruments Inc., NY, USA).
Electron microscopy
A root fragment of Li. uniflora from the rhizo-sandwich was washed in tap water, air dried on a silicon waver and analyzed by high-vacuum (13 pA) SEM (Versa 3D DualBeam; FEI, Hillsboro, Oregon, USA) operated in 5-kV mode.
Electric potential profiling
Electrical potentials were measured with a self-made microelectrode (Damgaard, Copenhagen K, Denmark; Risgaard-Petersen and Nielsen 2014), custom made millivoltmeter (Aarhus University, Aarhus C, Denmark) and 16-bit A/D converter (ADC-216; Unisense A/S, Aarhus N, Denmark). On days 16 and 23 of the incubation period, a rhizo-sandwich was transferred to a smaller water tank (36 × 15 × 37 cm3). The measurement was carried out at 15°C after acclimatization overnight and a prolonged light phase of several hours. A micromanipulator (Unisense, Aarhus N, Denmark) was used to profile in step sizes of 100 µm on day 16 and 50 µm on day 23 controlled with the software SensorTracePro (Unisense, Aarhus N, Denmark). The electrical potential microelectrode was manually adjusted in a horizontal motion to measure depth profiles in different distances away from the root (Fig. S3A, Supporting Information). Electric potential depth profiles were measured in a randomized order from bottom (32 mm depth) to top (5 mm above sediment) against a general-purpose reference electrode (REF201 Red Rod electrode; Radiometer Analytical, Copenhagen, Denmark). Profiles were recorded after shutting down the illumination and the water pump. Data processing included the discarding of data points below 30.5 mm to ensure the same depth of all profiles for plotting and the subtraction of the mean water-phase electric potential from all data points of each profile. Extracting the normalized data points of certain depths from all profiles on a respective day allowed plotting of the normalized electric potential as a function of distance away from the root.
RESULTS
Oxygen dynamics in the rhizosphere
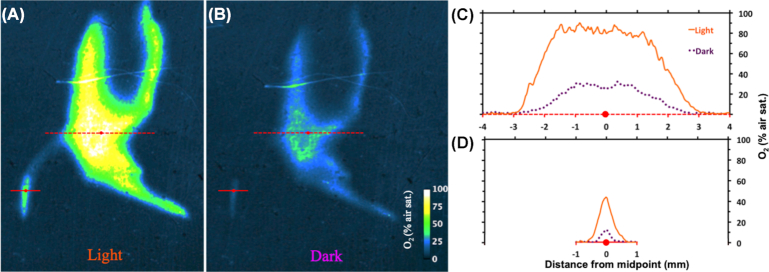
The rhizo-sandwich (Fig. S1, Supporting Information) was a set-up designed to investigate oxygen release from the roots. Parts of the rhizosphere of Li. uniflora were oxygenated throughout light and dark phases. O2 concentrations reached up to 90% saturation, where several roots were close together and up to 44% at a single root (Fig. 1). In the case of the root bundle, O 2 concentrations decreased from 90% saturation to anoxic conditions over 2.6 mm. The gradient for a single root decreased from 40% saturation to anoxic conditions within a distance of 0.6 mm. After 1 h in the dark, concentrations dropped to steady-state conditions of 12% and 30% O2 air saturation for the single root and the denser root bundle, respectively. After 40 min illumination, the maximal steady-state oxygen concentrations were reached again. Control samples of the root-sandwich revealed that the oxidation of the rhizosphere, indicated by red iron-hydroxide precipitations, solely originated from oxygen release of intact roots (Fig. S2B,C, Supporting Information).
Figure 1.
Oxygen dynamics in the rhizosphere of Li. uniflora between light (A) and dark (B) conditions. (C) Concentration profile crossing multiple roots (red dotted line, 8 mm). (D) Concentration profile crossing a single root (straight red line, 2 mm).
Furthermore, planar optode imaging showed that the rhizosphere of S. europaea was oxygenated with concentrations up to 68% air saturation during light exposure (Fig. S5A, Supporting Information).
Distribution of cable bacteria in the rhizosphere
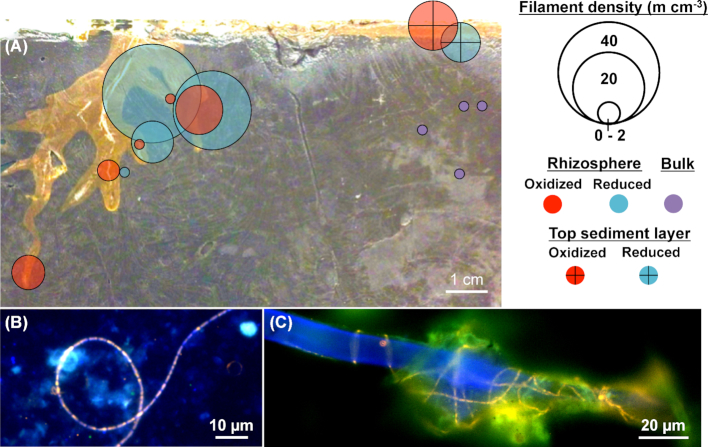
The rhizo-sandwich further allowed precise sampling of the rhizosphere of Li. uniflora. Cable bacteria were enriched above the background density in bulk sediments in the reduced zone near the top (7 m/cm3) and the roots (reduced rhizosphere) (2–39 m/cm3) as well as in the oxidized sediment at the top (11 m/cm3) and rhizosphere (2–10 m/cm3) (Fig. 2, Fig. S4, Supporting Information). Cable bacteria in the rhizosphere were observed as single filaments and as aggregates of multiple filaments, intertwined with root hairs (Fig. 2B and C). In contrast, the root-sandwich allowed us to determine the undisturbed spatial arrangement of cable filaments in the rhizosphere as FISH analysis was carried out directly on the microscope slide of the root-sandwiches. Cable bacteria were found next to roots of Lo. cardinalis (Fig. 3) and Li. uniflora (Fig. S2D, Supporting Information). Interestingly, cable filaments were patchily distributed rather than forming a continuous pattern, showing multiple hotspots along the root.
Figure 2.
FISH images and distribution of cable bacteria in the rhizo-sandwich of Li. uniflora. (A) Differentiation between oxidized (red) and reduced (blue) rhizospheres, oxidized (crossed red) and reduced (crossed blue) top sediment layers and bulk sediments (purple). The redox status was estimated from the shading of sediment (bright = oxidized, dark = reduced). The circle midpoints correspond to the sampling location and the circle areas to the cable bacteria density expressed in m/cm3. (B and C) FISH micrographs of cable filaments alone and intertwined with a root hair found in the reduced rhizospheres. Micrographs from DAPI staining (blue) were superimposed with FISH images hybridized with probe DSB706 specific for Desulfobulbaceae labeled with Cy3 (red) and probe EUB-MIX targeting most bacteria labeled with Atto-488 (green).
Figure 3.
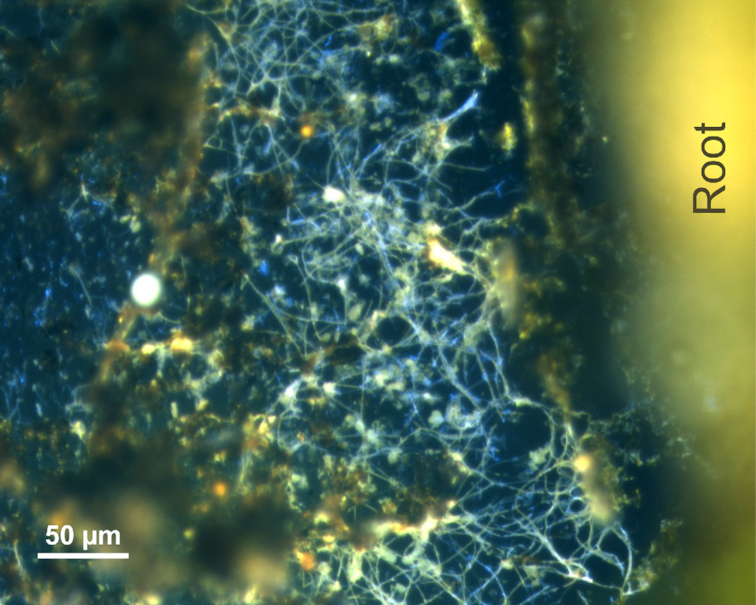
Overlay of micrographs from DAPI and FISH images of cable bacteria next to a root of Lo. cardinalis in the root-sandwich. Probe DSB706 specific for Desulfobulbaceae labeled with Cy3 (red) and probe FlidDSB194 specific for groundwater cable bacteria labeled with 6-Fam (green) were chosen for hybridization.
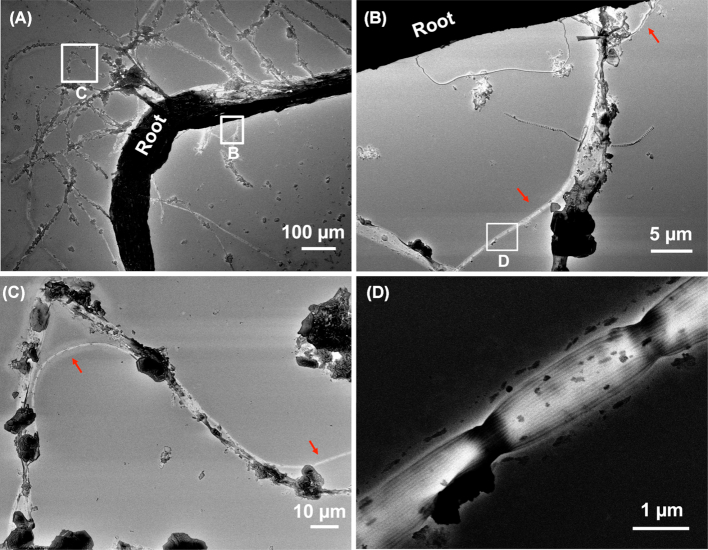
Furthermore, SEM images of washed root fragments from the rhizo-sandwich of Li. uniflora showed cable filaments being captured and intertwined in root hairs (Fig. 4B and C). The typical distinct ridges running along the filaments (Fig. 4D) confirmed the identity of the filaments as cable bacteria.
Figure 4.
SEM images of cable filaments in close association with a Li. uniflora root and root hairs. (A) Electron micrograph of a Li. uniflora root with root hairs. (Band C) Magnifications of squares in (A). Red arrows indicate cable filaments. (D) Magnification of the square in (B), showing ridges of the cable filament.
Cable filaments in the rhizosphere of S. europaea varied in diameter from 0.6 µm up to 2 µm (Fig. S5B, Supporting Information). Cable filaments were also found in the rhizosphere of rice plants (Fig. S5C, Supporting Information).
Electric potential profiling
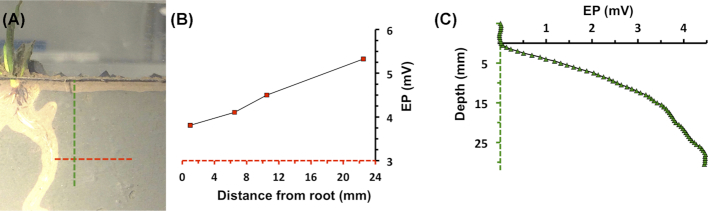
In order to assess the build-up of an electric field around the roots, electric potential profiles were recorded at different distances from the root of Li. uniflora in the rhizo-sandwich. Consistent with the cable bacteria distribution in the rhizosphere, an increase of 1.5 mV over a distance of 21.5 mm was measured on day 16, indicating that cable bacteria are active around the roots (Fig. 5B). On day 23, the electric potential increased by 3.4 mV over a distance of 8 mm from the root (Fig. S3C, Supporting Information). A distinct electric potential minimum was observed as the sensor passed close by the bending root at a depth of 20–26 mm (Figure S3B, Supporting Information). Probably, the sensor tip stroked the root, as it actually hit the root and broke in a subsequent effort to profile 1 mm closer. Furthermore, the electric potential increased with depth in all profiles (Fig. 5C, Fig. S3B, Supporting Information).
Figure 5.
Electric potential (EP) as a function of distance from the root and depth on day 16. (A) Picture of the Li. uniflora root, which was targeted for electric potential profiling. (B) Electric potential at different distances from the root at a depth of 23.5 mm. The red dotted line corresponds to the length of the red dotted line in (A). (C) Electric potential depth profile at a distance of 6 mm from the root. The green dotted line corresponds to the length of the green dotted line in (A).
DISCUSSION
In our experiments, we found cable bacteria next to the roots of the freshwater plant Li. uniflora, where molecular oxygen produced redox gradients (Figs 1 and 2). This oxygen release from roots is in accordance with earlier reports that Li. uniflora intensively oxygenates the rhizosphere (Christensen, Revsbech and Sand-Jensen 1994; Kofoed et al. 2012). Interestingly, the cable bacteria distribution in the rhizosphere indicated that most of the cells were located in the reduced sediment next to the oxidized rhizosphere. Similar spatial distributions of cable bacteria have been reported for marine sediments where the highest number of cable bacteria cells can be found in the suboxic zone beneath the oxic, top sediment layer (Larsen et al. 2015; van de Velde et al. 2016). In addition, cable bacteria were present in the rhizosphere of rice, one of the most important food crops, and S. europaea, a halophyte growing in coastal areas, where a high input of organic matter into sediments facilitates sulfide production by sulfate-reducing bacteria. The rhizosphere of rice (Larsen, Nielsen and Schramm 2015) and S. europaea (Fig. S5A, Supporting Information) gets oxygenated and therefore fulfills the basic prerequisite for a habitat of cable bacteria.
Cable bacteria were oriented laterally next to the roots of Lo. cardinalis (Fig. 3) and Li. uniflora (Fig. S2D, Supporting Information), which released oxygen along the entire roots. This spatial orientation of the cable bacteria suggests that they exploited sulfide sources in the bulk sediment. In contrast, Martin et al. (2018) found cable bacteria filaments along the surface of seagrass roots, where oxygen release was restricted to the root tips. The predominant source of sulfide for cable bacteria along these seagrass roots could have been sulfate reduction stimulated by the release of organic compounds from the roots, which possibly promoted a rather longitudinal orientation of the filaments along the surface of the roots.
In order to investigate a potential direct connection of cable bacteria to the root, SEM was performed with a washed root fragment of Li. uniflora. Despite earlier reports that Li. uniflora lacks root hairs (Søndergaard and Laegaard 1977; Farmer 1985; Beck-Nielsen and Vindbæk Madsen 2001), root hairs were clearly visible in our incubations (Fig. 4). Cable filaments were present along the root hairs and eventually pointing towards the root, suggesting a tight attachment. The SEM pictures suggest a close spatial interaction between the cable bacteria and roots or root hairs, which might be an adaption to small-scale, local redox gradients of oxygen and sulfide. The close interaction with root hairs and a possible direct connection to the root is supported by the observation that cable bacteria were predominantly found in the root hair region of seagrass roots (Martin et al. 2018). A direct connection of cable bacteria to surfaces was also shown in bioelectric systems where they were attaching with one end of the filament to an anode, forming a network of pilus-like nanofilaments (Reimers et al. 2017).
Upon dark–light shifts, the oxygen front around the roots changed with a speed of 0.3 mm/h, as shown for a single root of Li. uniflora. Cable bacteria filaments can grow centimeters in length (Pfeffer et al. 2012), which would span this whole fluctuating redox zone in the rhizosphere and allow for continuous sulfide oxidation around the root even though the redox gradients change. However, cable bacteria have a gliding motility of up to 2.9 mm/h and could also adapt to the diurnally moving oxygen gradients by chemotaxis (Bjerg et al. 2016).
Taken together, examples such as seagrass (Martin et al. 2018), S. europaea, Li. uniflora, Lo. cardinalis and rice highlight the capability of cable bacteria to exploit the rhizosphere of aquatic plants in sulfide-rich marine, but also in freshwater sediments, where cable bacteria might be involved in a cryptic sulfur cycle (Risgaard-Petersen et al. 2015) (Fig. 6).
Figure 6.
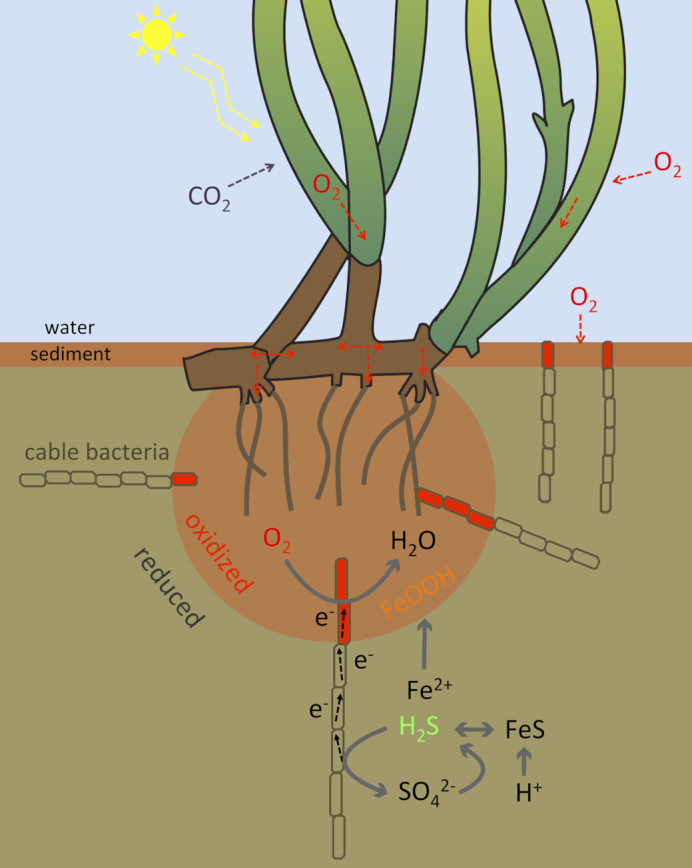
Conceptual diagram of cable bacteria in the rhizosphere. Cable bacteria reduce the oxygen released from the plant roots and oxidize sulfide. The sulfide gets replenished from local sulfate reduction, diffusion from deeper sediment layers and dissolution of iron sulfides promoted by the acidity-producing sulfide oxidation. The dissolved ferrous iron diffuses towards the oxygen-releasing roots, where it precipitates to iron oxide.
Beyond the mere presence, we also showed the activity of cable bacteria in the rhizosphere of Li. uniflora. Recent studies have shown that the activity of cable bacteria can be inferred from gradients of electric potential, which was measured by vertically pushing electric potential electrodes into the top sediment layer (Damgaard; Risgaard-Petersen and Nielsen 2014; Nielsen and Risgaard-Petersen 2015). An increase in electric potential in a horizontal manner at centimeter distances away from the roots was measured, indicating the establishment of an electric field caused by the activity of cable bacteria (Fig. 5B, Fig. S3C, Supporting Information). Hence, cable bacteria conduct electrons from the sulfidic zone to the rhizosphere, most likely by the oxidation of sulfide in the bulk sediment and the reduction of oxygen released from the roots. In addition, the sediments of the rhizo-sandwiches showed changes in electric potentials at the sediment surface (Fig. 5C, Fig. S3B, Supporting Information), indicating the activity of cable bacteria at the water–sediment interface. This implies that the dominant parameters determining the distribution of the cable bacteria in the rhizosphere are the geochemical gradients of sulfide and oxygen, although other plant-derived factors cannot be excluded.
The H2S oxidation of cable bacteria in the rhizosphere might lead to a protection of the plant root from exposure to the toxic sulfide and lead to a faster oxidation reaction compared to a pure abiotic chemical oxidation of sulfide by oxygen (Luther et al. 2011). Furthermore, the activity of cable bacteria strongly impacts the geochemistry of the sediment in the rhizosphere, potentially influencing nutrient cycling (Sulu-Gambari et al. 2016) and promoting iron-oxide precipitation (Risgaard-Petersen et al. 2012). Iron oxides react with free H2S by oxidizing H2S to elemental sulfur or by precipitation of FeS (Seitaj et al. 2015). Hence, cable bacteria may contribute to both immediate and long-term protection of roots against toxic, free sulfide.
In conclusion, our data indicate that cable bacteria are ubiquitous and active in the rhizosphere of aquatic plants where they take advantage of the electron acceptor oxygen released from the roots. The electric metabolism of cable bacteria presumably has a strong impact on rhizosphere geochemistry.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ian P.G. Marshall for valuable comments on the manuscript; Laurine Burdorf for instrumental advice; Lars B. Pedersen for producing the electric potential microelectrodes; Britta Poulsen and Pia B. Jensen for help with the visualization of cable bacteria; Andreas Schramm, Jesper T. Bjerg, Ugo Marzocchi, Nils Risgaard-Peterson, Lars R. Damgaard, Thomas Boesen and Ronny Mario Baaske for valuable discussions; and Caroline Surau for the determination of sediment parameters. We thank the Rønbjerg Microsensor Analysis in the Environmental Sciences course and especially Søren D. Nielsen, Andreas Greve, Andrea C. Sepúlveda and Elisa Merz for help with the S. europaea planar optode measurements. We acknowledge the provision of the plant illustration from Catherine Collier, Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/imagelibrary/).
FUNDING
This work was supported by the Poul Due Jensen Foundation, the Danish National Research Foundation [DNRF136] and the ERC advanced grant EcOILogy [Nr. 666 952].
Conflicts of interest. None declared.
REFERENCES
- Amann RI, Binder BJ, Olson RJ et al.. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM et al.. Pathways of aeration and the mechanisms and beneficial effects of humidity- and venturi-induced convections in Phragmitesaustralis (Cav.) Trin. ex Steud. Aquat Bot. 1996;54:177–97. [Google Scholar]
- Beck-Nielsen D, Vindbæk Madsen T. Occurrence of vesicular–arbuscular mycorrhiza in aquatic macrophytes from lakes and streams. Aquat Bot. 2001;71:141–8. [Google Scholar]
- Bjerg JT, Damgaard LR, Holm SA et al.. Motility of electric cable bacteria. J Appl Environ Microbiol. 2016;82: 3816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen KE, Koren K, Moßhammer M et al.. Seagrass-mediated phosphorus and iron solubilization in tropical sediments. Environ Sci Technol. 2017;51:14155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen KE, Siboni N, Nielsen DA et al.. Seagrass rhizosphere microenvironment alters plant‐associated microbial community composition. Environ Microbiol. 2018;20:2854–64. [DOI] [PubMed] [Google Scholar]
- Burdorf LD, Tramper A, Seitaj D et al.. Long-distance electron transport occurs globally in marine sediments. Biogeosciences. 2017;14:683. [Google Scholar]
- Christensen PB, Revsbech NP, Sand-Jensen K. Microsensor analysis of oxygen in the rhizosphere of the aquatic macrophyte Littorellauniflora (L.) Ascherson. Plant Physiol. 1994;105:847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daims H, Brühl A, Amann R et al.. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22:434–44. [DOI] [PubMed] [Google Scholar]
- Damgaard LR, Risgaard-Petersen N, Nielsen LP. Electric potential microelectrode for studies of electrobiogeophysics. J Geophys Res Biogeosci. 2014;119:1906–17. [Google Scholar]
- Farmer AM. The occurrence of vesicular-arbuscular mycorrhiza in isoetid-type submerged aquatic macrophytes under naturally varying conditions. Aquat Bot. 1985;21:245–9. [Google Scholar]
- Jensen SI, Kühl M, Priemé A. Different bacterial communities associated with the roots and bulk sediment of the seagrass Zosteramarina. FEMS Microbiol Ecol. 2007;62:108–17. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Zhang S, Klausen LH et al.. In vitro single-cell dissection revealing the interior structure of cable bacteria. Proc Natl Acad Sci. 2018;115:8517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoed MV, Stief P, Hauzmayer S et al.. Higher nitrate-reducer diversity in macrophyte-colonized compared to unvegetated freshwater sediment. Syst Appl Microbiol. 2012;35:465–72. [DOI] [PubMed] [Google Scholar]
- Lamers LP, Govers LL, Janssen IC et al.. Sulfide as a soil phytotoxin—a review. Front Plant Sci. 2013;4:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Borisov SM, Grunwald B et al.. A simple and inexpensive high resolution color ratiometric planar optode imaging approach: application to oxygen and pH sensing. Limnol Oceanogr. 2011;9:348–60. [Google Scholar]
- Larsen M, Santner J, Oburger E et al.. O2 dynamics in the rhizosphere of young rice plants (Oryza sativa L.) as studied by planar optodes. Plant Soil. 2015;390:279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Nielsen LP, Schramm A. Cable bacteria associated with long-distance electron transport in New England salt marsh sediment. Environ Microbiol Rep. 2015;7:175–9. [DOI] [PubMed] [Google Scholar]
- Lenzewski N, Mueller P, Meier RJ et al.. Dynamics of oxygen and carbon dioxide in rhizospheres of Lobeliadortmanna–a planar optode study of belowground gas exchange between plants and sediment. New Phytol. 2018;218:131–41. [DOI] [PubMed] [Google Scholar]
- Loy A, Lehner A, Lee N et al.. Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. J Appl Environ Microbiol. 2002;68:5064–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther GW, Findlay A, MacDonald D et al.. Thermodynamics and kinetics of sulfide oxidation by oxygen: a look at inorganically controlled reactions and biologically mediated processes in the environment. Front Microbiol. 2011;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz W, Amann R, Ludwig W et al.. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol. 1992;15:593–600. [Google Scholar]
- Marozava S, Mouttaki H, Müller H et al.. Anaerobic degradation of 1-methylnaphthalene by a member of the Thermoanaerobacteraceae contained in an iron-reducing enrichment culture. Biodegradation. 2018;29:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BC, Bougoure J, Ryan MH et al.. Oxygen loss from seagrass roots coincides with colonisation of sulphide-oxidising cable bacteria and reduces sulphide stress. ISME J. 2018;13:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzocchi U, Trojan D, Larsen S et al.. Electric coupling between distant nitrate reduction and sulfide oxidation in marine sediment. ISME J. 2014;8:1682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Bosch J, Griebler C et al.. Long-distance electron transfer by cable bacteria in aquifer sediments. ISME J. 2016;10:2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen LP, Risgaard-Petersen N. Rethinking sediment biogeochemistry after the discovery of electric currents. Annu Rev Mar Sci. 2015;7:425–42. [DOI] [PubMed] [Google Scholar]
- Pernthaler J, Glöckner F-O, Schönhuber W et al.. Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. J Microbiol Methods. 2001;30:207–26. [Google Scholar]
- Pfeffer C, Larsen S, Song J et al.. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491:218. [DOI] [PubMed] [Google Scholar]
- Reimers CE, Li C, Graw MF et al.. The identification of cable bacteria attached to the anode of a benthic microbial fuel cell: evidence of long distance extracellular electron transport to electrodes. Front Microbiol. 2017;8:2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risgaard-Petersen N, Revil A, Meister P et al.. Sulfur, iron-, and calcium cycling associated with natural electric currents running through marine sediment. Geochim Cosmochim Acta. 2012;92:1–13. [Google Scholar]
- Risgaard-Petersen N, Kristiansen M, Frederiksen RB et al.. Cable bacteria in freshwater sediments. J Appl Environ Microbiol. 2015;81:6003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risgaard-Petersen N, Damgaard LR, Revil A et al.. Mapping electron sources and sinks in a marine biogeobattery. J Geophys Res Biogeosci. 2014;119:1475–86. [Google Scholar]
- Sand-Jensen K, Prahl C, Stokholm H. Oxygen release from roots of submerged aquatic macrophytes. Oikos. 1982;38:349–54. [Google Scholar]
- Seitaj D, Schauer R, Sulu-Gambari F et al.. Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins. Proc Natl Acad Sci. 2015;112:13278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søndergaard M, Laegaard S. Vesicular–arbuscular mycorrhiza in some aquatic vascular plants. Nature. 1977;268:232–3. [Google Scholar]
- Sulu-Gambari F, Seitaj D, Meysman FJR et al.. Cable bacteria control iron-phosphorus dynamics in sediments of a coastal hypoxic basin. Environ Sci Technol. 2016;50:1227–33. [DOI] [PubMed] [Google Scholar]
- Trojan D, Schreiber L, Bjerg JT et al.. A taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema. Syst Appl Microbiol. 2016;39:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Velde S, Lesven L, Burdorf LD et al.. The impact of electrogenic sulfur oxidation on the biogeochemistry of coastal sediments: a field study. Geochim Cosmochim Acta. 2016;194:211–32. [Google Scholar]
- Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteriaIn: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH(eds). The Prokaryotes. New York: Springer, 1992, 3352–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.