Abstract
Background
Myxoid liposarcoma (ML) exhibits a special sensitivity to trabectedin (T) and radiation therapy (RT). Preclinical data suggest a synergistic effect. We aimed to study safety, feasibility and activity of the administration of pre-operative concurrent T and RT in patients affected by localized resectable ML.
Methods
Patients received 3 cycles (C) of T in combination with RT (45 Gy) in 25 fractions (1.8 Gy/fraction). Dose Levels for T were: − 1 (1.1 mg/m2), 0 (1.3 mg/m2) and 1 (1.5 mg/m2). Primary endpoint was safety; antitumor activity was assessed by RECIST and Choi criteria. This study is registered at ClinicalTrials.gov, number NCT02275286. The phase 1 part of the study is complete and phase 2 is ongoing.
Findings
From February 2015 to May 2016, 14 patients (M/F 7/7), median age 36 years (range 24–70) and median tumor size 12.5 cm (range 7–17 cm), were enrolled. One dose limiting toxicity (G3 transaminitis) occurred at Level 0 and one (sepsis due to catheter infection) at Level 1. All patients completed RT. Five patients achieved PR (36%), 8 SD (57%), 1 distant PD (7%) by RECIST, while 12 achieved PR (86%), 1 SD (7%) and 1 distant PD (7%) by Choi criteria. Twelve patients underwent surgery. Median viable residual tumor was 5% (0–60).
Interpretation
T in combination with RT showed a favorable safety profile and antitumor activity in localized ML. T dose of 1.5 mg/m2 is the recommended dose for the phase 2 study, which is ongoing.
Funding
This study was partially supported by Pharmamar.
Keywords: Sarcoma, Myxoid Liposarcoma, Trabectedin, Radiotherapy, Chemotherapy, Neoadjuvant, Prognosis, Survival
Research in context
Evidence before this study
We searched PubMed for published preclinical or clinical studies about the combination of trabectedin (T) and radiotherapy (RT) in sarcomas from January 1997 to June 2018. We used the following search terms: “sarcoma”, “soft tissue sarcoma”, “myxoid liposarcoma”, “trabectedin”, “radiotherapy”, “neoadjuvant chemotherapy”, “neoadjuvant radiotherapy”, “phase I study”, “phase II study”. We identified only 2 preclinical studies suggesting a synergistic activity between the use of T and RT, based on the fact that T typically causes S blockade and G2M arrest and the latter is the most sensitive cell cycle phase to radiation treatment. There was no single published clinical study testing the feasibility and activity of this combination in patients affected by soft tissue sarcoma or localized myxoid liposarcoma.
Added value of this study
This is the first-in-human clinical study showing the feasibility of the concurrent administration of T and RT in patients affected by localized myxoid liposarcoma. No cumulative toxicity was shown and no treatment related deaths were recorded. The recommended T dose for the phase 2 study was 1.5 mg/m2, which is the same dose registered for the use of T alone. In addition 85% of the patients had Choi partial response and 75% had < than 10% residual visible tumor in the surgical specimen. A confirmatory phase 2 trial is ongoing.
Implications of all the available evidence
Neoadjuvant combined chemo-radiation therapy has been extensively explored in localized. STS. A recent randomized trial showed a benefit of neoadjuvant AI versus a histology-driven chemotherapy in terms of OS and RFS, thus pointing to efficacy of neoadjuvant treatment as such in a subset of STS. In the myxoid liposarcoma stratum of this trial, the interim analysis showed the non-inferiority of T, with a much better toxicity profile. This study is still recruiting in the myxoid liposarcoma stratum and is expected to complete the accrual in a year. It is thus possible that the efficacy of T be confirmed to be not inferior to that of AI. The results of this phase I study provide the basis for the combination of T and RT for the phase II part, since no relevant toxicity was observed and both radiological and pathologic assessments of response indicate a synergy between both modalities. In the near future T combined with radiation therapy may become a standard neoadjuvant therapy for a subset of localized ML.
Alt-text: Unlabelled Box
1. Introduction
Myxoid liposarcoma (ML) is a rare soft tissue sarcoma (STS) subtype, accounting for 30% of all Liposarcoma and 10% of all STS [1], [2]. It arises predominantly in the extremities of young adults and is characterized by a balanced translocation, most commonly t(12;16)(q13;p11) fusing FUS (also named as TLS) with CHOP (also named as DDIT3) and rarely t(12;22)(q13;q12), where EWS (Ewing's Sarcoma protein) substitutes for its homologous FUS [3]. The classic myxoid variant shows low cellularity, conspicuous vascular network and myxoid stroma, whereas the cellular variant shows high cellularity made up of closely packed roundish cells, little or no intervening stroma and a capillary pattern not easy to be visualized. Diagnosis of cellular variant requires the presence of a cellular component exceeding 5% [2], [4]. This amount is of prognostic relevance as within the ML spectrum, the 5-year survival varies between 20 and 70% and the cellular variant falls in the shortest figures.
Evidence of a significant activity of both anthracycline and trabectedin (T) as well as radiotherapy (RT) has been consistently reported in patients affected by metastatic ML [5], [6], [7].
A phase II study on neoadjuvant T in 23 patients affected by localized resectable ML showed a 24% response rate by RECIST and a 13% pathological complete response in the surgical specimen with a very favorable toxicity profile [8].
Some preclinical data of the synergistic activity of RT and T have been reported [9] based on the fact that T typically causes S blockade and G2/M arrest and it is well known that this latter is the most sensitive cell cycle phase to radiation treatment [10].
Aim of the present study was to test the safety, feasibility and activity of the administration of pre-operative T with concurrent RT in patients affected by localized resectable ML. This study included also a separate group of metastatic STS patients, where the same was tested to treat metastatic STS to the lung not suitable to surgical resection. We present herein data from the phase 1 part of the study limited to the cohort of localized ML patients. The phase 2 part of the study is ongoing.
2. Methods
2.1. Study Design and Participants
This is an international, open label, phase 1 study aimed at testing the safety and feasibility of the concurrent administration of T and RT and finding the appropriate T dose to use in the phase 2 part of the study. Patients were enrolled in 4 centres in Spain, 1 in Italy and 2 in France.
Patients were eligible if they were aged between 18 and 70 years; had a histologically proven and centrally reviewed (before inclusion) diagnosis of localized resectable ML, originating in an extremity or trunk wall, with a longest diameter ≥ 5 cm if deeply or 10 cm if superficially located relative to the investing fascia; had an Eastern Cooperative Oncology Group performance status equal or less than 1; had adequate baseline bone marrow (white blood cell count > 3000 cells per μL, neutrophil > 1500 cells per μL, platelets > 100,000 platelets per μL, and haemoglobin > 10 g/L), renal function (creatinine < 1·6 mg/dL), hepatic function (total bilirubin ≤ upper normal value [UNV], transaminase and phosphatase alkaline ≤ 2·5 the UNV), creatine phoshokinase (≤ 2·5 the UNV) and cardiac function (left ventricular ejection fraction > 50%).
Patients were ineligible if they had unresectable disease with limb sparing surgery or distant metastases; had disease located outside the extremities or trunk wall; had other malignancies within the past 5 years, with the exception of carcinoma in situ of cervix and basocellular skin cancers treated with eradicating intent; had previous T or RT involving the tumor bed; had serious psychiatric disease; had medical disease limiting survival to less than 2 years; had cardiovascular diseases resulting in a New York Heart Association Functional Status of 2 or higher; or had uncontrolled bacterial, viral, or fungal infection. Other inclusion and exclusion criteria can be found in the appendix.
The trial protocol and all amendments were approved by the appropriate institutional review board or independent ethics committee at each trial centre. The trial was done in accordance with the provisions of the Declaration of Helsinki. All patients provided written informed consent before enrolment. The full protocol is available in the appendix.
2.2. Procedures
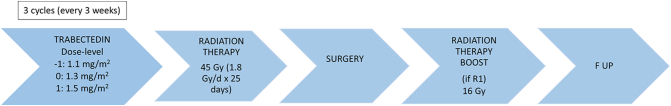
The dose escalation rules proceeded according to the traditional 3 + 3 design [11] being the prespecified dose-limiting toxicity (DLT) as follows: neutrophil count < 0.5 × 109/L during ≥ 5 days, febrile neutropenia, platelet count < 50 × 109/L and non-haematological toxicity of grade 3 or above, excluding nausea/vomiting without appropriate antiemetic treatment and grade 3 transaminitis if not leading to T delay. Biochemistry and full blood count was performed on day 1 and weekly, in the two first cycles at least. Patients received 3 cycles of T in combination with RT (total dose of 45 Gy) in 25 fractions (1.8 Gy/fraction). For those patients with micro or macroscopic positive margins after surgery, a boost of post-op RT (total dose 16 Gy) in 8 fractions (2 Gy/fraction) was allowed (Fig. 1).
Fig. 1.
Outline of the trial design.
T was administered intravenously as a 24 h infusion every 3 weeks. The planned dose-levels for T were as follows: − 1 (1.1 mg/m2), 0 (1.3 mg/m2) and 1 (1.5 mg/m2). Oral dexamethasone 4 mg was administered 24 h and 12 h before chemotherapy administration and i.v. dexametasone 20 mg was given 30 min before treatment, as previously described to minimize toxicity [12]. Administration of T through a central venous catheter was mandatory, due to fibrotic phlebitis encountered during peripheral administration. The starting dose-level for T, 1.3 mg/m2 was chosen based on previous accumulated experience, since this dose had shown activity in the context of myxoid liposarcoma.
RT started within 1 h after completion of the first T infusion (cycle 1 day 2), and was given daily for 25 days. Patients were treated with 3D conformal radiotherapy (3D-CRT) or intensity modulated radiotherapy (IMRT). Gross tumor volume (GTV) included gross tumor defined in the planning CT or contrast enhanced MRI T1 images. Clinical target volume (CTV) included GTV plus longitudinal margins (proximal and distal) of 5 cm and a radial margin of 2 cm. If after applying these margins the field extended beyond an intact fascia, the field could be shortened to include the entire compartment. Planning target volume (PTV) included the CTV plus an additional margin to compensate for organ motion and set-up uncertainties. Typically, this was CTV + 0 .5cm.
In the interval period, the tests were repeated at the discretion of the treating physician. We graded toxic effects using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Acute T mediated cardiac toxicity was not expected at protocol specified dose. However, an intermediate LVEF evaluation was mandatory within 2 weeks after last day of RT treatment with the same method as in the initial evaluation. If a decrease > 15% was observed the patient was to be withdrawn from the study. If a patient experienced a grade > 1 toxicity and did not recover to grade ≤ 1 after a maximum 3-weeks delay, the patient was to be withdrawn from the study.
Cardiac toxicities grade ≥ 4 were considered SUSAR (suspected unexpected serious adverse reaction) and should have been reported in less than 24 h.
We assessed response according to Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 [13] and Choi criteria [14] at the time of surgery with contrast-enhanced MRI or CT scan, or both, in all patients. A central radiological review of responses was performed in all cases at the Fondazione IRCCS Istituto Nazionale dei Tumori – Milan – Italy.
Surgery was planned 3–4 weeks after the administration of last preoperative cycle and not until 4 weeks after the end of preoperative RT. Following the resection, pathologic specimens were mapped in tumor sections to estimate the histologic changes and viable tumor after neoadjuvant treatment.
We followed up patients every 4 months with chest and abdominal CT and MRI or CT of the affected site.
2.3. Outcomes
Aim of the study was to confirm that the combination of T plus RT is feasible and safe and shows potential synergic activity with low toxicity.
The primary end-point was to describe the safety profile of the combination of T plus RT in order to find the recommended T dose for the phase II part of the study.
Secondary end-points were radiological response, measured by RECIST 1.1 and Choi criteria, and pathological response; recurrence free survival (RFS), disease free survival (DFS) and overall survival (OS); quality of Life measured by QLQ-C30 EORTC questionnaire.
2.4. Statistical Analysis
We did a 3 + 3 dose escalation with review of available safety before initiating the next dose level. Sample size for dose escalation was based on the 3 + 3 design rules with three to six patients per dose level. Three patients were treated at a given dose level. If at least 2 patients were observed to have dose-limiting toxicity (DLT), the prior dose level was defined as the maximum tolerable dosage (MTD) (unless only 3 patients have been treated at that level, in which case it is the tentative MTD). If 0 of the 3 patients were observed to have DLT, the dose level was escalated one step for the next cohort of 3 patients, and the process continued as above. If exactly 1 of the 3 patients treated showed DLT, 3 additional patients were treated at the current dose level. If none of these additional 3 patients showed DLT, the dose level was escalated for the next cohort of 3 patients, and the process continues as above; otherwise, the prior dose level was defined as the MTD.
The dose-escalation levels were: − 1: Trabectedin 1.1 mg/m2; 0: Trabectedin 1.3 mg/m2 and 1: Trabectedin 1.5 mg/m2. The starting dose-level was 1.3 mg/m2 and it was designed a de-escalation dose (− 1) of 1.1 mg/m2 in the case of observing more than one DLT over 6 patients in the starting dose, and one escalation dose (1) at 1.5 mg/m2, the ceiling dose recommended for trabectedin, in case of finding ≤ 1 DLT in the starting dose. It was not allowed de-escalating or escalating the dose on an individual basis, beside the defined dose reductions according to the protocol.
Dose reductions were done taking into account the highest toxicity in the prior cycle. Only two dose reductions were allowed in the same patient, except in cases of evident clinical benefit for the patient however, discussion with and approval by the steering committee (SC) and the Sponsor were mandatory. Once a dose reduction had taken place, re-escalation was not permitted in subsequent cycles. Every dose-reduction would imply decrease the dose in 0.2 mg/m2.
Haematological toxicity: With any of the following toxicities reduced one dose level:
-
•
Neutropenia < 0,5 × 109/l, for more than 5 days
-
•
Febrile neutropenia
-
•
Thrombocytopenia < 25 × 109/l
Non-haematological toxicity: With any of the following toxicities reduced one dose level:
-
•
Vomiting ≥ grade 3 in spite of adequate treatment
-
•
Transaminases elevation ≥ grade 3, not recovering to grade 1 previous to T administration.
-
•
Alkaline phosphatase (liver fraction) or bilirubin ≥ grade 1 at any time during treatment.
-
•
Other toxicities ≥ grade 3.
Safety and efficacy analyses were done on all patients receiving at least one cycle of trabectedin.
Treatment related adverse events (AEs) were those reported as possibly or probably related to treatment by the treating physician. Analysis AEs included any unfavorable and unintended sign (including abnormal laboratory findings), symptom or disease temporally associated with T and/or RT.
Time to event variables (RFS, DFS and OS) were measured from the date of surgery for RFS, and from onset of trial therapy for DFS and OS and were estimated according to the Kaplan–Meier method.
This study is registered with ClinicalTrials.gov, number NCT02275286
2.5. Role of the Funding Source
The TRASTS trial was an international academic trial designed by the members of the steering committee of the trial who were appointed by the National Cooperative Sarcoma groups. No pharmaceutical company had any role in study design, data collection, data analysis, data interpretation, or writing of the report. PharmaMar provided the study drug T and a small support for data management. Data were collected by investigators and associated site personnel, analyzed by statisticians working in the independent trial centre of the Spanish Sarcoma Group (GEIS), and interpreted by the members of the steering committee. The corresponding author had final responsibility for the decision to submit for publication.
3. Results
From February 2015 to May 2016, 14 patients (M/F 7/7) with median age of 36-years (range 24–70) and median tumor size of 12.5 cm (range 7–20 cm) were enrolled. Clinical and pathological characteristics of the study cohort are listed in Table 1.
Table 1.
Patients and treatment characteristics.
| Global series (n = 14) | 1.3 mg/m2 dose (n = 7) | 1.5 mg/m2 dose (n = 7) | |
|---|---|---|---|
| Median age (range) | 36 (24–71) | 31 (24–71) | 53 (28–68) |
| Sex (M/F) | 7 (50%)/7 (50%) | 4 (57%)/3 (43%) | 3 (43%)/4 (57%) |
| ECOG PS - 0 - 1 |
9 (62%) 5 (38%) |
5 (71%) 2 (29%) |
4 (57%) 3 (43%) |
| Grade - 1 - 3 |
11 (79%) 3 (21%) |
7 (100%) 0 (0%) |
4 (57%) 3 (43%) |
| Location - Thigh - Leg - Buttock |
10 (71%) 3 (21%) 1 (7%) |
6 (86%) 0 (0%) 1 (14%) |
4 (57%) 3 (43%) 0 |
| Median size mm. (range) | 130 (70–200) | 130 (30–170) | 110 (57–200) |
| Depth - Deep - Superficial |
14 (100%) 0 |
7 (100%) 0 |
7 (100%) 0 |
| Radiotherapy dose - 45 G - 50 Gy |
13 (93%) 1 (7%) |
7 (100%) 0 |
6 (86%) 1 (14%) |
| Surgery - Yes - No (n = 2) |
12 (86%) 2 (14%)a |
6 (86%) 1 (14%) |
6 (86%) 1 (14%) |
| Surgical margin - Free - Positive |
7 (58%) 5 (42%) |
4 (67%) 2 (33%) |
3 (50%) 3 (50%) |
| CHOP rearrangement - Positive - Not available |
12 (86%) 2 (14%)b |
6 (86%) 1 (14%) |
6 (86%) 1 (14%) |
2 patients did not undergo surgery, one because developed metastatic disease during neoadjuvant therapy and one because developed a sepsis due to central venous catheter infection and was treated by definitive RT.
2 not evaluable for limited material on the biopsy and lack of residual tumor on the surgical specimen.
7 patients received T at dose Level 0 and 7 patients at Level 1. All patients completed the 3 planned cycles of T, but one who had a sepsis after cycle 1 and received definitive RT. The median total dose of T was 2.6 mg per cycle in both cohorts (range 2.11–2.85 in dose level 0 and 2.4–3.2 in dose level 1). Seven patients were allowed to be enrolled in each dose-level, instead of the prespecified six, because one patient had an early treatment discontinuance, and another did not undergo surgery. Even though DLT analysis was based on first cycle, it was considered that at least 6 patients had to complete the whole treatment program for a better safety assessment. No dose reductions were observed in the enrolled patients. There were no treatment related deaths.
All underwent RT to a median total dose of 45 Gy (range 45–50 Gy). One DLT (G3 transaminitis) occurred at Level 0 and another (sepsis due to catheter infection) at Level 1. Overall the combination of T and RT was well tolerated. Regardless of the dose attribution the most common grade 3/4 AE was ALT elevation (n = 6, 43%). In addition gammaGT elevation, neutropenia, anemia, epithelitis and sepsis were observed in 1 patient each (7%). Other common grade 1/2 AEs were nausea (n = 7, 50%) and vomiting (n = 3, 21%), fatigue (n = 6, 43%) and anorexia (n = 3, 21%), skin disorders (n = 4, 28%) and AST (n = 6, 43%) and gammaGT (n = 3, 21%) elevation. The complete list of side effects and grade is shown in Table 2, overall and by dose level. Confidence interval for the major toxicities is reported in Table 3.
Table 2.
Haematological and non-haematological toxicity.
| Any grade |
Grade 3–4 |
Grade 1–2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of adverse event (n = 14) | Any grade N (%) |
Dose level 0 (1.3 mg/m2) |
Dose level 1 (1.5 mg/m2) |
Grade 3–4 N (%) |
Dose level 0 (1.3 mg/m2) |
Dose level 1 (1.5 mg/m2) |
Grade 1–2 N (%) |
Dose level 0 (1.3 mg/m2) |
Dose level 1 (1.5 mg/m2) |
| Haematological | |||||||||
| Anemia | 11 (78.5) | 6 (42.8) | 5 (35.7) | 1 (7.1) | 1 (7.1) | 0 (0) | 10 (71.4) | 5 (35.7) | 5 (35.7) |
| Neutropenia | 8 (57.1) | 2 (14.3) | 6 (42.8) | 2 (14.3) | 0 (0) | 2 (14.3) | 6 (42.8) | 2 (14.3) | 4 (28.5) |
| Lymphopenia | 8 (57.1) | 4 (28.5) | 4 (28.5) | 1 (7.1) | 1 (7.1) | 0 (0) | 7 (50.0) | 3 (21.4) | 4 (28.5) |
| Leukopenia | 6 (42.8) | 1 (7.1) | 5 (35.7) | 0 (0) | 0 (0) | 0 (0) | 6 (42.8) | 1 (7.1) | 5 (35.7) |
| Thrombocytopenia | 3 (21.4) | 1 (7.1) | 2 (14.3) | 0 (0) | 0 (0) | 0 (0) | 3 (21) | 1 (7.1) | 2 (14.3) |
| Non-haematological | |||||||||
| ALT/GPT increased | 12 (85.7) | 7 (50.0) | 5 (35.7) | 7 (50) | 6 (42.8) | 1 (7.1) | 5 (35.7) | 1 (7.1) | 4 (28.5) |
| AST/GOT increased | 12 (85.7) | 7 (50.0) | 5 (35.7) | 0 (0) | 0 (0) | 0 (0) | 12 (85.7) | 7 (50.0) | 5 (35.7) |
| Nausea | 7 (50.0) | 4 (28.5) | 3 (21.4) | 0 (0) | 0 (0) | 0 (0) | 7(50.0) | 4 (28.5) | 3 (21.4) |
| Fatigue | 6 (42.8) | 3 (21.4) | 3 (21.4) | 0 (0) | 0 (0) | 0 (0) | 6 (42.8) | 3 (21.4) | 3 (21.4) |
| Skin disorders (dermatitis, epitelitis, erythema) | 5 (35.7) | 2 (14.3) | 3 (21.4) | 1 (7.1) | 1 (7.1) | 0 (0) | 4 (28.5) | 1 (7.1) | 3 (21.4) |
| GGT increased | 10 (71.4) | 6 (42.8) | 4 (28.5) | 1 (7.1) | 1 (7.1) | 0 (0) | 9 (64.2) | 5 (35.7) | 4 (28.5) |
| Anorexia | 4 (28.5) | 2 (14.3) | 2 (14.3) | 0 (0) | 0 (0) | 0 (0) | 4 (28.5) | 2 (14.3) | 2 (14.3) |
| CPK increased | 4 (28.5) | 2 (14.3) | 2 (14.3) | 0 (0) | 0 (0) | 0 (0) | 4 (28.5) | 2 (14.3) | 2 (14.3) |
| Vomiting | 3 (21.4) | 1 (7.1) | 2 (14.3) | 0 (0) | 0 (0) | 0 (0) | 3 (21.4) | 1 (7.1) | 2 (14.3) |
| Diarrhea | 2 (14.3) | 1 (7.1) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 1 (7.1) | 1 (7.1) |
| Dyspepsia | 2 (14.3) | 1 (7.1) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 1 (7.1) | 1 (7.1) |
| Epigastralgia | 2 (14.3) | 2 (14.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 2 (14.3) | 0 (0) |
| Alkaline phosphatase increased | 2 (14.3) | 0 (0) | 2 (14.3) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 0 (0) | 2 (14.3) |
| Sepsis | 1 (7.1) | 0 (0) | 1 (7.1) | 1 (7.1) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) |
| Catheter related infection | 1 (7.1) | 0 (0) | 1 (7.1) | 1 (7.1) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) |
| Abdominal pain | 1 (7.1) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) | 1 (7.1) |
| Dizziness | 1 (7.1) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (7.1) | 0 (0) |
| Headache | 1 (7.1) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) | 1 (7.1) |
| Myalgia | 1 (7.1) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (7.1) | 0 (0) |
| Haemoglobinuria | 1 (7.1) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) | 1 (7.1) |
| Limb cramps | 1 (7.1) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (7.1) | 0 (0) |
| Hypersialorrea | 1 (7.1) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (7.1) | 0 (0) |
| Venous injury | 1 (7.1) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (7.1) | 0 (0) |
Table 3.
Overall 95% Confidence Interval of the major toxicities.
| Type of adverse event (n = 14) | Any grade N (%; 95%CI) |
Grade 3–4 N (%; 95%CI) |
Grade 1–2 N (%; 95%CI) |
|---|---|---|---|
| Haematological | |||
| Anemia | 11 (78; 54–100) | 1 (7; 0–23) | 10 (71; 44–98) |
| Neutropenia | 8 (57; 27–87) | 2 (14; 0–35) | 6 (43; 13–72) |
| Lymphopenia | 8 (57; 27–87) | 1 (7; 0–23) | 7 (50; 20–80) |
| Leukopenia | 6 (43; 13–72) | 0 (0) | 6 (43; 13–72) |
| Thrombocytopenia | 3 (21; 0–46) | 0 (0) | 3 (21; 0–46) |
| Non-haematological | |||
| ALT/GPT increased | 12 (86; 65–100) | 7 (50; 20–80) | 5 (36; 7–64) |
| AST/GOT increased | 12 (86; 65–100) | 0 | 12 (86; 65–100) |
| Nausea | 7 (50; 20–80) | 0 | 7(50; 20–80) |
| Fatigue | 6 (43; 13–72) | 0 | 6 (43; 13–72) |
| Skin disorders (dermatitis, epitelitis, erythema) | 5 (36; 7–64) | 1 (7; 0–23) | 4 (29; 1–56) |
| GGT increased | 10 (71; 44–98) | 1 (7; 0–23) | 9 (64; 36–93) |
| Anorexia | 4 (28; 1–56) | 0 | 4 (28; 1–56) |
| CPK increased | 4 (29; 1–56) | 0 | 4 (29; 1–56) |
All patients were evaluable for centralized assessment of response by RECIST and Choi criteria: 5 achieved PR (36%), 8 SD (57%), 1 distant PD (7%) by RECIST, while 12 achieved PR (86%), 1 SD (7%) and 1 distant PD (7%) by Choi criteria (Fig. 2).
Fig. 2.
Waterfall plot of radiological responses after treatment by Choi criteria, including both tumor size and density information. *Patient with progressive disease due to new distant lesions while achieving CHOI partial response on primary tumor.
12/14 patients underwent surgery. All had a macroscopic complete resection. Microscopic surgical margins were negative (R0) in 7 patients and positive (R1) in 5. Two patients were not operated, one because developed metastatic disease during neoadjuvant therapy and one because developed a sepsis due to central venous catheter infection and was treated by definitive RT.
Median visible residual tumor in the surgical specimen was 5% (0–60) with 9/12 patients (75%) with ≤ 10% visible remaining tumor. Three of them (3/12, 25%) had a complete pathological response, with no visible residual tumor (Fig. 3).
Fig. 3.
Panel a and d are examples of non-treated low grade and high grade myxoid liposarcoma respectively. Panels b, c, e and f are examples of different patterns of pathological response observed after the combination of trabectedin and radiotherapy: mature adipocytic differentiation (panel b), necrosis (panel c), sclero-hyalinosis (panel e) and areas of fibrosis with inflammatory infiltrate, vascular reactive proliferation and haemosideraphages (panel f). All changes can coexist in the same case.
Median follow-up from surgery was 26 months (range 24–41). One patient developed local and distant progression at the end of the neoadjuvant treatment and one patient developed distant metastases 15 months after surgery. This latter recurred in the central nervous system and finally died 5 months later. This case was the only registered death of this series.
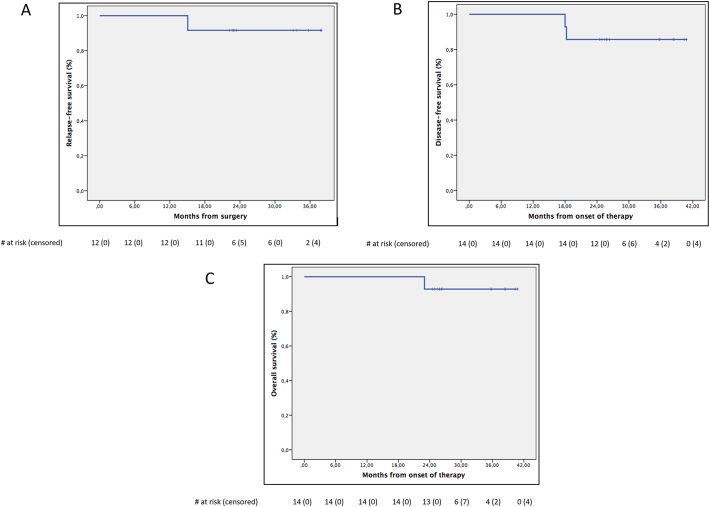
The corresponding RFS, DFS and OS at 3-yr were 92% (95%CI: 76–100), 86% (95%CI: 67–100) and 93% (95%CI: 79–100), respectively (Fig. 4).
Fig. 4.
Relapse-free survival of the 12 operated patients (panel A); disease-free survival (panel B) and overall survival (panel C) of the 14 included patients.
4. Discussion
In this phase 1 study in patients affected by localized ML, the neoadjuvant administration of concurrent T and RT was well tolerated. No cumulative toxicity was shown and no treatment-related deaths were observed. The recommended T dose for the phase 2 study was 1.5 mg/m2, which is the same dose approved for T alone. In addition, both radiological and pathological responses were promising. A confirmatory phase 2 trial is ongoing.
This is the first-in-human study of the combination of T and RT. Both T and RT are known to have exceeding activity in patients affected by ML. However, while preclinical data about the concurrent administration of the two modalities are available, no clinical studies had been performed so far. Of note the dose of RT was slightly lower (45 Gy) than the one conventionally used in sarcomas in the preoperative setting (50 Gy). A study specifically addressing to which extent the dose of RT can be reduced in ML without compromising activity is presently ongoing (DOREMY trial, NCT02106312). The next question to answer may well be if the addition of T can be of any help also in this perspective.
The use of preoperative chemo-radiotherapy is increasingly employed in localized high-risk STS of the extremities and trunk wall, as it theoretically allows covering both the local and systemic risks [15], [16], [17], [18], [19], [20], while helping maximize local control in a proportion of challenging cases. This is of particular relevance when function preservation is a goal, or the tumor is of borderline resectability and subsequent recurrences may directly lead to death [21]. Indeed, the preoperative delivery of anthracycline + ifosfamide (AI) and RT was proven to be feasible in an Italian and Spanish Sarcoma Group phase 3 randomized study comparing the use of 3 and 5 cycles of AI [22]. Other single-arm studies are available [23], [24], [25]. However this combination was consistently shown to be more toxic as compared to the administration of AI alone. A marked thrombocitopenia in as many as 35% of the patients along with the other expected haematological toxicities was observed. Nonetheless the dose intensity of AI was maintained. Of note the administration of the 2 treatments together proved to offset the negative prognostic impact of planned positive surgical margins on outcome [26], [27].
As far as the efficacy of neoadjuvant chemotherapy on the systemic risk is concerned, at an interim analysis, another randomized study [28] comparing neoadjuvant AI versus a neoadjuvant histology-driven chemotherapy showed a statistically significant benefit in RFS and OS in favor of AI, thus pointing to a distinct effect of neoadjuvant chemotherapy as such in localized STS. If the final analysis confirms these results, neoadjuvant chemotherapy may become standard treatment in a subset of localized STS patients. However, in the same trial, T was the histology-driven chemotherapy for ML, and at the moment it is the only stratum in which the two arms were superimposable with regard to RFS and OS. T had a much better toxicity profile. This study is still recruiting in the ML stratum and is expected to complete the accrual in a year. It is thus possible that the efficacy of T be confirmed to be not inferior to that of AI.
If so, in the near future T combined with radiation therapy may become a standard neoadjuvant therapy for a subset of localized ML. For the moment, this Phase 1 study actually showed the feasibility of the combination of T with pre-operative radiation therapy.
Other studies have explored so far the combination of RT with chemotherapy in localized STS. A phase 1 study on preoperative continuous infusion Adriamycine over 5 days for 5 courses and concurrent RT to a total dose of 50 Gy was performed on 27 patients affected by localized high risk STS of an extremity or trunk wall. The treatment was fairly well tolerated, but 30% of the patients developed a ≥ 3 skin toxicity, along with the expected haematological side effects [29]. This regimen has not been further developed, mainly due to the observed skin toxicity and the use of a single agent regimen, which is not the standard chemotherapy approach used in localized high risk STS when a decision for an adjuvant/neoadjuvant chemotherapy is taken. More recently a phase 1 study on Gemcitabine administered 5 times and concurrent RT to a total dose of 50 Gy was performed. The treatment was well tolerated. No significant ≥ 3 skin toxicity was observed. The main side effect was myelosuppression, which did not prevent the patients to complete the preoperative treatment [30]. A formal phase 2 study to assess the activity of this regimen was warranted, but no results are available as of yet. However Gemcitabine is not active in ML and therefore its use in combination with RT in this disease would not be the first choice.
With regard to the combination of RT with targeted therapies, the combination of Vascular Endothelial Growth Factor Receptor (VEGFR) inhibitors and RT has also been studied in the latter years [31], [32], [33]. Sunitinib, Pazopanib or Sorafenib and combined RT to a total dose of 50 Gy have been tested in phase I studies, in patients affected by high risk STS in an extremity or trunk wall. The combination has proven to be feasible. However grade ≥ 3 haematological and hepatic toxicities were observed in up to 50% of the patients. In addition the effect of VEGFR inhibitors against the metastatic risk of localized high risk STS is far from being demonstrated and a limited activity in advanced liposarcoma has consistently been reported for all these compounds. Their use is mainly intended at enhancing RT activity. A confirmatory phase II study on the combination of Pazopanib and RT is presently ongoing.
Another approach explored the combination of intratumoral injection of hafnium oxide nanoparticles (NBTXR3; a radioenhancer) and RT in a phase 1 study in 22 patients affected by STS in an extremity or trunk wall [34]. The treatment proved to be feasible, with limited local toxicity. A promising pathological response rate was observed. A confirmatory phase 3 study has recently been completed. However this modality is not intended to impact the metastatic and survival risk as the activity of both modalities is limited to the site of the primary tumor.
As a matter of fact, in the 14 patients of this Phase 1 component of the trial, an impressive activity of the combination of T with RT was observed, especially when radiological response was measured by modified Choi criteria [14], [35] (Fig. 2), known to better predict both pathological response and final outcome. Indeed the 75% rate of very good pathological response (≤ 10% residual visible tumor) looks very promising. These results compare favorably with previous experiences using either T or RT alone as neoadjuvant therapies in localized ML [7], [8]. However, if the phase II part of this study confirmed the promising activity reported herein, a comparative trial would be needed to demonstrate the superiority of T plus RT over either single therapeutic modality in localized ML.
Acknowledgments
Acknowledgements
The authors would like to thank Melissa Fernández, Ana Arcas, Patricio Ledesma, Emanuela Marchesi, Lorella Rusi and Julien Gautier for clinical trial management tasks and Dr. Carlo Morosi and Dr. Moreno Marino for central radiology reviews. The authors would also like to thank the Grupo Espaniol por la Investigacion in Sarcoma (GEIS), the Italian Sarcoma Group (ISG) and the French Sarcoma Group (FSG) for supporting the study and PharmaMar for funding trial organizational and logistics costs.
Authors' Contribution
A. Gronchi, J.Martin, JY Blay: conception and design of the study, acquisition and interpretation of the data, manuscript drafting, final approval of the version to be published and agreement to be accountable for all aspect of the work.
N. Hindi, J. Cruz, A. Lopez Pousa, A. Italiano, R. Alvarez, I. Rincon, C. Sangalli, JL Perez Aguiar, J. Romero, C. Morosi, MP Sunyach, R. Sanfilippo, C. Romagnosa, D. Ranchere-Vince, AP Dei Tos, PG Casali: data acquisition and interpretation of the data, critical revise of the manuscript draft, final approval of the version to be published and agreement to be accountable for all aspect of the work.
A. Gutierrez: data analysis and interpretation, manuscript drafting, final approval of the version to be published and agreement to be accountable for all aspect of the work.
Conflict of Interest
Dr. Alvarez reports grants and personal fees from PHARMAMAR, during the conduct of the study; personal fees and other from PHARMAMAR, personal fees from LILLY, personal fees from NOVARTIS, outside the submitted work;
Dr. Blay reports grants and personal fees from PHARMAMAR, during the conduct of the study;
Dr. Casali reports non-financial support from GEIS, during the conduct of the study; grants and personal fees from PharmaMar, personal fees from Deciphera Pharmaceuticals, personal fees from Eisai, grants and personal fees from Eli Lilly, personal fees from Nektar Therapeutics, grants and personal fees from Pfizer, grants from Amgen, grants from AROG, grants from Bayer, grants from Blueprint, grants from Daiichi Sankyo, grants from Epizyme, grants from Glaxo SK, grants from Novartis, outside the submitted work;
Dr. Cruz reports personal fees, non-financial support and other from PHARMAMAR, personal fees, non-financial support and other from NOVARTIS, personal fees, non-financial support and other from PFYZER, personal fees, non-financial support and other from LILLY, personal fees, non-financial support and other from ROCHE, personal fees from AMGEN, personal fees and non-financial support from ASTRA ZENECA, personal fees, non-financial support and other from EISAI, outside the submitted work;
Dr. Dei Tos reports personal fees from PharmaMar, outside the submitted work;
Dr. Gronchi reports personal fees from Novartis, personal fees from Pfizer, personal fees from Bayer, grants and personal fees from Lilly, personal fees and other from Nanobiotix, grants, personal fees and other from Pharmamar, outside the submitted work;
Dr. Hindi reports grants from Pharmamar, during the conduct of the study; grants, personal fees and non-financial support from Pharmamar, outside the submitted work;
Dr. Martin-Broto reports grants from LILLY, other from NOVARTIS, grants and other from PHARMAMAR, other from EISAI, outside the submitted work;
Dr. Sanfilippo reports grants and personal fees from Pharmamar, outside the submitted work;
Dr. Romagosa reports grants from Grupo GEIS, during the conduct of the study; other from Farmamar, outside the submitted work;
All other authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.03.007.
Appendix A. Supplementary Data
The full study protocol.
References
- 1.Antonescu C.R., Ladany M. Myxoid liposarcoma. In: Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F., editors. WHO classifications of tumors of soft tissue and bone. IARC; Lyon: 2013. pp. 39–41. [Google Scholar]
- 2.Fiore M., Grosso F., Lo Vullo S. Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2007;109(12):2522–2531. doi: 10.1002/cncr.22720. [DOI] [PubMed] [Google Scholar]
- 3.Panagopoulos I., Hoglund M., Mertens F. Fusion of the EWS and CHOP genes in myxoid liposarcoma. Oncogene. 1996;12:489–494. [PubMed] [Google Scholar]
- 4.Antonescu C.R., Tschernyavsky S.J., Decuseara R. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7(12):3977–3987. [PubMed] [Google Scholar]
- 5.Jones R.L., Fisher C., Al-Muderis O., Judson I.R. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer. 2005;41(18):2853–2860. doi: 10.1016/j.ejca.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Grosso F., Jones R.L., Demetri G.D. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8(7):595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 7.Chung P.W., Deheshi B.M., Ferguson P.C. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer. 2009;115(14):3254–3261. doi: 10.1002/cncr.24375. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A., Bui B.N., Bonvalot S. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol. 2012;23(3):771–776. doi: 10.1093/annonc/mdr265. [DOI] [PubMed] [Google Scholar]
- 9.Simoens C., Korst A.E., De Pooter C.M. In vitro interaction between ecteinascidin 743 (ET-743) and radiation, in relation to its cell cycle effects. Br J Cancer. 2003;89(12):2305–2311. doi: 10.1038/sj.bjc.6601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero J., Zapata I., Cordoba S. In vitro radiosensitisation by trabectedin in human cancer cell lines. Eur J Cancer. 2008;44(12):1726–1733. doi: 10.1016/j.ejca.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Le Tourneau C., Lee J.J., Siu L.L. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosso F., Dileo P., Sanfilippo R. Steroid premedication markedly reduces liver and bone marrow toxicity of trabectedin in advanced sarcoma. Eur J Cancer. 2006;42:1484–1490. doi: 10.1016/j.ejca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Stacchiotti S., Collini P., Messina A. High-grade soft-tissue sarcomas: tumor response assessment–pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251:447–456. doi: 10.1148/radiol.2512081403. [DOI] [PubMed] [Google Scholar]
- 15.Casali P.G., Abecassis N., Bauer S. ESMO guidelines committee and EURACAN. Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv51–iv67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 16.von Mehren M., Randall R.L., Benjamin R.S. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(5):536–563. doi: 10.6004/jnccn.2018.0025. [DOI] [PubMed] [Google Scholar]
- 17.Gronchi A., Jones R.L. The value of neoadjuvant chemotherapy in localized high risk soft tissue sarcoma of the extremities and trunk. JAMA Oncol. 2018;4(9):1167–1168. doi: 10.1001/jamaoncol.2018.1392. [DOI] [PubMed] [Google Scholar]
- 18.George S., Wagner A.J. Low levels of evidence for neoadjuvant chemotherapy to treat soft-tissue sarcoma. JAMA Oncol. 2018;4(9):1169–1170. doi: 10.1001/jamaoncol.2018.1403. [DOI] [PubMed] [Google Scholar]
- 19.Gronchi A. Towards the standard use of (neo)adjuvant chemotherapy in selected localized soft tissue sarcoma at high risk of relapse: are we finally getting there? Eur J Cancer. 2018;101:251–253. doi: 10.1016/j.ejca.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin R.S. Adjuvant and neoadjuvant chemotherapy in soft tissue sarcomas: a personal point of view. Tumori Journal. 2017;103(3):213–216. doi: 10.5301/tj.5000628. [DOI] [PubMed] [Google Scholar]
- 21.Gronchi A., Lo Vullo S., Colombo C. Extremity soft tissue sarcoma in a series of patients treated at a single institution. the local control directly impacts survival. Ann Surg. 2010;251:512–517. doi: 10.1097/SLA.0b013e3181cf87fa. [DOI] [PubMed] [Google Scholar]
- 22.Palassini E., Ferrari S., Verderio P. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian sarcoma group/Grupo Español de Investigación en sarcomas randomized clinical trial: three versus five cycles of full-dose Epirubicin plus Ifosfamide. J Clin Oncol. 2015;33(31):3628–3634. doi: 10.1200/JCO.2015.62.9394. [DOI] [PubMed] [Google Scholar]
- 23.Greto D., Loi M., Saieva C. Safety of concurrent adjuvant radiotherapy and chemotherapy for locally advanced soft tissue sarcoma. Tumori Journal. 2018;104:322–329. doi: 10.1177/0300891618765565. [DOI] [PubMed] [Google Scholar]
- 24.Pennington J.D., Eilber F.C., Eilber F.R. Long-term outcomes with ifosfamide-based hypofractionated preoperative chemoradiotherapy for extremity soft tissue sarcomas. Am J Clin Oncol. 2018;41(12):1154–1161. doi: 10.1097/COC.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 25.Kraybill W.G., Harris J., Spiro I.J. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: radiation therapy oncology group trial 9514. Cancer. 2010;116(19):4613–4621. doi: 10.1002/cncr.25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gronchi A., Verderio P., De Paoli A. Quality of surgery and neoadjuvant combined therapy in the ISG-GEIS trial on soft tissue sarcomas of limbs and trunk wall. Ann Oncol. 2013;24(3):817–823. doi: 10.1093/annonc/mds501. [DOI] [PubMed] [Google Scholar]
- 27.Gundle K.R., Kafchinski L., Gupta S. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue sarcoma resection. J Clin Oncol. 2018;36(7):704–709. doi: 10.1200/JCO.2017.74.6941. [DOI] [PubMed] [Google Scholar]
- 28.Gronchi A., Ferrari S., Quagliuolo V. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: a randomized clinical trial from the Italian sarcoma group (ISG), the Spanish sarcoma group (GEIS), the French sarcoma group (FSG) and the polish sarcoma group (PSG) Lancet Oncol. 2017;18:812–822. doi: 10.1200/JCO.19.03289. [DOI] [PubMed] [Google Scholar]
- 29.Pisters P.W., Patel S.R., Prieto V.G. Phase I trial of preoperative doxorubicin-based concurrent chemoradiation and surgical resection for localized extremity and body wall soft tissue sarcomas. J Clin Oncol. 2004;22(16):3375–3380. doi: 10.1200/JCO.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Tseng WW, Zhou S, To CA, et al. Phase 1 adaptive dose-finding study of neoadjuvant gemcitabine combined with radiation therapy for patients with high-risk extremity and trunk soft tissue sarcoma. [DOI] [PMC free article] [PubMed]
- 31.Jakob J., Simeonova A., Kasper B. Combined sunitinib and radiation therapy for preoperative treatment of soft tissue sarcoma: results of a phase I trial of the German interdisciplinary sarcoma group (GISG-03) Radiat Oncol. 2016;11:77. doi: 10.1186/s13014-016-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas R.L., Gelderblom H., Sleijfer S. A phase I study on the combination of neoadjuvant radiotherapy plus pazopanib in patients with locally advanced soft tissue sarcoma of the extremities. Acta Oncol. 2015;54(8):1195–1201. doi: 10.3109/0284186X.2015.1037404. [DOI] [PubMed] [Google Scholar]
- 33.Canter R.J., Borys D., Olusanya A. Phase I trial of neoadjuvant conformal radiotherapy plus sorafenib for patients with locally advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2014;21(5):1616–1623. doi: 10.1245/s10434-014-3543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonvalot S., Le Pechoux C., De Baere T. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin Cancer Res. 2017;23(4):908–917. doi: 10.1158/1078-0432.CCR-16-1297. [DOI] [PubMed] [Google Scholar]
- 35.Stacchiotti S., Verderio P., Messina A. Tumor response assessment by modified Choi criteria in localized high-risk soft tissue sarcoma treated with chemotherapy: an exploratory analysis on a phase III trial. Cancer. 2012;118(23):5857–5866. doi: 10.1002/cncr.27624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full study protocol.