Abstract
The type of commensal microorganisms can influence the efficiency of sputum decontamination for TB diagnosis. A basic characterization of contaminants from LJ contaminated media showed that Gram positive Spore Forming Bacteria (SFB) were the major contaminants. This study aims to identify the species of this contaminants and to evaluate the effectiveness of VCNT at 10 µg of vancomycin to reduce mycobacterial culture contamination mainly linked to SFB. Fifty-three SFB isolated between February 2016 and May 2017 were used. The effectiveness of LJ with VCNT at 10 µg of Vancomycin were evaluated with sputum collected in the same period. SFB had been stored at −20 °C and identified after subculture onto 5% sheep blood Columbia agar and incubated at 37 °C during 24 h. Bacteria cells and isolated colonies were described. API 50CH/B was performed and MALDI-TOF MS was used for external quality control. Thirty- five (66%) isolates representing 4 genera (Bacillus, Paenibacillus, Brevisbacillus and Lysinibacillus) including 10 species were identified. The most important species were Bacillus cereus (30%) and Bacillus licheniformis (21%). Eighteen (34%) isolates were non-reactive Bacillus. The overall contamination rate on LJ with VCNT at 10 µg of vancomycin was statistically lower than which without VCNT (18.7% versus 43.8%) (p = 0.01). The most important SFB identified were B. cereus and B. licheniformis. Almost all identified strains were similar to those currently isolated in fermented traditional food suggesting in part food related contaminants. VCNT containing 10 µg of vancomycin is a good alternative method to reduce mycobacterial culture contamination.
Subject terms: Microbiology techniques, Clinical microbiology
Introduction
Culture on solid media remains the gold standard for detecting TB and drug sensitivity testing1,2. However, the effectiveness of culture systems is greatly undermined by contamination with other bacteria and fungi3–5. The sputum culture process on LJ media, include a step of decontamination in order to eliminate contaminating microorganisms while protecting mycobacteria6. But the type of the contaminant microorganisms can influence the efficiency of the decontamination7. Indeed, the presence of an external envelope for Gram negative bacteria, of a wax coat for mycobacteria or a sporale coat for spore forming bacteria (SFB) lead to a variability of sensibility to biocides within the microorganisms8.
The literature reports little and different data about contaminants from LJ media. Studies carried out in Ireland, in 2011, and in Uganda in 2014, showed that a Gram negative bacteria (Pseudomonas aeruginosa (47.2%), Serratia marcescens (13%)), and Gram positive bacteria excluding SFB (Staphylococcus spp (50%) and Streptococcus spp (16%)), were respectively the main contaminant species isolated9,10. A few studies involved mycobacterial culture from different specimen (sputum, liquid gastric) on different media (liquid and solid) reported the presence of SFB among contaminants in mycobacterial cultures9,11–14. But possibly due to the difference in the context, none of them mentioned SFB as major contaminants. Recently in 2017, a basic characterization of sputum culture contaminants in Burkina-Faso, showed for the first time that SFB from the genus of Bacillus and related were the major residual contaminants15. But no specific identification of SFB species has been carried out on these studies, excepted that of McClean et al.9. However, knowing the species to which these contaminants belong would provide information on their likely origin (maybe for prevention), and would help to understand and to resolve the contamination issue.
These contaminants, particularly resistant to biocides due to their ability to sporulate give for the first time, a stronger justification for high contamination rate of mycobacterial culture in Burkina Faso and also maybe in most resource limited sittings. Fortunately, vancomycin, generally inactive on mycobacteria is active against SFB16,17, therefore, it was a potential candidate to include in the antibiotic combination used as a selective supplement for culture media. Limited data have indicated that culture with mycobacterial Growth Indicator Tube (MGIT) supplemented with the Vancomycin, Amphotericin B and Nalidixic acid (VAN) combination and culture with Selective Kirchner medium supplemented with Vancomycin 10 mg/l (SKV) showed important reduction of culture contamination18,19. However, these studies were limited to liquid medium, less used in resources limited sitting because of the high costs associated with them. Therefore, we believe that sputum culture for mycobacteria, using the popular LJ medium, supplemented with Vancomycin at 10 µg, Colistin, Nystatin and Trimethoprim (VCNT) could contribute to reduce the culture contamination. The use of VCNT is doubly advantageous because of its availability in most African laboratories, and the specificity of its antibiotic combination that targets both SFB and other Gram negative, Gram Positive bacteria and fungi (knowing the poly-microbial character of sputum).
The objective of this study was to identify the species of SFB,to evaluate the effectiveness of VCNT to reduce mycobacterial culture contamination rate and to ensure the tolerance of mycobacteria and the susceptibility of SFB to VCNT.
Results
Phenotypic characterization of Spore forming Bactria
Morphology
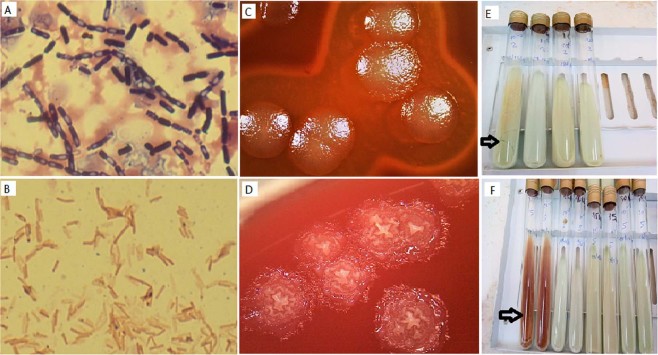
Globally, microscopic examination of SFB colonies and cells showed 8 different morpho-groups. Their represented different size and forms of colonies, hemolytic and non-hemolytic, different size of rod-shaped bacteria, producing endospores of different forms, at different positions in bacteria cells (Table 1). All the isolates were catalase positive. The most important of which were the morpho-groups 1 and 2 with respectively 16 (30.18%) and 11 (20.75%) isolates (Table 1). The group 1 bacteria were Gram positive, large motile rods, are alone, grouped in pairs and in chain with ellipsoidal and cylindrical, central and subterminal spores which do not deforming the bacteria cells (Fig. 1A). They grow large, smooth, grey, regular and hemolytic colonies (Fig. 1C). The group 2 bacteria were Gram positive, middle or little motile rods, alone, in pairs, and in chain sometimes with ellipsoidal and cylindrical, central and subterminal spore which do not deforming the bacteria cells (Fig. 1B). They grow middle, rough creamy, irregular and non- haemolytic colonies (Fig. 1D).
Table 1.
Phenotypic characteristics of spore forming bacteria strains isolated from contaminated LJ media.
| Bacteria group | Colony description | Cell morphology | Gram | Spore | Cat* | oxy ** | No. (%) |
|---|---|---|---|---|---|---|---|
| Group 1 | Large, smooth, grey, regular, Haemolytic | Motile rods alone, in pairs and in chain | Gram positive | + Ellipsoidal and cylindrical, Central and subterminal, not warping | + | + | 16 (30.18) |
| Group 2 | Middle, rough. creamy, irregular, non- hemolytic | Motile rods alone and in pairs and in chain | Gram positive | + Ellipsoidal and cylindrical, Central and subterminal, not warping | + | +/− | 11 (20.75) |
| Group 3 | Middle, smooth, creamy, regular, | Motile rods alone and in pairs | Gram positive | + Ellipsoidal, subterminal, warping | + | + | 3 (5.66) |
| Group4 | Large, mucosal, irregular, non- hemolytic | Motile rods alone and in pairs | Gram positive | +Ellipsoidal, subterminal, not warping | + | + | 2 (3.77) |
| Group5 | Large, smooth, grey, regular, Non-Haemolytic | Motile rods alone, in pairs and in chain | Gram positive | + Ellipsoidal and cylindrical, Central and subterminal, not warping | + | + | 1 (1.8) |
| Group6 | Small, smooth, creamywhite, regular, non- hemolytic | Motile rods alone and in pairs and in chain | Gram positive | + Ellipsoidal, Central and subterminal, not warping | + | + | 1 (1.8) |
| Group 7 | Large, mucous, transparent, irregular, non- hemolytic | Motile rods alone and in pairs | Gram positive | + Ellipsoidal, sub terminal, not warping | + | + | 1 (1.8) |
| Group 8 | Middle/ Small, smooth, creamy, regular, non- hemolytic | Motile rods alone and in pairs | Gram variable | + Ellipsoidal or round, Subterminal and terminal, warping | + | + | 18(33.9 318) |
| Total | 53 (100) |
Cat*: Catalase test.
Oxy**: oxidase test.
Figure 1.
B. cereus and B. licheniformis cells, colonies and contamination aspect. B. cereus: Gram-positive rods, alone, in pairs and in chain with unwarping endospores representing bacteria cells of the group1 (A); Large, smooth, grey, regular, and Hemolytic colonies after 24 h of growth on 5% sheep blood agar (C); contamination aspect on LJ media (E). B. licheniformis: Gram-positive rods, alone and in pairs (B); middle, rough. Creamy/grey, irregular and non- hemolytic colonies after 24 h of growth on 5% sheep blood agar (D); contamination aspect on LJ media (F).
Identification
Biochemical identification performed with the API 50 CHB system identified 35 of the 53 strains representing 3 genera (Bacillus, Paenibacillus and Brevibacillus) including 8 species. Thirty-four (64%) isolates were identified with more than 60% of similarity, one (2%) isolate with 58% of similarity, and 18 (33.96%) isolates which were unable to ferment any of the 49 carbohydrates present in the API 50CH/B strip were identified as non-reactive (Table 2). External quality control with the MALDI-TOF showed 87% (13/15) of accordance with the API 50CH/B. Only one discordance on specie level (B. firmus 58% similarity, identified as B. cereus by MALDI-TOF) was observed and one supplementary species was identify as Lysinibacillus fusiformis for one non-reactive isolate (Table 2). Finally, this study indicated the presence of 10 species (B. cereus, B. licheniformis, Paenibacillus thiaminolyticus, Paenibacillus alvei, B. Subtilis/amyloliquefaciens, Brevibacillus laterosporus, B. firmus, B. anthracis, B. vallismortis and Lysinibacillus fusiformis) (Table 2). The most important were B. cereus (30%) and B. licheniformis (21%). Around 69% of isolates from confirmed TB cases was formally identified against 55% for those from negative TB cases.
Table 2.
Identification of species of spore forming bacteria isolates.
| API 50 CH/B | Isolates Nb. | Carbohydrate fermented Nb. | Similarity % (Nb.) | MALDI-Tof | score | |
|---|---|---|---|---|---|---|
| Bacteria species | Bacteria species | Isolates Nb. | ||||
| B. Cereus1 | 1 | 12 | 94.10(1) | B. Cereus | 4 | [2.00; 2.39] |
| B.Cereus1 | 11 | 13 | 71.40 (2), 72.80 (9) |
|||
| B. Cereus2 | 1 | 9 | 92.40(1) | B. cereus | 3 | |
| B. Cereus2 | 1 | 10 | 79.80(1) | |||
| B. Cereus2 | 2 | 11 | 79 .3(1), 88 (1) |
|||
| B. Licheniformis | 5 | 25 | 99.90 (3), 66.10 (2) |
Licheniformis | 4 | [2.3; 2.47] |
| B. Licheniformis | 2 | 26 | 99.80 (2) | |||
| B. Licheniformis | 2 | 27 | 99.90 (1), | |||
| B. Licheniformis | 2 | 28 | 99.90 (3) 100 (1) |
|||
| Paenibacillus thiaminolyticus | 1 | 21 | 99.60 (1) | Paenibacillus thiam. Paenibacillus thiam./B. vallismortis* Paenibacillus thiam./B. cereus* | 1 | [2.10; 2.17] |
| Paenibacillus thiaminolyticus | 1 | 23 | 99.90 (1) | 1 | ||
| Paenibacillus alvei | 1 | 26 | 99.90 (1) | 1 | ||
| B. Subtilis/amylo | 2 | 24 | 98.8 (1), 99.5 (1) |
Not identified | 1 | NA |
| B. anthracis | 1 | 10 | 83.10 (1) | Not identified | 1 | NA |
| B. firmus** | 1 | 6 | 57.70 (1) | B. Cereus** | 1 | 2 |
| Brevibacillus laterosporus | 1 | 10 | 76.6 | Not identified | 1 | NA |
| Not reactive | 18 | 0 | NA | Lysinibacillus fusiformis | 1 | 2.15 |
*Two mixed species were found in MALDI-Tof identification on the two isolates marked in bold and containing Paenibacillus thiaminolyticus.
**Different identifications were obtained from API and MALDI-TOF for the isolate marked in bold.
LJ with VCNT effectiveness on clinical sputum culture contamination
The overall contamination rate on LJ with VCNT at 10 µg of vancomycin was statistically lower than which with LJ without VCNT (18.7% versus 43.8%) (p = 0.01). Negative culture was two time as great on LJ with VCNT as on LJ without VCNT (12. 5% versus 6. 2%) (Table 3).
Table 3.
Comparison of sputum culture result on LJ with or without VCNT.
| Sputum culture | LJ without VCNT | Total % | Chi2 | p | |||
|---|---|---|---|---|---|---|---|
| Nb of Not contaminated | Nb of contaminated | ||||||
| LJ with VCNT | Nb of Not contaminated | Pos | Nég | 4.9 | 0.011 | ||
| Pos | 13 | 1 | 8 | 68.8 | |||
| Nég | 2 | 1 | 1 | 12.5 | |||
| Nb of Contaminated | 1 | 0 | 5 | 18.8 | |||
| Total % | 50 | 6.2 | 43.8 | 100 | |||
Legend:
Nb: Number.
Mycobacterium tolerance and spore forming Bacteria susceptibility to VCNT
Mycobacterium tolerance to VCNT
The CFUs number was uncountable (confluents and semi-confluents colonies) at 10−1 dilution for three (3/7) isolates of mycobacteria, as well as the BCG. For the remaining 4 isolates of which the CFUs was countable, the mean CFUs on LJ without VCNT and LJ with VCNT at 10, 15 and 30 µg of vancomycin were not different (mean CFUs = 95.5, 64, 44.5 and 33.5 respectively with p = 0.34, 0.34 and 0.32 respectively).
CFUs were countable for all isolates of mycobacteria at 10−3 dilution. The mean CFUs on LJ with VCNT at 10 µg of vancomycin compared to control were not significantly different (45.43 vs 31.14, p = 0.56; IC 95: −37.15-65.73), but it differ for LJ with VCNT at 15 and 30 µg of vancomycin (45.43 vs 16.85 and vs 3.71 respectively; p = 0.03 and p = 0.00 respectively).
Spore forming bacteria susceptibility to VCNT
After three days of incubation, 12/15 (80%) Bacillus have contaminated LJ without VCNT. We noted that any (0/15) Bacillus has contaminated LJ with VCNT at any concentration and inoculum dilution. After 7 days of incubation, all LJ without VCNT were contaminated. One B. cereus and one non-reactive Bacillus have contaminated LJ with VCNT at 10 µg of vancomycin for inoculum diluted at 10−1 and 10−3. This represented a low contamination rate of 13% that compared to our standard contamination rate was statistically different (13% vs 40%; p = 0.035; IC95: 3.73-37.88). One non-reactive Bacillus have also contaminated LJ with VCNT at 15 µg of vancomycin for both dilution (contamination rate: 7%). Compared to our standard contamination rate, the difference was statistically significant (p = 0.00; IC 95: 1.18-29.8). After 14 days of incubation, 3/15 Bacillus (two B. cereus and one non-reactive B.) have contaminated LJ with VCNT at 10 µg of vancomycin for both dilution (contamination rate of 20%). Compared to our standard contamination rate, the difference was not statistically significant (p = 0.11, IC: 7.04–45.18). No Bacillus have contaminated LJ with VCNT at 30 µg of vancomycin and the control Escherichia coli ATCC25922 (sensible to VCNT) have not grown on any LJ with VCNT. The general appearance of contaminants on media was characteristic of Bacillus groups. The B. cereus group was characterized by a stratal growth of brown-yellow color with a large liquefaction of media (cracked medium), those of B. licheniformis were also in a stratal growth, with a raspberry red pigment and a less significant liquefaction of the media (respectively Fig. 1E and Fig. 1F), the Paenibacillus were characterized by the turquoise blue turn of the media without liquefaction.
Discussion
The objective of this study was to identify the species of SFB isolated as a major contaminants from LJ media during pulmonary tuberculosis (TB) diagnosis, to evaluate the effectiveness of VCNT to reduce mycobacterial culture contamination rate and to ensure the tolerance of mycobacteria and the susceptibility of SFB to VCNT.
The phenotypical description show that our major isolates are group 1 and group2. They are corresponding respectively to bacteria cells of group1 (sub- group A) and group 1 (sub- group B) described by Drobniewski et al., in 1993 representing B. cereus group and B. subtilis group20. According to Drobniewski, these group of bacillus are the most frequently isolated in clinic, so their isolation in sputum is not an exceptional occurrence.
The API 50CH/B identification indicate the presence of 8 species which the most important were B. cereus (30%) and B. licheniformis (21%) representing respectively morpho-group1 and 2 of Table 1. One important fact is that the positive reactions at 24 h for all B. licheniformis strains, are transformed to false-negative at 48 hours of incubation. Since many studies described the natural production of alkaline proteases by B. licheniformis strains21, this phenomenon is probably due to the neutralization of the acid by alkaline proteases produced. The API 50CH/B results were not in complete agreement with external quality control. Indeed, one SFB identified with low similarity rate (58%) as B. firmus by API50 CH/B was identified as B. cereus by MALDI-TOF-MS. According to current taxonomy, this specie is belong to B. subtilis group and discrimination with B. cereus should be made without trouble, but it is not the case here. Morphologically, Bacteria cell of this isolate looked like B. cereus but it fermented only 6 carbohydrates instead of 13 as the majority of B. cereus. Molecular identification would be necessary for clear identification. API 50CH/B failed to identify 18 (33%) heterogeneous isolates. Any of these strains has not fermented the 49 carbohydrates of the API 50CH / B and consequently, no similarity in species of Bacillus and related was able to be established. According to their morphology group 8 (Table 1), these bacteria belong to group II and group III of the classification of Drobniewski20. Unfortunately, almost all of these isolates was not analyzed with MALDI-TOF-MS method, what would maybe allowed to refine the identification of these non- reactive group of Bacillus and related. In spite of these limits, we were able to highlight the major spore forming bacteria.
B. cereus (30%) and B. licheniformis (21%) were the most important isolates identified in this study. These results were different to those of Ireland, and Uganda which respectively showed that Gram negatives bacteria (Pseudomonas aeruginosa 47.2% and Serratia marcescens 13%) and Gram positives bacteria (Staphylococcus spp 50% and Streptococcus spp 16%) were the major isolated contaminants9,10. Only 2% of B. Licheniformis was identified in Ireland study9. This difference could be explain by climatic, geographical and ethnic conditions (nutrition, genetic, immunities which lead to variability of the oral microbiome)22, mainly for Ireland study where the climate was tempered oceanic and ethnic conditions were more different to ours compared to Uganda closed to us. Indeed, the main nutritional condition is a strong argument for our results because we noticed that Bacillus spp are usually reported as starter in fermented foods, and are largely used in African and particularly in Burkina Faso diet23–27. So their isolation in sputum culture in our country, should not be exceptional.
We noted a wide similarity (74%) between the species that we identified and those identified as a starter in fermented foods in Burkina-Faso. It was substantively B. subtilis B. licheniformis, B. cereus, B. firmus and B. borstelensis25,26. This indicates that food may plays at least in part, an important role in sputum culture contamination by transferring these microorganisms in oropharynx or in pulmonary ecosystems. Even if we don’t know the interactions which can facilitate this colonization, we know that their isolation in contaminated LJ media is linked to contributing factors. Indeed, the use of sodium hydroxide during decontamination process of sputum could be the way of chemo-resistant microorganism’s selection. In fermented foods the long boiling time of Parkia biglobosa and Hibiscus sabdariffa seeds during Bikalga and soumbala (fermented foods) preparation are the ways of these thermos-tolerant microorganisms selection.
Our results raises one important question: the real role of the presence in sputum of B. cereus, B. licheniformis and Subtilis the most frequently bacillus implicated in human infection28. Many arguments suggest that the precautionary principle must be applied and that clinicians and clinical microbiologists must take care, instead of considering these bacteria as being simply contaminants. First, already in 1993, Drobniewski et al. asserted that most of the important strains of Bacillus isolated clinically belonged to Cereus and Subtilis group20 and therefore in the specific case, this presence maybe is not fortuitous. Indeed, Bacillus cereus is well known as a cause of food poisoning, and B. licheniformis has occasionally been isolated from cases of food associated illness29,30. In addition to food poisoning, B. cereus and B. licheniformis in recent years have been increasingly implicated in a wide range of infections including abscesses, bacteremia/septicemia, wound and burn infections, ear infections, endocarditis, meningitis, ophthalmitis, osteomyelitis, peritonitis, and respiratory and urinary tract infections in both immunologically compromised and immunocompetent individuals28,30–34. Moreover, paenibacillus and brevisbacillus identified also in our study were sporadically isolated in clinical sample (Blood, urine, drainage, bronchial aspirate.17,35,36. Lastly, B. anthracis, one of the most pathogenic Bacillus, involved in human infections, was also identified on one isolate belonging to TB negative case. Secondly, although it is not the objective of this current study, characters of virulence as hemolysis and protease secretion were highlighted on most of our isolate. Finally, current evidence suggests that the lung microbiome is a potential modifiable risk factor for TB infection and disease37. All this arguments suggest a possible association between these SFB (particularly B. cereus, B. licheniformis, Paenibacillus B. anthracis) and the pulmonary TB or pneumonia in general. Future studies are necessary to confirm this hypothesis.
Our second objective was to check how to overcome of these chemo-resistant contaminants in the sputum culture process by using VCNT at 10 µg of vancomycin and to ensure proper mycobacterial tolerance and susceptibility of SFB to VCNT. The sputum culture on LJ with VCNT at 10 µg of vancomycin showed a significantly lower contamination rate and a better recovery rate of mycobacteria compared to that on LJ without VCNT. Although widely used in classic bacteriology laboratories, VCNT to our knowledge has never been used as a selective supplement in LJ media. However, another selective supplement (VAN: Vancomycin, nalidixic acid and nystatin) containing vancomycin at 10 µg has been used with liquid media for gastric aspiration culture for mycobacteria, and has similarly shown a significant reduction of culture contamination18. Despite the small number of samples tested, this check suggests that the selective LJ medium with VCNT could be used for the isolation of mycobacteria from sputum, mainly for laboratories that are facing high levels contamination linked to SFB. However, the contamination rate remains above the acceptable rates (5–10%). Therefore, it was important to study the tolerance of mycobacteria and the susceptibility of SFB to VCNT from the perspective to increase the concentration of VCNT.
Compared to control, the mycobacteria showed good tolerance at all concentrations of VCNT for inoculum diluted at 10−1 (p ≥ 0.32), But for those diluted at 10−3, this tolerance is maintained only for the VCNT at 10 µg of vancomycin but not for the VCNT at 15 or 30 µg of vancomycin (respectively: p = 0.56; p = 0.03 and p = 0.00). This other check suggests that using the LJ with VCNT at 10 µg of vancomycin would not affect the isolation of mycobacteria, as opposed to the LJ with VCNT at 15 or 30 µg of vancomycin, where there might be a real risk for mycobacterial recovery especially for paucibacillary sputum. However, the use of VCNT at 15 µg vancomycin could be considered for strongly positive smears.
For SFB susceptibility, our result showed increasing susceptibility of SFB depending on the VCNT concentration. Thus, contamination rates on LJ with VCNT at 10 µg of vancomycin, were 0%, 13% and 20% after 3, 7 and 14 days of incubation respectively, 0%, 6% and 6% for LJ with VCNT at 15 µg of vancomycin and 0% for LJ with VCNT at 30 µg of vancomycin for any incubation time. Even if LJ with VCNT at 15 µg or 30 µg of vancomycin allow better reduction of contamination, the results of tolerance and susceptibility taken together, suggest that the concentration of VCNT at 10 µg of vancomycin is that which allows both optimal preservation of mycobacteria and optimal removal of SFB. This concentration of VCNT allow an important reduction of contamination linked to SFB (20%) after 14 days of incubation compared to our standard contamination rate (around 40%). Nevertheless, the observed level of resistance to VCNT induces contamination rates above acceptable levels recommended by the WHO (5–10%). In fact, this method could just be used as an alternative one until the development of a more efficient methods. One of the potential candidates could be a method that initiate germination, blocks sporulation and would therefore target the vegetative form which is the Achilles heel of SFB.
When the incubation time of SFB susceptibility testing did not exceed three days, all the isolates were susceptible to VCNT at 10 µg of vancomycin. This results was similar to studies on clinical Bacillus and on African traditional food starters38–40. But increased incubation time appears to reduce effectiveness of VCNT at 10 µg of vancomycin. This is a concern, but contamination is expected to stabilize after 14 days of incubation, because this deadline will allow sufficient time for the majority of mycobacteria to begin to grow. We noted that the contamination appearances were specific, particularly for B. cereus, B. licheniformis and Paenibacillus. This specificity of the appearance of contamination could serve as a tool for predicting the types of contaminants.
In summary, this check indicated that VCNT at 10 µg of vancomycin is a good alternative to reduce contamination of sputum culture, particularly those linked to SFB. Increasing the VCNT concentration for reducing contamination is not an option for paucibacillary sputum but could be used for strongly positive smears. A multicenter evaluation is necessary to sharpen and confirm all these results mainly for resource-limited settings where contamination issue are frequent.
Conclusion
The identification of isolated SFB from contaminated LJ media belonging to the Bacillus, Paenibacillus, Brevibacillus and Lysinibacillus genera. The most important were B. cereus and B. licheniformis. The most important identified strains were similar to those currently isolated in fermented traditional food suggesting at least in part food-related contaminants. The involvement of similar isolates in human infections suggests that care must be taken about their real role in sputum. The use of selective LJ with VCNT containing 10 µg of vancomycin to reduce culture contamination is a good alternative method pending the development of a more efficient method.
Materials and Methods
Ethics statement and biosecurity
Isolates of SFB, M. tuberculosis and sputum used in this study were obtained from previous study carried out in Bacteriology laboratory of Centre MURAZ research institute12. Briefly, these isolates were collected after culture of sputum collected at Centre regional de lutte antituberculeuse (CRLAT), without use of antiseptic mouth rinsing as described previously12. Sputum for VCNT evaluation were remaining samples collected from adult patients after informed consent at CRLAT during the same study12. This latter protocol was approved by the Institutional Ethics Committee of Centre MURAZ research institute (N° 2017-03/MS/SG/CM/CEI). All work were done under a Class 2 Biosafety Cabinets (BSC Class II) and Personal Protective Equipment (PPE) have been used as recommended by the WHO for laboratories in countries with limited resources that cultivate mycobacteria41.
Subculture and morphological characterization
Fifty-tree SFB strains isolated from contaminated sputum culture on LJ media were used. Around 79% (42/53) of isolate was collected from confirmed TB cases, and 21% (11/ 53) from negative TB cases with coughing more than two weeks. These isolates, collected between February 2016 and May 2017 were stored at −20 °C in liquid media, consisting of skim milk, glycerol, glucose, and tryptone soya broth (STGG). Isolates were piqued onto 5% sheep-blood Columbia agar (Liofilchem®,roseta d. Abruzzi TE- Italy), and incubated for 24 hours in aerobic conditions at 37 °C. In order to obtain isolated colonies, the obtained colonies were diluted at 10−3 with sterile distilled water after a suspension at 0.5 McFarland, and were plated onto 5% sheep-blood Columbia agar for 24 hours in aerobic conditions at 37 °C. Isolated colonies were described, and the bacteria motility was determined after microscope observation of bacteria cells between slide and cover glass. Cells from smear of the colonies were described after Gram and Malachite green staining. Catalase test (ID color Catalase, Biomérieux SA, Lyon, Marcy-l’étoile/France) as well as the oxidase one (Oxydase test Disc, Liofilichem s.r.l Roseto D.A- Italy) were done.
Spores forming bacteria identification
API 50 CH strip (bioMerieux, Marcy-L’Etoile, France) intended for the identification of Bacillus and related genera were performed according to the indications of the manufacturer. This ready-to-use medium allows the fermentation of the 49 carbohydrates and allow to identify around 29 species of Bacillus and related genera. A 24 hours grown bacterial colony from 5% sheep-blood Columbia agar was suspended at 2 McFarland in the CHB/E medium (bioMerieux, Marcy- l’Etoile, France) and inoculated on each strips. The carbohydrates fermentation product an acid metabolites which produce a decrease in the pH (after 24 to 48 hours of incubation at 37 °C) detected by the change in color of the indicator. The obtained biochemical profile were used for isolates identification. API test kit results were interpreted using the Analytical Profile Index (API) database of the Apiweb software (software BioMérieux, Marcy l’Etoile, France, version1.2.1). To identify an organism, the APIweb software compares the profiles obtained with those of the taxa in the database and assigns a score to each test. The strain of Bacillus spizizenii/ATCC/6633 was used for the internal quality control of the identification.
External quality control were done with 15 isolates identified by Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) in Marseille (France).
Grown bacterial colony from solid media was applied directly on a MALDITOF steel target plate in quadruplicate (Bruker Daltonics, Wissembourg, France) and a matrix solution was used to overlay the sample and allowed to co-crystallize. The plate was then loaded into the Microflex LT MALDI-TOF Mass Spectrometer (Bruker Daltonics) for spectra acquisition using MALDI Biotyper automation. Identification score criteria were performed as recommended by Bruker Daltonics: A score of 2.000 indicated species level identification; a score of 1.700–1.999 indicated identification to the genus level and a score of <1.700 was interpreted as misidentification.
LJ with VCNT effectiveness on clinical sputum culture contamination
Thirty two sputum had been collected from 19 tubercular detected adult patients between December 2016 and January 2017 were used. Thirteen patients provided each 2 sputum and 6 patients provided 1 sputum. Collected sputum were stored at +4 °C−+8 °C in the refrigerator and were then transported within 72 h on ice packs to Centre MURAZ laboratory for culture processing.
After decontamination by standard methods with 4% NaOH, as previously described41, 100 µl of each specimen was inoculated onto 2 LJ without VCNT and 2 LJ supplemented with VCNT at10 µg of vancomycin. All inoculated media were incubated at 37 °C and exanimated at day three and seven mainly for contamination, and every week during 90 days for colonies. The criteria for contaminated culture were: (1) any change in colour or consistency of culture media, (2) development of any liquid or film on the culture media, and/or (3) presence of non-mycobacterial colonies on culture media, on the base of colony morphology and/or ZN staining.
Mycobacteria tolerance and spores forming bacteria susceptibility to VCNT
Theses experimentations were realized between 24 November 2018 and 19 December 2018 with isolates of mycobacteria and SFB collected during a study realized between February 216 and May 201715.
Mycobacteria tolerance to VCNT
Eight (8) isolates of mycobacteria were used including one live attenuated Bacillus Calmette Guerin strain as positive control (BCG 00002462 ref: KSV-026/17; Green Signal, India) and seven (7) clinical isolates obtained during the research activity of the mycobacteria Research Unit, Centre Muraz Bobo-Dioulasso, Burkina-Faso. In addition, sterile distilled H2O was used as negative control. The colonies gathered from LJ media, transferred to a 15-mm tube containing several glass beads (20 a 30 beads) and 3 ml of distilled water, were vortexed and a suspension calibrate to 0.5 Mac Farland using sterile H2O and a 580-nm cell densitometer (Densimat; BioMerieux, Marcy-L’Etoile, France) is realized in order to obtain a 106 CFU/ml. From the 0.5 McFarland suspension, inoculum were diluted at 10−1 and 10−3 for each isolate and then, 100ul volume (containing around 104 CFU for dilution at 10−1 and around 102 CFUs for dilution at 10−3) were inoculated in duplicate onto LJ without or with VCNT at different concentration of vancomycin. LJ media were prepared according to manufacturer guidelines and supplemented with VCNT to make a final concentration of 10, 15 and 30 µg/ml of vancomycin (SR0091E, Oxoid Ltd, wade Road, Basingstoke, Hants,RG24 8PW, UK) before coagulating in Frio cell (Fisher Bioblok scientifique). The inoculated tubes were incubated at 37 °C and were checked visually by the naked eye every 72 h for colonies during 28 days. Ziehl-Neelsen staining of colonies was used to confirm the presence of Acid Fast Bacillus (AFB). For each isolates, colonies were counted on duplicate tubes, at deferent concentration of VCNT and the mean count were recorded.
Spores forming bacteria susceptibility to VCNT
Fifteen isolates including three B. cereus, three B. Licheniformis, three Paenibacillus, three non-reactive Bacillus, one B. subtilis one Brevibacillus were used. In addition, one E. coli ATCC25922 and sterile distilled H2O as positive and negative control respectively were used. The treatment of these isolates before inoculating onto LJ media with or without VCNT was the same as mycobacteria isolates except that beads were not used for the preparation of the bacillary suspension. The inoculated LJ with or without VCNT were checked visually by the naked eye at three, seven and fourteen days for contamination. Because of stratal contamination, CFUs counting is not possible on LJ contaminated media. Thus, we determine the contamination rate at each concentration of VCNT by reporting the ratio of SFB that have grown on LJ with VCNT on the total number of inoculated SFB at both dilution.
Statistical analysis
Excel 2013 were used for data entry and OpenEpi 3 for data analysis. The contamination rate of clinical sputum inoculated onto LJ without or with VCNT at 10 µg of vancomycin were compared using McNemar test (2 × 2 tables) or Fischer’s exact test where appropriate. Wilson t test were used to compare mean of CFUs of LJ with VCNT at different concentration of vancomycin to control (LJ without VCNT). The proportions of SFB which have contaminated LJ with VCNT at different concentration was compared with binomial test to standard contamination rate in our laboratory (around 40%). Values of p < 0.05 were considered statistically significant.
Acknowledgements
The authors thank: the National Tuberculosis Program and the CRLAT, particularly Koutou Félicité, Edouard Kalmogo for their kind cooperation during the isolates collection; the Center. MURAZ bacteriology and virology teams, particularly Dr Thérese Kagoné, Dr Soumeya. Ouangraoua, Eli Kabre, Kobo Gnada, Moumini Nouctara and for frank collaboration during study. This study was supported by Strategic Network on Tuberculosis of Tropical Medicine Institute, Antwerp.
Author Contributions
Conception and design study: H.H., N.M., G.D., A.K., J.D.T. and L.S. Performed the experiments: A.K., O.Z. and D.Z. Analyzed and interpretation of the data: A.K., J.D.T., D.Z. and S.A. Drafting the article: A.K. and H.H. Revising it: H.H., A.S., G.A.O., M.K.G., J.D.T., N.M. and L.S. Final approval of the version: All authors read and agreed to the final version of this manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jacobus, H. W., & Jaime, R. Conventional diagnostic methods in Tuberculosis 2007 from basic science to patient care (eds Palomino, J. C., Leao, S. C. & Ritacco, V.) 401–420, Available from, www.tuberculosistextbook.com (2007).
- 2.Alli OA, et al. Direct molecular detection of Mycobacterium tuberculosis complex from clinical samples - An adjunct to cultural method of laboratory diagnosis of tuberculosis. North Am J Med Sci. 2011;3:281–288. doi: 10.4297/najms.2011.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray, P. R., Baron, E. J., Pfaller, M. A., Tenover, F. C. & Yolken, R. H. Manual of clinical microbiology (17è ed. Washington, D.C. ASM Press) (1999).
- 4.Peres RL, et al. Evaluation of oral antiseptic rinsing before sputum collection to reduce contamination of Mycobacterial cultures. J Clin Microbiol. 2011;49:3058–3060. doi: 10.1128/JCM.00541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asmar S, Drancourt M. Chlorhexidine decontamination of sputum for culturing Mycobacterium tuberculosis. BMC Microbiol. 2015;15:155. doi: 10.1186/s12866-015-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO). Laboratory services in tuberculosis control. (ed. WHO) available: http://whqlibdoc.who.int/hq/1998/WHO_TB_98.258_(part3).pdf, Geneva, Switzerland (1998).
- 7.Prescott, L. M., Harley, J. P., & Klein, D. A. Le control des microorganismes par les agents physiques et chimiques in Microbiologie (2ème éds Française Prescott, L. M., Harley, J. P., & Klein, D) 136–151 (de Boeck, 2003).
- 8.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClean M, et al. Identification and characterization of breakthrough contaminants associated with the conventional isolation of Mycobacterium tuberculosis. J Med Microbiol. 2011;60:1292–8. doi: 10.1099/jmm.0.030619-0. [DOI] [PubMed] [Google Scholar]
- 10.Kassaza K., Orikiriza P., Llosa A., Bazira J., Nyehangane D., Page A.-L., Boum Y. Lowenstein-Jensen Selective Medium for Reducing Contamination in Mycobacterium tuberculosis Culture. Journal of Clinical Microbiology. 2014;52(7):2671–2673. doi: 10.1128/JCM.00749-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keilty RA. A Study of the Cultivation of the Tubercle Bacillus Directly from the Sputum by the Method of Petroff. J. Exp. Med.,, xxii. 1915;612:11. doi: 10.1084/jem.22.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothlauf MV, George L, Brown GL, Blair EB’. Isolation of Mycobacteria from Undecontaminated Specimens with Selective 7H10 Medium. Journal of Clinical Microbiology. 1981;13:76–79. doi: 10.1128/jcm.13.1.76-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew S, Paramasivan CN. Use of vancomycin in the culture of Mycobacterium tuberculosis from gastric lavage. Indian J Med Res. 2001;113:125–128. [PubMed] [Google Scholar]
- 14.Outo J, et al. Comparative evaluation of BACTEC MGIT 960 with BACTEC 9000 MB and LJ for isolation of mycobacteria in The Gambia. J Infect Dev Ctries. 2008;2:200–205. doi: 10.3855/jidc.263. [DOI] [PubMed] [Google Scholar]
- 15.Kabore Antoinette, Tranchot-Diallo Juliette, Sanou Adama, Hien Hervé, Daneau Géraldine, Gomgnimbou Michel Kireopori, Meda Nicolas, Sangaré Lassana. Why oral antiseptic mouth rinsing before sputum collection cannot reduce contamination rate of mycobacterial culture in Burkina-Faso. African Health Sciences. 2019;19(1):1321. doi: 10.4314/ahs.v19i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinstein E, Keynan Y. Vancomycin revisited – 60 years later. Public Healf. 2014;2:217–224. doi: 10.3389/fpubh.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celandroni F, et al. Identification and Pathogenic Potential of Clinical Bacillus and Paenibacillus Isolates. PLoS ONE. 2016;11:e0152831. doi: 10.1371/journal.pone.0152831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chulhun LC, et al. Reduction of Contamination of Mycobacterial Growth Indicator Tubes with a Modified Antimicrobial Combination. Journal of Clinical Microbiology. 2002;40:3845–3847. doi: 10.1128/JCM.40.10.3845-3847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew S, Paramasivan CN, Datta M, Prabhakar R. Vancomycin for controlling contamination of selective Kirchner’s liquid medium in the culture of gastric lavage for tubercle bacilli. Indian J Med Res. 1995;102:152–155. [PubMed] [Google Scholar]
- 20.Drobniewski FA. Bacillus cereus and Related Species. Clin Microbiol Rev. 1993;6:324–338. doi: 10.1128/CMR.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrero MA, Castro GR, Abate CM, Baigorõ MD, Siñeriz F. Thermostable alkaline proteases of Bacillus licheniformis MIR 29: isolation, production and characterization. Appl Microbiol Biotechnol. 1996;45:327–332. doi: 10.1007/s002530050691. [DOI] [Google Scholar]
- 22.Li J, et al. Comparative analysis of the human saliva microbiome from different climate zones: Alaska, Germany, and Africa. BMC Microbiol. 2014;14:316. doi: 10.1186/s12866-014-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanni AI. The needs of optimization of African fermented foods and beverages. Int J Food Microbiol. 1993;18:85–95. doi: 10.1016/0168-1605(93)90213-Z. [DOI] [PubMed] [Google Scholar]
- 24.Sanni AI, Onilude AA, Fadahunsi IF, Ogubanwo ST, Afolabi RO. Selection of starter cultures for the production of ugba, a fermented soup condiment. Eur. Food Res. Technol. 2002;215:176–180. doi: 10.1007/s00217-002-0520-3. [DOI] [Google Scholar]
- 25.Ouoba LII, Parkouda C, Diawara B, Scotti C, Varnam AH. Identification of Bacillus spp from Bikalga, fermented seeds of Hibiscus sabdariffa: phenotypic and genotypic characterization. J Appl Microbiol. 2008;104:122–13. doi: 10.1111/j.1365-2672.2007.03550.x. [DOI] [PubMed] [Google Scholar]
- 26.Savadogo A, Ilboudo AJ, Gnankine O, Traore AS. Numeration and Identification of thermotolerant endospore-forming Bacillus from two fermented condiments Bikalga and Soumbala. Adv. Environ. Biol. 2011;5:960–2967. [Google Scholar]
- 27.Ouoba LII, Diawara B, Jespersen L, Jakobsen M. Antimicrobial activity of Bacillus subtilis and Bacillus pumilus during the fermentation of African locust bean (Parkia biglobosa) for Soumbala production. J. Appl. Microbiol. 2007;102:963–970. doi: 10.1111/j.1365-2672.2006.03156.x. [DOI] [PubMed] [Google Scholar]
- 28.Turnbull, P. C. B. Bacillus in Medical Microbiology. (4th edition Baron, S.) Galveston (TX): University of Texas Medical Branch at Galveston; Chapter 15, https://www.ncbi.nlm.nih.gov/books/NBK7699/ (1996).
- 29.Logan NA. Bacillus and relatives in foodborne illness. J. of Appl Microbiol. 2011;112:417–429. doi: 10.1111/j.1365-2672.2011.05204.x. [DOI] [PubMed] [Google Scholar]
- 30.Bottone EJ. Bacillus cereus, a Volatile Human Pathogen. Clin. Microbiol. Rev. 2010;2:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweany HC, Pinner M. A pathogenic subtilis bacillus from a patient with chronic tuberculosis. Infect. Dts. 1925;37:340–343. [Google Scholar]
- 32.Jeon YL, et al. Combined Bacillus licheniformis and Bacillus subtilis infection in a patient with esophageal perforation. J Med Microbiol. 2012;61:1766–1769. doi: 10.1099/jmm.0.042275-0. [DOI] [PubMed] [Google Scholar]
- 33.Pearson HE. Human Infections Caused by Organisms of the Bacillus Species. Am J Clin Pathol. 1970;53:506–515. doi: 10.1093/ajcp/53.4.506. [DOI] [PubMed] [Google Scholar]
- 34.Yuichiro S, et al. Bacillus cereus pneumonia in an immunocompetent patient: a case report. JA clin. Rep. 2017;3:25. doi: 10.1186/s40981-017-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frankard J, et al. Bacillus cereus pneumonia in a patient with acute lymphoblastic leukemia. Eur J Clin Microbiol Infect Dis. 2004;23:725–728. doi: 10.1007/s10096-004-1180-y. [DOI] [PubMed] [Google Scholar]
- 36.Kim KK, et al. Paenibacillus sputi sp. nov., isolated from the sputum of a patient with pulmonary disease. Int J Syst Evol Microbiol. 2010;60:2371–2376. doi: 10.1099/ijs.0.017137-0. [DOI] [PubMed] [Google Scholar]
- 37.Wood MR, Yu EA, Mehta S. The Human Microbiome in the Fight against tuberculosis. Am. J. Trop. Med. Hyg. 2017;96:1274–1284. doi: 10.4269/ajtmh.16-0581,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda M, et al. Clinical characteristics and antimicrobial susceptibility of Bacillus cereus blood stream infections. Ann Clin Microbiol Antimicrob. 2015;14:43. doi: 10.1186/s12941-015-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adimpong DB, et al. Antimicrobial Susceptibility of Bacillus Strains Isolated from Primary Starters for African Traditional Bread Production and Characterization of the Bacitracin Operon and Bacitracin Biosynthesis. Appl. Environ. Microbiol. 2012;78:79037914. doi: 10.1128/AEM.00730-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.International Union Against Tuberculosis and Lung Disease. Priorities for Bacteriology Services in Low-Income Countries. Available from, http://www.theunion.org/what-we-do/publications/technical/priorities-for-tuberculosis-bacteriology-services-in-low-income-countries (2007)
- 41.Zingué D, et al. Advantages and drawbacks of expectoration decontamination methods for tuberculosis and anti-tuberculosis drug resistance diagnosis. Ann Biol Clin. 2013;71:283–291. doi: 10.1684/abc.2013.0815. [DOI] [PubMed] [Google Scholar]