Abstract
The present study investigated the effect of arylsulfonyl indoline-benzamide (ASIB) on neovascular glaucoma in the mice model in vivo. In the mice model of glaucoma, ASIB treatment significantly (P < 0.05) increased PDGF-B-positive cell count in the corneal tissues. ASIB treatment at 5, 10, 15 and 20 mg/kg doses raised the level of PDGF-B mRNA in the mice cornea by 2.3-, 3.8-, 5.4- and 5.5-fold, respectively. Pre-treatment of the glaucoma mice with ASIB leads to inhibition of TNF-α and IL-6 production. In the glaucoma mice, treatment with ASIB leads to a marked decrease in the level of NOD2 mRNA and protein. ASIB treatment caused a significant decrease in the glaucoma-induced up-regulation of NF-κB p65 activation. The phosphorylation of NF-κB p65 was almost completely inhibited in the glaucoma mice on treatment with 15 mg/kg dose of ASIB. ASIB exhibited inhibitory effect on glaucoma-induced inflammatory cytokine and oxidative factor damage in the mice. It caused up-regulation of PDGF expression and down-regulated NF-κB activation. Therefore, ASIB can be of therapeutic significance for neovascular glaucoma treatment. However, more studies need to be performed to fully understand the molecular mechanism of ASIB in glaucoma treatment.
Keywords: Neovascular glaucoma, Intraocular, Optic nerve, Pro-inflammatory
Introduction
Neovascular glaucoma is a disease of the eyes leading to blindness, intractable disease and is difficult to manage (Sohan 2007). It is one of the serious ophthalmic diseases, is detected generally in the old aged people and is a cause of blindness in high percentage of the population (Worley and Grimmer-Somers 2011; Wong et al. 2011; Yanagi et al. 2011). The main factor responsible for the development and progression of glaucoma is high intraocular pressure which is associated with optic nerve shrinking and loss of ganglion cells in the retina (Worley and Grimmer-Somers 2011; Wong et al. 2011; Yanagi et al. 2011). There are no chances of vision restoration following glaucoma because of the loss of ganglion cells. At present, the disease is generally treated by the transplantation of stem cells; however, in most of the cases, the transplanted cells are unable to undergo specialised differentiation (Worley and Grimmer-Somers 2011; Hayreh 2007). Therefore, protection of neuronal damage by inhibition of inflammatory cytokines and oxidant radicals in patients with glaucoma is considered to be the best approach for development of treatment strategy.
The production of pro-inflammatory cytokine, tumour necrosis factor-α (TNF-α) and mediators of inflammation such as interleukin (IL)-6 and -8 during the infection and under oxidative stress leads to immune system modulation. TNF-α and IL-6 play very important role in the regulation of network of the cytokines in the body (Parrillo et al. 1990; Pinsky et al. 1993). The secretion of cytokines in higher concentration is associated with the enhancement of vascular permeability, development of coagulopathy and also leading cause of multiple organ dysfunction (Blackwell and Christman 1996).
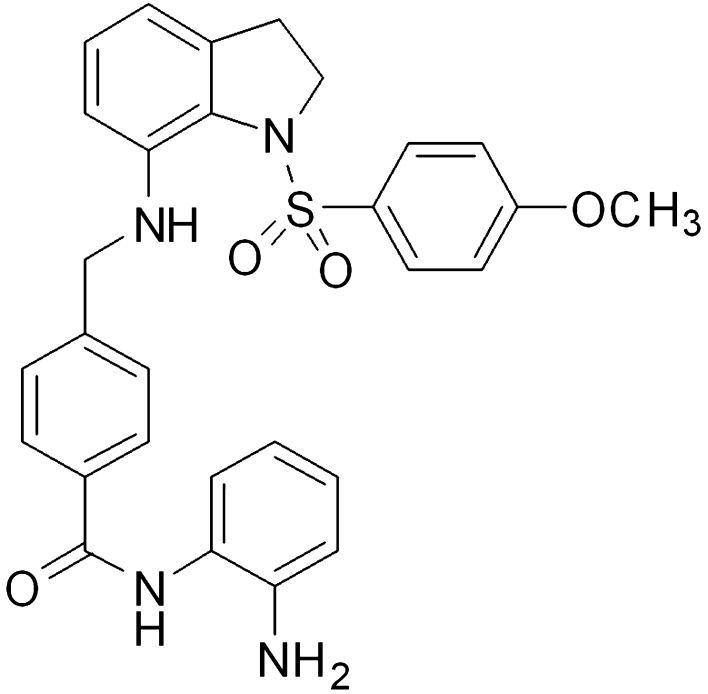
Platelet-derived growth factors (PDGF) are produced in several types of cells and have been found to attach to their specific receptor (PDGFR) (Ishii et al. 2006). Study has revealed that administration of PDGFs exogenously plays a vital role in the protection of neuronal degeneration (Ishii et al., 2006). It is believed that PDGFs are associated with neuronal development and their maintenance (Ishii et al. 2006). The process of embryogenesis and pathological changes in the adults are linked with the expression of several members belonging to PDGF family (Bergsten et al. 2001). Transmission of the nerve impulse and axonal growth is regulated by the expression of PDGFs (Wu et al. 2010a, b). In the rat model, PDGF-B has been found to enhance glial cell mitosis and protection denegation of the nervous system (Janmaat et al. 2010). In the present study, effect of novel molecule, arylsulfonyl indoline-benzamide (ASIB; Fig. 1), on neovascular glaucoma in the mice model in vivo was investigated.
Fig. 1.
Chemical structure of arylsulfonyl indoline-benzamide
Materials and methods
Animal model and approval
A total of sixty C57BL/6J mice (5 months old) were obtained from the Experimental Animal Center, Soochow University (Suzhou, China). The mice were acclimatised to the laboratory environment 1-week before the actual start of experiment. All the mice were kept in animal house caged singly at 30 °C temperature and 60% humidity with 12-h light/dark cycles. The mice were provided free access to standard laboratory food and water. Approval for the study was obtained from the Ethics Committee for Care and Animal Research, Department of Ophthalmology (Second Hospital of Jilin University, Changchun, China). The experimental procedures were carried in accordance with the guidelines issued from Ethics Committee for Care and Animal Research.
Establishment of glaucoma mice model
The glaucoma was induced in mice after anesthetization with ketamine (50 mg/kg) and xylazine (10 mg/kg). The mice were then subjected to laser irradiation to the eyes twice with an in-between time gap of 2 h using slit lamp. The laser irradiation involved 65 pulses of 2 s each under diode input of 532 nm. The mice in the control group were subjected to anesthetization but left without laser irradiation. Neovascular glaucoma induction in mice was confirmed by recording intraocular pressure using Icare® Tonolab tonometer (Icare Finland Oy, Espoo, Finland). At 24 h of laser irradiation, intraocular pressure increased to ~ 22 mmHg in comparison to ~ 7–9 mmHg in normal mice. The mice were pre-treated with 5, 10, 15 and 20 mg/kg doses of ASIB 1 h before the surgery through intraperitoneal route.
Immunohistochemical assay
On day 8 of surgery, the mice were killed using ketamine (50 mg/kg) and xylazine (10 mg/kg) anaesthesia to collect the corneal tissues. The tissues after saline washing were treated with 200 ml of fixative which contained 4% paraformaldehyde in 0.1 M PBS at a pH of 7.5 for 3 h. Then the tissues were put into the 30% phosphate-buffered sucrose, embedded in paraffin and subsequently mounted onto the slides subjected to poly-l-lysine pre-coating. The tissues were washed with PBS and then incubated for 45 min with 1% bovine serum albumin. Then incubation of the tissues was performed at 4 °C with primary antibodies against PDGF-B (dilution 1:100) and 1% BSA overnight. The slides after PBS washing were subjected to incubation for 2 h with biotinylated goat anti-rabbit secondary antibody (dilution 1:200). Visualisation of the immunolabelling was performed using 0.05 DAB and 0.3% H2O2 in PBS. Before mounting of tissues under coverslips with Permount™, dehydration was performed using ethyl alcohol and xylene. The pathological changes in tissues were observed using H&E staining.
Western blot analysis
The corneal tissues were treated with lysis buffer containing Tris–hydrochloride (12 mM), EDTA (1.2 mM), and sucrose (275 mM) at a pH of 7.5, aprotinin (17 μg/ml), leupeptin (6 μg/ml), PhCH2SO2F (0.2 mM), sodium fluoride (1.2 mM) and Na3VO4 (1.2 mM). The concentration of proteins in lysates after centrifugation was determined using bicinchoninic acid (BCA) kit (Sigma-Aldrich). The protein resolution was performed by electrophoresis on SDS-PAGE with 10% Tris–glycine gel and then transferred to polyvinylidene membranes. The protein bands were incubated overnight at 4 °C with rabbit polyclonal antibody against p-NF-κB p65 (dilution 1:1000; obtained from Cell Signaling Technology, Danvers, MA, USA). The bands after washing two times with Tris-buffered saline and Tween 20 (TBST) were subjected to incubation for 2 h with horseradish peroxidase-conjugated secondary antibodies (dilution 1:1000; from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature. Visualisation of the immunoreactive bands was performed by incubation with lumiGLO reagent (Cell Signaling Technologies, Inc.) in accordance with the manual protocol. The protein bands were quantified in relation to GAPDH protein expression.
Real-time qPCR assay
The total RNA from corneal tissues was extracted by the TRIzol® reagent kit in accordance with the instructions from the supplier. The spectrophotometer was used for the determination of RNA concentration and 2 μg of samples was utilised for the synthesis of complementary DNA. The reaction was performed using the mixture 4 μl of 5X RT buffer, 2.5 μmol/l oligodeoxythymidylic acid oligo(dt), 5 mmol/l deoxyribonucleotide triphosphate (dNTP) and 20 U of RNase inhibitor. Annealing of the hexamers was carried out for 10 min at 65 °C followed by the addition of M-MLV reverse transcriptase (200 U). Then the mixture was subjected to incubation for 1 h at 45 °C and the reaction was quenched by heating for 15 min to 75 °C. The reaction mixture used for RT-qPCRs consisted of complementary DNA (4 μl), SYBR®-Green qPCR mix (40 μl), 5 U TaqDNA polymerase (0.5 μl) and 22 pmol/μl PDGF primer (0.5 μl). Denaturation of the DNA was performed for 4 min at 94 °C and amplification involved 45 rounds of PCR (the sequence consisted of denaturation for 15 s at 94 °C, annealing for 35 s at 62 °C and extension for 25 s at 73 °C). The fluorescence was recorded at 73 °C. The primers used were as follows: PDGF-B: forward, 5-CTCCATCC GCTCCTTTGATGACCTT-3 and backward, 5-CAGCTCAGC CCCATCTTCGTCTA-3; GAPDH: forward, 5-GGTGGACCTCATGGCCTACAT-3 and backward, 5-GCCTCTCTCTTGCTCTCAGTATCCT-3; NOD2 forward, ATC CCT CGG TTA CTA TGT TG; backward, GCT TCC TGA ATA CTC CTC CT.
Enzyme-linked immunosorbent assay (ELISA)
The retinal tissues after washing with PBS were homogenised using ice-cold solution of homogenate buffer. The homogenization buffer consisted of HEPES (10 mM; pH 7.7), potassium chloride (10 mM), magnesium chloride (2 mM), EDTA (0.1 mM), dithiothreitol (1.0 mM) and phenylmethanesulfonyl fluoride (0.5 mM). The centrifugation of homogenate was carried out for 20 min at 4000×g at 4 °C. The collected supernatants were stored under liquid nitrogen until use in the further experiment. Analysis of the TNF-α and IL-6 was performed using commercially available kits in accordance with the instructions from manufacturer (TNF-α ELISA kit; Diaclone, Besançon, France; IL-6 Rat ELISA kit, cat. no. KRC0061; BioSource Europe SA, Nivelles, Belgium). The level of TNF-α and IL-6 in tissues was presented as units per pg.
Statistical analysis
The data were analysed statistically using SPSS software, version 15.0 (SPSS Inc., Chicago, IL, USA). The values are represented as the average ± standard deviation (SD). The Student’s t test and one-way analysis of variance (ANOVA) were used for the determination of differences among various groups. The differences were taken to be statistically significant at P < 0.05.
Results
ASIB increases PDGF-B expression in glaucoma mice
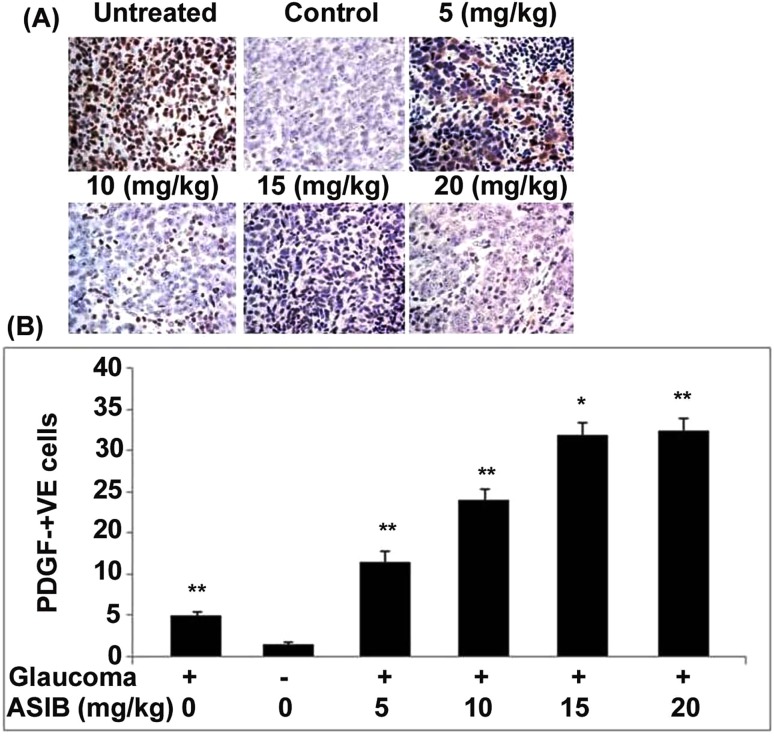
In the mice of sham control and untreated groups, DAB staining showed the presence of only few PDGF-B-positive cells in the corneal tissues. ASIB treatment of the mice with glaucoma significantly (P < 0.05) increased PDGF-B-positive cell count in the corneal tissues (Fig. 2a). ASIB treatment increased the PDGF-B-positive cell count from 5 to 15 mg/kg doses but then it remained constant. These findings suggested that the effect of ASIB on increasing PDGF-B cell population was maximum at 15 mg/kg doses. The PDGF-B-positive cell count was found to be almost equal in the mice treated with 15 and 20 mg/kg doses of ASIB. The effect of ASIB on expression of PDGF-B in mice corneal tissues was also confirmed using RT-qPCR. A significant increase in the level of PDGF-B mRNA in mice corneal tissues was caused by treatment with ASIB (Fig. 2b). ASIB treatment at 5, 10, 15 and 20 mg/kg doses raised the level of PDGF-B mRNA in mice cornea by 2.3-, 3.8-, 5.4- and 5.5-fold, respectively.
Fig. 2.
Increase in PDGF-B expression by ASIB. The mice pre-treated with ASIB were induced glaucoma and then PDGF-B expression was assessed by a immunohistochemical staining and b RT-PCR assays. Images were captured at × 250. *P < 0.05 and **P < 0.01 vs. untreated mice
ASIB inhibits pro-inflammatory cytokines in glaucoma mice
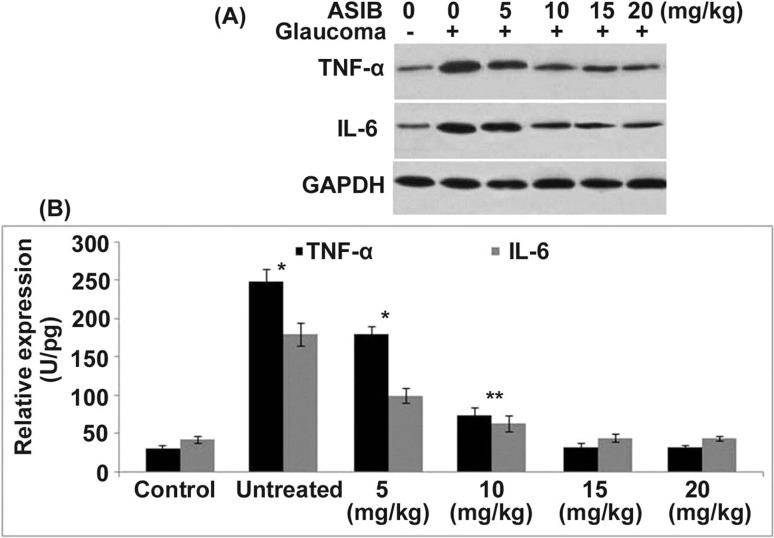
The level of TNF-α and IL-6 in the glaucoma mice corneal tissues was significantly (P < 0.05) higher than those of the normal control group (Fig. 3). Pre-treatment of the glaucoma mice with ASIB leads to inhibition of TNF-α and IL-6 production. The inhibitory effect of ASIB on the production of TNF-α and IL-6 in mice corneal tissues was dose based. Although a significant decrease in the production of TNF-α and IL-6 was caused by ASIB from 5 mg/kg dose, the effect was maximum at 15 mg/kg concentration.
Fig. 3.
Effect of ASIB on TNF-α and IL-6. Glaucoma was induced in mice following ASIB pre-treatment. Production of TNF-α and IL-6 in mice corneal tissues was determined by a ELISA and b western blotting assays. *P < 0.05 and **P < 0.01 vs. untreated mice
ASIB suppresses NOD2 level in glaucoma mice
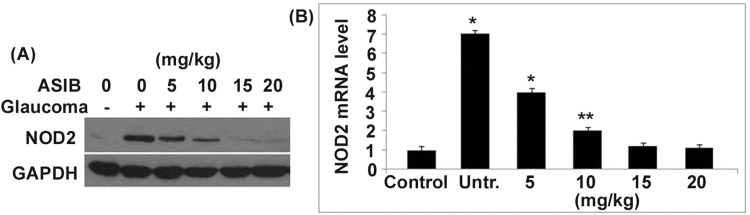
The level of NOD2 mRNA and protein in the corneal cells of glaucoma mice was significantly (P < 0.05) higher than those of the normal control group (Fig. 4). In the glaucoma mice, treatment with ASIB leads to a marked decrease in the level of NOD2 mRNA and protein in a dose-based manner. The level of NOD2 mRNA and protein was almost completely inhibited in the glaucoma mice on treatment with 15 mg/kg dose of ASIB.
Fig. 4.
Effect of ASIB on level of NOD2 mRNA in glaucoma mice. The NOD2 mRNA and protein levels in the glaucoma mice were analysed by a western blot and b RT-PCR assays. The presented values are average ± SD of three experiments. *P < 0.05 and **P < 0.01 vs. untreated mice
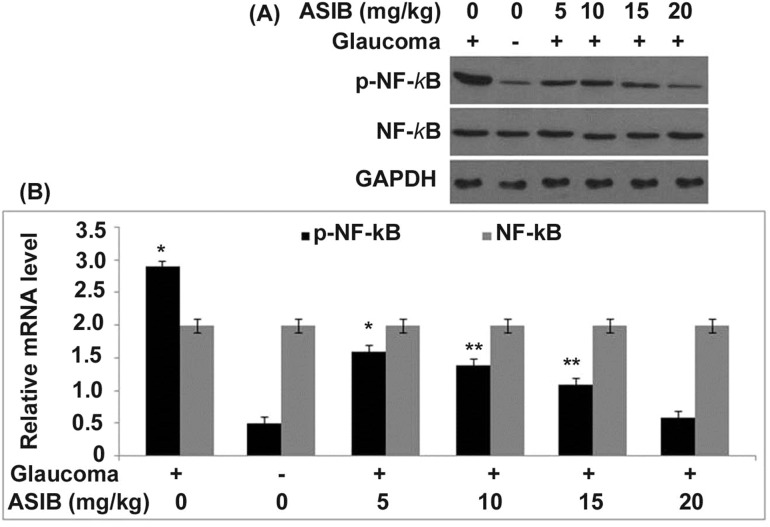
ASIB down-regulated NF-κB p65 phosphorylation in glaucoma mice
The activation of NF-κB p65 was markedly up-regulated in the corneal tissues of glaucoma mice than those in the normal group (Fig. 5). ASIB treatment caused a significant decrease in the glaucoma-induced up-regulation of NF-κB p65 activation. A dose-based reduction in glaucoma-induced up-regulation of NF-κB p65 activation was caused in mice on treatment with ASIB. The phosphorylation of NF-κB p65 was almost completely inhibited in the glaucoma mice on treatment with 15 mg/kg dose of ASIB.
Fig. 5.
Down-regulation of NF-κB activation by ASIB. The activation of NF-κB in mice was analysed by a western blotting and b calculation of values was performed in relation to density of β-actin. *P < 0.05 and **P < 0.01 vs. untreated mice
Discussion
Neovascular glaucoma is caused due to the development of abnormal blood vessels on iris leading to blocking of water drainage from the frontal portion of the eyeball. The present study investigated the effect of arylsulfonyl indoline-benzamide (ASIB) on neovascular glaucoma in the mice model in vivo. ASIB exhibited inhibitory effect on glaucoma-induced inflammatory cytokine and oxidative factor damage in the mice. It caused up-regulation of PDGF expression and down-regulated NF-κB activation. Therefore, ASIB can be of therapeutic significance for neovascular glaucoma treatment.
In adult human beings, members of PDGF family are involved in the process of embryogenesis and various pathologies (Enge et al. 2003). The compatibility of PDGF-B is good enough with the central nervous system development and in response of astroglial cells to the injury (Enge et al. 2003). HIV-1 Tat-induced toxicity of the neurons is protected by the exogenous administration of PDGF. In the present study, DAB staining showed the presence of only few PDGF-B-positive cells in the corneal tissues of the mice of sham control and untreated groups. ASIB treatment of the mice with glaucoma significantly (P < 0.05) increased PDGF-B-positive cell count in the corneal tissues. The increase in PDGF-B-positive cell count by ASIB treatment was maximum at 15 mg/kg doses and then it remained constant. The increase in PDGF-B by ASIB in the mice with glaucoma suggested the potential of the tissues to repair and regain their function.
Suppression of the pro-inflammatory factor, TNF-α and related mediators of inflammation like IL-6 and prostaglandin E2 by chemotherapeutic agents have been shown to be of significance in the treatment of various disorders (Kim et al. 2015; Zhu et al. 2013; Han et al. 2011; Sun et al. 2009). In the rat model, inflammation is inhibited by down-regulation of TNF-α and interleukin-6 on treatment with drug candidates (Wu et al. 2008; Wu et al. 2010a, b). The present study investigated the effect of ASIB on secretion of pro-inflammatory cytokines and mediators of inflammation in the mice corneal cells. The study demonstrated that ASIB treatment of the mice decreased glaucoma-induced up-regulation of TNF-α and IL-6 levels. The inhibitory effect of ASIB on production of TNF-α and IL-6 in mice corneal tissues was dose based. Although a significant decrease in production of TNF-α and IL-6 was caused by ASIB from 5 mg/kg dose, the effect was maximum at 15 mg/kg concentration.
NF-κB, which is an inducible nuclear transcription factor, plays an important role in the regulation of transcription of genes associated with different functions (Hotchkiss et al. 2003; Arnalich et al. 2000). The genes which encode TNF-α and IL-6 pro-inflammatory factors and mediators are also regulated by NF-κB (Hotchkiss et al. 2003; Arnalich et al. 2000). The increased mortality rate because of various disorders is believed to be associated with the constant NF-κB activation (Hotchkiss et al. 2003). Suppression of NF-κB activation has been found to exhibit beneficial effect (Feng et al. 2006a, b). The present study showed markedly higher activation of NF-κB p65 in the corneal tissues of glaucoma mice. ASIB treatment caused a significant decrease in the glaucoma-induced up-regulation of NF-κB p65 activation. The phosphorylation of NF-κB p65 was almost completely inhibited in the glaucoma mice on treatment with 15 mg/kg dose of ASIB.
NOD2 exhibits its effect by transmitting signals to receptor-interacting protein 2 which subsequently activates NF-κB-induced pro-inflammatory response (Hasegawa et al. 2008; Magalhaes et al. 2011). Failure of NOD2 to regulate NF-κB activation due to polymorphism in the genes which encode NOD2 leads to early mortality (Brenmoehl et al. 2007). In the present study, NOD2 mRNA and protein levels in the corneal cells of glaucoma mice were significantly (P < 0.05) higher. ASIB treatment of the mice with glaucoma leads to marked decrease in the level of NOD2 mRNA and protein.
Conclusion
ASIB exhibited inhibitory effect on glaucoma-induced inflammatory cytokine and oxidative factor damage in the mice. It caused up-regulation of PDGF expression and down-regulated NF-κB activation. Therefore, ASIB can be of therapeutic significance for neovascular glaucoma treatment. However, more studies need to be performed to fully understand the molecular mechanism of ASIB in glaucoma treatment.
References
- Arnalich F, Garcia-Palomero E, López J, Jiménez M, Madero R, Renart J, Vázquez JJ, Montiel C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun. 2000;68:1942–1945. doi: 10.1128/IAI.68.4.1942-1945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten E, Uutela M, Li X, et al. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- Brenmoehl J, Herfarth H, Glück T, Audebert F, Barlage S, Schmitz G, Froehlich D, Schreiber S, Hampe J, Schölmerich J, et al. Genetic variants in the NOD2/CARD15 gene are associated with early mortality in sepsis patients. Intensive Care Med. 2007;33:1541–1548. doi: 10.1007/s00134-007-0722-z. [DOI] [PubMed] [Google Scholar]
- Enge M, Wilhelmsson U, Abramsson A, et al. Neuron-specific ablation of PDGF-B is compatible with normal central nervous system development and astroglial response to injury. Neurochem Res. 2003;28:271–279. doi: 10.1023/A:1022421001288. [DOI] [PubMed] [Google Scholar]
- Feng X, Ren B, Xie W, Huang Z, Liu J, Guan R, Duan M, Xu J. Influence of hydroxyethyl starch 130/0.4 in pulmonary neutrophil recruitment and acute lung injury during polymicrobial sepsis in rats. Acta Anaesthesiol Scand. 2006;50:1081–1088. doi: 10.1111/j.1399-6576.2006.01113.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Yan W, Liu X, Duan M, Zhang X, Xu J. Effects of hydroxyethyl starch 130/0.4 on pulmonary capillary leakage and cytokines production and NF-kappaB activation in CLP-induced sepsis in rats. J Surg Res. 2006;135:129–136. doi: 10.1016/j.jss.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Han NR, Kim HM, Jeong HJ. Inactivation of cystein-aspartic acid protease (caspase)-1 by saikosaponin A. Biol Pharm Bull. 2011;34:817–823. doi: 10.1248/bpb.34.817. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Núñez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 2008;27:373–383. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res. 2007;26:470–485. doi: 10.1016/j.preteyeres.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Oya T, Zheng L, et al. Mouse brains deficient in neuronal PDGF receptor-beta develop normally but are vulnerable to injury. J Neurochem. 2006;98:588–600. doi: 10.1111/j.1471-4159.2006.03922.x. [DOI] [PubMed] [Google Scholar]
- Janmaat ML, Heerkens JL, de Bruin AM, Klous A, de Waard V, de Vries CJ. Erythropoietin accelerates smooth muscle cell-rich vascular lesion formation in mice through endothelial cell activation involving enhanced PDGF-BB release. Blood. 2010;115:1453–1460. doi: 10.1182/blood-2009-07-230870. [DOI] [PubMed] [Google Scholar]
- Kim SO, Park JY, Jeon SY, Yang CH, Kim MR. Saikosaponin a, an active compound of Radix Bupleuri, attenuates inflammation in hypertrophied 3T3-L1 adipocytes via ERK/NF-κB signaling pathways. Int J Mol Med. 2015;35:1126–1132. doi: 10.3892/ijmm.2015.2093. [DOI] [PubMed] [Google Scholar]
- Magalhaes JG, Lee J, Geddes K, Rubino S, Philpott DJ, Girardin SE. Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands. Eur J Immunol. 2011;41:1445–1455. doi: 10.1002/eji.201040827. [DOI] [PubMed] [Google Scholar]
- Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- Sohan SH. Neovascular glaucoma. Prog Ret Eye Res. 2007;26:470–485. doi: 10.1016/j.preteyeres.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Cai TT, Zhou XB, Xu Q. Saikosaponin A inhibits the proliferation and activation of T cells through cell cycle arrest and induction of apoptosis. Int Immunopharmacol. 2009;9:978–983. doi: 10.1016/j.intimp.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Wong VH, Bui BV, Vingrys AJ. Clinical and experimental links between diabetes and glaucoma. Clin Exp Optom. 2011;94:4–23. doi: 10.1111/j.1444-0938.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- Worley A, Grimmer-Somers K. Risk factors for glaucoma: what do they really mean? Aust J Prim Health. 2011;17:233–239. doi: 10.1071/PY10042. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Lin YH, Chu CC, Tsai YH, Chao JC. Curcumin or saikosaponin A improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J Med Food. 2008;11:224–229. doi: 10.1089/jmf.2007.555. [DOI] [PubMed] [Google Scholar]
- Wu QH, Chen WS, Chen QX, Wang JH, Zhang XM. Changes in the expression of platelet-derived growth factor in astrocytes in diabetic rats with spinal cord injury. Chin Med J (Engl) 2010;123:1577–1581. [PubMed] [Google Scholar]
- Wu SJ, Tam KW, Tsai YH, Chang CC, Chao JC. Curcumin and saikosaponin A inhibit chemical-induced liver inflammation and fibrosis in rats. Am J Chin Med. 2010;38:99–111. doi: 10.1142/S0192415X10007695. [DOI] [PubMed] [Google Scholar]
- Yanagi M, Kawasaki R, Wang JJ, Wong TY, Crowston J, Kiuchi Y. Vascular risk factors in glaucoma: a review. Clin Exp Ophthalmol. 2011;39:252–258. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Luo C, Wang P, He Q, Zhou J, Peng H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp Ther Med. 2013;5:1345–1350. doi: 10.3892/etm.2013.988. [DOI] [PMC free article] [PubMed] [Google Scholar]