Abstract
Chitosan oligosaccharides have been reported to inhibit various tumors. However, the water-soluble marine plant oligosaccharide alginate oligosaccharide (AOS) has only rarely been reported to have anti-cancer effects. Moreover, the inhibitory effect of AOS on prostate cancer and the underlying molecular mechanism remain unknown. This study shows that AOS inhibited cell growth, which was consistent with the attenuation of α2,6-sialylation modification. Furthermore, AOS inhibited ST6Gal-1 promoter activity and thus affected transcriptional processes. In addition, AOS could activate the Hippo/YAP pathway and block the recruitment of both the coactivator YAP and c-Jun. Furthermore, YAP interacted with the transcription factor c-Jun and regulated the transcriptional activity of the downstream target ST6Gal-1 gene. Consistent with in vitro data, AOS suppressed the tumorigenicity of prostate cancer cells via the Hippo/YAP pathway in vivo. In summary, these data indicate that AOS slows the proliferation of prostate cancer and provides a basis for the healthy function of kelp in traditional cognition.
Subject terms: Cancer prevention, Glycobiology, Glycobiology
Introduction
Prostate cancer is a significant public disease across the world and the most common solid tumor diagnosed in males in the United States. Furthermore, it is a severe disease with high incidence rate and fatality1. Prostate cancers are typically characterized by high androgen levels and during initial stages, prostate cancer responds to hormonal intervention therapies. However, with the emergence of androgen-independence, the tumor becomes more advanced, which leads to castration-resistant prostate cancers. This is a lethal form of prostate cancer, which has no effective treatment to date2. Currently, the standard therapies for castration-resistant prostate cancer include hormone therapy, chemotherapy, and radiation. However, such treatments cannot inhibit tumor metastasis and pose high toxicity to normal tissues in patients3. Therefore, effective drugs for the treatment of prostate cancer are a top priority. Furthermore, despite tremendous advances in surgery, chemotherapy, and drug therapy, the incidence of prostate cancer is still increasing due to a tendency of recurrence and metastasis4. Therefore, identifying the molecular mechanism underlying the process of prostate cancer development will be helpful for both diagnosis and treatment.
Alginate oligosaccharide (AOS) consists of β-d-mannuronic acid (mannuronic acid) and α-l-guluronic acid (guluronic acid) linked via 1,4-glycosidic bonds. It is a water-soluble functional oligomer5, and is derived from brown algae6. Furthermore, it has been regarded as a non-toxic and biodegradable polymer, and has a bright prospect for biomedical applications7. The antioxidant property of AOS has received significant attention5,8. This type of oligosaccharide possesses additional beneficial characteristics such as anti-inflammatory activity5 and bacteriostatic function9. The most unique feature is its antineoplastic activity10,11. Little is known about the relationship between AOS and the development of prostate cancer. Therefore, this study investigated whether AOS could impact the growth and proliferation of prostate cancer cells.
Glycosylation plays an essential role in many biological processes such as immune surveillance and tumor progression12,13. Sialic acid (SA) is a derivative of the nine-carbon monosaccharide family, in which terminal monosaccharides are attached to a glycan chain14,15. Sialylation is closely associated with many cellular functions, such as cell adhesion, signal recognition, and protein stability16,17. The sialyltransferase (ST) family is a group of sialylation synthases, consisting of 20 members that have been divided into β-galactoside α2,3-sialyltransferases (ST3GalI–VI), β-galactoside α2,6-sialyltransferases (ST6Gal-I and II), GalNAc α2,6-sialyltransferases (ST6Gal-NAcI–VI), and α2,8-sialyltransferases (ST8SIAI–VI) families18. The sialyltransferase that catalyzes α2,6-linked SA, especially ST6Gal-1, is the main sialyltransferase among these. β-galactoside α2,6-sialyltransferase 1 (ST6Gal-1) adds an α2,6-linked SA to the N-glycans of specific receptors19. High expression of ST6Gal-1 has been reported to be related to malignant tumor invasion and metastasis20,21. Previous studies have reported that ST6Gal-1 is upregulated in several cancer types, including many colon carcinomas19, liver cancer22, and prostate cancer23. However, the intricate relationship between AOS and ST6Gal-1 and the molecular mechanisms underlying prostate cancer progression still remain poorly understood.
This study further explored a critical role that AOS may play in the modulation of prostate cancer cell growth both in vitro and in vivo. This study further investigated whether AOS inhibits the growth and proliferation of prostate cancer cells through the sialylation of N-glycans, mediated by ST6Gal-1 on the cell surface. The results showed that AOS had a significant anti-tumor effect and inhibited the expressions of ST6Gal-1 both in mRNA levels and protein levels. Furthermore, the apoptosis rates of ST6Gal-1 overexpressing cells increased significantly when compared to the control group in both presence and absence of AOS. In addition, in pathway perspective, AOS triggered the activation of the Hippo/YAP signaling pathway. In summary, the results of this study indicate that AOS could modulate the expression of ST6Gal-1 via the Hippo/YAP pathway and play a fundamental role in prostate cancer cell growth and proliferation.
Results
Anti-proliferation effects of AOS in human prostate cancer cells
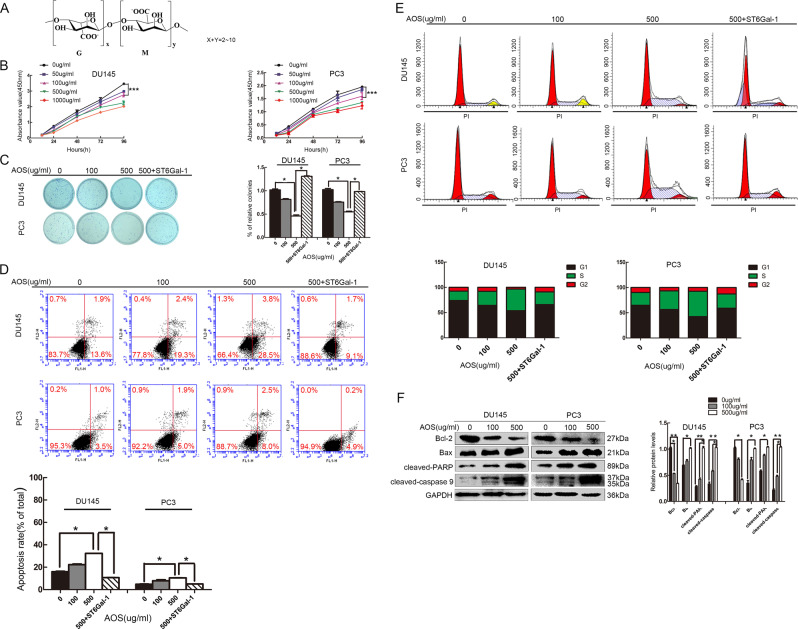
The chemical structure of AOS is shown in Fig. 1a. Previous experiments have shown that AOS has no apparent cytotoxicity to human normal cells (Supplementary Fig. S1). To examine the effects of AOS on cancer cell growth, human prostate cancer DU145 and PC-3 cell lines were treated with various concentrations of AOS (0, 50, 100, 500, and 1000 µg/ml) for 24 h, and then viable cells were determined via CCK-8 assay. As shown in Fig. 1b, AOS treatment inhibited DU145 and PC-3 cell proliferation. Medication with lower concentrations of AOS (100 and 500 µg/ml) resulted in growth inhibition without cell death after 24 h of treatment. In addition, a colony-formation assay was used to verify cell proliferation changes. AOS treatment also decreased DU145 and PC-3 cell colony formation (Fig. 1c). To study the possible function of AOS in modulating apoptosis in prostate cancer cells, Annexin V-FITC/PI staining was used. As shown in Fig. 1d, the apoptosis rates of prostate cancer cells increased in the presence of AOS. Accordingly, AOS triggered cell cycle arrest during the S phase (Fig. 1e). Next, the effects of apoptotic-related proteins were examined via Western blot analysis. Exposure to different concentrations of AOS resulted in increased levels of cleaved PARP, cleaved caspase-9, Bax protein and an inhibition of the Bcl-2 protein level (Fig. 1f). These results indicate that AOS could suppress the proliferation ability of DU145 and PC-3 cells.
Fig. 1. AOS inhibits cell growth in vitro.
a Structure of AOS. b, c Cell viability with AOS treatment was detected by both CCK-8 assay and colony-formation assay. Relative cell colony-formation rates of DU145 and PC-3 cells from three independent experiments. CCK-8 assay showed that the (50, 100, 500, and 1000 µg/ml for 24 h) measurements differed (*P < 0.05). d Induction of apoptosis by AOS. DU145 and PC-3 cells were treated with 100 and 500 µg/ml AOS for 24 h and upregulated by ST6Gal-1. The rates of apoptosis were determined by flow cytometry analysis of Annexin V-FITC/PI. e Cell cycle distribution analysis showed that the rate of the S phase was higher in AOS-treatment cells than in the control group, while ST6Gal-1 overexpression rescued the phenomenon of cycle arrest. f Regulation of apoptosis-related proteins by AOS. DU145 and PC-3 cells were treated with or without 100 and 500 µg/ml AOS for 24 h. Then, total proteins were extracted, and the expression levels of Bcl-2, Bax, cleaved caspase-9, and cleaved PARP proteins were analyzed by western blot. Results are representative of three independent experiments (*P < 0.05)
AOS treatment suppressed DU145 and PC-3 cell migration and invasion ability in vitro
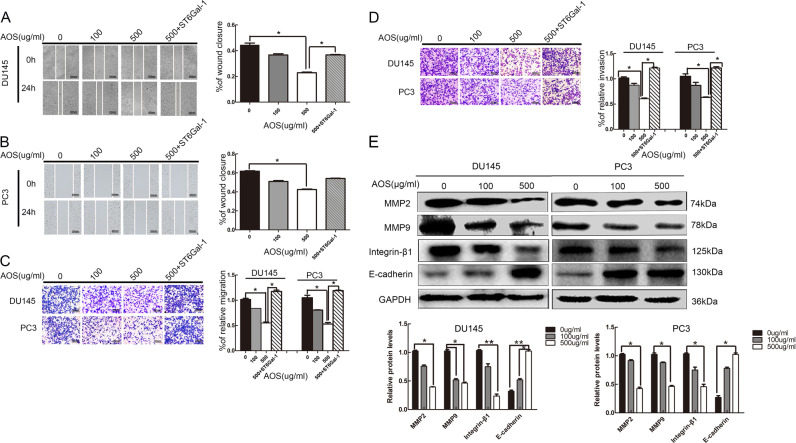
To investigate the effects of AOS on the migration and invasion abilities of prostate cancer cells, wound healing and transwell assays were used. The changes in migration ability were studied before and after AOS treatment in both DU145 and PC-3 cells. The results showed that drug treatment inhibited the migration ability of DU145 and PC-3 cells (Fig. 2a, b). Furthermore, transwell migration assays were conducted to analyze the migration ability of DU145 and PC-3 cells, and results were identical as those of the wound-healing assay (Fig. 2c). Moreover, Matrigel-invasion assay was also conducted and it showed that after AOS treatment, invasion ability of prostate cancer cells was suppressed (Fig. 2d). Moreover, the effects of migration-related proteins were examined. The results indicated that different concentrations of AOS contributed to MMP2 and MMP9 downregulation in prostate cancer cells (Fig. 2e). In summary, these results demonstrated that AOS treatment suppressed the migration and invasion abilities of both DU145 and PC-3 cells.
Fig. 2. AOS reduces the migration and invasion of DU145 and PC-3 cells in vitro.
a, b Migration behaviors of DU145 and PC-3 cells with AOS treatment and upregulated ST6Gal-1 expression explored via wound-healing assay. Cells were pretreated with 0, 100, and 500 µg/ml AOS for 24 h followed by subjecting to the wound-healing assay for another 24 h. The closure of denuded area of the AOS-treated group was calculated and then normalized with that of the control. c Transwell assays showed that drug treatment inhibited the DU145 and PC-3 cell migration ability and overexpression of ST6Gal-1 increased the migration rates of DU145 and PC-3 cells. d A similar experimental procedure as described above was performed except that the Matrigel was precoated onto the upper membrane of the transwell. e Regulation of metastasis associated proteins by AOS. DU145 and PC-3 cells were treated with or without 100 and 500 µg/ml AOS for 24 h, and then, total proteins were extracted. The expression levels of MMP2 and MMP9 proteins were analyzed by Western blot. Results represent the mean ± SD of the expression levels from three independent experiments. (*P < 0.05 compared to cells without AOS treatment)
Effect of AOS on the expression profile of sialyltransferase gene and downregulation of ST6Gal-1 expression in human prostate cancer cells
To explore the effect of AOS on the expression of sialyltransferase genes in the human prostate cancer cell line DU145, the mRNA expression levels of sialyltransferase genes were examined. As shown in Fig. 3a–d, the transcription levels of ST3GAL3, ST6GAL1, ST6GALNAC5, and ST8SIA4 were different after treatment with AOS. The mRNA levels of ST6GAL1, ST6GALNAC5, and ST8SIA4 were significantly decreased. Higher expression levels of ST6GAL1 were observed and the difference in the role of AOS was obvious (2.93-fold), whereas ST3GAL3, ST6GALNAC5, and ST8SIA4 expression levels were not high. In summary, these results implied that the ST6GAL1 gene was highly expressed in prostate cancer cells and the effect of AOS on ST6GAL1 differed significantly.
Fig. 3. Differential expression of sialyltransferase gene and suppression of ST6Gal-1 expression by AOS in prostate cancer.
a–c mRNA levels of the sialyltransferase (ST) gene family without or with 500 µg/ml AOS administration in DU145 cells determined by real-time quantitative PCR (qPCR) and normalized for GAPDH. d Relative ≥2-fold intensity ratios of the ST genes observed. e–i Determination of ST6Gal-1 expression in both DU145 and PC-3 cells after 100 and 500 µg/ml AOS treatment by qPCR (e, f), western blot (g, h), and lectin blot (i). Cells following ST6Gal-1 overexpression transfection were detected by lectin blot (i). Data represent the mean ± SD of the expression levels of three independent experiments (*P < 0.05)
Previous studies have shown that ST6Gal-1 plays a fundamental role in growth, migration, and invasion of prostate cancer PC-3 and DU145 cells23. The corresponding assays associated with the role of ST6Gal-1 in tumor growth are shown in Supplementary Materials. AOS exerts a clear impact on ST6Gal-1 in the human prostate cancer cell line DU145 (Fig. 3b). Therefore, the expression of sialyltransferase ST6Gal-1 encoded by the ST6GAL1 gene was evaluated to elucidate the suppression function of AOS to ST6Gal-1. As shown in Fig. 3e, f, AOS decreased the relative mRNA levels of ST6Gal-1. Furthermore, western blot analysis showed that ST6Gal-1 protein levels decreased correspondingly (Fig. 3g, h). Accordingly, SNA lectin blot indicated that α2,6-linked SA was decreased after AOS treatment (Fig. 3i). Furthermore, ST6Gal-1 overexpression rescued the reduction of SNA lectin staining caused by AOS (Fig. 3i). Therefore, these data indicate that ST6Gal-1 might be a targeting molecule, which contributed to AOS-suppressed proliferation, migration, and invasion of DU145 and PC-3 cells.
Activation of the Hippo/YAP signaling pathway by AOS
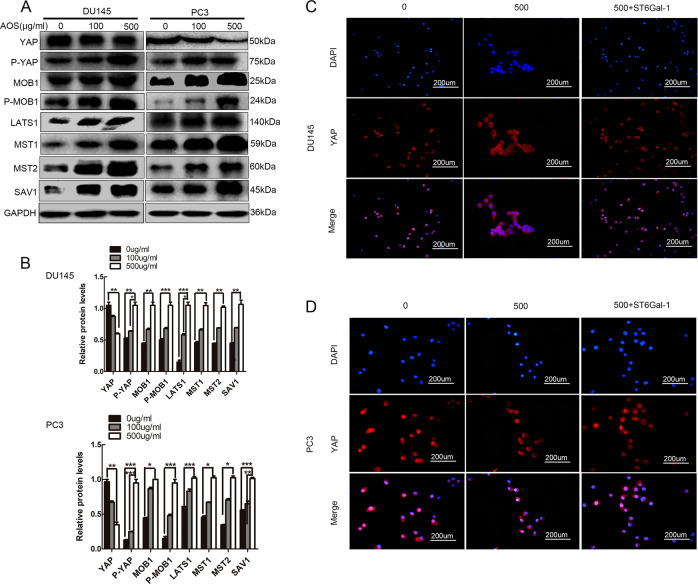
It has been reported that the Hippo/YAP signaling pathway plays a central role in the progression of tumorigenicity24. Therefore, this study investigated whether this pathway was involved in AOS-mediated prostate cancer cell proliferation, migration, and invasion. The expression levels of Hippo family members, including a panel of kinases (MST1/2 and LATS1) and adaptor proteins (SAV1 and MOB1), and downstream transcriptional factor YAP were investigated by Western blot analysis. As shown in Fig. 4a, b, relatively high levels of MST1/2, LATS1, SAV1, and MOB1 were found in AOS-treated cells. However, expression of the oncogene YAP was decreased in DU145 and PC-3 cells in response to AOS treatment. These observations clearly indicate that AOS could promote the activation of the Hippo/YAP pathway in prostate cancer cells. Additionally, immunofluorescence experiment showed increased expression and accumulation of YAP in the cytoplasm, i.e., the promotion of YAP transfer from the nucleus to the cytoplasm in response to AOS treatment (Fig. 4c, d). In summary, these findings suggest that AOS might be associated with the Hippo/YAP pathway and activated the Hippo signaling pathway in human prostate cancer cells.
Fig. 4. Activation of the Hippo/YAP signaling pathway by AOS.
a DU145 and PC-3 cells were treated with or without 100 and 500 µg/ml AOS for 24 h. Then, the total proteins were extracted and the main protein components of the Hippo/YAP signaling pathway were subjected to western blotting using antibodies as indicated. b Quantification of protein levels was performed by densitometry. The expression of GAPDH was used as internal standard. Results are representative of three independent experiments (*P < 0.05). c, d Immunofluorescence imaging analysis was used to monitor YAP release from the nuclear into the cytosol contributed by AOS, and then, released from the cytosol into the nuclear upon overexpression of ST6Gal-1. Representative figures are shown. *P < 0.05
Overexpression of ST6Gal-1 rescues the activation of the Hippo/YAP signaling pathway in DU145 and PC-3 cells
To further verify that AOS may affect the development of prostate cancer by regulating the expression of ST6Gal-1, cells that had been treated with 500 μg/ml AOS were transfected with ST6Gal-1 overexpression vectors. Reintroduction of ST6Gal-1 in AOS-induced cells significantly modified the levels of Hippo signaling-related proteins expression. Clonogenic capacity and apoptotic ability, migration, and invasion abilities were rescued by ST6Gal-1 overexpression (Figs. 1c–e and 2a–d). In addition, AOS-induced S-phase arrest was also attenuated (Fig. 1f). Immunofluorescence results showed that ST6Gal-1 overexpression rescued the transfer of YAP, which was conditioned by AOS, which relocated from the nucleus to the cytoplasm and back to the nucleus (Fig. 4c, d). Moreover, the level of phosphorylated YAP returned to the original level compared to AOS-mediated groups (Fig. 5a, b). YAP overexpression stimulated the expression of ST6Gal-1 (Fig. 5c–f). These outcomes indicate that upregulation of ST6Gal-1 might rescue the proliferation, migration, and invasion abilities of both DU145 and PC-3 cells by restraining the activation of the Hippo/YAP pathway facilitated by AOS.
Fig. 5. Upregulation of ST6Gal-1 inhibits activation of the Hippo/YAP pathway in prostate cancer cells in response to AOS treatment.
a, b DU145 and PC-3 cells treated by 500 µg/ml AOS were transfected with ST6Gal-1 overexpression vectors, and expression levels of Hippo/YAP pathway signaling molecules were analyzed by western blot. c–f YAP overexpression plasmids were transfected to observe the expression level of ST6Gal-1 in DU145 and PC-3 cells. Relative protein intensities were determined with Image Lab software (Bio-Rad). *P < 0.05
Synergistic interaction between YAP and c-Jun plays a role in the AOS-mediated inhibitory effect on ST6Gal-1 gene expression
Bioinformatics predicted that the c-Jun transcription factor is located upstream of the ST6Gal-1 promoter and upregulated ST6Gal-1 gene expression. This study evaluated the effect of AOS on transcriptional activity of the ST6Gal-1 promoter. The results of the dual-luciferase reporter gene assay indicated the inhibition of AOS to ST6Gal-1 promoter activity and the core functional area was located at nucleotides −308/+1 upstream of the ST6Gal-1 promoter (Fig. 6a). Furthermore, Fig. 6b shows a schematic diagram of the c-Jun response element located at nucleotides −308/+1 upstream of the ST6Gal-1 promoter region. Examination of the ST6Gal-1 promoter region found one putative c-Jun-binding site. Individual mutation of this putative c-Jun-binding site indicated that the transcription factor c-Jun was involved in the regulation of ST6Gal-1 promoter activity (Fig. 6c). Furthermore, upregulated ST6Gal-1 was detected by anti-c-Jun antibody chromatin immunoprecipitation (CHIP) assay. As depicted in Fig. 6d, reduction of c-Jun interaction at the c-Jun response element located at (nucleotides −308/+1) upstream of the ST6Gal-1 promoter in AOS-treated cells was concentration dependent. These studies indicated that AOS could decrease the recruitment of c-Jun into the upstream response region of the ST6Gal-1 promoter in prostate cancer cells.
Fig. 6. AOS inhibits ST6Gal-1 promoter activity and the transcription factor c-Jun binds to ST6Gal-1 promoter with coactivator YAP.
a Prostate cancer cells were treated with or without AOS for 24 h. The cells were then collected and promoter activity was analyzed using the ST6Gal-1 luciferase promoter reporter construct. b Schematic diagram representing the c-Jun transcription factor located at the upstream of ST6Gal-1 promoter region. c One putative c-Jun-binding site located at nucleotides −308/+1 in the upstream of ST6Gal-1 promoter region is shown. This putative c-Jun-binding site is important for ST6Gal-1 promoter activity. The putative c-Jun-binding site was mutated. The luciferase activity was measured in the presence or absence of AOS. d CHIP from prostate cancer cells was performed with both control IgG and c-Jun antibodies as indicated. The presence of ST6Gal-1 promoter was detected by PCR. e YAP overexpression plasmids were transfected to evaluate the expression level of c-Jun in both DU145 and PC-3 cells. Relative protein intensities were determined with the Image Lab software (Bio-Rad). *P < 0.05. f Co-location of both YAP and c-Jun proteins inside the prostate cancer cells was observed by cell immunofluorescence. g Co-immunoprecipitation (Co-IP) of YAP and c-Jun from both DU145 and PC-3 cells
This study focused on validating the associations between YAP and c-Jun via co-immunoprecipitation (Co-IP) (Fig. 6g). In addition, cells were treated with 0, 100, and 500 μg/ml AOS for 24 h and YAP was upregulated by transfection with pcDNA3.1/YAP plasmid. Relative protein levels of c-Jun were obtained by Western blot and the results are illustrated in Fig. 6e. Furthermore, co-location of YAP and c-Jun proteins inside the prostate cancer cells was observed by cell immunofluorescence. Both YAP and c-Jun were mainly localized in the nucleus (Fig. 6f). In summary, these results validated the interaction between YAP and c-Jun played a major role in the AOS-mediated inhibitory effect on ST6Gal-1 gene expression in prostate cancer cells.
AOS treatment attenuates prostate cancer cell tumorigenicity in nude mice via the Hippo/YAP pathway in vivo
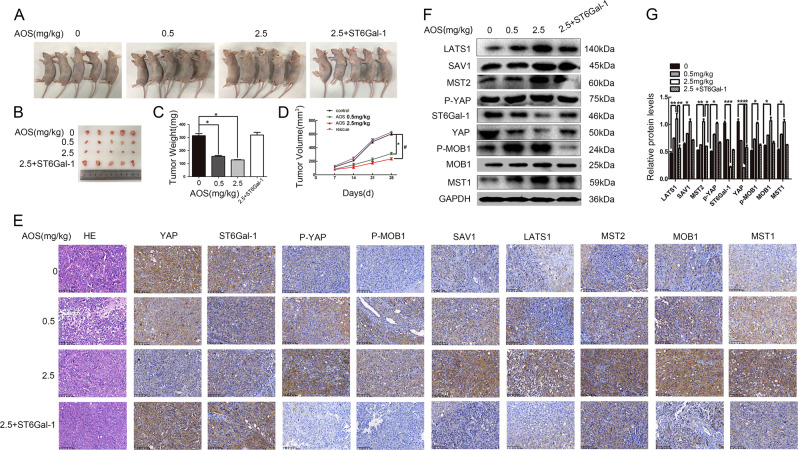
To investigate the effect of AOS on tumor development, a subcutaneous engraftment assay was conducted in nude mice and AOS was administered consecutively for 21 d by intraperitoneal injection once per day at doses of 0.5 and 2.5 mg/kg. The mice were then sacrificed. As shown in Fig. 7a, b, AOS inhibited the tumor size and growth rate of DU145 cells in vivo. Accordingly, tumor weights and volumes of the control group increased compared to AOS treatment groups (Fig. 7c, d). Furthermore, the ST6Gal-1 overexpressing group counteracted the AOS-induced inhibition of tumor growth in vivo. Similarly, the weight loss was prevented by ST6Gal-1 overexpression. To further investigate whether the Hippo/YAP pathway was involved in the suppression of tumor growth by AOS in vivo, the expression of related proteins was evaluated in tumor tissues by western blot. As shown in Fig. 7f, g, the expression levels of Hippo signaling molecules had changed compared to those of control cells. Interestingly, as shown in Fig. 7e, IHC analysis showed that AOS resulted in an activation of the Hippo/YAP pathway. Reintroduction of ST6Gal-1 in AOS-medication DU145 cells rescued the expression of ST6Gal-1 and Hippo signaling-related proteins at both the protein and tissue levels, respectively (Fig. 7e–g). Therefore, these findings indicate that AOS treatment suppressed tumor evolvement in prostate cancer cells through the Hippo/YAP signaling pathway in vivo.
Fig. 7. AOS inhibits the prostate tumor formation in nude mice through the Hippo/YAP pathway.
a Morphological images of tumors that were engrafted and AOS treated. b Solid tumors were isolated from nude mice. c, d Average tumor weights (c) and sizes (d) were measured in different groups. e, f Western blot assay (e) and IHC (f) were used to detect the expression levels of the main signaling molecules of the Hippo/YAP in tumor tissues. *P < 0.05
Materials and methods
Alginate oligosaccharide (AOS)
AOS was provided by Heng Yin from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences. AOS is a marine plant oligomer that is obtained by enzymatic hydrolysis of sodium alginate and consists of mannuronic acid (M), guluronic acid (G), or a heterozygous fragment of both. The chemical structure of AOS is shown in Fig. 1a.
Cell lines and cell culture
Human prostate cancer DU145 and PC-3 cells were purchased from the Cell Bank of the Shanghai Life Science Institution, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium supplied with 10% fetal bovine serum in a humidified incubator with 5% CO2 and maintained at 37 °C. Both cell lines used in this study were authenticated by short tandem repeat (STR) profiling (by Shanghai Biowing Applied Biotechnology).
Cell survival assays by cell counting kit-8
Cell viability was determined using the Cell Counting Kit-8 (CCK-8) assay. Prostate cancer cells were cultured in 96-well plates at a density of 4000 cells per well and treated with a series of different doses of AOS for 24, 48, 72, and 96 h. Then, the AOS-containing medium was removed, CCK-8 solution was diluted with RPIM 1640 medium (at a dilution of 1:10) and 110 µl of system reagent was added to each well. Cells were incubated for 2 h and the absorbance at 450 nm was measured with a microplate reader (Thermo Fisher Scientific, USA).
Colony-formation assay
DU145 and PC-3 cells at the logarithmic growth phase were digested into a single-cell suspension with a trypsin-EDTA (Gibco) solution, and then seeded into six-well culture plates (Corning, NY, USA) at a density of 2000 cells per well. After adherence, cells were treated with AOS (0, 100, and 500 µg/ml) for 24 h. Subsequently, a further group overexpressed ST6Gal-1 after treatment with 500 µg/ml AOS. After the AOS-containing medium was removed and replaced with fresh medium, cells were incubated at 37 °C with 5% CO2 for 14 d. The surviving cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet, and then, the plates were photographed. The total number of colonies (50 cells per colony) was counted by using the software Image J.
Wound-healing assay
Cells were seeded in six-well plates at a density of 5 × 105 cells per well. When cells had grown more than 90% confluence, the cell monolayer was wounded with a 10 µl sterile pipette tip, ensuring that all wounds had the same width at the beginning. The grouping of cells was as described above. Then, the culture medium was removed and plates were washed three times using PBS. Finally, cells that had migrated to the wounded area were observed using a microscope (Olympus, CA) and images were analyzed to determine the percentage of wound closure.
Transwell migration and invasion assay
The Costar Transwell System (8-μm pore size polycarbonate membrane, 6.5-mm diameter, Corning, USA) was used to evaluate both cell migration and invasion. Both DU145 and PC-3 were divided into four groups (0, 100, 500, 500 + ST6) and were resuspended in 200 µl serum-free RPMI-1640 medium at a density of 3 × 104 per well. These were added into the upper chambers and 500 μl complete medium was added to the lower chambers. Then, cells were allowed to migrate for 24 h, at which point cells on the top of the membrane were washed with PBS and removed via cotton swab. Membranes were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Images of the membranes were obtained, counted in three non-overlapping fields, and photographed. In addition, cells were counted three times with a random approach by Image-Pro Plus 6.0 software. Similar to the above-mentioned experiment, for the invasion assay, the upper chambers were coated with 40 μl Matrigel (diluted 1:8). As soon as the Matrigel solidified 30 min later, treated DU145 and PC-3 cells were seeded in the upper chambers and cultured for 24 h.
Cell cycle analysis
Cells were fixed in 70% cold ethanol overnight at −20 °C, washed, and then PBS was added to the suspended cells. Subsequently, cells were stained by adding propidium iodide (50 µg/ml) combined with RNase A (50 µg/ml) and this mixture was then incubated for 1 h at 37 °C. The cell cycle distribution of DU145 and PC-3 was analyzed by flow cytometer (BD Biosciences). At least 106 cells were acquired per sample.
Flow cytometry analysis for apoptosis
In prostate cancer cells, AOS-induced apoptosis was measured by flow cytometry. In addition, the Annexin V-FITC/PI apoptosis detection kit (Dojindo Laboratories, JAPAN) was used to analyze the apoptosis rate. At least 1 × 106 DU145 and PC-3 cells were treated with AOS (0, 100, 500 µg/ml, and 500 µg/ml + ST6) for 24 h, then collected by centrifugation at 900 × g for 3 min, and washed with cold PBS three times. 1 × 106 cells were resuspended in 500 µl Annexin V Binding buffer containing 5 µl Annexin V-FITC and PI solutions. Next, cells were incubated at room temperature for 15 min in darkness. Finally, cells were analyzed by flow cytometry (BD Biosciences) within 1 h.
Lectin blot analysis
Proteins extracted from cell lysis buffer, containing 30 µg of protein, were exposed to 10% sodiumdodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). One of the resulting gels was stained with Coomassie Brilliant Blue (CBB) while the other gel was transferred to a PVDF membrane for subsequent experiments. The membrane was blocked in 5% skim milk for 3 h at room temperature and then incubated with biotin-labeled SNA (1:2000, Vector) for 1 h. Next, the PVDF membrane was washed with Tris-buffered saline, containing Tween 20 (pH 7.4) and incubated with diluted horseradish peroxidase (HRP)-labeled streptavidin (1:8000, ZSGB-BIO) for 1 h at room temperature. Blots were visualized by enhanced chemiluminescence (ECL) kit (Advansta, Menlo Park, CA, USA).
Immunohistochemical analysis (IHC)
Tissue samples were fixed overnight in 4% paraformaldehyde to obtain paraffin-embedded sections. The sections were deparaffinized using xylene and rehydrated using an alcohol gradient. The antigen was repaired with sodium citrate, and then immersed in 3% H2O2 for 10 min to remove endogenous catalase. The slides were washed with PBS and blocked with goat serum for 15 min. Next, the sections were incubated overnight at 4 °C using anti-ST6Gal-1 (1:70, Proteintech, 14355–1-AP), anti-LATS1 (1:80, Proteintech, 17049-1-AP), anti-SAV1 (1:80, Abcam, ab230265), anti-MST1 (1:80, Proteintech, 22245-1-AP), anti-MST2 (1:50, ABGENT, AP7923a), anti-YAP (1:200, Cell Signaling Technology, 8418), anti-p-YAP (1:1250, Cell Signaling Technology, 13008), anti-MOB1 (1:80, Proteintech, 12790-AP-1), and anti-p-MOB1 (1:50, Cell Signaling Technology, 8699) antibodies. After washing with PBS, the PBS surrounding the tissue was wiped dry and then biotinylated secondary antibody was added. The mixture was incubated at 37 °C for 30 min. The sections were then treated with DAB, counterstained with hematoxylin, dehydrated with an alcohol gradient, dewaxed with xylene, dried and sealed with a neutral gum, and observed under a microscope.
Western blot analysis
Proteins were isolated by SDS-PAGE and blotted onto a PVDF membrane. Membranes were blocked with 5% milk and incubated with specific primary antibodies, following the same method and incubated with peroxidase-conjugated secondary antibodies. The bands were visualized by an ECL kit (Advansta, Menlo Park, CA, USA). Subsequently, protein grayscale analysis was conducted using Gel-Pro software. The following antibodies were used: ST6Gal-1 (1:1000, Proteintech, 14355–1-AP), p-YAP (Ser127; 1:1000, Cell Signaling Technology, 13008), YAP (1:1000, Cell Signaling Technology, 8418), LATS1 (1:1000, Cell Signaling Technology, 3477), MST1 (1:1000, Cell Signaling Technology, 3682), SAV1 (1:1000, Cell Signaling Technology, 13301), MST2 (1:1000, Cell Signaling Technology, 3952), MOB1 (1:1000, Cell Signaling Technology, 13730), p-MOB1 (1:1000, Cell Signaling Technology, 8699), and GAPDH (1:6000, Bioworld, AP0063).
Immunofluorescence and immunofluorescence colocalization
Cells were fixed with 4% paraformaldehyde for 20 min, and were then successively permeabilized and blocked with 0.1% Triton-X 100 and 2% BSA for 20 min. Then, cells were incubated overnight with sufficient YAP primary antibody (1:400, Invitrogen, PA1-46189). A Rhodamine (TRITC)-Conjugated Goat anti-Rabbit IgG (1:50, ZSGB-BIO, ZF-0316) was used at 37 °C for 1 h in the dark, and DAPI was used to stain nuclei for 5 min. Immunofluorescence images were obtained using a microscope (Olympus, CA). In agreement with the above-mentioned immunofluorescence colocalization experiment, the two primary antibodies YAP primary antibody (1:400, Invitrogen, PA1-46189) and rabbit anti-c-Jun (1:50, Invitrogen, MA5-15172) were simultaneously incubated. The secondary antibody of Rhodamine was incubated first, and the Fluorescein-Conjugated Goat anti-Rabbit IgG antibody was incubated second (1:50, ZSGB-BIO, ZF-0311).
Reverse transcription quantitative real-time PCR (RT-qPCR)
Total RNA was extracted from DU145 and PC-3 cells using RNAiso Plus (TaKaRa, 9108, CA). Reverse transcription was conducted from 1 µg total RNA, which was used to synthesize cDNA using a PrimeScriptTM RT reagent Kit with gDNA Eraser (TaKaRa, RR047A). Specific Primer sequences used for qPCR have been presented previously25. Real-time quantitative RT-PCR was performed in a 10 µl reaction volume containing 1 µl cDNA template. The reactions were performed in a TransStart Tip Green qPCR SuperMix system (Transgen, AQ141) and gene expression of the target mRNA was calculated by the 2−ΔΔCt method. The following real-time PCR parameters were used for all qPCR reactions: initial denaturation at 94 °C for 30 s, followed by 40 cycles of 5 s denaturation at 94 °C, 30 s annealing, and extension at 60 °C. Furthermore, all gene expression values were normalized to that of GAPDH in the same sample.
In vivo anti-tumor activity of AOS
Xenograft model
Athymic male BALB/c nude mice aged 4–6 weeks were obtained from the Animal Experiment Center of Dalian Medical University. Approximately 1 × 107 cells were mixed with 100 µl PBS and were then subcutaneously injected into the left side of each mouse and the number of mice is twenty. After 7 d, the nude mice gradually developed a tumor section. At this time, the diameter and length of the tumor were measured with a Vernier caliper every week, and the tumor volume was calculated using the following formula: 1/2 (length × width2).
Analysis of anti-tumor activity of AOS in vivo
Next, the effect of AOS on tumor cell growth and proliferation in vivo was confirmed. Different concentrations of AOS were consecutively administered for 21 d by intraperitoneal injection. The doses of AOS were selected based on the findings of our previous studies and because AOS at these doses could inhibit DU145 and PC-3 cell proliferation. The control group was injected with PBS at an amount of 100 µl per day. The mice were euthanized when the size and weight of the tumor had retained a certain level for about 5 weeks. Finally, the fresh tissue was fixed in 4% paraformaldehyde for more than 24 h. After the process of harvesting, dehydration, etc., the tissue specimen was embedded in paraffin, sliced, and stained with H&E.
Transient transfection
DU145 and PC-3 cells were transfected with pcDNA3.1/ST6Gal-1 and Lipofectamine 2000 TM (Invitrogen, CA, USA) was used according to the manufacturer’s instructions. The recombinant pcDNA3.1/ST6Gal-I vector was constructed as previously described26. Therefore, DU145 and PC-3 cells were transiently transfected with pcDNA3.1/ST6Gal-1 plasmid to rescue the inhibition of AOS on cancer cells. After 24 h of transfection, the rescued cells were used for further experiments. Similarly, prostate cancer cells were transiently transfected with pcDNA3.1/YAP plasmid to verify the expression of c-Jun protein.
Construction of the ST6Gal-1 promoter truncated reporter gene vector and Luciferase reporter assay
DU145 and PC-3 cells were transiently co-transfected with 1 µg ST6Gal-1 promoter region firefly luciferase reporter plasmids and 2 ng pRL-TK luciferase (Promega Corporation, Madison, WI, USA). Lipofectamine2000TM (Invitrogen, CA, USA) was considered as an internal control with or without AOS in 24-well plates. The ST6Gal-1 promoter region truncation sequence is shown in Supplementary Fig. S2. Cell extracts were prepared 24 h after transfection. The luciferase activity was measured using the Dual-Luciferase® Reporter Assay System (Promega E1910) according to the manufacturer’s protocol.
Co-immunoprecipitation assay (Co-IP)
Due to the well-recognized functions of ST6Gal-1 and YAP in cell growth and proliferation, coupled with previous data demonstrating the binding of the transcription factor c-Jun to the ST6Gal-1 promoter, it is essential to assess the interaction between proteins of YAP and c-Jun. The Pierce™ Co-Immunoprecipitation Kit (Thermo Scientific, 26149) was used to implement the endogenous immunoprecipitation assay. According to the manufacturer’s instructions, cells were lysed at 4 °C in IP Lysis/Wash Buffer. 10–75 µg of affinity-purified c-Jun antibody acted as bait protein for coupling by adjusting the volume to 200 µl, using sufficient ultrapure water and 20X Coupling Buffer to produce 1× Coupling Buffer. The antibody was immobilized onto an AminoLink Plus Coupling Resin for 2 h at room temperature. Subsequently, cell lysates were added to the Pierce control agarose resin and incubated at 4 °C for 30 min to 1 h. Then, the proteins with the above AminoLink Plus Coupling Resin were immunoprecipitated at 4 °C overnight, followed by two washes with Elution Buffer. Then, the resin-containing bead-antibody complex and protein lysate were suspended. The proteins in the supernatant were separated from the resin by centrifugation at 1000g for 3 min, followed by three washes with Elution Buffer. Finally, YAP was used as prey protein and immunoprecipitation was analyzed by western blot, following previously described steps.
Chromatin immunoprecipitation (CHIP) assay
CHIP assay was conducted using the EpiQuik™ Chromatin Immunoprecipitation Kit (Epigentek, P-2002) following the manufacturer’s instructions. Briefly, at the beginning of the procedures, antibodies were bound to the assay plate. The antibodies included: 1 µl of Normal Mouse IgG as negative control, 1 µl of Anti-RNA Polymerase II as positive control, and 2–4 µg of each antibody of interest. The strip wells were covered with Parafilm M and incubated at room temperature for 60–90 min. Furthermore, the cell extracts were prepared as described in the next steps. DU145 and PC-3 cells were added to 9 ml fresh culture medium containing 1% formaldehyde (final concentration) and then incubated at 37 °C for 10 min on a rocking platform (50–100 rpm). The fixation reaction was quenched by adding glycine; then, the DNA was sheared into small fragments by sonication, so that the length of the sheared DNA was between 200 and 1000 base pairs. Next, the required volume of the supernatant was diluted with CHIP Dilution Buffer and transferred to a new 1.5 ml vial. 5 µl of the diluted supernatant containing the digested chromatin was removed to a 0.5 ml vial, labeled as “input DNA”. Subsequently, the corresponding antibodies were incubated with the supernatant at room temperature (22–25 °C) for 60–90 min on an orbital shaker (50–100 rpm). Then, the DNA was purified using this kit (Epigentek, P-2002). In the immunoprecipitated DNA, the relative abundance of the DNA sequence from the ST6Gal-1 promoter region was analyzed by PCR. The following primer sequences were used: 5′-TCCTGCTCAGAACAAAGTGAC-3′ (forward) and 5′-ATCTTTTGCAGCCTAGGGAT-3′ (reverse).
Statistical analysis
Quantitative data were presented as mean ± standard deviation (SD). Statistical significance was estimated by a two-tailed Student’s t-test and analysis of variance (ANOVA). SPSS version 13.0 software was used. The mean values of two groups were considered significantly different at *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
This study described that the novel marine oligosaccharide AOS (identified from brown algae), exhibited an anti-proliferative effect and blocked the tumor progression via induction of cell cycle arrest and apoptosis on human prostate cancer cells both in vitro and in vivo.
Abnormal proliferation and metastasis are considered as the two leading causes of malignant cancer-related deaths27,28. It has been reported that a specific concentration of AOS can effectively inhibit the growth and proliferation of osteosarcoma11. However, the effect of AOS on the malignant phenotype of prostate cancer cells has not been reported. The results show that the promotion of growth and proliferation as well as the induction of apoptosis are possibly vital mechanisms with which AOS achieves cancer suppression. In addition, at non-cytotoxic concentrations, AOS inhibited both the migration and invasion of DU145 and PC-3 cells (Fig. 2). These results suggest that AOS may have preventive and therapeutic effects on progression and metastasis of prostate cancer.
Aberrant sialylation has been reported to be closely associated with malignant phenotypes of cells, including invasiveness and tumorigenicity29. Overexpression of specific sialyltransferase levels is an important reason for tumorigenesis30,31, especially ST6Gal-1 sialyltrasferse, which catalyzes α2,6-linked sialylation22. A previous report has shown that ST6Gal-1 played an important role in the proliferation, migration, and invasion of prostate cancer cells23. In the current study, a decrease of ST6Gal-1 was observed upon AOS treatment at mRNA, protein, and glycan levels in DU145 and PC-3 cells (Fig. 3). This suggests that AOS acts on prostate cancer cells by affecting the expression of ST6Gal-1 and causing changes in SA.
ST6Gal-1-mediated α2,6-linked sialylation is important in cancer progression. Accumulating evidence demonstrated that ST6Gal-1 is overexpressed in colon cancer32,33, breast cancer34, liver cancer35, cervical cancer36, and other diseases37. Therefore, ST6Gal-1 has become an important diagnostic marker and therapeutic target for the detection and treatment of human cancer. In fact, changes in the promoter portion of the gene result in the modulation of gene expression regulation. This study showed that AOS downregulated ST6Gal-1 expression at a transcriptional level in DU145 and PC-3 cells (Fig. 3). At the transcriptional level, extensive results detail the complex regulatory networks that control ST6Gal-1 mRNA expression, such as, Slug38, HNF139, and Sp131 transcription factors. Bioinformatics predicted that the transcription factor c-Jun binds to the ST6Gal-1 promoter region.
C-Jun is a member of the activation protein (AP1) family and is an oncogene that can be immediately and transiently expressed under the action of gonadotropins, growth factors, phorbol esters, and neurotransmitters40. It not only binds to AP1 family members, but also plays a biological role in the form of AP141, and can also participate in the regulation of gene transcription as a transcription factor42. Various types of stimulation such as drugs and ultraviolet irradiation can induce c-Jun activation43. Activated c-Jun participates in various physiological processes such as proliferation and apoptosis of tumor cells by regulating target gene transcription44. A previous study showed that the interaction between KLF5 and c-Jun promoted Angiotensin II-induced suppression of p21 expression in vascular smooth muscle cells45. This study showed that administration of AOS significantly suppressed the expression of c-Jun, which in turn attenuated the interaction of the c-Jun transcription factor onto the promoter region of ST6Gal-1. This led to the downregulation of ST6Gal-1 mRNA expression, and subsequently inhibited DU145 and PC-3 cell proliferation, migration, and invasion.
The Hippo/YAP signaling pathway is highly conserved in mammals, with core components including MST1/2, SAV1, LATS1/2, MOB1, and YAP/TAZ. In addition, YAP is the major downstream effector of the Hippo/YAP signaling pathway in mammals46. Furthermore, YAP is significant in prostate cancer cells47. As a transcriptional coactivator, YAP has no DNA-binding domain and therefore cannot directly bind to DNA. Consequently, the transcriptional expression of target genes needs to be regulated by DNA-binding transcription factors such as members of the TEAD1-4 family, Smad4, RUNX1/2, p63/p73, and ErbB448. This study showed the interaction between transcription factor YAP and the transcriptional coactivator c-Jun and then regulated the transcriptional process of the promoter in prostate cancer (Fig. 6). Moreover, it has been reported that inhibition of YAP expression was sufficient to impair migration and invasion of PC-3 cancer cells49. Here, we showed that AOS activated the Hippo/YAP pathway in both DU145 and PC-3 cells (Fig. 4). The total YAP level decreased and lower nucleus YAP levels were a final result of alleviated nucleus location due to YAP phosphorylation (Ser127) and the decrease of total YAP level. However, it to be further elucidated whether AOS (as a small molecule) is dissolved by cells and whether there is any difference in the anti-tumor effect between AOS mixture and AOS monomer. Furthermore, whether AOS enters cancer cells to exert tumor suppressive effects, and what upstream signals and receptors of AOS act on the Hippo signaling pathway also remains to be further elucidated. Moreover, the reliance on DU145 and PC3 cells, limits the potential translational relevance of the results. Both cell lines represent the AR-null subtype of metastatic prostate cancer. This issue will be further studied.
In summary, we have provided the first evidence that AOS inhibits prostate cancer cell proliferation via changes in SA and by affecting the expression of ST6Gal-1. Moreover, these effects manifested at a non-cytotoxic concentration of AOS and attenuated the proliferation, migration, and invasion of human prostate cancer cells through suppression of the Hippo/YAP/c-Jun pathway. These findings provide insights regarding the mechanisms of AOS-mediated anti-proliferation action. Furthermore, the problem of toxic side effects on normal cells cannot be ignored; therefore, the development of the application potential of AOS to increase its selective efficacy against tumor cells is a very worthwhile research topic.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31470799), the Natural Science Foundation of Liaoning Province (20170540288), the Special Fund of Dalian city for Distinguished Young Scholars (2017RJ07), and the National Key R&D Program of China (2017YF0200900). And this work was supported by Liaoning Provincial Program for Top Discipline of Basic Medical Sciences. None of these funding sources had any role in writing the manuscript nor in the decision to submit for publication. The authors attest that they have not been paid to write this article by a company or any other agency. The alginate oligosaccharide was provided by Heng Yin of the Dalian Institute of Chemical Physics, Chinese Academy of Sciences.
Authors’ contributions
H.Y. performed, analyzed data, and wrote the manuscript. H.Y. and W.S.J. designed the experiments. Y.H. provided alginate oligosaccharide. Y.X. provided the tissue slices. All authors were involved in writing this paper and approved the submission and publication. In Fig. 1, H.Y. generated the data. Y.H. provided the structure of alginate oligosaccharide. In Fig. 2, H.Y. generated the data. Z.L. supported the data analysis. In Figs. 3, 4, 5, and 6, H.Y. assembled the data. In Fig. 7, Y.X. generated the immune-histochemistry data.
Data availability
Data that supports this research publication has been shown in the article and Supplementary Information.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal care and experimental procedures were performed in accordance with a protocol approved by the Institutional Animal Care and Ethics Committee of Dalian Medical University (AEE18033). In addition, all animal experiments in the present study were consistent with the National Institutes of Health guide for the care and use of laboratory animals.
Footnotes
Edited by A. Stephanou
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41419-021-04425-w
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/9/2021
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41419-021-04425-w
Contributor Information
Heng Yin, Email: yinheng@dicp.ac.cn.
Shujing Wang, Email: wangshujing@dlmedu.edu.cn.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-019-1560-y).
References
- 1.Zaorsky NG, Raj GV, Trabulsi EJ, Lin J, Den RB. The dilemma of a rising prostate-specific antigen level after local therapy: what are our options? Semin. Oncol. 2013;40:322–336. doi: 10.1053/j.seminoncol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Valerio M, Emberton M, Eggener SE, Ahmed HU. The challenging landscape of medical device approval in localized prostate cancer. Nat. Rev. Urol. 2015;108:377–403. doi: 10.1038/nrurol.2015.289. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari MS, Petrylak DP, Hussain M. Clinical trials in metastatic prostate cancer–has there been real progress in the past decade? Eur. J. Cancer. 2005;41:941–953. doi: 10.1016/j.ejca.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2015;120:2824–2838. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- 5.Guo JJ, et al. Alginate oligosaccharide prevents acute doxorubicin cardiotoxicity by suppressing oxidative stress and endoplasmic reticulum-mediated apoptosis. Marine Drugs. 2016;14:231. doi: 10.3390/md14120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang. Y, et al. Alginate oligosaccharide indirectly affects toll-like receptor signaling via the inhibition of microRNA-29b in aneurysm patients after endovascular aortic repair. Drug Des. Dev. Ther. 2017;11:2565–2579. doi: 10.2147/DDDT.S140206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tusi SK, Khalaj L, Ashabi G, Kiaei M, Khodagholi F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials. 2011;32:5438–5458. doi: 10.1016/j.biomaterials.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Guo JJ, et al. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress-mediated apoptosis. Drug Des. Dev. Ther. 2017;11:2387–2397. doi: 10.2147/DDDT.S142118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pritchard MF, et al. A low molecular weight alginate oligosaccharide disrupts pseudomonal microcolony formation and enhances antibiotic effectiveness. Antimicrob. Agents Chemother. 2017;61:AAC.00762–17. doi: 10.1128/AAC.00762-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, et al. The marine-derived oligosaccharide sulfate MS80, a novel TGF-β1 inhibitor, reverses TGF-β1-induced epithelial-mesenchymal transition and suppresses tumor metastasis. J. Pharmacol. Exp. Ther. 2016;359:54–61. doi: 10.1124/jpet.116.234799. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, et al. Alginate oligosaccharide DP5 exhibits antitumor effects in osteosarcoma patients following surgery. Front. Pharmacol. 2017;8:623. doi: 10.3389/fphar.2017.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer C, et al. Panobinostat reduces hypoxia-induced cisplatin resistance of non-small cell lung carcinoma cells via HIF-1alpha destabilization. Eur. J. Cancer. 2015;14:4. doi: 10.1186/1476-4598-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kailemia MJ, Park D, Lebrilla CB. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2016;409:395–410. doi: 10.1007/s00216-016-9880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen MN, et al. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–546. doi: 10.1002/pmic.201300387. [DOI] [PubMed] [Google Scholar]
- 15.Crespo HJ, Lau JT, Videira PA. Dendritic cells: a spot on sialic acid. Front. Immunol. 2013;4:491. doi: 10.3389/fimmu.2013.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki O, Abe M, Hashimoto Y. Sialylation by β-galactoside α-2,6-sialyltransferase and N-glycans regulate cell adhesion and invasion in human anaplastic large cell lymphoma. Int. J. Oncol. 2015;46:973–980. doi: 10.3892/ijo.2015.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranjan A, Kalraiya RD. α 2,6 Sialylation associated with increased β1,6-branched N-oligosaccharides influences cellular adhesion and invasion. J. Biosci. 2013;38:867–876. doi: 10.1007/s12038-013-9382-z. [DOI] [PubMed] [Google Scholar]
- 18.Badr HA, et al. Harnessing cancer cell metabolism for theranostic applications using metabolic glycoengineering of sialic acid in breast cancer as a pioneering example. Biomaterials. 2017;116:158–173. doi: 10.1016/j.biomaterials.2016.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swindall AF, et al. ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 2013;73:1368–1378. doi: 10.1158/0008-5472.CAN-12-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz MJ, et al. The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res. 2016;76:3978–3988. doi: 10.1158/0008-5472.CAN-15-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antony P, et al. Epigenetic inactivation of ST6GAL1 in human bladder cancer. BMC Cancer. 2014;14:901. doi: 10.1186/1471-2407-14-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, et al. α2,6-Sialylation mediates hepatocellular carcinoma growth in vitro and in vivo by targeting the Wnt/β-catenin pathway. Oncogenesis. 2017;6:e343. doi: 10.1038/oncsis.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Wei A, et al. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3β/β-catenin signaling pathway. Oncotarget. 2016;7:65374–65388. doi: 10.18632/oncotarget.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X, et al. TAp63 suppresses mammary tumorigenesis through regulation of the Hippo pathway. Oncogene. 2017;36:2377–2393. doi: 10.1038/onc.2016.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Q, et al. Modification of α2,6-sialylation mediates the invasiveness and tumorigenicity of non-small cell lung cancer cells in vitro and in vivo via Notch1/Hes1/MMPs pathway. Int. J. Cancer. 2018;143:2319–2330. doi: 10.1002/ijc.31737. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, et al. ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget. 2018;7:51955–51964. doi: 10.18632/oncotarget.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao YW, Hua XW, Chen X, Wu FG. Platinum-doped carbon nanoparticles inhibit cancer cell migration under mild laser irradiation: multi-organelle-targeted photothermal therapy. Biomaterials. 2018;183:30–42. doi: 10.1016/j.biomaterials.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Wu G, Liu J, Wu Z, Wu X, Yao X. MicroRNA-184 inhibits cell proliferation and metastasis in human colorectal cancer by directly targeting IGF-1R. Oncol. Lett. 2017;14:3215. doi: 10.3892/ol.2017.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, et al. Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol. Cell. Proteomics. 2014;13:520–536. doi: 10.1074/mcp.M113.034025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastas. Rev. 2012;31:501–518. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, et al. β-Galactoside α2,6-sialyltranferase 1 promotes transforming growth factor-β-mediated epithelial-mesenchymal transition. J. Biol. Chem. 2014;289:34627–34641. doi: 10.1074/jbc.M114.593392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian L, et al. α 2,6-linked sialic acid serves as a high-affinity receptor for cancer oncolytic virotherapy with Newcastle disease virus. J. Cancer Res. Clin. Oncol. 2017;143:2171–2181. doi: 10.1007/s00432-017-2470-y. [DOI] [PubMed] [Google Scholar]
- 33.Dall’Olio F, et al. Beta-galactoside alpha 2,6 sialyltransferase in human colon cancer: contribution of multiple transcripts to regulation of enzyme activity and reactivity with Sambucus nigra agglutinin. Int. J. Cancer. 2000;88:58. doi: 10.1002/1097-0215(20001001)88:1<58::aid-ijc9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.dos-Santos PB, et al. Eduardo Isidoro Carneiro Beltrão. Lectin histochemistry reveals SNA as a prognostic carbohydrate-dependent probe for invasive ductal carcinoma of the breast: a clinicopathological and immunohistochemical auxiliary tool. Int. J. Clin. Exp. Pathol. 2015;7:2337–2349. [PMC free article] [PubMed] [Google Scholar]
- 35.Yu S, et al. Caveolin‐1 up‐regulates integrin α2,6‐sialylation to promote integrin α5β1‐dependent hepatocarcinoma cell adhesion. FEBS Lett. 2013;587:782. doi: 10.1016/j.febslet.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Lópezmorales D, Reyesleyva J, Santoslópez G, Zenteno E, Vallejoruiz V. Increased expression of sialic acid in cervical biopsies with squamous intraepithelial lesions. Diagn. Pathol. 2010;5:1–5. doi: 10.1186/1746-1596-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaburagi T, Kizuka Y, Kitazume S, Taniguchi N. The inhibitory role of α2,6-sialylation in adipogenesis. J. Biol. Chem. 2017;292:2278. doi: 10.1074/jbc.M116.747667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz MJ, et al. Abstract 3327: The tumor associated sialyltransferase ST6Gal-I promotes a cancer stem cell phenotype and upregulates stem-related transcription factors. Cancer Res. 2017;76:3978–3988. doi: 10.1158/0008-5472.CAN-15-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, et al. Transcriptional regulation of human beta-galactoside alpha2,6-sialyltransferase (hST6Gal I) gene in colon adenocarcinoma cell line. Biochem. Biophys. Res. Commun. 2003;307:1070–1074. doi: 10.1016/s0006-291x(03)01314-7. [DOI] [PubMed] [Google Scholar]
- 40.Singh VP, Katta S, Kumar S. WD-repeat protein WDR13 is a novel transcriptional regulator of c-Jun and modulates intestinal homeostasis in mice. BMC Cancer. 2017;17:148. doi: 10.1186/s12885-017-3118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thakur N, et al. TGFβ-induced invasion of prostate cancer cells is promoted by c-Jun-dependent transcriptional activation of Snail1. Cell Cycle. 2014;13:2400–2414. doi: 10.4161/cc.29339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukey MJ, Kai SG, Erickson JW, Wilson KF, Cerione RAJNC. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat. Commun. 2016;7:11321. doi: 10.1038/ncomms11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfundt R, et al. In situ demonstration of phosphorylated c‐jun and p38 MAP kinase in epidermal keratinocytes following ultraviolet B irradiation of human skin. J. Pathol. 2001;193:248–255. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH780>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 44.Krestnikova N, Stulpinas A, Imbrasaite A, Sinkeviciute G, Kalvelyte AV. JNK implication in adipocyte-like cell death induced by chemotherapeutic drug cisplatin. J. Toxicol. Sci. 2015;40:21–32. doi: 10.2131/jts.40.21. [DOI] [PubMed] [Google Scholar]
- 45.He M, Han M, Zheng B, Shu YN, Wen JKJJOB. Angiotensin II stimulates KLF5 phosphorylation and its interaction with c-Jun leading to suppression of p21 expression in vascular smooth muscle cells. J. Biochem. 2009;146:683–691. doi: 10.1093/jb/mvp115. [DOI] [PubMed] [Google Scholar]
- 46.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol. Cell. Biol. 2015;35:1350. doi: 10.1128/MCB.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collak FK, Demir U, Ozkanli S, Kurum E, Zerk PE. Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension. Cancer Biol. Med. 2017;14:405–413. doi: 10.20892/j.issn.2095-3941.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that supports this research publication has been shown in the article and Supplementary Information.