Abstract
HIV-1 subtypes associate with differences in transmission and disease progression. Thus, the existence of geographic hotspots of subtype diversity deepens the complexity of HIV-1/AIDS control. The already high subtype diversity in Portugal seems to be increasing due to infections with sub-subtype A1 virus. We performed phylogenetic analysis of 65 A1 sequences newly obtained from 14 Portuguese hospitals and 425 closely related database sequences. 80% of the A1 Portuguese isolates gathered in a main phylogenetic clade (MA1). Six transmission clusters were identified in MA1, encompassing isolates from Portugal, Spain, France, and United Kingdom. The most common transmission route identified was men who have sex with men. The origin of the MA1 was linked to Greece, with the first introduction to Portugal dating back to 1996 (95% HPD: 1993.6–1999.2). Individuals infected with MA1 virus revealed lower viral loads and higher CD4+ T-cell counts in comparison with those infected by subtype B. The expanding A1 clusters in Portugal are connected to other European countries and share a recent common ancestor with the Greek A1 outbreak. The recent expansion of this HIV-1 subtype might be related to a slower disease progression leading to a population level delay in its diagnostic.
Subject terms: Bioinformatics, HIV infections
Introduction
The Human immunodeficiency virus 1 (HIV-1) pandemic is characterized by an extensive genetic diversity of the pathogen, a consequence of high viral replication, recombination, and mutation rates1,2. Among the four existing HIV-1 groups3 only the M group has a global distribution. This group is consensually divided into several subtypes (A-D, F-H, J, K), sub-subtypes (A1 to A4 and F1 and F2) and an increasing number of recombinant forms4,5. The genetic diversity among HIV-1 subtypes may cause different disease progression rates6–9, advantages in specific transmission routes10,11, different capacities to evade the immune response12,13 and was associated with specific differential therapy outcomes14–17. Moreover, HIV-1 subtypes surveillance proved to be an important tool to reconstruct the history of an epidemic over time18,19.
The global distribution of HIV-1 subtypes has been heterogeneous but well compartmentalized20. However, in recent decades the HIV-1 subtype geographic distribution pattern became more complex21–23. The number of non-B infections is increasing24–30 in Western and Central Europe (WCE), where subtype B has long been predominant. The infections by non-B subtypes in this region were commonly linked to immigrants from other geographic locations19,21. Nevertheless, the transmission of previously rare subtypes is increasing among native individuals25,31–35.
Portugal has one of the highest numbers of new HIV infections among Western European countries and an atypical HIV-1 subtype diversity due to the high prevalence of G subtype25,27,36. When studying new HIV-1 infections in Europe from 2002 to 2005, Abecasis et al. highlighted Portugal as the European country with more non-B subtype infections (60.8%)19. A recent study25 of a Portuguese cohort from the region of Minho (Northwest) reported the predominance of G and B subtypes followed by recombinant forms. The authors also showed the existence of non-B and non-G transmission clusters, related to the increasing number of new infections by A1 and F1 sub-subtypes25.
In the present study, we performed a multicenter characterization of the HIV-1 sub-subtype A1 infections in Portugal. Using a phylogenetic approach, we inferred regional and multinational transmission chains while estimating their geographic and temporal origins.
Results
Characterization of the phylogenetic relationship among study sequences
The phylogenetic representation (Fig. 1) demonstrated that 80% (52 of 65) of the A1 sub-subtype virus isolated in 14 Portuguese hospitals clustered together in a main A1 clade (MA1, n = 61). Most of the study sequences (96%, 50 of 52) within MA1 were isolated from individuals of Portuguese nationality. MA1 also included sequences obtained from public databases isolated in Spain, France, and United Kingdom (10%, 5 of 61). MA1 was nested in the phylogeny with most of the sequences isolated in WCE.
Figure 1.
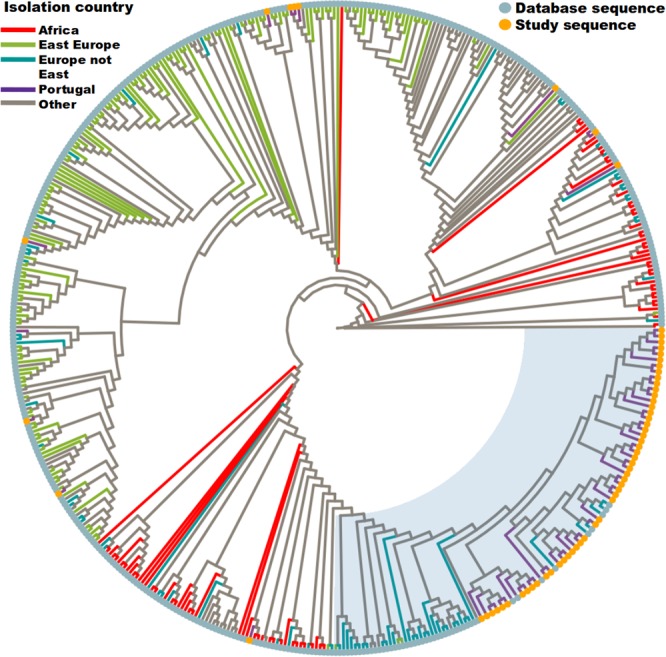
Phylogenetic representation of the A1 sequences isolated in Portugal and closely related sequences from databases. Circular cladogram representation of the Maximum likelihood tree (Supplementary Fig. 1) with the study A1 sequences and the closely related database sequences (n = 490). Branch colours indicate the geographical origin of the sequences. The tip points indicate if the taxa are an A1 sequence from this study or a database sequence. Most of this study sequences cluster together in a well-defined area of the phylogeny (52 of 65), here further mentioned as the main A1 cluster (MA1). The light grey background encompasses the MA1 and closely related sequences used for further analyses (n = 99, aLRT SH-like branch support = 0.95).
The remaining sub-subtype A1 isolates sequenced in this study (13 of 65) are scattered over the tree. From which, five clustered with sequences isolated in African countries. Among those are four sequences isolated from African immigrants (Supplementary Table 1). The other eight, including three sequences isolated from Ukrainian nationals, were highly related to isolates from East Europe (see Supplementary Table 1).
Overall, these results suggest that there were several introductions of sub-subtype A1 viruses in Portugal, from at least three different geographic regions: Europe not East; Africa; and Eastern Europe. However, most of the sampled infections (>80%) were caused by a monophyletic introduction of a sub-subtype A1 virus.
Most recent common ancestor characterization
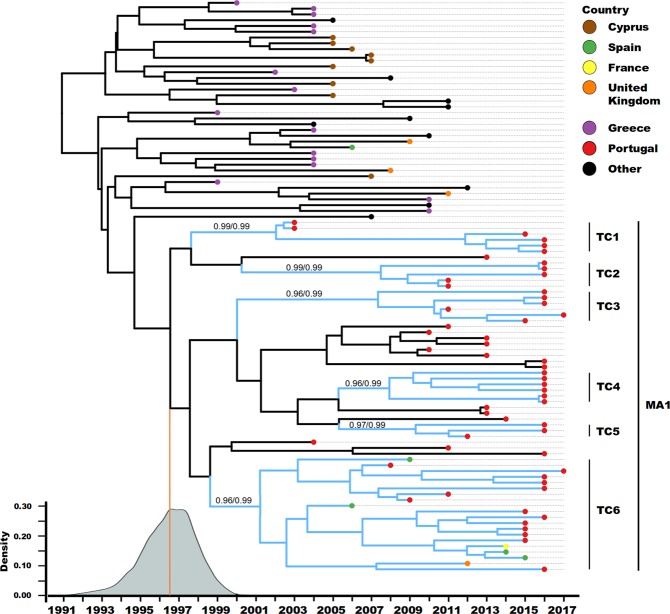
To better understand the most recent history of the sub-subtype A1 spread in Portugal the MA1 and closely related database sequences (total n = 99) were analyzed. This dataset is mainly composed by isolates from Western (France, Portugal, Spain, and United Kingdom) and Southern (Cyprus and Greece) European countries (Fig. 2). The estimate for the date of the most recent common ancestor (MRCA) of the MA1 is mid 1996 (95% highest posterior density (HPD): 1993.6–1999.2). The taxa outside the MA1 but closely related to it, sharing and older MRCA (1990.9; 95 HPD: 1986.8–1994.6), were almost exclusively sampled in Greece and Cyprus. Therefore, this data demonstrates the European ancestry of the MA1 viruses expanding in Portugal.
Figure 2.
Evolution of the study sub-subtype A1 transmission chain through its most recent history in several European countries. Maximum clade credibility tree summarised from the output trees from the BEAST analyses (n = 99). The tip point colours represent the country where the samples were collected. The blue branch lines indicate that the respective HIV-1 sequence belong to a transmission cluster. The vertical black bars demonstrate the taxa included in each transmission cluster and the main A1 clade (MA1). The aLRT SH-like branch support and posterior probability (pp) of each of the transmission clusters is displayed (aLRT/pp). The density plot in the bottom left refers to the time of the most recent common ancestor of the MA1. The country of sampling of the taxa labelled as others is: USA (3); Belgium (3); Sweden (1); Australia (1); The Netherlands (1), and Kuwait (1).
Transmission clusters characterization
From the 61 taxa in the MA1, 46 (~75%) are part of six well-delimited transmission clusters (TC1 to TC6, Table 1). TC5 is the smallest with 3 sequences, followed by TC2 with 5, TC1, TC3 and TC4 with 6. Interestingly, TC6 included 20 sequences. Eight sequences in all the clusters lacked demographic information. Among those with available data, two sequences were isolated from females and 36 from males. The self-reported transmission route for all transmission clusters is 100% sexual, being the majority (59%) men who have sex with men (MSM). However, in TC5 and TC2 self-reported heterosexual transmission dominated, while in TC4 only MSM is reported. These results suggest a strong association of the MA1 transmission clusters with males and MSM route of transmission.
Table 1.
Characterization of the six transmission clusters identified in the main A1 cluster.
| Cluster | No of individuals | Route of transmission (n) | Sampling date | Time of MRCA | Cluster Depth | Sample origin | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Unknown | Mean | Range | Mean | 95% HPD | ||||
| TC1 | 6 | 4 | 0 | 2 | MSM (3), Heterosexual (1), Unknown (2) | 2011.7 | 2003–2016 | 2002 | 2000.8–2002.9 | 14 | Multicentera |
| TC2 | 5 | 3 | 2 | 0 | MSM (1), Heterosexual (4) | 2014 | 2011–2016 | 2007.5 | 2004.9–2009.8 | 8.5 | Multicentera |
| TC3 | 6 | 6 | 0 | 0 | MSM (4), Heterosexual (2) | 2015.2 | 2011–2017 | 2007.3 | 2004.2–2010.0 | 9.6 | Multicentera |
| TC4 | 6 | 6 | 0 | 0 | MSM (6) | 2016 | 2016–2016 | 2007.9 | 2005.0–2010.7 | 8.1 | Multicentera |
| TC5 | 3 | 3 | 0 | 0 | MSM (1), Heterosexual (2) | 2014.7 | 2012–2016 | 2009.3 | 2006.9–2011.4 | 6.7 | Multicentera |
| TC6 | 20 | 14 | 0 | 6 | MSM (12), Heterosexual (2), Unknown (6) | 2013.5 | 2006–2017 | 2001.2 | 1998.8–2003.2 | 15–8 | Multicentera, Multinationalb |
aSamples from more than one of the 14 Portuguese hospital centers.
bFrance, Portugal, Spain, and United Kingdom.
All the transmission clusters (TC) in this study are multicenter and TC6 is multinational. Three transmission clusters (2, 3, and 5) are composed by isolates from the cities of Braga and Porto, in the northern region of Portugal. The same pattern is observed for the central region of Portugal, with isolates from Aveiro, Coimbra, Lisbon and Setúbal in TC1 and TC4. This result suggests some level of regional compartmentalization in the transmission of MA1 viruses in Portugal. However, TC6, with 20 taxa, includes isolates from several Portuguese regions (North: Braga, Centre: Lisbon and Setúbal; South: Faro) and isolates from France, Spain, and the United Kingdom. This supports that the transmission history of this cluster is not restricted to Portugal.
The estimates for the time of the MRCA for each cluster were at the first two decades of the 2000s. The cluster depth (diagnostic date of the most recent sample minus the time of MRCA) showed that transmission of TC 1, 3, and 6 are expected to be ongoing for at least one decade. It is also of notice that all the transmission clusters have at least one taxon isolated in the years of 2016 or 2017, suggesting the emergent character of these transmission clusters.
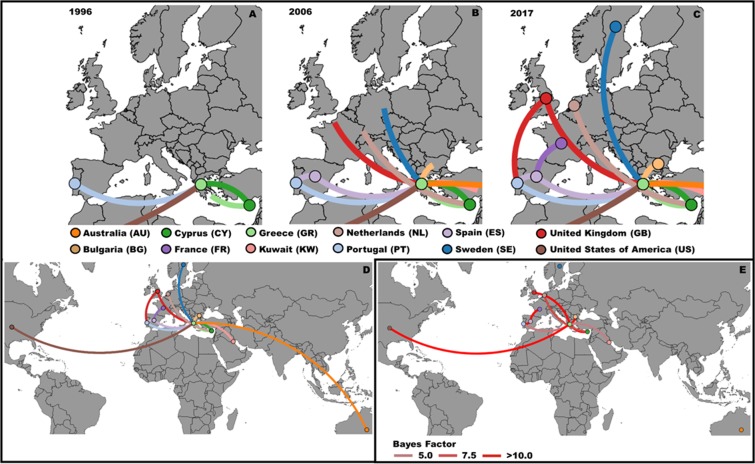
Phylogeographic root and discrete rates among countries
A phylogeographic analysis was performed using the country of sample collection as a discrete trait (Fig. 3, Supplementary Video). Greece had the highest posterior probability for the tree root (pp = 0.84). Nevertheless, most of the countries in the analysis are in the 95% HPD interval for the tree root. A total of 6 (ES-PT, CY-GR, GB-GR, GR-US, ES-FR, BG-GR) pairwise rates of diffusion between locations have a strong support value (Bayes factor >1037, Fig. 3E, Supplementary Table 2). Greece is the most common location among well supported rates of diffusion, being present in four. These rates highlight that several geographic locations, at a given point of the transmission history were not only receivers but also donors.
Figure 3.
Phylogeographic evolution of the Main A1 clade. Geographical display of the phylogeny built using BEAST with the country of the sequence origin as discrete trait. (A) In the year of 1996 this transmission history reached Western Europe, arriving to Portugal. (B) After a decade, in 2006, viruses from this clade are expected to be found in several countries from different geographic regions. Some countries, like Portugal, started now being donors and not only receivers in this transmission history. (C) In 2017 the spread among several European countries is established. (D) Global representation of this transmission history; (E) Rates between country pairs with Bayes Factor superior to 5.
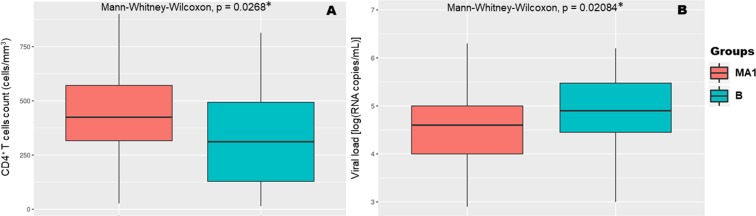
CD4+ T-cell levels and amino acid variant characterization
To investigate the association of MA1 viruses with CD4+ T-cell counts or viral load a comparison with a matched control group was performed. The groups are composed by individuals infected with MA1 viruses (n = 50, 11 cases were excluded due to lack of clinical data) and subtype B (n = 42), matching for gender, age and proportion of ambiguous sites (PAS - as a surrogate of time of infection38–41). The values for the CD4+ T-cell counts and viral load of the two groups are statistically different (p < 0.05, Mann-Whitney-Wilcoxon Test, Fig. 4A,B). To investigate if the viral genotype could contribute to these findings the prevalence of the existing amino acid variants was compared between the two groups. For 15 amino acid sites in the protease and 19 in the reverse transcriptase there are marked differences (Bonferroni correction of Fisher Exact Test, p < 0.05) in the proportion of the variants when comparing MA1 vs. B (Supplementary Tables 3 and 4). Interestingly, some of these sites (protease: 14, 20, 35, 36, 37, 41, 57, 63, 64, 71, and 89; reverse transcriptase: 207) were previously reported to influence viral fitness (Supplementary Tables 3 and 4)42–46. We evaluated if there were associations between the amino acid variants and CD4+ T-cell counts or viral load. The results were not statistically significant. Of note, the amino acid sites 14, 36, 71 and 89 in the protease and the site 207 in the reverse transcriptase were associated with differences in the viral load and CD4+ T-cell counts before adjustment for multiple comparisons (Supplementary Tables 5 and 6).
Figure 4.
Comparison of infection progression outcomes between the MA1 study group and the control group (B subtype). There were statistically significant differences between the two study groups regarding CD4+ T-cell counts (A) and viral load (B). *At a significance level of 0.05 using the Mann-Whitney-Wilcoxon Test (p < 0.05).
Discussion
Our results demonstrate that the sub-subtype A1 viruses circulating in Portugal had three distinct historical and geographic origins: related to the Eastern European outbreak; isolates from the African continent; and the emergent Greek-Cyprus epidemic. A similar pattern was observed by Lai A. et al.30 when characterizing the sub-subtype A1 epidemic in Italy. These authors concluded that sub-subtype A1 entered Italy originated from three geographic locations30. We found no evidence of an evolutionary relationship between the A1 sequences from that study30 and the sequences herein reported. Thus, the introductions of sub-subtype A1 viruses in Portugal and Italy are likely to be independent.
Only a minority of A1 sequences isolated in Portugal was found in migrants and clustered with sequences isolated from their countries of origin. These cases are likely to be infections acquired abroad and with limited spread in Portugal. In contrast, the Greece-Cyprus introduction resulted in a large transmission chain established among Portuguese natives, the MA1. The sub-subtype A1 has been increasing in prevalence in Greece47, representing 30% of the new diagnosis between 2002 and 200519. This expansion continued26,48 making sub-subtype A1 responsible for most of HIV-1 infections in some Greek cohorts, suggesting a possible advantage to previous established subtypes. The high prevalence of sub-subtype A1 in Greece may have been the preponderant factor for the establishment of the link between Portugal and Greece suggested by our analyses. Therefore, our results highlight an additional case of increasing number of infections by a non-B subtype in an European country unrelated with migrants from outside Europe.
The characterization of the six transmission clusters made evident that the main clade of sub-subtype A1 infections in Portugal is related to MSM route of transmission among Portuguese. Moreover, this characterization revealed that there was some level of compartmentalization of the HIV-1 transmission in Portugal at a region level. However, TC6 encompassed sequences isolated in several countries, indicating a larger geographical spread. The disparity seen in TC5, composed only by males but mainly reporting heterosexual transmission route can be explained by the previously described MSM under-reporting49,50. Furthermore, “missing-links” can still exist, related to unavailable data that could help explain this disparity. Except for TC4, all the transmission clusters have sequences isolated from a range of years superior to six. This shows that our approach was successful in characterizing the most recent history of these clusters. Our previous work studying HIV-1 infections from all subtypes in the region of Minho (Portugal) from the years 2000 to 201225 showed a statistical significant increase in the detection of A1 infections. In the present work we characterized a monophyletic clade (MA1) with six transmission clusters, an MRCA not older than 2000 and less than 16 years of cluster depth. Together these results demonstrate that the transmission history of the sub-subtype A1 in Portugal occurred mainly in the last two decades.
We believe that even if older samples were available the dating of the MRCA of the MA1 as 1996 and the beginning of the transmission clusters in the 2000s would not change significantly. These estimations are in accordance to what was previously described by Carvalho A. et al.25 about an A1 transmission chain, and the report by Esteves A. et al.51 of sub-subtype A1 virus circulation in Portugal in the late 90s (1997 to 2001).
The indication of Greece as the geographic location of origin for the spread of these sub-subtype A1 viruses in other European countries highly depends on the data available in public databases. However, our aim was to characterise the most recent history of these A1 sequences in Europe. We cannot exclude the possibility of missing data being brought to light showing that there were intermediate steps in the transmission not visible in our analyses.
Upon introduction of a HIV-1 subtype to a novel region its capacity to become established and lead to the formation of large transmission chains is somewhat elusive. The sub-subtype A1 was previously characterised as a viral genotype that could lead to a slow disease progression in different host populations7–9,52–55. Here we observed a statistically significant difference between the CD4+ T-cell counts, and viral load of patients followed in Portugal and infected by virus of the MA1 compared to subtype B. Given that the two groups were normalised for factors known to influence CD4+ T-cell counts and viral load, these results suggest that the infection by MA1 viruses may lead to a lower viral load and a slower decrease of the CD4+ T-cell count when compared with infections by a virus of the predominant subtype.
A total of 24 sites, 15 in protease and 19 in reverse transcriptase, showed statistically significant differences in the amino acid proportions between subtype groups. Of those sites, 12 were previously associated with changes in the viral fitness (Supplementary Tables 3 and 4). When comparing several protease mutations, M. Parera et al.42 demonstrated that modifications in sites 14, 20, 35, 36, 37, 41, 57, 63, 64, 71 and 89 had an impact in this protein catalytic efficiency. The substitution M36I was also reported as having an impact in the virion maturation43. The protease site 63 had been associated with compensatory mutations with replication benefits44. Variations in the amino acid at protease position 64 were previously associated with differences in the viral replication capacity45. The reverse transcriptase site 207 has been associated with viral fitness alterations46. Nevertheless, in our study we found no statistically significant association between these sites and viral load or CD4+ T-cell counts. To our knowledge, the remaining 12 sites were not previously associated with impact in the viral fitness. We cannot conclude that changes in these sites can influence the natural history of HIV-1 infection, further studies need to be conducted. Moreover, sites that we did not explore, like the C2V3 sequence in HIV-1 envelope56, may have a strong impact in the CD4+ T-cell counts that we cannot evaluate in the present study. Nonetheless, given the previous reports of a slower CD4+ T-cells depletion caused by sub-subtype A1 virus7–9,52–55 a better understanding of the genetic characteristics of this HIV-1 sub-subtype is necessary. With our characterization, we provide the basis for a more focused work tackling the clinical significance of sub-subtype A1 genetic diversity and its impact on the European HIV-1 epidemic.
The prevalence of HIV-1 subtypes is changing21–23. We need to better understand the causes behind these changes, and their impact on the HIV-1 infection. In recent years sub-subtype A1 became extremely common in Greece26,48. Now it is increasing among new infections in Portugal, and potentially other Western European countries. Further HIV-1 surveillance studies are required to evaluate this phenomenon and elucidate its consequences.
Methods
Study Population
The inclusion criteria for this study were: (i) availability of partial HIV-1 genome sequence; (ii) infection with sub-subtype B or A1 virus; (iii) absence of previous antiretroviral treatment upon sampling. Data was collected from HIV-1 infected patients followed at Hospital de Braga from 2007 until 2017 and from other 13 Portuguese hospital centres taking part in the BEST HOPE surveillance study from 2016 until 2017 (Supplementary Table 7). For all cases matching the criteria the following patient secondary data was collected: self-reported transmission route; gender; birth year; nationality; self-reported country of infection; date of the viral sample collection for sequencing; CD4+ T-cell count at sampling; viral load at sampling. The collection of the patient’s data was performed anonymously after approval by the ethic comities of each healthcare institution.
Sequencing
Viral RNA was extracted from plasma using MagNA Pure total nucleic acid isolation kits (Roche Applied Science). DNA sequencing, from the reverse transcriptase PCR amplicons was performed with the TrugeneTM HIV-1 genotyping system (Siemens Healthcare Diagnostics) and ViroSeqTM genotyping system (Abbott Molecular). The sequenced portion corresponds to the pol region, from position 2253 to 3554 (with some variability related to the used primers). The HIV-1 positions in this study refer to the HXB2 HIV-1 reference genome (GenBank: K03455.1). All multiple sequence alignments were performed using MUSCLE v3.857. The sequences obtained in this study were made available in GenBank (accession numbers in Supplementary Table 8) or are part of the BEST-HOPE program database.
Database queries
Two databases were queried: the NCBI full nucleotide collection and the HIV reference sequence database (http://www.hiv.lanl.gov/) using BLAST58,59. From each of 65 A1 sequences obtained in this study three different input queries were constructed: (i) full sequence; (ii) protease region; (iii) reverse transcriptase region. Each database query generated 10 outputs. We excluded duplicates, results missing collection date or geographic origin, and sequences showing evidence of recombination60. Applying these criteria 425 database sequences were selected for this study. Codons related to major antiretroviral drug resistance61 were excluded from the multiple sequence alignments prior to phylogenetic analysis.
Phylogenetic analysis
An alignment of 490 sequences was used to make a phylogenetic reconstruction using PhyML v3.062. The best fitting substitution model was GTR + G4 + I, determined by PhyML SMS63. Tree search started from 10 random trees using SPR and NNI methods. The tree with the best likelihood value was selected. For the in-detail study of the most recent history of the sub-subtype A1 in Portugal the main A1 clade, composed by 52 of the study sequences (52 of 65, 80%) and by the most closely related database sequences (total n = 99) was further explored by the following analysis. The maximum likelihood tree was built with the parameters described above. This phylogenetic representation was used to infer the correlation between genetic distance and time, using TempEst v1.564, and to characterize the transmission clusters. The same phylogeny was inferred for a near full-length alignment (for those sequences with available data) to compare topological alterations (Supplementary Fig. 2). Bayesian evolutionary and phylogeographic analyses were performed using BEAST v1.865,66, with BEAGLE library v2.167. The site model used in all the BEAST analyses was GTR + G4 + I for two different codon partitions (1 + 2, 3). According to path and stepping-stone sampling68–70, the coalescent skygrid model with an uncorrelated relaxed clock showed the best fit (Supplementary Table 9). Three different runs (random seeds), of 30 million generations, converged to similar values. Outputs were analyzed with Tracer v1.6 to ensure all parameters had an effective sampling size (ESS) superior to 300. The three multiple tree output files were combined, using LogCombiner v1.865 and used to build the maximum clade credibility tree with mean heights using TreeAnnotator v1.865, excluding 10% burn-in. The skygrid plot was also created (Supplementary Fig. 3). In the phylogeographic analysis the sampling country was used as a discrete trait71, with a total of 12 different discrete locations (Fig. 3).
Definition of transmission cluster and tree visualization
The criteria for the definition of a clade as a transmission cluster, chosen after performing a sensitivity analysis of the relevant factors (Supplementary Table 10), were: likelihood ratio test (aLRT) SH-like branch support ≥0.95 (estimated with PhyML v3); branch posterior probability ≥0.99 (estimated with BEAST v1.8); mean cluster genetic distance <0.003 substitutions per site; and maximum genetic distance <0.05 substitutions per site. MEGA v7.072 was used for genetic distance calculation.
For the visualization and manipulation of the trees in this study the software FigTree v1.4 and the R packages ggtree v1.10.573 and APE v5.074 were used. The phylogeographic representation was created with SpreaD375. The countries locations were plotted as their geographic center.
Statistical analysis
To investigate associations between the infection with the most transmitted sub-subtype A1 virus clade in Portugal (identified as MA1) and the levels of CD4+ T-cells and viral load two groups were compared: i) cases infected with sub-subtype A1 virus from MA1 (n = 50, 11 cases were excluded due to lack of clinical data); ii) cases infected with the geographically most prevalent subtype (B) virus (n = 42). All corresponding to treatment naive individuals. The B group was created from the 170 available subtype B cases (Supplementary Table 11) to match the MA1 group in factors known to influence the disease progression: gender (p > 0.05, Fisher’s exact test), age (p > 0.05, Mann-Whitney-Wilcoxon Test), and proportion of ambiguous sites (PAS) (p > 0.05, Mann-Whitney-Wilcoxon Test). PAS was used to normalize the time after infection as it was shown to positively correlate with this variable38–41.
To study the association of amino acid variants with these two groups, their prevalence was compared using Fisher’s exact test, at a 0.05 significance level. To infer the association of amino acid variants with changes in the CD4+ T-cell counts or viral load, the sequences from the two previously mentioned groups (MA1 and B, n = 92) were grouped according to the amino acid variant present in each site. The groups for each variant for each amino acid position were compared (Mann-Whitney-Wilcoxon Test and T-test, at a 0.05 significance level, using Bonferroni correction for multiple comparisons). The statistical tests were performed using R v3.4.376.
Supplementary information
Acknowledgements
Supported by FEDER, COMPETE, and FCT by the projects NORTE-01-0145-FEDER-000013, POCI-01-0145-FEDER-007038 and IF/00474/2014; FCT PhD scholarship PDE/BDE/113599/2015; FCT contract FCT IF/00474/2014; European Funds through grant BEST HOPE (project funded through HIVERA, grant 249697) and by FCT PTDC/DTP-EPI/7066/2014. Global Health and Tropical Medicine Center are funded through FCT (UID/ Multi/04413/2013). We would like to acknowledge all the patients and health care professionals from the Portuguese hospitals that contributed in some way to this study.
Author Contributions
P.M.M.A. and N.S.O. conceived the work, performed and interpreted the analyses and wrote the paper; A.C. and M.P. gather and curated data and participated in the discussions of the findings; A.B.A. critically assessed the methods and results and participated in the discussion of the findings.
Competing Interests
The authors declare no competing interests.
Footnotes
*A list of other contributors can be seen in the Acknowledgments section
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nuno S. Osório, Email: nosorio@med.uminho.pt
BEST-HOPE study group:
Domítilia Faria, Raquel Pinho, José Ferreira, Paula Proença, Sofia Nunes, Margarida Mouro, Eugénio Teófilo, Sofia Pinheiro, Fernando Maltez, Maria José Manata, Isabel Germano, Joana Simões, Olga Costa, Rita Corte-Real, António Diniz, Margarida Serrado, Luís Caldeira, Nuno Janeiro, Guilhermina Gaião, José M. Cristino, Kamal Mansinho, Teresa Baptista, Perpétua Gomes, Isabel Diogo, Rosário Serrão, Carmela Pinheiro, Carmo Koch, Fátima Monteiro, Maria J. Gonçalves, Rui Sarmento e Castro, Helena Ramos, Joaquim Oliveira, José Saraiva da Cunha, Vanda Mota, Fernando Rodrigues, Raquel Tavares, Ana Rita Silva, Fausto Roxo, Maria Saudade Ivo, José Poças, Bianca Ascenção, Patrícia Pacheco, Micaela Caixeiro, Nuno Marques, Maria J. Aleixo, Telo Faria, Elisabete Gomes da Silva, Ricardo Correia de Abreu, and Isabel Neves
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43420-2.
References
- 1.Hemelaar J. Implications of HIV diversity for the HIV-1 pandemic. J. Infect. 2013;66:391–400. doi: 10.1016/j.jinf.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Aldrich C, Hemelaar J. Global HIV-1 diversity surveillance. Trends Mol. Med. 2012;18:691–694. doi: 10.1016/j.molmed.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Plantier J-C, et al. A new human immunodeficiency virus derived from gorillas. Nat. Med. 2009;15:871–872. doi: 10.1038/nm.2016. [DOI] [PubMed] [Google Scholar]
- 4.Robertson DL, et al. HIV-1 nomenclature proposal. Science. 2000;288:55–65. doi: 10.1126/science.288.5463.55d. [DOI] [PubMed] [Google Scholar]
- 5.Peeters M, D’Arc M, Delaporte E. The origin and diversity of human retroviruses. AIDS Reviews. 2014;16:23–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Kaleebu P, et al. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J. Infect. Dis. 2002;185:1244–50. doi: 10.1086/340130. [DOI] [PubMed] [Google Scholar]
- 7.Easterbrook, P. J. et al. Impact of HIV-1 viral subtype on disease progression and response to antiretroviral therapy. J. Int. AIDS Soc. 13 (2010). [DOI] [PMC free article] [PubMed]
- 8.Kiwanuka N, et al. Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J. Infect. Dis. 2008;197:707–713. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, et al. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J. Infect. Dis. 2007;195:1177–80. doi: 10.1086/512682. [DOI] [PubMed] [Google Scholar]
- 10.Renjifo B, et al. Preferential in-utero transmission of HIV-1 subtype C as compared to HIV-1 subtype A or D. AIDS. 2004;18:1629–36. doi: 10.1097/01.aids.0000131392.68597.34. [DOI] [PubMed] [Google Scholar]
- 11.John-Stewart GC, et al. Subtype C Is associated with increased vaginal shedding of HIV-1. J. Infect. Dis. 2005;192:492–6. doi: 10.1086/431514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serwanga J, et al. Frequencies of Gag-restricted T-cell escape ‘footprints’ differ across HIV-1 clades A1 and D chronically infected Ugandans irrespective of host HLA B alleles. Vaccine. 2015;33:1664–72. doi: 10.1016/j.vaccine.2015.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartolo I, et al. Origin and epidemiological history of HIV-1 CRF14_BG. PLoS One. 2011;6:e24130. doi: 10.1371/journal.pone.0024130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abecasis AB, et al. Protease mutation M89I/V is linked to therapy failure in patients infected with the HIV-1 non-B subtypes C, F or G. AIDS. 2005;19:1799–806. doi: 10.1097/01.aids.0000188422.95162.b7. [DOI] [PubMed] [Google Scholar]
- 15.Abecasis AB, et al. Investigation of baseline susceptibility to protease inhibitors in HIV-1 subtypes C, F, G and CRF02_AG. Antivir. Ther. 2006;11:581–9. [PubMed] [Google Scholar]
- 16.Camacho RJ, Vandamme A-M. Antiretroviral resistance in different HIV-1 subtypes: impact on therapy outcomes and resistance testing interpretation. Curr. Opin. HIV AIDS. 2007;2:123–9. doi: 10.1097/COH.0b013e328029824a. [DOI] [PubMed] [Google Scholar]
- 17.Brenner B, et al. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS. 2003;17:F1–5. doi: 10.1097/00002030-200301030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Yebra G, et al. Analysis of the history and spread of HIV-1 in Uganda using phylodynamics. J. Gen. Virol. 2015;96:1890–1898. doi: 10.1099/vir.0.000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abecasis, A. B. et al. HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology10 (2013). [DOI] [PMC free article] [PubMed]
- 20.Hemelaar J. The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 2012;18:182–192. doi: 10.1016/j.molmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Beloukas A, et al. Molecular epidemiology of HIV-1 infection in Europe: An overview. Infect. Genet. Evol. 2016;46:180–189. doi: 10.1016/j.meegid.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Castley A, et al. A national study of the molecular epidemiology of HIV-1 in Australia 2005-2012. PLoS One. 2017;12:e0170601. doi: 10.1371/journal.pone.0170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis AM, et al. Rising prevalence of non-B HIV-1 subtypes in North Carolina and evidence for local onward transmission. Virus Evol. 2017;3:vex013. doi: 10.1093/ve/vex013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai A, et al. HIV-1 subtype F1 epidemiological networks among Italian heterosexual males are associated with introduction events from South America. PLoS One. 2012;7:e42223. doi: 10.1371/journal.pone.0042223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho A, et al. Analysis of a local HIV-1 epidemic in portugal highlights established transmission of non-B and non-G subtypes. J. Clin. Microbiol. 2015;53:1506–14. doi: 10.1128/JCM.03611-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoniadou Z-A, et al. Short communication: molecular epidemiology of HIV type 1 infection in northern Greece (2009–2010): evidence of a transmission cluster of HIV type 1 subtype A1 drug-resistant strains among men who have sex with men. AIDS Res. Hum. Retroviruses. 2014;30:225–232. doi: 10.1089/aid.2013.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteves A, et al. Molecular epidemiology of HIV type 1 infection in Portugal: high prevalence of non-B subtypes. AIDS Res. Hum. Retroviruses. 2002;18:313–325. doi: 10.1089/088922202753519089. [DOI] [PubMed] [Google Scholar]
- 28.Thomson MM, et al. Rapid Expansion of a HIV-1 Subtype F Cluster of Recent Origin Among Men Who Have Sex With Men in Galicia, Spain. J. Acquir. Immune Defic. Syndr. 2012;59:e49–e51. doi: 10.1097/QAI.0b013e3182400fc4. [DOI] [PubMed] [Google Scholar]
- 29.Paraskevis D, et al. Molecular characterization of HIV-1 infection in Northwest Spain (2009–2013): Investigation of the subtype F outbreak. Infect. Genet. Evol. 2015;30:96–101. doi: 10.1016/j.meegid.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Lai A, et al. HIV-1 A1 Subtype Epidemic in Italy Originated from Africa and Eastern Europe and Shows a High Frequency of Transmission Chains Involving Intravenous Drug Users. PLoS One. 2016;11:e0146097. doi: 10.1371/journal.pone.0146097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabeni L, et al. Recent Transmission Clustering of HIV-1 C and CRF17_BF Strains Characterized by NNRTI-Related Mutations among Newly Diagnosed Men in Central Italy. PLoS One. 2015;10:e0135325. doi: 10.1371/journal.pone.0135325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yebra G, et al. Most HIV type 1 non-B infections in the Spanish cohort of antiretroviral treatment-naïve HIV-infected patients (CoRIS) are due to recombinant viruses. J. Clin. Microbiol. 2012;50:407–13. doi: 10.1128/JCM.05798-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand D, et al. Characteristics of patients recently infected with HIV-1 non-B subtypes in France: a nested study within the mandatory notification system for new HIV diagnoses. J. Clin. Microbiol. 2014;52:4010–6. doi: 10.1128/JCM.01141-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaix M-L, et al. Increasing HIV-1 non-B subtype primary infections in patients in France and effect of HIV subtypes on virological and immunological responses to combined antiretroviral therapy. Clin. Infect. Dis. 2013;56:880–7. doi: 10.1093/cid/cis999. [DOI] [PubMed] [Google Scholar]
- 35.Dauwe K, et al. Characteristics and spread to the native population of HIV-1 non-B subtypes in two European countries with high migration rate. BMC Infect. Dis. 2015;15:524. doi: 10.1186/s12879-015-1217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira NR, et al. Characterization of HIV-1 subtypes in a Portuguese cohort. J. Int. AIDS Soc. 2014;17:19683. doi: 10.7448/IAS.17.4.19683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffreys, H. The Theory of Probability (Oxford, 1961).
- 38.Shankarappa R, et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 1999;73:10489–502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kouyos RD, et al. Ambiguous nucleotide calls from population-based sequencing of HIV-1 are a marker for viral diversity and the age of infection. Clin. Infect. Dis. 2011;52:532–9. doi: 10.1093/cid/ciq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragonnet-Cronin M, et al. Genetic diversity as a marker for timing infection in HIV-infected patients: evaluation of a 6-month window and comparison with BED. J. Infect. Dis. 2012;206:756–64. doi: 10.1093/infdis/jis411. [DOI] [PubMed] [Google Scholar]
- 41.Andersson E, et al. Evaluation of sequence ambiguities of the HIV-1 pol gene as a method to identify recent HIV-1 infection in transmitted drug resistance surveys. Infect. Genet. Evol. 2013;18:125–31. doi: 10.1016/j.meegid.2013.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parera M, Fernandez G, Clotet B, Martinez MA. HIV-1 Protease Catalytic Efficiency Effects Caused by Random Single Amino Acid Substitutions. Mol. Biol. Evol. 2006;24:382–387. doi: 10.1093/molbev/msl168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa MGS, et al. Impact of M36I polymorphism on the interaction of HIV-1 protease with its substrates: insights from molecular dynamics. BMC Genomics. 2014;15(Suppl 7):S5. doi: 10.1186/1471-2164-15-S7-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suñé C, Brennan L, Stover DR, Klimkait T. Effect of polymorphisms on the replicative capacity of protease inhibitor-resistant HIV-1 variants under drug pressure. Clin. Microbiol. Infect. 2004;10:119–26. doi: 10.1111/j.1469-0691.2004.00832.x. [DOI] [PubMed] [Google Scholar]
- 45.Ng OT, et al. HIV type 1 polymerase gene polymorphisms are associated with phenotypic differences in replication capacity and disease progression. J. Infect. Dis. 2014;209:66–73. doi: 10.1093/infdis/jit425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu J, Whitcomb J, Kuritzkes DR. Effect of the Q207D mutation in HIV type 1 reverse transcriptase on zidovudine susceptibility and replicative fitness. J. Acquir. Immune Defic. Syndr. 2005;40:20–3. doi: 10.1097/01.qai.0000172369.82456.36. [DOI] [PubMed] [Google Scholar]
- 47.Paraskevis D, et al. Increasing prevalence of HIV-1 subtype A in Greece: estimating epidemic history and origin. J. Infect. Dis. 2007;196:1167–1176. doi: 10.1086/521677. [DOI] [PubMed] [Google Scholar]
- 48.Davanos N, Panos G, Gogos CA, Mouzaki A. HIV-1 subtype characteristics of infected persons living in southwestern Greece. HIV. AIDS. (Auckl). 2015;7:277–283. doi: 10.2147/HIV.S90755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Laar TJ, et al. Phylogenetic evidence for underreporting of male-to-male sex among human immunodeficiency virus-infected donors in the Netherlands and Flanders. Transfusion. 2017;57:1235–1247. doi: 10.1111/trf.14097. [DOI] [PubMed] [Google Scholar]
- 50.Spindler H, Salyuk T, Vitek C, Rutherford G. Underreporting of HIV transmission among men who have sex with men in the Ukraine. AIDS Res. Hum. Retroviruses. 2014;30:407–8. doi: 10.1089/aid.2013.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esteves A, et al. Spreading of HIV-1 subtype G and envB/gagG recombinant strains among injecting drug users in Lisbon, Portugal. AIDS Res. Hum. Retroviruses. 2003;19:511–517. doi: 10.1089/088922203766774568. [DOI] [PubMed] [Google Scholar]
- 52.Kiwanuka N, et al. HIV-1 viral subtype differences in the rate of CD4 + T-cell decline among HIV seroincident antiretroviral naive persons in Rakai district, Uganda. J. Acquir. Immune Defic. Syndr. 2010;54:180–4. doi: 10.1097/QAI.0b013e3181c98fc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Touloumi G, et al. Impact of HIV-1 subtype on CD4 count at HIV seroconversion, rate of decline, and viral load set point in European seroconverter cohorts. Clin. Infect. Dis. 2013;56:888–97. doi: 10.1093/cid/cis1000. [DOI] [PubMed] [Google Scholar]
- 54.Ssemwanga D, et al. Effect of HIV-1 subtypes on disease progression in rural Uganda: a prospective clinical cohort study. PLoS One. 2013;8:e71768. doi: 10.1371/journal.pone.0071768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amornkul PN, et al. Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS. 2013;27:2775–86. doi: 10.1097/QAD.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ringe R, et al. Unique C2V3 sequence in HIV-1 envelope obtained from broadly neutralizing plasma of a slow progressing patient conferred enhanced virus neutralization. PLoS One. 2012;7:e46713. doi: 10.1371/journal.pone.0046713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altschul SF, et al. Basic Local Alignment Search Tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 59.Morgulis A, et al. Database indexing for production MegaBLAST searches. Bioinformatics. 2008;24:1757–1764. doi: 10.1093/bioinformatics/btn322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz A-K, et al. jpHMM: Improving the reliability of recombination prediction in HIV-1. Nucleic Acids Res. 2009;37:W647–W651. doi: 10.1093/nar/gkp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin. Infect. Dis. 2006;42:1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guindon SS, et al. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 63.Lefort V, Longueville J-E, Gascuel O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017;6:461–464. doi: 10.1093/molbev/msx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. 2016;2:vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drummond AJ, Suchard Ma, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferreira MAR, Suchard MA. Bayesian analysis of elapsed times in continuous-time Markov chains. Can. J. Stat. 2008;36:355–368. doi: 10.1002/cjs.5550360302. [DOI] [Google Scholar]
- 67.Ayres DL, et al. BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics. Syst. Biol. 2012;61:170–173. doi: 10.1093/sysbio/syr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baele G, et al. Improving the accuracy of demographic and molecular clock model comparison while accommodating phylogenetic uncertainty. Mol. Biol. Evol. 2012;29:2157–2167. doi: 10.1093/molbev/mss084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baele G, Li WLS, Drummond AJ, Suchard MA, Lemey P. Accurate model selection of relaxed molecular clocks in Bayesian phylogenetics. Mol. Biol. Evol. 2013;30:239–243. doi: 10.1093/molbev/mss243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baele G, Lemey P, Suchard MA. Genealogical Working Distributions for Bayesian Model Testing with Phylogenetic Uncertainty. Syst. Biol. 2016;65:250–264. doi: 10.1093/sysbio/syv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lemey P, Rambaut A, Drummond AJ, Suchard MA. Bayesian phylogeography finds its roots. PLoS Comput. Biol. 2009;5:e1000520. doi: 10.1371/journal.pcbi.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 74.Paradis E, Claude J, Strimmer K. Bioinformatics. 2004. APE: Analyses of Phylogenetics and Evolution in R language; pp. 289–290. [DOI] [PubMed] [Google Scholar]
- 75.Bielejec F, et al. SpreaD3: Interactive Visualization of Spatiotemporal History and Trait Evolutionary Processes. Mol. Biol. Evol. 2016;33:2167–2169. doi: 10.1093/molbev/msw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.R Core Team. R: A Language and Environment for Statistical Computing (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.