Abstract
Recurrent urinary tract infection (rUTI) continues to challenge pediatric care providers. The diagnosis of an rUTI can be difficult, especially in young febrile children. Antibiotic resistance rates continue to rise, which limits oral treatment options. Prophylactic antibiotics are used commonly to manage rUTI, but their use increases the risk of rUTI with antibiotic-resistant strains without significantly reducing renal scarring. Alternative therapies for rUTI include probiotics and anthocyanidins (eg, cranberry extract) to reduce gut colonization by uropathogens and prevent bacterial adhesion to uroepithelia, but efficacy data for these treatments are sparse. The future of rUTI care rests in addressing the following contemporary issues: best diagnostic practices, risk factors associated with rUTI, and the prevention of recurrent infection. In this review, we summarize the state of the art for each of these issues and highlight future studies that will aim to take an alternative approach to managing rUTI.
Keywords: antibiotic resistance, diagnosis, probiotics, recurrent UTI
Urinary tract infection (UTI) is one of the most common types of infection in children [1]. A subset of children experience recurrent UTI (rUTI) and pose a management challenge for all clinicians. In this review, we focus on the risk factors for rUTI and strategies for preventing and better managing it.
EPIDEMIOLOGY OF UTI
Although the exact cost of rUTI management is not known, the aggregate hospital costs of all pediatric UTI management exceeds $520 million every year [1, 2]. In children, the rate of rUTI after the first episode is 13.6% (ie, incidence rate of 0.12 per person-year) [3]. rUTI is associated with school absenteeism, a need for parental leave, frequent visits to health care providers, and a likely increase in healthcare costs.
GUIDELINES FOR DIAGNOSING rUTI
rUTI is defined as an onset of UTI symptoms after resolution of a previous UTI. The rate of renal scarring increases significantly after the third UTI [4].
Diagnosing recurrent episodes is similar to that of the sentinel episode and should involve reviewing clinical features and the results of urinalysis and bacterial culture [5]. Clinical features include dysuria, urinary frequency, irritability, fever, nausea, and poor feeding. The presence of nitrites and leukocyte esterase in a urinalysis and presence of white blood cells (WBCs) as determined by microscopy are the most widely used parameters for diagnosis [6]. The conversion of dietary nitrates to nitrites by uropathogens in the bladder takes approximately 4 hours. Therefore, the traditional nitrite test is not sensitive for children, particularly infants, who empty their bladders frequently [7]. The leukocyte esterase test is approximately 94% sensitive when used in the context of clinical UTI; however, its specificity is low (74%) [6].
A comparison of enhanced urinalysis (manual microscopic examination of uncentrifuged urine) and automated urinalysis was conducted on 703 urine samples collected by clean catch or catheterization from children aged 0 to 19 years (median, 10 months) [8]. A positive enhanced urinalysis result was defined as ≥10 WBCs/mm3 and the presence of any bacteria per 10 oil immersion fields on a Gram-stained smear. A positive automated urinalysis result was defined as ≥2 WBCs per high power field (hpf) and the presence of any bacteria detected by the machine. The positive predictive values (PPVs) and sensitivities for pyuria were similar between the 2 methods. However, the sensitivities and PPVs for bacteriuria to detect a single organism culture of ≥50000 colony-forming units (CFU)/mL were superior in the enhanced urinalysis. When pyuria as determined by automated urinalysis was combined with bacteriuria as determined by Gram-stain analysis, the PPVs and sensitivities were similar to those of the enhanced urinalysis. Therefore, combining these 2 methods might increase sample throughput without decreasing their sensitivity and PPV. Another study also found that urinalysis results can be influenced by concentration of the urine [9]. The results of a Chaudhari et al study [9] suggested that urine concentration alters diagnostic criteria and that 3 WBCs/hpf when urine is dilute (specific gravity, <1.015) or 6 WBCs/hpf when urine is concentrated (specific gravity, >1.015) should be considered a positive result. A urine culture that results in a single pathogen with ≥100000 CFU/mL from a midstream sample or ≥50000 CFU/mL from a sample obtained via catheter or suprapubic collection is considered diagnostic for UTI when associated with appropriate symptoms and a suggestive urinalysis result. A single pathogen culture that results in ≥10000 CFU/mL in infants with symptoms consistent with a UTI and evidence of inflammation in urinalysis can also be considered diagnostic for UTI [10].
PREDISPOSING FACTORS ASSOCIATED WITH rUTI
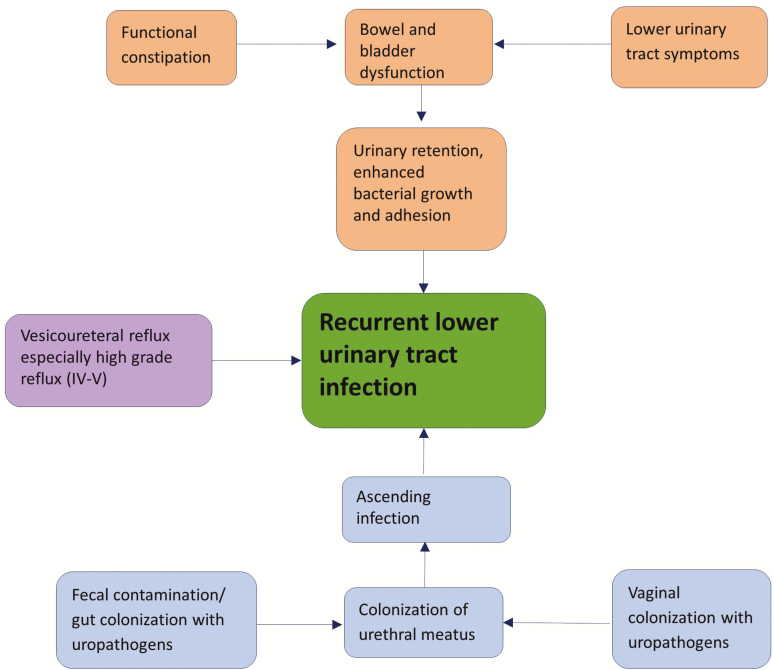
Figure 1 provides an overview of the predisposing factors associated with rUTI.
Figure 1.
Overview of risk factors associated with recurrent urinary tract infection.
Bowel and Bladder Dysfunction
Bowel and bladder dysfunction (BBD) is an underdiagnosed yet common pediatric condition [11]. BBD is a combination of lower urinary tract symptoms (LUTSs) and bowel disorders, including constipation and/or encopresis, in patients with no known neurological abnormality [12]. Overactive bladder that results from detrusor overactivity is the most common form of LUTSs in children and is marked with increased frequency of daytime urination and nocturia with or without incontinence [12]. Voiding postponement, another common LUTS, occurs when a patient delays urination (associated with withholding maneuvers such as leg-crossing and the “pee-pee dance”), which leads to urgency and incontinence because of the overfilled bladder [12, 13]. Such patients frequently also have constipation because of delayed stooling habits. Other LUTSs include underactive bladder caused by detrusor underactivity, which results in the need to strain during urination, dysfunctional voiding caused by habitual contraction of the bladder sphincter and pelvic floor, and bladder neck dysfunction, which refers to delayed or impaired bladder opening that results in reduced urine flow despite increased bladder pressure [12]. These conditions together can result in postvoid urine retention, which provides a rich medium for bacterial replication and adherence and increased risk of rUTI [14].
A review of 2 longitudinal studies, the Randomized Intervention for Children With Vesicoureteral Reflux (RIVUR) trial [15] and the Careful Urinary Tract Infection Evaluation (CUTIE) study [16], found that the most frequent urinary symptoms in children with BBD were urgency (85%), withholding maneuvers (80%), and daytime wetting (63%) [17]. The most common bowel disorders were painful defecation (39%) and having fewer than 3 bowel movements per week (8%). Overall 22% of the patients evaluated in both studies combined met the criteria for constipation. The results of this review also suggested that BBD is an independent risk factor for rUTI and therefore should be considered part of the clinical evaluation.
Constipation, also a component of BBD, occurs in up to one-third of girls with rUTI [18]. Constipation causes urinary stasis as a result of compression of the bladder and elongation of the urethra by fecal retention, which reduces urinary flow and promotes pathogen adherence [19]. Some children with constipation are found also to have renal pelvic dilation even in the absence of anatomical abnormalities or infection [20].
Vesicoureteral Reflux
A retrospective analysis found that grades IV and V vesicoureteral reflux (VUR), age between 2 and 6 years, and Caucasian race are associated with rUTI [3], whereas lower grades of VUR are not. However, another prospective cohort study found a higher rate of recurrence in children with any grade of VUR than in those with no VUR [16]. Among children with any grade of VUR, those who were less than 24 months old or were diagnosed with constipation were at a higher risk of rUTI.
Gut Colonization With Uropathogens
The gastrointestinal and vaginal tracts are well established reservoirs of uropathogens [21]. Czaja et al [22] found a significant increase in gut-derived uropathogens in the periurethral area in women in the days that preceded a UTI episode. Another study noted that rUTI in infants are usually caused by the same organism as that which caused the previous episode, which suggests a local reservoir for the bacterial strain [23].
Male Circumcision and Female Vaginal Microbial Colonization
Other predisposing factors include uncircumcised status in male neonates and antibiotic use. Among healthy male infants less than 3 months old, there is a greater than 10-fold increase in the incidence of UTI among those who are uncircumcised [3, 24]. Normal vaginal colonization with H2O2-producing strains of Lactobacillus occurs well before the onset of menarche [25]. The use of antibiotics can alter the normal vaginal flora and result in a predominance of enteric bacteria and an increased risk of rUTI [26].
MICROBIOLOGY
Uropathogenic Escherichia coli (UPEC) causes 70% to 80% of all UTIs [1, 3]. Other organisms involved in UTI are enteric bacteria such as Klebsiella spp, Proteus spp, and Enterococcus spp and vaginal colonizers such as Ureaplasma spp and Mycoplasma spp [3]. Pseudomonas aeruginosa is an uncommon uropathogen, but it has been associated with rUTI, VUR, and other renal abnormalities and therefore should be considered a possible cause of infection in this population [27]. Clonal evaluation of uropathogens from urine and rectal swabs in patients with a UTI has shown that the gut is a major reservoir of these bacteria [28]. In addition, colonization of the periurethral area by UPEC increases in the days that precede an rUTI [22].
STRATEGIES FOR PREVENTING rUTI
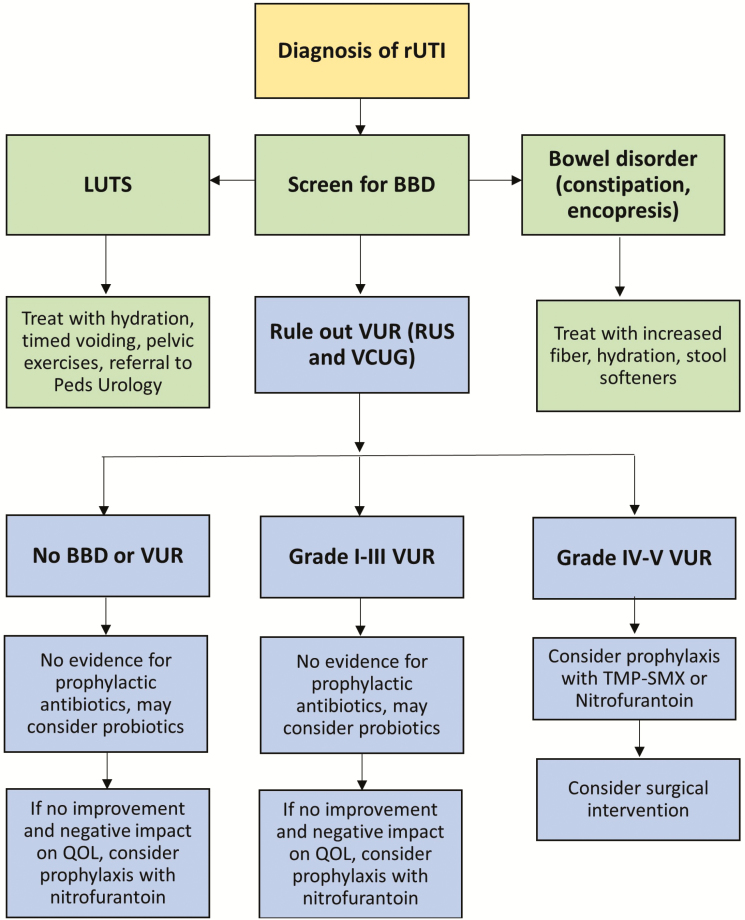
Figure 2 provides an overview of the recommended management of rUTI.
Figure 2.
Recommended management of recurrent urinary tract infection. Abbreviations: BBD, bowel and bladder dysfunction; LUTS, lower urinary tract symptoms; QOL, quality of life; RUS, renal ultrasound; rUTI, recurrent urinary tract infection; TMP-SMX, trimethoprim-sulfamethoxazole; VCUG, voiding cystourethrogram; VUR, vesicoureteral reflux.
Diagnosis and Treatment of BBD
Standardized questionnaires for diagnosing BBD in a primary care setting are available. Two such questionnaires were developed by Farhat et al [29] (Dysfunctional Voiding Score System) and Afshar et al [30] (Vancouver Symptom Score for Dysfunctional Elimination Syndrome). Both of them are based on quantitative and qualitative assessments of constipation, daytime and nighttime wetting, urgency, and difficulty in voiding or defecating. If BBD is suspected in a patient with rUTI, physicians can recommend maintenance of a urination and stooling diary, typically for 7 to 14 days, to provide objective data regarding frequency of urination, fluid intake, voided volume, presence of incontinence, frequency and physical characteristics of bowel movements, and any associated encopresis [31, 32]. Some authors have recommended maintaining a diary for 48 to 72 hours only for increased compliance [13]. An objective measurement of bowel movements can be made using the Bristol stool chart [33].
Treatment of BBD should include managing constipation with adequate hydration, an increase in fiber intake, and use of stool softeners [34]. Polyethylene glycol 3350 is the most commonly used stool softener, and it has been found to be effective and safe in the pediatric population [35]. Some LUTS, such as overactive bladder and voiding postponement, can be managed by behavioral changes, including using a combination of adequate hydration, timed voiding, and pelvic floor training using Kegel exercises or diaphragmatic breathing [36, 37]. Immediate-release (IR) (Ditropan) and extended-release (ER) (Ditropan XL) formulations of oxybutynin, an antimuscarinic agent, are also approved for use in children with overactive bladder. Although IR oxybutynin has been in clinical use for many years, pediatric data have been extrapolated largely from studies of adults [38]. ER oxybutynin was shown to have a greater efficacy than the IR form in studies with a relatively limited sample size [39, 40]. Some of this effect might be related to better adherence because of fewer adverse effects such as gastrointestinal disturbances, dry eyes and mouth, sleep difficulty, and blurred vision [40]. Referral to a pediatric urology specialist for voiding cystometry and/or biofeedback therapy using urodynamic studies should be considered also [41].
Antibiotics
Traditional strategies for preventing the recurrence of UTI, especially in children, have relied on prolonged use of antibiotics. However, several studies that compared prophylactic antibiotic use with a “just-in-time” approach found a limited effectiveness of prophylaxis in reducing renal scarring, which is the primary justification for its use, especially among patients with no or low-grade VUR [42, 43]. The RIVUR clinical trial randomly assigned more than 600 children to receive either trimethoprim-sulfamethoxazole (TMP-SMX) or placebo for 2 years and found an approximately 50% reduction in the rate of rUTI, irrespective of the severity of VUR, with a number needed to treat of 8 (ie, 5840 antibiotic doses to prevent a single recurrence) [15]. However, 63% of recurrences in the prophylaxis group were a result of TMP-SMX–resistant E coli vs 19% in the placebo group, which is concerning given the rapid rise in antimicrobial resistance in recent years. Prophylaxis did not reduce the incidence of renal scarring [44]. The Swedish reflux trial, which enrolled 203 children with grade III or IV VUR, also found a significant decrease in the rate of rUTI among girls who received TMP-SMX prophylaxis and in those who underwent endoscopic treatment compared to those who underwent clinical observation [45]. However, the authors also reported that prophylaxis did not reduce the incidence of renal scarring, and antimicrobial resistance increased significantly in the treatment group. A recent meta-analysis of 7 studies with a total sample size of 1427 participants revealed no significant difference in renal scarring in the antibiotic prophylaxis and control groups [46]. It is important to note also that these studies focused on antibiotic prophylaxis in children with VUR and not children with a history of rUTI.
Conclusive data to support the use of prophylactic antibiotics, even in neonates with known antenatal hydronephrosis or children with neurogenic bladder, are lacking [47, 48]. Subinhibitory concentrations of certain antibiotics, such as ciprofloxacin and gentamicin, might in fact result paradoxically in upregulation of bacterial cell surface adhesins in common uropathogens such as E coli and Staphylococcus saprophyticus, which results in denser biofilm formation [49].
Nitrofurantoin, a drug with minimal systemic absorption, has been shown to have an efficacy similar to that of TMP-SMX in prophylaxis against rUTI [50]. However, the use of nitrofurantoin results in a risk of subsequent resistance that is significantly lower than that with the use of TMP-SMX. Adverse effects occurred more frequently in children treated with nitrofurantoin than in those treated with TMP-SMX but consisted predominantly of gastrointestinal symptoms. Therefore, in this era of rising antimicrobial resistance, it might be prudent to use nitrofurantoin as the prophylactic antibiotic of choice when necessary.
Probiotics
Given the rise in antimicrobial resistance and evidence of the adverse effects of long-term use of antibiotics on the commensal flora, probiotics such as Lactobacillus spp and the yeast Saccharomyces boulardii have garnered new interest as long-term therapy for rUTI. Probiotics are generally thought to exert their effect though the production of antimicrobial products [51, 52], competition with uropathogens for iron (which is essential for several bacterial functions) [53], and occupation of the epithelial space to prevent adherence of uropathogenic bacteria [54].
Over the past decade, several studies measured the effects on rUTI of probiotics with or without antibiotics. In 1 trial, 120 children with established VUR who had undergone TMP-SMX prophylaxis in the preceding year were randomly assigned to receive Lactobacillus acidophilus or to continue TMP-SMX prophylaxis during the second year of follow-up [55]. The rates of rUTI were similar between the 2 groups. In a follow-up trial, 128 infants diagnosed with primary VUR were randomly assigned to receive either the probiotic Lactobacillus acidophilus or TMP-SMX for 1 year [56]. The rates of rUTI in the probiotic and antibiotic prophylaxis groups were not statistically different. In a retrospective study of 191 infants with acute pyelonephritis and normal urinary tract anatomy, including no VUR, prophylaxis with Lactobacillus led to an 8.2% incidence rate of rUTI in the 6-month follow-up period, which was similar to the 10% rate in the antibiotic group and significantly lower than the 20.6% rate in the no-prophylaxis group [57]. All of the infants with rUTI in the antibiotic group were infected with a TMP-SMX–resistant strain of bacteria, compared to 25% in the probiotic group and 41% in the no-prophylaxis group, which further shows the negative consequences of chronic antibiotic prophylaxis.
S boulardii is a nonpathogenic yeast that is able to establish itself in the colon rapidly and has antagonistic activity against pathogens [58, 59]. In children, once-daily treatment with 5 billion CFU of S boulardii was effective in significantly decreasing the burden of gut colonization with E coli [60]. Because gut colonization with uropathogens increases the risk of rUTI, S boulardii might play a role in reducing recurrent episodes.
Cranberry Juice
Recent work found that cranberry extract prevents adhesion of UPEC to uroepithelial cells in a dose-dependent manner [61]. This effect is mediated by the following 2 main components of cranberry: fructose, which inhibits UPEC adherence by type 1 fimbriae, and anthocyanidins, which inhibit adherence by pyelonephritis fimbriae (p-fimbriae) [62].
Among clinical trials performed specifically in the pediatric population, cranberry products modestly reduced the incidence of rUTI in children with normal urinary anatomy. In a review of 8 trials that used cranberry juice or products for rUTI prophylaxis in healthy children or infants, only 4 trials found a significant reduction in the incidence of rUTI [63]. Most of these studies included <50 patients. However, 1 study enrolled 263 children between the ages of 1 and 16 years with normal urinary anatomy or grade I or II VUR [64]. The total number of UTI episodes per year was significantly lower in the cranberry group than in the placebo group, but the proportions of children who had >1 recurrence were the same. Most studies have concurred that cranberry juice and preparations are safe for use in all age groups [65]. Poor palatability of the cranberry products might have an adverse effect on compliance.
Authors of studies in adult patients have recommended a daily dose of 300 mL of cranberry juice to achieve a reduction in the incidence of rUTI [66]. A pediatric study that showed the effectiveness of cranberry juice used a dose of 5 mL of juice per kilogram of body weight, up to 300 mL, per day for a 6-month period [64].
Male Circumcision
Bacterial colonization in the foreskin (prepuce) has been associated with UTI in male infants less than 3 months of age [67]. Circumcision decreases the risk of UTI, especially among neonates [68]. A study of 2000 circumcised and 1000 uncircumcised neonates over a 15-month period reported no UTI episodes in the circumcised group, whereas 2% of those in the uncircumcised group experienced a UTI [69]. A 2012 American Association of Pediatrics policy statement also indicated that the health benefits of elective circumcision of male infants (reduction in the incidence of UTI and sexually transmitted disease) outweigh the associated risks [70].
Surgical Intervention
Surgery to correct VUR plays a significant role in preventing rUTI. The success rate for endoscopic surgery for VUR grades II, III, IV, and V ranges from 60% to 90% and depends on the VUR grade and the absence of associated BBD [71]. The success rate for open neoureterocystostomy or ureteral reimplantation, the gold standards for surgical intervention, is 95% irrespective of VUR grade or BBD [72]. In addition, children with low-grade VUR (grades I–III) have an approximately 40% chance of spontaneous resolution within their first year of life [73].
FUTURE AND ONGOING RESEARCH
Vaccines
Two vaccines are available currently in Europe for use in treating rUTI, Solco-Urovac (Legacy Pharmaceuticals, Switzerland) and Uro-Vaxom (Om-pharma, Portugal). Solco-Urovac is a whole-cell inactivated vaccine used as a vaginal suppository that contains 6 E coli strains and 1 strain each of Proteus mirabilis, Morganella morganii, Enterococcus faecalis, and Klebsiella pneumoniae [74]. An intramuscular formulation of Solco-Urovac was used in a trial on 10 otherwise healthy girls (5–11 years of age) with rUTI [75]. These girls were vaccinated 3 times at weekly intervals and then received a booster 6 months later. The total number of UTI episodes among all the participants decreased from 34 in the 6 months before enrollment to 13 over the 1-year follow-up period. Uro-Vaxom is an oral capsule that contains a lyophilized mixture of membrane proteins from 18 UPEC isolates. In a randomized controlled trial in 112 women, participants were given either the vaccine or a daily placebo for 3 months and were followed for another 3 months [76]. By the end of the 6-month study period, 67% of the participants in the vaccine group had no episodes of rUTI, whereas this rate was 22% in the placebo group [76]. Uro-Vaxom is currently part of the European Association of Urology guidelines for alternative treatment of UTI in adults. Both these vaccines are not licensed for use in the United States.
Ideal strategies for vaccinating against rUTI would be to target bacterial factors involved in the establishment and maintenance of bladder colonization. Vaccines that target FimH, the type I pilus adhesin that plays a critical role in bacterial adhesion to urothelial cells, have shown some promise. In 1 study, the FimH antigen copurified with its chaperone protein, FimC, was used to vaccinate cynomolgus monkeys, which resulted in significant protection against infection with type I pili expressing UPEC [77]. This vaccine is being developed by Sequoia Sciences (St. Louis, Missouri) [78].
Small-Molecule Inhibitors of Pathogenic UPEC Factors
Several small-molecule inhibitors of pathogenic UPEC factors have been developed. They generally act to decrease bacterial virulence, which enables the host immune system to eradicate the bacteria. Mannose derivatives, called mannosides, inhibit the binding of FimH adhesin on type I pili to the mannosylated receptors on the uroepithelial cells by the formation of a FimH–oligomannose complex [79]. These molecules were found to be effective in the treatment of chronic infection in a murine model and had synergistic activity with conventional antibiotics such as TMP-SMX. Other small molecules in development target the chaperone–usher pathway (CUP) pili [80], which are ubiquitous among UPEC strains and play a major role in adhesion and in evasion of host immunity.
CONCLUSIONS
Pediatric rUTI continues to pose a significant challenge to medical providers and the healthcare system. Accurate diagnosis is vital for preventing the inappropriate use of antibiotics. BBD increases residual urine and urinary stasis, which allow for greater bacterial adherence and rUTI. Therapeutic management of rUTI traditionally has included the use of prophylactic antibiotics; however, there is strong evidence that this approach does not prevent renal scarring but does increase the risk of antibiotic resistance, which suggests that alternative strategies, including the use of probiotics and management of BBD, might be more prudent. With a better understanding of the etiology and pathogenesis of rUTI, researchers are investigating agents that can interfere with bacterial adhesion and intracellular growth.
Notes
Acknowledgments. M. A. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis.
Disclaimer. The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Financial support. M. A. received support from the National Institutes of Health (grants K08AI123524 and K12-HD043494) and the Derfner Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Foxman B. The epidemiology of urinary tract infection. Infect Dis Clin North Am 2014; 28:1–13. [DOI] [PubMed] [Google Scholar]
- 2. Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol 2010; 25:2469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conway PH, Cnaan A, Zaoutis T, et al. Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials. JAMA 2007; 298:179–86. [DOI] [PubMed] [Google Scholar]
- 4. Jodal U. The natural history of bacteriuria in childhood. Infect Dis Clin North Am 1987; 1:713–29. [PubMed] [Google Scholar]
- 5. Roberts KB; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011; 128:595–610. [DOI] [PubMed] [Google Scholar]
- 6. Echeverry G, Hortin GL, Rai AJ. Introduction to urinalysis: historical perspectives and clinical application. Methods Mol Biol 2010; 641:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Kunin CM, DeGroot JE. Sensitivity of a nitrite indicator strip method in detecting bacteriuria in preschool girls. Pediatrics 1977; 60:244–5. [PubMed] [Google Scholar]
- 8. Shah AP, Cobb BT, Lower DR, et al. Enhanced versus automated urinalysis for screening of urinary tract infections in children in the emergency department. Pediatr Infect Dis J 2014; 33:272–5. [DOI] [PubMed] [Google Scholar]
- 9. Chaudhari PP, Monuteaux MC, Bachur RG. Urine concentration and pyuria for identifying UTI in infants. Pediatrics 2016; 138:e20162370. [DOI] [PubMed] [Google Scholar]
- 10. Swerkersson S, Jodal U, Åhrén C, et al. Urinary tract infection in infants: the significance of low bacterial count. Pediatr Nephrol 2016; 31:239–45. [DOI] [PubMed] [Google Scholar]
- 11. Halachmi S, Farhat WA. Interactions of constipation, dysfunctional elimination syndrome, and vesicoureteral reflux. Adv Urol 2008; 828275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Children’s Continence Society. J Urol 2014; 191:1863–5.e13. [DOI] [PubMed] [Google Scholar]
- 13. Santos JD, Lopes RI, Koyle MA. Bladder and bowel dysfunction in children: an update on the diagnosis and treatment of a common, but underdiagnosed pediatric problem. Can Urol Assoc J 2017; 11:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang SJ, Tsai LP, Hsu CK, Yang SS. Elevated postvoid residual urine volume predicting recurrence of urinary tract infections in toilet-trained children. Pediatr Nephrol 2015; 30:1131–7. [DOI] [PubMed] [Google Scholar]
- 15. Hoberman A, Greenfield SP, Mattoo TK, et al. ; RIVUR Trial Investigators Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med 2014; 370:2367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics 2015; 136:e13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaikh N, Hoberman A, Keren R, et al. Recurrent urinary tract infections in children with bladder and bowel dysfunction. Pediatrics 2016; 137(1):e20152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loening-Baucke V. Urinary incontinence and urinary tract infection and their resolution with treatment of chronic constipation of childhood. Pediatrics 1997; 100:228–32. [DOI] [PubMed] [Google Scholar]
- 19. Blethyn AJ, Jenkins HR, Roberts R, Verrier Jones K. Radiological evidence of constipation in urinary tract infection. Arch Dis Child 1995; 73:534–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Averbeck MA, Madersbacher H. Constipation and LUTS—how do they affect each other?Int Braz J Urol 2011; 37:16–28. [DOI] [PubMed] [Google Scholar]
- 21. Russo TA, Stapleton A, Wenderoth S, et al. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis 1995; 172:440–5. [DOI] [PubMed] [Google Scholar]
- 22. Czaja CA, Stamm WE, Stapleton AE, et al. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J Infect Dis 2009; 200:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jantunen ME, Saxén H, Salo E, Siitonen A. Recurrent urinary tract infections in infancy: relapses or reinfections?J Infect Dis 2002; 185:375–9. [DOI] [PubMed] [Google Scholar]
- 24. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008; 27:302–8. [DOI] [PubMed] [Google Scholar]
- 25. Hickey RJ, Zhou X, Settles ML, et al. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 2015; 6:e00097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lidefelt KJ, Bollgren I, Nord CE. Changes in periurethral microflora after antimicrobial drugs. Arch Dis Child 1991; 66:683–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bitsori M, Maraki S, Koukouraki S, Galanakis E. Pseudomonas aeruginosa urinary tract infection in children: risk factors and outcomes. J Urol 2012; 187:260–4. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto S, Tsukamoto T, Terai A, et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol 1997; 157:1127–9. [PubMed] [Google Scholar]
- 29. Farhat W, Bägli DJ, Capolicchio G, et al. The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol 2000; 164:1011–5. [DOI] [PubMed] [Google Scholar]
- 30. Afshar K, Mirbagheri A, Scott H, MacNeily AE. Development of a symptom score for dysfunctional elimination syndrome. J Urol 2009; 182:1939–43. [DOI] [PubMed] [Google Scholar]
- 31. Abrams P, Klevmark B. Frequency volume charts: an indispensable part of lower urinary tract assessment. Scand J Urol Nephrol Suppl 1996; 179:47–53. [PubMed] [Google Scholar]
- 32. Santos J, Varghese A, Williams K, Koyle MA. Recommendations for the management of bladder bowel dysfunction in children. Pediatr Therapeut 2014; 4:191. doi: 10.4172/2161-0665.1000191. [Google Scholar]
- 33. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32:920–4. [DOI] [PubMed] [Google Scholar]
- 34. Rowan-Legg A; Canadian Paediatric Society, Community Paediatrics Committee Managing functional constipation in children. Paediatr Child Health 2011; 16:661–70. [PMC free article] [PubMed] [Google Scholar]
- 35. Nurko S, Youssef NN, Sabri M, et al. PEG3350 in the treatment of childhood constipation: a multicenter, double-blinded, placebo-controlled trial. J Pediatr 2008; 153:254–61, 261.e1. [DOI] [PubMed] [Google Scholar]
- 36. Allen HA, Austin JC, Boyt MA, et al. Initial trial of timed voiding is warranted for all children with daytime incontinence. Urology 2007; 69:962–5. [DOI] [PubMed] [Google Scholar]
- 37. Zivkovic V, Lazovic M, Vlajkovic M, et al. Diaphragmatic breathing exercises and pelvic floor retraining in children with dysfunctional voiding. Eur J Phys Rehabil Med 2012; 48:413–21. [PubMed] [Google Scholar]
- 38. Ramsay S, Bolduc S. Overactive bladder in children. Can Urol Assoc J 2017; 11:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Arendonk KJ, Knudson MJ, Austin JC, Cooper CS. Improved efficacy of extended release oxybutynin in children with persistent daytime urinary incontinence converted from regular oxybutynin. Urology 2006; 68:862–5. [DOI] [PubMed] [Google Scholar]
- 40. Youdim K, Kogan BA. Preliminary study of the safety and efficacy of extended-release oxybutynin in children. Urology 2002; 59:428–32. [DOI] [PubMed] [Google Scholar]
- 41. Combs AJ, Glassberg AD, Gerdes D, Horowitz M. Biofeedback therapy for children with dysfunctional voiding. Urology 1998; 52:312–5. [DOI] [PubMed] [Google Scholar]
- 42. Craig JC, Simpson JM, Williams GJ, et al. ; Prevention of Recurrent Urinary Tract Infection in Children With Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT) Investigators Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med 2009; 361:1748–59. [DOI] [PubMed] [Google Scholar]
- 43. Pennesi M, Travan L, Peratoner L, et al. ; North East Italy Prophylaxis in VUR Study Group Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics 2008; 121:e1489-94. [DOI] [PubMed] [Google Scholar]
- 44. Hewitson TD. Fibrosis in the kidney: is a problem shared a problem halved?Fibrogenesis Tissue Repair 2012; 5:S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brandström P, Esbjörner E, Herthelius M, et al. The Swedish reflux trial in children: III. Urinary tract infection pattern. J Urol 2010; 184:286–91. [DOI] [PubMed] [Google Scholar]
- 46. Hewitt IK, Pennesi M, Morello W, Ronfani L, Montini G. Antibiotic prophylaxis for urinary tract infection-related renal scarring: a systematic review. Pediatrics 2017; 139:e20163145. [DOI] [PubMed] [Google Scholar]
- 47. Braga LH, Mijovic H, Farrokhyar F, et al. Antibiotic prophylaxis for urinary tract infections in antenatal hydronephrosis. Pediatrics 2013; 131:e251-61. [DOI] [PubMed] [Google Scholar]
- 48. Clarke SA, Samuel M, Boddy SA. Are prophylactic antibiotics necessary with clean intermittent catheterization? A randomized controlled trial. J Pediatr Surg 2005; 40:568–71. [DOI] [PubMed] [Google Scholar]
- 49. Goneau LW, Hannan TJ, MacPhee RA, et al. Subinhibitory antibiotic therapy alters recurrent urinary tract infection pathogenesis through modulation of bacterial virulence and host immunity. mBio 2015; 6: e00356-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brendstrup L, Hjelt K, Petersen KE, et al. Nitrofurantoin versus trimethoprim prophylaxis in recurrent urinary tract infection in children. A randomized, double-blind study. Acta Paediatr Scand 1990; 79:1225–34. [DOI] [PubMed] [Google Scholar]
- 51. Asahara T, Shimizu K, Nomoto K, et al. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun 2004; 72:2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klaenhammer TR. Bacteriocins of lactic acid bacteria. Biochimie 1988; 70:337–49. [DOI] [PubMed] [Google Scholar]
- 53. Grosse C, Scherer J, Koch D, et al. A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol Microbiol 2006; 62:120–31. [DOI] [PubMed] [Google Scholar]
- 54. Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 2003; 17:741–54. [DOI] [PubMed] [Google Scholar]
- 55. Lee SJ, Shim YH, Cho SJ, Lee JW. Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatr Nephrol 2007; 22:1315–20. [DOI] [PubMed] [Google Scholar]
- 56. Lee SJ, Lee JW. Probiotics prophylaxis in infants with primary vesicoureteral reflux. Pediatr Nephrol 2015; 30:609–13. [DOI] [PubMed] [Google Scholar]
- 57. Lee SJ, Cha J, Lee JW. Probiotics prophylaxis in pyelonephritis infants with normal urinary tracts. World J Pediatr 2016; 12:425–9. [DOI] [PubMed] [Google Scholar]
- 58. McFarland LV, Bernasconi P. Saccharomyces boulardii: a review of an innovative biotherapeutic agent. Microb Ecol Health Dis 1993; 6:157–71. [Google Scholar]
- 59. Czerucka D, Dahan S, Mograbi B, et al. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun 2000; 68:5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Akil I, Yilmaz O, Kurutepe S, et al. Influence of oral intake of Saccharomyces boulardii on Escherichia coli in enteric flora. Pediatr Nephrol 2006; 21:807–10. [DOI] [PubMed] [Google Scholar]
- 61. Rafsanjany N, Senker J, Brandt S, et al. In vivo consumption of cranberry exerts ex vivo antiadhesive activity against FimH-dominated uropathogenic Escherichia coli: a combined in vivo, ex vivo, and in vitro study of an extract from vaccinium macrocarpon. J Agric Food Chem 2015; 63:8804–18. [DOI] [PubMed] [Google Scholar]
- 62. Hisano M, Bruschini H, Nicodemo AC, Srougi M. Cranberries and lower urinary tract infection prevention. Clinics (Sao Paulo) 2012; 67:661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Durham SH, Stamm PL, Eiland LS. Cranberry products for the prophylaxis of urinary tract infections in pediatric patients. Ann Pharmacother 2015; 49:1349–56. [DOI] [PubMed] [Google Scholar]
- 64. Salo J, Uhari M, Helminen M, et al. Cranberry juice for the prevention of recurrences of urinary tract infections in children: a randomized placebo-controlled trial. Clin Infect Dis 2012; 54:340–6. [DOI] [PubMed] [Google Scholar]
- 65. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2008; (10): CD001321. [DOI] [PubMed] [Google Scholar]
- 66. Avorn J, Monane M, Gurwitz JH, et al. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA 1994; 271:751–4. [DOI] [PubMed] [Google Scholar]
- 67. Arshad M, Seed PC. Urinary tract infections in the infant. Clin Perinatol 2015; 42:17–28, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schoen EJ, Colby CJ, Ray GT. Newborn circumcision decreases incidence and costs of urinary tract infections during the first year of life. Pediatrics 2000; 105:789–93. [DOI] [PubMed] [Google Scholar]
- 69. Simforoosh N, Tabibi A, Khalili SA, et al. Neonatal circumcision reduces the incidence of asymptomatic urinary tract infection: a large prospective study with long-term follow up using Plastibell. J Pediatr Urol 2012; 8:320–3. [DOI] [PubMed] [Google Scholar]
- 70. American Academy of Pediatrics, Task Force on Circumcision. Circumcision policy statement. Pediatrics 2012; 130:585–6.22926180 [Google Scholar]
- 71. Rensing A, Austin P. The diagnosis and treatment of vesicoureteral reflux: an update. Open Urol Nephrol J 2015; 8:96–103. [Google Scholar]
- 72. Marshall S, Guthrie T, Jeffs R, et al. Ureterovesicoplasty: selection of patients, incidence and avoidance of complications. A review of 3527 cases. J Urol 1977; 118:829–31. [DOI] [PubMed] [Google Scholar]
- 73. Wildbrett P, Schwebs M, Abel JR, et al. Spontaneous vesicoureteral reflux resolution in children: a 10-year single-centre experience. Afr J Paediatr Surg 2013; 10:9–12. [DOI] [PubMed] [Google Scholar]
- 74. Uehling DT, Hopkins WJ, Elkahwaji JE, et al. Phase 2 clinical trial of a vaginal mucosal vaccine for urinary tract infections. J Urol 2003; 170:867–9. [DOI] [PubMed] [Google Scholar]
- 75. Nayir A, Emre S, Sirin A, et al. The effects of vaccination with inactivated uropathogenic bacteria in recurrent urinary tract infections of children. Vaccine 1995; 13:987–90. [DOI] [PubMed] [Google Scholar]
- 76. Magasi P, Pánovics J, Illés A, Nagy M. Uro-Vaxom and the management of recurrent urinary tract infection in adults: a randomized multicenter double-blind trial. Eur Urol 1994; 26:137–40. [DOI] [PubMed] [Google Scholar]
- 77. Langermann S, Möllby R, Burlein JE, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 2000; 181:774–8. [DOI] [PubMed] [Google Scholar]
- 78. O’Brien VP, Hannan TJ, Nielsen HV, Hultgren SJ. Drug and vaccine development for the treatment and prevention of urinary tract infections. Microbiol Spectr 2016 Feb; 4; doi: 10.1128/microbiolspec.UTI-0013-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cusumano CK, Pinkner JS, Han Z, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med 2011; 3:109ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Greene SE, Pinkner JS, Chorell E, et al. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 2014; 5:e02038. [DOI] [PMC free article] [PubMed] [Google Scholar]