Abstract
Objective
The aim of the present study was to investigate the patterns of use and prognostic significance of adjuvant chemotherapy (CT) for patients with stage IC ovarian granulosa cell tumors (GCTs).
Methods
We identified patients with stage IC GCTs diagnosed between 2004 and 2015 in the National Cancer Data Base (NCDB). Logistic regression was performed to identify variables independently associated with chemotherapy administration. Overall survival (OS) was evaluated for patients diagnosed between 2004 and 2014 following generation of Kaplan-Meier curves and compared with the log-rank test. A Cox model was constructed to control for known confounders.
Results
A total of 492 patients with stage IC GCTs were identified, of which 166 (33.7%) received CT. Tumor size > = 10 cm (OR: 1.85, 95% CI: 1.21, 2.82) was independently associated with the administration of CT. There was no difference in OS between patients who did (n = 145) and did not (n = 282) receive CT, p = 0.52; 5-yr OS rates were 93.7% and 91.6% respectively. After controlling for patient age (<50 vs ≥50 years), tumor size and performance of lymphadenectomy (LND), the administration of CT was not associated with a survival benefit (HR: 1.07, 95% CI: 0.52, 2.21).
Conclusions
Approximately one in three patients with stage IC GCTs received CT in the NCDB, however CT was not associated with a survival benefit.
Keywords: Ovary, Tumor, Chemotherapy sex cord-stromal, Granulosa cell
Highlights
-
•
In a cohort of patients with stage IC GCTs rate of adjuvant chemotherapy use was 33.7%.
-
•
Tumor size > = 10 cm was associated with the administration of chemotherapy.
-
•
Adjuvant chemotherapy was not associated with a survival benefit.
1. Introduction
Ovarian granulosa cell tumors (GCTs) are rare malignancies representing <7% of all ovarian tumors. Most patients present with stage I disease (Ray-Coquard et al., 2014; Schultz et al., 2016) while late recurrences, even 10 years from the initial diagnosis are not infrequent; emphasizing the importance of a long-term follow-up (Mangili et al., 2013). Surgical excision is the mainstay of treatment, while the necessity of lymphandenectomy (LND) for apparent early stage disease has not been established (Nasioudis et al., 2017). Given the excellent survival rates of patients with early-stage disease, and the indolent course of GCTs the role of adjuvant chemotherapy for this group has yet to be established (Ray-Coquard et al., 2014). Due to the lack of high-quality evidence, there is significant variation in practice. Certain providers reserve adjuvant chemotherapy for patients with high-risk characteristics such as large tumor size, high mitotic count and stage IC disease (Ray-Coquard et al., 2014). Currently, the National Comprehensive Cancer Network guidelines recommend that either observation or adjuvant chemotherapy can be offered to patients with stage IC SCSTs (Morgan Jr et al., 2016). However, a recent analysis of the MITO network that included 40 patients with stage IC disease failed to demonstrate a benefit for those who received chemotherapy (Mangili et al., 2016). The aim of this study was to evaluate the patterns of utilization and outcomes of adjuvant chemotherapy for patients with stage IC GCTs, using a large multi-institutional, hospital-based database.
2. Materials and methods
The National Cancer Data Base was accessed and patients diagnosed between 2004 and 2015 with an ovarian granulosa cell tumor (ICD-O-3 histology codes: 8620/3-622/3) were identified. Those with substage IC were selected for further analysis. Patients without information on the administration of chemotherapy were excluded from the present study. Fig. 1 depicts the patient selection flowchart. Demographic and clinico-pathological information were extracted from the de-identified NCDB dataset. For the purpose of analysis, reporting facility type was divided into academic/research and other. Reporting facility type is suppressed by NCDB for patients aged <40 years. Year of diagnosis was also mathematically categorized into 3-yr intervals. Demographic and clinico-pathological characteristics were compared with the chi-square and Mann-Witney U tests. Logistic regression was performed to identify factors independently associated with the administration of adjuvant chemotherapy. For any patient to be included in the survival analysis, a minimum of 1 month of follow-up was required. In the present study, overall survival (OS) was defined as the number of months elapsed from tumor diagnosis to the date of death or last-follow up. In the NCDB, vital status and months from cancer diagnosis to the date of last contact or death are not available for cases diagnosed in 2015, as such survival analyses were restricted to cases diagnosed in 2014 and earlier. OS was evaluated following generation of Kaplan-Meier curves and comparisons were performed with the log-rank test. A sensitivity analysis was performed following stratification by performance of LND and tumor size. A Cox multivariate model was constructed to control for a priori selected confounders known to be associated with survival in patients with GCTs. All statistical analysis was performed with the SPSS v.24 statistical package (IBM Corp. Armonk, NY), and the alpha level of statistical significance was set at 0.05.
Fig. 1.
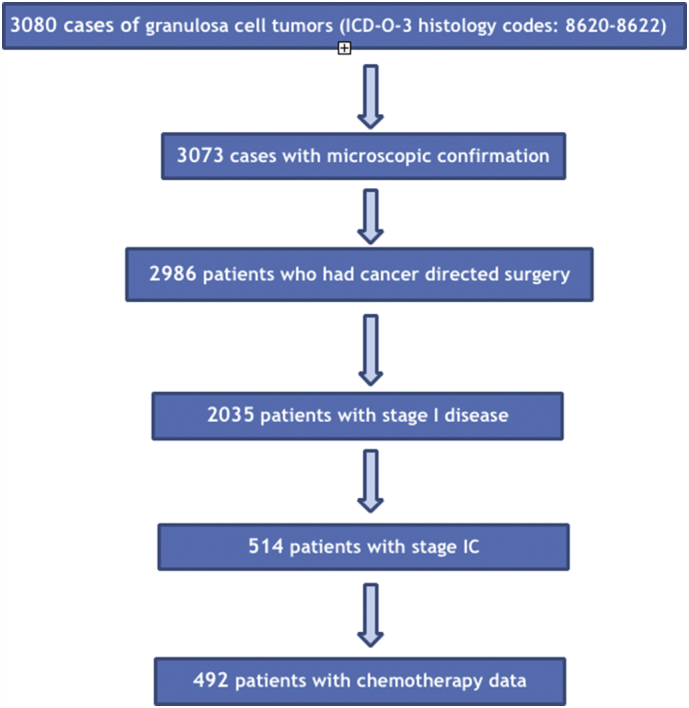
Patient selection flowchart.
3. Results
A total of 492 patients with stage IC GCTs who met the inclusion criteria were identified. Median patient age was 49.5 years (range: 7–90 years, IQR: 21). The majority were of White race (70.3%) followed by Black (24.2%) and had private insurance (63%). Presence of medical co-morbidities as assessed by the Charlson-Deyo index score (defined as a score > 0) was infrequent (17.5%). Regarding tumor size, 45.9% of tumors were ≥ 10 cm in size. A total of 260 (52.8%) patients underwent LND. Information on the type of reporting facility was available for 365 (74.2%) cases; 29.3% were managed at academic institution. Tumor grade was available only for 128 patients; 42.2% had grade 1 tumors, while 34.4% and 23.4% had grade 2 and 3 tumors respectively.
A total of 166 (33.7%) patients received adjuvant chemotherapy. Based on available information, a multi-agent chemotherapy regimen was administered in 94.6% (157/166) of patients. Median interval between surgery and chemotherapy administration was 49.5 days (n = 164, IQR: 34.75). Among, patients who did not receive chemotherapy; 17 were deemed ineligible due to patient-related risk factors while 36 patients were offered chemotherapy but refused, and for the remainder chemotherapy was not offered. The percent of patients receiving chemotherapy increased over time, from 22.7% to 31.5% to 36.2% and 39.2% over each respective 3-year interval from 2007 to 2009 to 2013–2015 (p = 0.056). In addition, patients who received chemotherapy were younger (median age was 48 vs 51 years, p = 0.005) and more likely to have private insurance (p = 0.006) and be managed in non-academic facility (p = 0.041). Higher chemotherapy administration rates were noted for those who had tumors > = 10 cm in size (p = 0.033). No differences were noted based on patient race (p = 0.37), personal history of another tumor (p = 0.18), performance of LND (p = 0.11), performance of hysterectomy (p = 0.79) or the presence of medical co-morbidities (p = 0.21). For the 128 patients with known tumor grade, rate of adjuvant chemotherapy use for those with grade 1 (n = 54), grade 2 (n = 44) and grade 3 (n = 30) were 22.2%, 25% and 33.3% respectively, p = 0.53. Table 1 summarizes the clinico-pathological characteristics of patients with stage IC ovarian SCSTs stratified by the administration of chemotherapy. After controlling for year of diagnosis and type of insurance, and tumor size ≥10 cm (OR: 1.85, 95% CI: 1.21, 2.82), and management in a non-academic facility (OR: 1.78, 95% CI: 1.10, 2.88) were significantly associated with the administration of CT while patient age was not.
Table 1.
Clinicopathological characteristics of patients with stage IC ovarian granulosa cell tumors stratified by the administration of adjuvant chemotherapy (CT).
| No CT | CT | p value | |
|---|---|---|---|
| Age (median) | 51 yrs. | 48 yrs. | 0.005 |
| Age (yrs) <=30 31–40 41–50 51–60 60+ |
22 (6.7%) 66 (20.2%) 73 (22.4%) 72 (22.1%) 93 (28.5%) |
24 (14.5%) 29 (17.5%) 44 (26.5%) 42 (25.3%) 27 (16.3%) |
0.004 |
| Medical co-morbidities No Yes |
264 (81%) 62 (19%) |
142 (85.5%) 24 (14.5%) |
0.21 |
| History of other tumor No Yes |
290 (89%) 36 (11%) |
154 (92.8%) 12 (7.2%) |
0.18 |
| Race White Non-white/Unknown |
225 (69%) 101 (31%) |
121 (72.9%) 45 (27.1%) |
0.37 |
| Year of diagnosis 2004–2006 2007–2009 2010–2012 2013–2015 |
68 (20.9%) 74 (22.7%) 88 (27%) 96 (29.4%) |
20 (12%) 34 (20.5%) 50 (30.1%) 62 (37.3%) |
0.056 |
| Reporting facility Type Academic Other Unknown |
107 (32.8%) 142 (43.6%) 77 (23.6%) |
37 (22.3%) 79 (47.6%) 50 (30.1%) |
0.041 |
| Insurance Private Medicaid/Medicare/Government Uninsured/Unknown |
190 (58.3%) 105 (32.2%) 31 (9.5%) |
120 (72.3%) 39 (23.5%) ^ |
0.006 |
| Median Income* <38,000 $ 38,000–47,999 $ 48,000–62,999 $ > = 63,000 $ |
71 (22%) 75 (23.2%) 94 (29.1%) 83 (25.7%) |
35 (21.1%) 44 (26.5%) 46 (27.7%) 41 (24.7%) |
0.89 |
| LND Yes No/Unknown |
164 (50.3%) 162 (49.7%) |
96 (57.8%) 70 (42.2%) |
0.11 |
| Hysterectomy& Yes No |
192 (63.4%) 111 (36.6%) |
95 (64.6%) 52 (35.4%) |
0.79 |
| Size < 10 cm > = 10 cm Unknown |
152 (46.6%) 139 (42.6%) 35 (10.7%) |
57 (34.3%) 87 (52.4%) 22 (13.3%) |
0.033 |
^ suppressed n < 10, * missing for 3 cases, &missing for 42 cases.
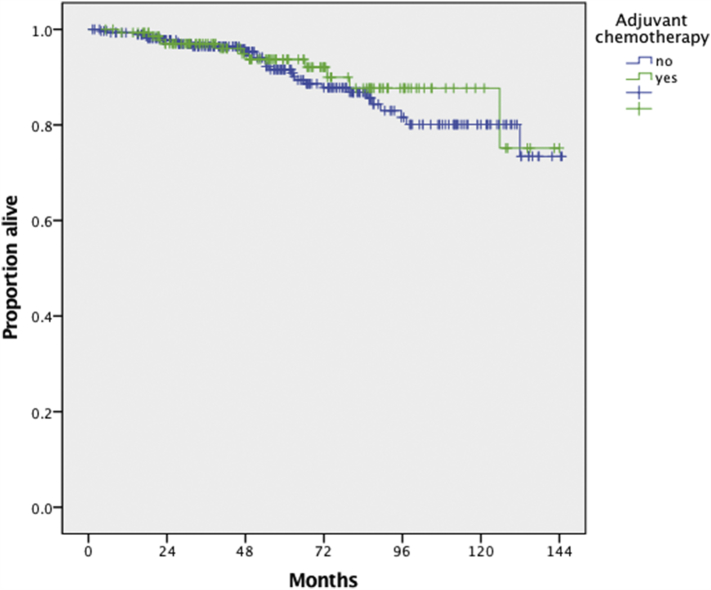
A total of 427 patients were included in the survival analysis. The median follow-up of the chemotherapy (n = 145) and observation (n = 282) groups were 57.3 and 61.5 months respectively. A total of 11 (7.6%) and 29 (10.3%) deaths were observed in the chemotherapy and observation groups respectively. Following the generation of Kaplan-Meir curves, 5-yr OS rates were 93.7% and 91.6% respectively and there was no difference in OS observed between the two groups (p = 0.52); (Fig. 2). After adjusting for patient age (<50 vs ≥50 years), tumor size (<10 vs ≥10 cm vs unknown) and the performance of LND, the administration of adjuvant chemotherapy was not associated with a survival benefit (HR: 1.07, 95% CI: 0.52, 2.21) (Table 2).
Fig. 2.
Overall survival of patients with stage IC ovarian granulosa cell tumors who did (n = 145) and did not (n = 282) receive adjuvant chemotherapy, p = 0.52 from log-rank test.
Table 2.
Multivariate analysis of overall survival of patients with stage IC ovarian granulosa cell tumors.
| Hazard ratio 95% CI | |
|---|---|
| Age <50 yrs. > = 50 yrs. |
Referent 3.68 (1.78, 7.63) |
| LND Yes No/Unkn |
Referent 2.43 (1.27, 4.63) |
| Tumor size <10 cm > = 10 cm Unknown |
Referent 2.37 (1.16, 4.81) 0.89 (0.25, 3.23) |
| Adjuvant chemotherapy No Yes |
Referent 1.07 (0.52, 2.21) |
Following stratification by performance of LND there was no difference in OS between the chemotherapy and observation groups among patients who did (p = 0.15) and did not (p = 0.32) receive LND. Similarly, following stratification by tumor size, there was no difference in OS between the chemotherapy and observation groups for patients with tumors > = 10 cm (p = 0.07) and < 10 cm (p = 0.28) in size.
4. Discussion
This study is based on a large cohort of patients with stage IC GCTs derived from a hospital-based database. Approximately one third of patients received adjuvant chemotherapy. An increase in the administration of chemotherapy was noted over time while large tumor size was independently associated with its administration. However, adjuvant chemotherapy failed to achieve any survival benefit even after controlling for confounders.
Given the rarity of GCTs the majority of prior studies evaluating the utility of adjuvant chemotherapy were derived from single institutional case experience and combined early stage and advanced stage disease patients in an effort to increase statistical power (Meisel et al., 2015; Park et al., 2012). Rupture of the ovarian surface epithelium or tumor involvement of the ovarian surface have been identified in multiple studies as a negative prognostic factor for patients with stage I disease (Auranen et al., 2007; Wilson et al., 2015). In large cohort of 160 patients with stage I granulosa cell tumors, a higher relapse rate was observed for patients with stage IC (43%) compared to those with stage IA disease (24%) (p = 0.02) (Wilson et al., 2015). To date, only two studies (Mangili et al., 2016; Wang et al., 2018) have investigated the role of adjuvant chemotherapy for patients with stage IC disease and both reported on disease-free survival (DFS). Similar to our results, an analysis of the Italian MITO-9 network failed to demonstrate an improvement in disease-free survival (DFS) for patients with stage IC granulosa cell tumors who received adjuvant chemotherapy (Mangili et al., 2016). In that study, the rate of adjuvant chemotherapy use was slightly lower, 22.5% (9/40 patients). After a median follow-up of 96 months, there was no difference in DFS between the observation and CT groups with a 5-yr DFS rates of 50% and 27% respectively (p = 0.4) (Mangili et al., 2016). In another retrospective study, Wang et al. (2018), analyzed 60 patients with stage IC granulosa cell tumors. In that study rate of adjuvant chemotherapy administration was 53.3%. After a median follow-up of 88 months no difference in DFS was noted between patients who did and did not receive adjuvant chemotherapy (87.5% and 76.3% respectively, p = 0.197). In addition, the number of chemotherapy cycles was not associated with relapse rate (Wang et al., 2018).
Given the indolent nature of GCTs, it is difficult to demonstrate a benefit to adjuvant chemotherapy since tumor relapse can occur even 10 years or more following initial treatment. On the other hand, chemotherapy can be associated with significant morbidity such a pulmonary fibrosis, myelotoxicity, and secondary malignancies. There is no level I or II evidence supporting its use for patients with stage I SCSTs, even in the presence of high-risk characteristics. As such, the decision to administer adjuvant chemotherapy should be individualized and involve extensive patient counseling. Traditionally the BEP regimen has been offered, however platinum-taxane combination is an acceptable alternative with less toxicity (Ray-Coquard et al., 2018).
Nevertheless, since relapse rates for patients with stage IC disease can be as high as 30–40%, there remains a need for novel adjuvant treatment options as well as biomarkers to identify patients at an increased risk for a relapse. The expansion of molecular techniques and the identification of a unique genetic mutational profile such as FOXL2 for granulosa cell tumors (Schultz et al., 2016) open new possibilities for the development of targeted and immune therapies. Recently, the presence of a mutation at the telomerase reverse transcriptase (TERT) gene was correlated with relapse risk and overall survival for patients with granulosa cell tumors (Pilsworth et al., 2018). Moreover, in vitro experiments indicate that tyrosine kinase inhibitors may have a role in the management of patients with granulosa cell tumors and merit further investigation (Jamieson and Fuller, 2015). Hormonal therapy can also be useful in the management of select patients with GCTs, especially those whose tumors express estrogen and progestin receptors (van Meurs et al., 2014)
The main strength of the present study is the fact that the data was derived from a hospital-based database and included patients managed in academic as well as non-academic centers limiting patient selection bias. However, several limitations should be noted. First, due to the lack of central pathology review possible tumor misclassifications cannot be excluded, while tumor grade was available for 128 patients. In addition, unmeasured factors such as patient functional status, specialty of treating physician and physician preference may have influenced the decision to administer adjuvant chemotherapy. While the vast majority of patients received a multi-agent regimen, specific details such as dosage, number of cycles and agents used were not available. Moreover, we could not discriminate between stage IC1, IC2 and IC3. However, given the rarity of GCTs the power of such sub-analyses would have been limited. Unfortunately, the NCDB does not collect data on tumor relapse, thus we could not calculate differences in PFS. Median follow-up of the present cohort was approximately 5 years, and as such, we cannot exclude the possibility that chemotherapy has an impact on 10 year survival rate. Lastly, specific details on the staging procedures performed and pre-operative imaging were not available, thus our population may have included inadequately staged (presumed stage IC) patients.
In this large cohort of patients with stage IC GCTs a temporal trend towards an increase in the administration of adjuvant chemotherapy was observed. Similar to previous studies, however, adjuvant chemotherapy was not associated with an overall survival benefit even after controlling for major confounders. Given the limitations of the present study, our results should not be regarded as definitive evidence but rather as hypothesis generating only. Analysis of data from international registries could aid in elucidating the optimal management of these patients. Due to the unclear benefit of current adjuvant chemotherapy in early disease, efforts should be focused on the development of novel therapies and evaluating these in international clinical trials. In the absence of definitive evidence decision to administer adjuvant chemotherapy should be individualized following discussion with the patient.
Conflicts of interest
No conflicts of interest to declare.
Research support
None.
Contributions
DN: conception, statistical analysis, crititical analysis, drafting/final editing.
EK: crititical analysis, drafting/final editing.
AH: crititical analysis, drafting/final editing.
RJ: crititical analysis, drafting/final editing.
RB: crititical analysis, drafting/final editing.
MM: crititical analysis, drafting, final editing.
NA: supervision, crititical analysis, drafting, final editing.
References
- Auranen A., Sundström J., Ijäs J., Grénman S. Prognostic factors of ovarian granulosa cell tumor: a study of 35 patients and review of the literature. Int. J. Gynecol. Cancer. 2007 Sep-Oct;17(5):1011–1018. doi: 10.1111/j.1525-1438.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Jamieson S., Fuller P.J. Tyrosine kinase inhibitors as potential therapeutic agents in the treatment of Granulosa cell Tumors of the ovary. Int. J. Gynecol. Cancer. 2015 Sep;25(7):1224–1231. doi: 10.1097/IGC.0000000000000479. [DOI] [PubMed] [Google Scholar]
- Mangili G., Ottolina J., Gadducci A. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br. J. Cancer. 2013 Jul 9;109(1):29–34. doi: 10.1038/bjc.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangili G., Ottolina J., Cormio G. Adjuvant chemotherapy does not improve disease-free survival in FIGO stage IC ovarian granulosa cell tumors: the MITO-9 study. Gynecol. Oncol. 2016 Nov;143(2):276–280. doi: 10.1016/j.ygyno.2016.08.316. [DOI] [PubMed] [Google Scholar]
- Meisel J.L., Hyman D.M., Jotwani A. The role of systemic chemotherapy in the management of granulosa cell tumors. Gynecol. Oncol. 2015 Mar;136(3):505–511. doi: 10.1016/j.ygyno.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.J., Jr., Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N. Ovarian cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2016 Sep;14(9):1134–1163. doi: 10.6004/jnccn.2016.0122. [DOI] [PubMed] [Google Scholar]
- Nasioudis D., Kanninen T.T., Holcomb K., Sisti G., Witkin S.S. Prevalence of lymph node metastasis and prognostic significance of lymphadenectomy in apparent early-stage malignant ovarian sex cord-stromal tumors. Gynecol. Oncol. 2017 May;145(2):243–247. doi: 10.1016/j.ygyno.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Jin K.L., Kim D.Y. Surgical staging and adjuvant chemotherapy in the management of patients with adult granulosa cell tumors of the ovary. Gynecol. Oncol. 2012 Apr;125(1):80–86. doi: 10.1016/j.ygyno.2011.12.442. [DOI] [PubMed] [Google Scholar]
- Pilsworth J.A., Cochrane D.R., Xia Z., Aubert G. TERT promoter mutation in adult granulosa cell tumor of the ovary. Mod. Pathol. 2018;31(7):1107–1115. doi: 10.1038/s41379-018-0007-9. (Jul) [DOI] [PubMed] [Google Scholar]
- Ray-Coquard I., Brown J., Harter P. GynecologicCancer InterGroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int. J. Gynecol. Cancer. 2014 Nov;24(9):S42–S47. doi: 10.1097/IGC.0000000000000249. [DOI] [PubMed] [Google Scholar]
- Ray-Coquard I., Morice P., Lorusso D., Prat J., Oaknin A., Pautier P., Colombo N. ESMO Guidelines Committee. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct 1;29(Supplement 4):iv1–iv18. doi: 10.1093/annonc/mdy001. [DOI] [PubMed] [Google Scholar]
- Schultz K.A., Harris A.K., Schneider D.T. Ovarian sex cord-stromal Tumors. J Oncol Pract. 2016 Oct;12(10):940–946. doi: 10.1200/JOP.2016.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meurs H.S., van Lonkhuijzen L.R., Limpens J., van der Velden J., Buist M.R. Hormone therapy in ovarian granulosa cell tumors: a systematic review. Gynecol. Oncol. 2014 Jul;134(1):196–205. doi: 10.1016/j.ygyno.2014.03.573. [DOI] [PubMed] [Google Scholar]
- Wang D., Xiang Y., Wu M. Is adjuvant chemotherapy beneficial for patients with FIGO stage IC adult granulosa cell tumor of the ovary? J Ovarian Res. 2018 Mar 27;11(1):25. doi: 10.1186/s13048-018-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.K., Fong P., Mesnage S. Stage I granulosa cell tumours: a management conundrum? Results of long-term follow up. Gynecol. Oncol. 2015 Aug;138(2):285–291. doi: 10.1016/j.ygyno.2015.05.011. [DOI] [PubMed] [Google Scholar]