Abstract
Paediatric traumatic brain injury (pTBI) is a leading cause of disability for children and young adults. Children are a uniquely vulnerable group with the disease process that occurs following a pTBI interacting with the trajectory of normal brain development. Quantitative MRI post-injury has suggested a long-term, neurodegenerative effect of TBI on the morphometry of the brain, in both adult and childhood TBI. Changes to the brain beyond that of anticipated, age-dependant differences may allow us to estimate the state of the brain post-injury and produce clinically relevant predictions for long-term outcome. The current review synthesises the existing literature to assess whether, following pTBI, the morphology of the brain exhibits either i) longitudinal change and/or ii) differences compared to healthy controls and outcomes. The current literature suggests that morphometric differences from controls are apparent cross-sectionally at both acute and late-chronic timepoints post-injury, thus suggesting a non-transient effect of injury. Developmental trajectories of morphometry are altered in TBI groups compared to patients, and it is unlikely that typical maturation overcomes damage post-injury, or even ‘catches up’ with that of typically-developing peers. However, there is limited evidence for diverted developmental trajectories being associated with cognitive impairment post-injury. The current review also highlights the apparent challenges to the existing literature and potential methods by which these can be addressed.
Keywords: Morphometry, Paediatric, Brain development, Traumatic brain injury, TBI
Abbreviation: STS, Superior temporal sulcus; TP, Temporal pole; FP, Frontal pole; PFC, Prefrontal cortex; OFC, Orbitofrontal cortex; TPJ, Temporoparietal junction; ACC, Anterior cingulate cortex; PCC, Posterior cingulate cortex; IPL, Inferior parietal lobule; SPL, Superior parietal lobule; dlPFC, Dorso-lateral prefrontal cortex; vmPFC, Ventro-medial prefrontal cortex; PPC, Posterior parietal cortex; IFG(−po), Inferior frontal gyrus (pars opercularis); MFG, Middle frontal gyrus; PARH, Parahipocampal gyrus; PCUN, Precuneus; pTRI, Pars triangularis; pORB, Pars orbitalis; pOPER, Pars opercularis; LOF, Lateral orbitofrontal gyris; MOG, Middle occipital gyrus; SFG, Superior frontal gyrus; STG, Superior temporal gyrus; MTG, Middle temporal gyrus; ITG, Inferior temporal gyrus; postC, Post central gyrus; preC, Pre central gyrus; FFG, Fusiform gyrus; OrbG, Orbital gyrus; LING, Lingual gyrus; CC, Corpus callosum; CSF, Cerebro spinal fluid; AG, Angular gyrus; OG, Occipital gyrus
Highlights
-
•
Paediatric TBI has lifelong consequences owing to alterations in brain morphometry.
-
•
Cross sectional differences (acute and late-chronic) are non-transient post-injury.
-
•
Longitudinal change altered by TBI; unclear if disrupted developmental trajectory.
-
•
Future challenges include sample heterogeneity, and effect of lesions on analyses.
-
•
Need to establish the role of TBI-related changes on long-term functional outcomes.
1. Introduction
Traumatic brain injury (TBI) is a leading cause of disability for both children and young adults (World Health Organization, 2006). Estimates of incidence are high for the 0–25 year old age group, with overall prevalence being estimated at approximately 30% of individuals experiencing a TBI by the time they reach young-adulthood (aged 25). Between the ages of 0–15 year olds there is an estimated incidence between 1.10 and 1.85 cases per hundred (McKinlay et al., 2008). Thus, many injuries occur to the still-developing brain (Wilde et al., 2012a). Unfortunately, the risk of poor neuropsychological and functional outcomes for those with mild to severe paediatric TBI (pTBI) is not clearly understood, especially due to the many factors upon which the likelihood of ongoing sequelae may be predicated (Babikian and Asarnow, 2009; Crowe et al., 2015; Irimia et al., 2017; Polinder et al., 2015).
In particular, the interaction between injury mechanisms and brain maturation in childhood may underpin the long-term neuropsychological effects of TBI. The impact and extent of ongoing neural changes associated with TBI is likely to have significant implications for children's later functioning. That is, the disease process that occurs following a pTBI necessarily interacts with the trajectory of normal brain development. Thus, the extent to which the injury alters that normal process may be an important factor to consider when trying to understand the apparent vulnerability of children's brains to early TBI and producing clinically relevant and reliable predictions for long-term outcomes. The current systematic review aims to investigate the interaction of injury and development by examining studies which have measured the effects of injury on the paediatric brain through MRI.
Alterations in brain structure occur after TBI but also as a part of normal development. TBI is defined as a neurological condition in which a traumatic external force to the brain leads to deformation of tissue, resulting in cellular or tissue damage which can cause transient or permanent functional impairment (Bigler, 2007, Bigler, 2016; Maxwell, 2012). TBI can result in the compromise of vasculature and physiology of the brain (Bigler, 2001) as well as resulting in trauma-induced cell loss (Bigler, 2013). This atrophy can vary in relation to injury factors such as mechanism, severity and pathology (Bigler, 2013; Cullen et al., 2011; Maxwell et al., 2010). This can be realised as changes to both brain volume (Bigler, 2016) and cortical thickness measures (Urban et al., 2017). Morphometric brain changes are also a feature of typical brain developing throughout childhood and adolescence (Batalle, Edwards and O'Muircheartaigh, 2018; Mills et al., 2016; Raznahan et al., 2011; Shaw et al., 2008). Non-linear trajectories of grey matter (GM) and white matter (WM) maturation are apparent in measures of volume (Giedd, 2004; Gilmore et al., 2007; Knickmeyer et al., 2008), gyrification patterning (Dubois et al., 2008) and cortical thickness (Herting et al., 2015; Nie et al., 2014; Whitaker et al., 2016), usually showing reductions over time, in line with models of synaptic pruning and myelination (Whitaker et al., 2016). This means that the morphometric atrophy and developmentally-inappropriate apoptosis (Urban et al., 2017; Wilde et al., 2005) due to pTBI is occurring in the context of an already changing, age and development-dependent brain (Bigler, 2016; Maxwell, 2012). Therefore, long term effects of injury are likely due to these interactions of age, neuroinflammation and neurodegenerative effects (Bigler, 2013; Johnson et al., 2013).
Bigler (2013) suggested that changes to the volumetrics of the brain, as measured by MRI, beyond that of anticipated age-dependant differences, may act as a biomarker of the state of health of the brain following pTBI. Previous reviews and investigations of quantitative MRI have also suggested a more long-term neurodegenerative effect of TBI on volumetry of the brain, in both adult and childhood TBI (Bigler, 2013; Cole et al., 2015; Keightley et al., 2014; Masel and DeWitt, 2010; Ross, 2011). Given the sensitivity of MRI-derived morphometry of the brain to typical development (as highlighted above), assessments of the brain using MRI post-TBI could prove to be key in understanding the potential long-term neurobehavioural and cognitive sequelae of pTBI (Bigler, 2013; Levin et al., 2008).
The brain can be uniquely vulnerable to the primary effects of TBI depending on the developmental stage at which the insult occurs (Anderson et al., 2011; Goldstrohm and Arffa, 2005; McCrory et al., 2004; Wilde et al., 2006). For example, the state of development of myelinated axons at the time of injury influences the response of tissues to brain injury (Adelson and Kochanek, 1998; Kochanek et al., 2000; Maxwell, 2012). Degeneration of nerve fibres following TBI occurs at a faster rate for unmyelinated versus myelinated cells (Maxwell, 2012; Staal and Vickers, 2011). Therefore, the early developing brain may be uniquely vulnerable in this way, with injuries occurring at different critical periods of development experiencing potentially very different functional trajectories (Anderson et al., 2011). In addition to potentially deleterious effects of a brain injury, it is also important to consider the potential of compensatory neural trajectories, through mechanisms such as neural plasticity, which may lead to restitution of function (Anderson et al., 2011; Bigler et al., 2010).
With this in mind, the current systematic review aimed to evaluate studies in which MRI-derived morphometry was measured in comparison to typical development, or longitudinally in paediatric patients following a TBI. In this vein, we chose to only include those studies that report on both patients and controls, thus excluding studies which only report on morphometry of patients. Whilst still informative, studies that just compare morphometry across injury severity cannot necessarily tease apart difference due to the injury and those expected differences due to typical development. A previous scoping review of studies investigated evidence of neurodegenerative change following TBI in children (Keightley et al., 2014). However, recent expansion of the literature in this field warrants a re-investigation.
The current systematic review aimed to answer the question; following paediatric brain injury, over a range of severities, does the morphology of the brain exhibit either i) longitudinal change and/or ii) differences compared to healthy controls. We then sought to determine whether there was evidence of a relationship between these changes or differences in morphology and cognitive outcomes.
2. Methods
2.1. Review strategy
Five sources were searched for the systematic review; Web of Science, Psycharticles, Cochrane Library, PubMED and Scopus. No limits on publication dates were applied. Three blocks of related search terms were used: block 1 for ‘paediatric’ terms, block 2 for ‘TBI’ terms and block 3 for ‘neuroimaging’ terms. Table 1 shows the full list of search terms for each block. Blocks were combined using the AND function for searching and terms within each block were combined with the OR function. The ‘neuroimaging’ block was left deliberately broad to capture studies where investigations of morphometry were carried out as a secondary outcome (i.e. alongside DTI investigations in Konigs et al. (2017)).
Table 1.
Blocks of search terms used to query publication databases in the review strategy.
| Block | Terms |
|---|---|
| Block 1 - Children | (pe$diatric OR infant OR child* OR Adolescen* OR youth OR teenage* OR young) |
| Block 2 - TBI | (TBI OR Trauma*-brain-injury OR brain-injur* OR brain NEAR/3 injury OR brain-insult OR DAI OR diffuse-axonal-injur* OR axonal-injur*) |
| Block 3 - Imaging | (MRI OR magnetic-resonance-imag* OR neuroimag*) |
Returned records from each database were combined and collated using Endnote (Tomson Reuters, 2013) and duplicate records were excluded. Publications were included in the synthesis if they; i) report on human participant data following non-penetrating TBI of any severity using; a. between groups analysis against an appropriate comparison group of either typically developing (TD) or orthopaedic injury (OI) controls or, b. within groups analysis investigating longitudinal change over time against controls, ii) presented isolated results of a paediatric sample (ages 0–19) at scanning, iii) presented original empirical quantification of the morphometry of the brain from T1-weighted (T1w) magnetic resonance images (MRI), and iv) written in English. Exclusion criteria included lack of control comparison group, reviews, conference abstracts, case studies, dissertations and/or book chapters.
Initial screening of abstracts for inclusion was conducted independently by two reviewers (DJK and KRE). Full-text articles of records identified by the two reviewers were independently assessed for inclusion by two reviewers (DJK and AGW) and consensus on eligibility was sought through discussion. Following identification of relevant records for inclusion, a further backwards (reference lists) and forwards (citations) search were conducted in the web of science platform to ensure identification of all relevant publications. This was done iteratively, i.e. new papers selected for inclusion were subjected to the same forwards and backwards searches, until no new publications were identified.
Information from the studies chosen for inclusion was systematically extracted into a pre-designed data pro-forma from full text articles by two reviewers (DJK and KRE). The following data were abstracted; citation details, country of origin, inclusion/exclusion criteria, design, study aim, MR imaging timepoint(s) relative to time of injury, patient sample (size, gender, injury severity, age at MRI, age at injury), control sample (size, gender, age at MRI, control comparison group (ie. TD (TD) vs OI (OI) samples)), neuroimaging characteristics (magnet strength, scan parameters, scale of region-of-interest (ROI; i.e. whole brain, ROI, voxel-wise), software, statistical design, morphometric measure(s) derived), results, and cognitive tests (tests administered, statistical approach, results). Where relevant and/or necessary, authors were contacted to request further information about the methodology or data.
2.2. Study quality
Assessment of study quality was conducted using the ‘Methodological Index for Non-Randomized Studies’ (MINORS; Slim et al., 2003) tool (full 12-item checklist). Assessment was conducted by a single reviewer (DJK). Studies were given a rating of 0 (not reported), 1 (reported but inadequately), 2 (reported adequately) or N/A if deemed to be not relevant to the study design. An average score was calculated across all non-N/A items to produce a continuous measure of quality from 0 to 2. High quality was identified as 1.51+, moderate as 1–1.5 and low as 0–0.99.
2.3. Data visualisation
Visualisation of dispersion of cross-sectional studies based upon sample characteristics of age at injury and injury-scan interval was achieved with the ggplot2 package in R (Wickham, 2009). This was to aid qualitative interpretation of the heterogeneity in the patient populations being tested. Details of the methodology used are included in appendix A.
2.4. Overlapping samples
Similar to Dennis et al. (2017a), we attempted to identify overlapping samples across the eligible studies presented for qualitative synthesis. Some studies clearly referenced other instances where the dataset was used in other published works. However, due to gaps in reporting of demographic characteristic or differences in the exact selection of participants used from a wider sample, we may have missed some of these overlaps. Despite data reuse, we report on all studies as the hypotheses tested were substantially different enough to warrant inclusion.
3. Results
3.1. Eligible studies
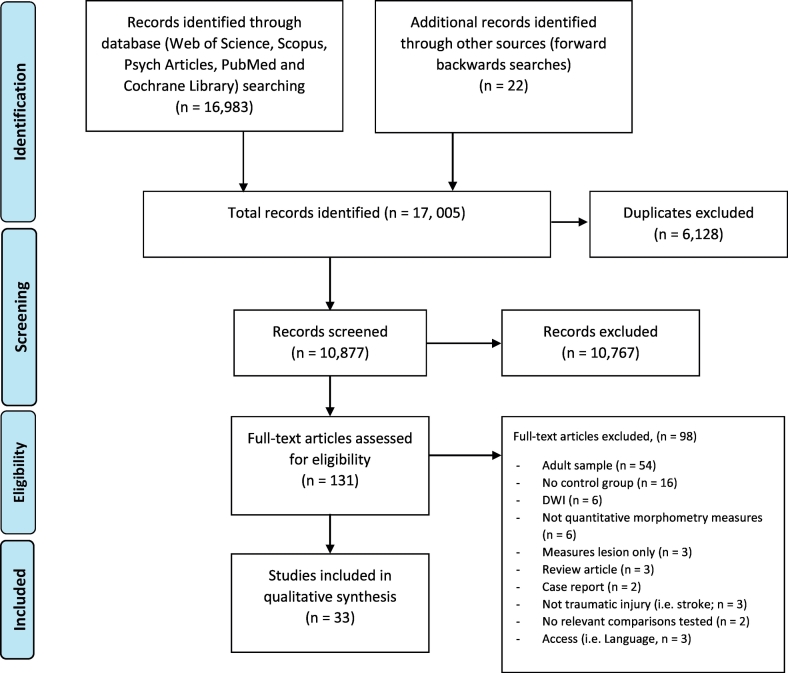
The search strategy (including forwards and backwards searches) was conducted on 15/11/17 and the initial search identified 17,005 articles over the five databases. Fig. 1 shows the PRISMA flowchart of this process. The iterative forwards and backwards searches concluded in two iterations (i.e. for the 2nd iteration, no new papers were identified).
Fig. 1.
PRISMA flowchart, modified from Moher et al. (2009)
Overall, 33 studies were deemed as meeting the inclusion criteria and were included in the narrative synthesis. Study characteristics of all eligible studies are reported in table 2 for cross-sectional studies and table 3 for longitudinal studies.
Of the included studies, two were rated as poor quality, 22 were rated as medium and nine as high. The individual ratings are reported in both Table 2 and Table 3. Many studies were rated low on items pertaining to items of ‘Unbiased assessment of study endpoint’ where there may have been a lack of blinding practices. Low ratings also occurred for all studies for the item of “Prospective calculation of the study size” due to lack of a-priori power calculations for sample size (Slim et al., 2003).
We were precluded from performing a formal quantitative meta-analysis because included studies utilised divergent approaches, both across dimensions of methods and anatomical partitions tested.
3.2. Cross-sectional studies
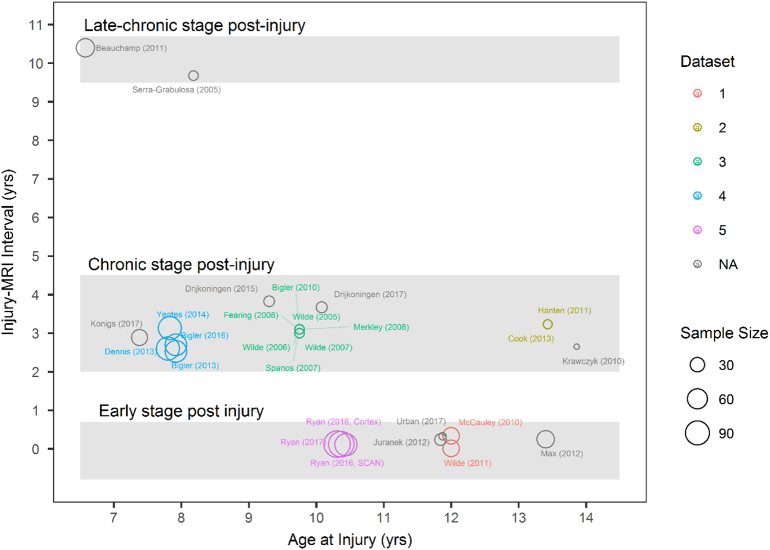
Twenty-seven studies investigated cross-sectional differences in morphology between paediatric TBI groups and controls. Fig. 2 plots the descriptive characteristics of these studies. Eligible studies sampled a range of ages at injury (meanpooled = 9.55,1 range of means = 6.58 years - 13.86 years). The distribution of pooled ages fits into a bell curve, with few investigating very early childhood and late adolescence. The sample sizes for the majority of studies are small, with the average sample size for eligible studies being 38.96 participants (SD = 29.74, range = 12–112). The majority of studies investigated samples that were scanned within the first five years post injury. The minimum mean time post injury for which MRI's were obtained was 4.0 days ±0.9 (Wilde et al., 2011), with the maximum mean being 10.4 years ±1.45 post injury (Beauchamp et al., 2011b). Table 2a lists all cross-sectional studies eligible for review and their sample demographics. Here we report on the most commonly replicated findings across studies. Table 2b. summarises the results from all individual, cross-sectional studies included in this section.
Fig. 2.
Descriptive plot of all eligible cross-sectional studies included for review. Studies are plotted based on mean age at injury of their sample against mean time between injury and MRI (years). Size of each point is proportional to the size of the TBI participant sample used in the study whilst the colour segregates clusters of studies which all use the same dataset of patients. To aid qualitative synthesis, studies were grouped into three major ‘bands’ of enquiry; i) an early stage (days to 1 year post-injury), ii) chronic stage (1–5 years post-injury) and iii) late chronic stage (9+ years post-injury). These band were qualitatively identified once studies where plotted in this way and are therefore based on the ‘natural’ grouping of the studies and therefore represent the current state of the literature.
Table 2a.
Study demographics for all cross-sectional studies included in the review.
| Reference | Sample and age (age at scanning; years, M ± SD) | Age at injury (years, M ± SD) | Time since injury (days/months/years, M ± SD) | Comparative Group and age at scan (years, M ± SD) | Study quality |
|---|---|---|---|---|---|
| Early stage (days to 1-year post injury) | |||||
| Urban et al. (2017), Canada | 13 Mild TBI, 12.2 years ±1.6, 13 M | Not reported | 120.69 days ±2.05 (range 90.07–240.27) | 14 TD controls, 12.6 years ±1.6, 14 M, (age and sex matched) | High (1.55) |
| Ryan et al. (2017), AUS | 57 Mild TBI, 10.80 years ±2.33, 13F, 44 M, 14 Mild complex TBI, 9.57 years ±2.43, 6F, 8 M, 26 Moderate TBI, 10.37 years ±2.58, 10F, 16F, 15 Severe TBI, 10.41 years ±3.10, 7F, 8 M |
Mild TBI, 10.67 ± 2.36, Mild complicated TBI, 9.47 years ±2.44, Moderate TBI, 10.33 years ±2.49, Severe TBI, 9.72 years ±3.01 |
42.28 days ± 29.53 | 43 TD controls, 10.41 years ±2.76, 19F, 24 M | High (1.73) |
| Ryan et al. (2016a), AUS | 67 Mild TBI, 10.54 years ±2.39, 19F, 48 M, 24 Moderate TBI, 10.37 years ±2.58, 10F, 14 M, 12 Severe TBI, 10.41 years ±3.10, 4F, 8 M |
Mild TBI, 10.44 ± 2.40, Moderate TBI, 10.26 years ±2.58, Severe TBI, 10.22 years ±3.08 |
42.29 days ± 29.53 | 34 TD controls, 10.41 years ±2.76, 13F, 21 M (matched on age, sex and SES) | High (1.73) |
| Ryan et al. (2016b), AUS | 53 Mild TBI, 13F, 40 M, 13 Mild complicated TBI, 5F, 8 M, 22 Moderate TBI, 9F, 13 M, 10 Severe TBI, 3F, 7 M (Age at scan not reported) |
Mild TBI, 10.69 ± 2.35, Mild complicated TBI, 9.65 years ±2.45, Moderate TBI, 10.37 years ±2.47, Severe TBI, 10.33 years ±3.25 | Mild TBI, 38.77 days ± 21.84, Mild complicated TBI, 37.62 days ± 17.91, Moderate TBI, 38.33 days ± 19.34, Severe TBI, 57.31 days ± 30.93 |
33 TD controls, 13F, 20 M (Age at scan not reported) | High (1.64) |
| Juranek et al. (2012), USA | 21 Moderate to Severe TBI, 12.08 years ±3.58 (range 6.5–16.4), 6F, 15 M | Not reported | Females 95.67 days ± 42.34, Males 84.47 days ± 39.73 | 20 OI Controls, 12.25 years ±2.79 (range 8–15.9), 7F, 15 M | High (1.64) |
| Max et al. (2012), USA | 27 Severe TBI, 7 Moderate TBI, 10 Complicated Mild, 14F, 30 M (some patients excluded for cortical thickness analysis due to quality) |
13.4 years ±3.0 | 3 months | 44 OI controls, 12.0 years ±2.6, 12F, 32 M | Medium (1.27) |
| Wilde et al. (2011), USA | 25 Severe TBI, 8 Moderate TBI, 7 Complicated Mild TBI, 12.1 years ±2.4 (range 7–17), 14F, 26 M |
Not reported | 4.0 days ± 0.9 | 41 OI controls, 13.5 years ±2.5 (range 7–17), 13F, 28 M | High (1.55) |
| McCauley et al. (2010), USA | 40 Moderate to severe TBI, 13.8 years ±2.5, 14F, 26 M | Range 7–17 years | 124.8 days ± 30.9 | 41 OI controls, 12.4 years ±2.4, 11F, 30 M | Medium (1.46) |
| Chronic stage (1–5 years post injury) | |||||
| Konigs et al. (2017), Netherlands | 20 Mild RF+ TBI, 10.5 years ±1.8, 7F, 13 M, 17 Moderate to Severe TBI, 10.0 years ±1.4, 7F, 10 M |
Mild TBI RF+ 7.7 years ±2.3, Moderate/Severe TBI 7.0 years ±1.9 |
Mild TBI RF+ 2.8 years ±1.1, Moderate/Severe TBI 3.0 years ±1.4 |
Traumatic injury controls, 10.2 years ±1.5, 15F, 12 M | Medium (1.33) |
| Drijkoningen et al. (2017), Belgium | 19 Moderate to Severe TBI 13 yea rs11month ± 3 ye ars1m (range 8y6m-18y11m), 10F, 9 M | 10 ye ars1month ± 3y3m | 3 years 8 months ± 3y3m | 30 TD controls, 14 years 10 months ± 2y2m (range 9y5m-17y3m), 17F, 13 M | Medium (1.18) |
| Bigler et al. (2016), Canada & USA | 82 Complicated Mild to Severe TBI, 72 scanned, refers to Bigler et al., 2013 for demographics | Not reported | 2.7 years | 61 OI controls, 52 scanned, refers to Bigler et al., 2013 for demographics (comparable on age and sex) | Poor (0.91) |
| Drijkoningen et al. (2015), Belgium | 18 Moderate to Severe TBI, 14 years 2 months ± 2 years 11 months, 9F, 9 M | range 3.0–15.6 | 3 years 10 months ± 3 years 3 month (range 0.3–10.8) | 30 TD controls, 14 years 2 months ± 2 years 11 months, 17F, 13 M | Medium (1.18) |
| Yeates et al. (2014), USA | 82 Complicated Mild to Severe TBI, 10.36 years ±1.50, 28F, 54 M | 7.83 years ±1.94 | range 12–63 months | 61 OI controls, 10.62 years ±1.68, 24F, 37 M | Medium (1.18) |
| Cook et al. (2013), USA | 15 Moderate to Severe TBI, 16.66 years ±2.22 (range 12.38–19.70), 7F, 8 M | 13.43 years ±2.35 (range 9.16–16.66) | 38.81 months ± 10.47 (range 11.32–52.96) | 13 TD controls, 16.87 years ±2.1 (range 13.19–19.94), 7F, 6 M | Medium (1.42) |
| Bigler et al. (2013), USA | 41 Complicated mild TBI, 10.67 years ±1.42, 32%F, 68%M, (only 32 used in quantitative neuroimaging), 11 Moderate TBI, 10.16 years ±1.35, 36%F, 64%M, (only 9 used in quantitative neuroimaging), 20 Severe TBI, 10.13 years ±1.61, 45%F, 55%M, (only 18 used in quantitative neuroimaging) |
Mild complicated TBI, 8.08 years ±1.87, Moderate TBI, 7.40 years ±1.74, Severe TBI, 7.85 years ±2.04 |
Mild complicated TBI, 2.59 years ±1.26, Moderate TBI, 2.77 years ±1.35, Severe TBI, 2.28 years ±1.14 |
61 OI controls, 10.66 years ±1.64, 42%F, 58%M | Medium (1.36) |
| Dennis et al. (2013), USA | 57 Mild to Moderate TBI, 10.5 years ±1.5, 19F, 38 M, 25 Severe TBI, 9.9 years ±1.5, 9F, 16 M | Mild to Moderate, 8.0 years ±1.9, Severe, 7.5 years ±2.1 | Mild to Moderate, 2.6 years ±1.2, Severe, 2.5 years ±1.2 | 61 OI controls, 10.6 years ±1.4, 24F, 37 M | Medium (1.36) |
| Hanten et al. (2011), USA | 15 Moderate to Severe TBI, 16.66 years ±2.22 (range 12.38–19.70), 7F, 8 M | 13.43 years ±2.35 (range 9.16–16.66) | 38.81 months ± 10.47 (range 11.32–52.96) | 13 TD controls, 16.87 years ±2.1 (range 13.19–19.94), 7F, 6 M | Medium (1.17) |
| Krawczyk et al. (2010), USA | 12 Moderate to severe TBI, 16.51 years ±2.14 (range 12.79–19.12, 5F, 7 M | Not reported | 2.65 years ±0.76 | 11 TD controls, 16.37 years ±1.89, 5F, 6 M | Medium (1.27) |
| Bigler et al. (2010), USA | 16 Moderate to Severe TBI, 12.9 years ±2.5 (range 9.0–16.8), 8F, 8 M | 9.75 years ±3.0 (range 3.7–13.8) | 3.1 years ±2.4 (range 1.0–10.1) | 16 TD controls, 12.8 years ±2.4 (range 9.0–16.4), 8F, 8 M | Medium (1.36) |
| Fearing et al. (2008) | 16 Moderate to Severe TBI, 12.9 years ±2.5 (range 9.0–16.8), 8F, 8 M | 9.75 years ±3.0 (range 3.7–13.8) | 3.1 years ±2.4 (range 1.0–10.1) | 16 TD controls, 12.8 years ±2.4 (range 9.0–16.4), 8F, 8 M (matched on ages, sex, ethnicity, handedness and maternal education) | High (1.64) |
| Merkley et al. (2008), USA | 16 Moderate to Severe TBI, 12.9 years ±2.5, 8F, 8 M (SAME AS Bigler et al., 2010) | 9.75 years ±3.0 | 3.1 years ±2.4 | 16 TD controls, 12.8 years ±2.4, 8F, 8 M | Poor (0.91) |
| Spanos et al. (2007), USA | 16 Moderate to Severe TBI, 12.9 years ±2.5 (range 9.0–16.8), 8F, 8 M (SAME AS Bigler et al., 2010) | Not reported | 3.1 years ±2.4 (range 1.0–10.1) | 16 TD controls, 12.8 years ±2.4 (range 9.0–16.4), 8F, 8 M (matched on ages, sex, ethnicity, handedness and maternal education) | Medium (1.27) |
| Wilde et al. (2007), USA | 16 Moderate to Severe TBI, 12 years 10 months ± 2 years 6 months (range 9–16 years 9 month), 8F, 8 M | Not reported | 3 years ±2 years 5 month (range 1-10 yr) | 16 TD controls, 12 years 10 months ± 2 years 5 months (range 9–16 years 5 months) | Medium (1.46) |
| Wilde et al. (2006), USA | 16 Moderate to Severe TBI, 12.9 years ±2.5 (range 9–16.8), 8F, 8 M | 9.75 years ±3.0 (range 3.7–13.8) | 3.1 years ±2.4 (range 1.0–10.1) | 16 TD controls, 12.8 years ±2.4 (range 9.0–16.4), 8F, 8 M (age and gender matched) | Medium (1.36) |
| Wilde et al. (2005), USA | 16 Moderate to Severe TBI, 12.9 years ±2.5 (range 9.0–16.8), 8F, 8 M | 9.75 years ±3.0 (range 3.7–13.8) | 3.1 years ±2.4 (range 1.0–10.1) | 16 TD controls, 12.8 years ±2.4 (range 9.0–16.4), 8F, 8 M | High (1.64) |
| Late chronic stage (9+ years post injury) | |||||
| Beauchamp et al. (2011b), AUS | 11 Mild TBI, 17.08 years ±3.77, 6F, 5 M, 26 Moderate TBI, 17.24 years ±3.60, 8F, 18 M, 12 Severe TBI, 16.34 years ±3.30, 4F, 8 M |
Mild TBI 7.04 years ±3.54, Moderate TBI 6.99 years ±3.18, Severe TBI 5.29 years ±2.77 | Mild TBI 10.04 years ±1.39, Moderate TBI 10.25 years ±1.44, Severe TBI 11.06 years ±1.44 | 20 TD controls (from NIH repository), 15.80 years ±1.94, 7F, 13 M (matched on age and gender) | Medium (1.17) |
| Serra-Grabulosa et al. (2005) | 16 Severe TBI, 17.88 years ±2.85, 2F, 14 M | 8.18 years ±3.65 | 9.68 years ±1.88 | 16 TD controls, 16.94 years ±3.21, 2F, 14 M, (Gender, age, education and parental SES matched) | Medium (1.09) |
Note. OI=Orthopaedic Injury, SES = socio-economic status
Table 2b.
Study findings for all cross-sectional studies included in the review.
| Reference | Magnet Strength | Methodology (software, statistical approach, anatomical-level) | Measure of interest | Variables controlled for | Findings |
|---|---|---|---|---|---|
| Early stage (days to 1 year post injury) | |||||
| Urban et al. (2017), Canada | 3 T | CIVET (GLM, Vertex-wise) | Cortical Thickness | None reported | Significantly thinner cortex found in TBI group compared to controls in the ldlPFC, right anterior IPL and posterior IPL (Cohen's d = 0.963, 1.152 and 1.002 respectively). |
| Ryan et al. (2017), AUS | 3 T | Freesurfer (MANOVA, Network ROI summed for DMN, CEN, SN, MN and MNEN) | Volume | Age at Scanning and ICV, SES and sex | Time between injury and MRI was not significantly related to any measure of global or regional volumes. Volume of DMN, CEN, SN, CCMN and MNEN all significantly differed as a function of group, with significant differences found between severe TBI and all other severity/control groups. vmPFC, PCC, IPL, hippocampus, dlPFC, PPC, TH, vlPFC, ACC, A, STS, TPJ, TP, IPL, iFG-po had reduced volumes in the severe group. |
| Ryan et al. (2016a), AUS | 3 T | FreeSurfer (ANCOVA, Network ROI summed for CSN) | Volume | Age and ICV | Significant effect of group on the volume of the total CSN, with smaller CSN for severe injury compared to control, moderate and mild groups. Of the CSN regions, only the severe group differed from controls in vmPFC, nucleus accumbens and ACC. |
| Ryan et al. (2016b), AUS | 3 T | Freesurfer (ANCOVA, global-brain and Network ROI summed for SBN) | Volume | ICV, age and SES | Across severity groups and controls, there was no multivariate effect of group on total brain, CC, WM and GM volumes. However, univariate effect of group was found on total WM volume and total SBN volume. SBN (specifically regions of STS, TP, mPFC, OFC, TPJ, cingulate, and insula) was significantly smaller only for severe TBI compared to controls. |
| Juranek et al. (2012), USA | 3 T | Freesurfer (ANOVA, ROI) | Volume | ICV | No main effect of TBI/OI group (or gender or hemisphere) on the volume of the amygdala or hippocampus. |
| Max et al. (2012), USA | 1.5 T | Freesurfer (MANCOVA, ROI) | Volume and Cortical Thickness | Age and ICV | No effect of group on structural volumes of cerebral GM and WM, cerebellar GM and WM, right and left frontal, right and left temporal, basal ganglia, amygdala, thalamus, corpus callosum and hippocampus. |
| Wilde et al. (2011), USA | 1.5 T | Freesurfer (GLM, ROI, Vertex-wise) | Volume and Cortical Thickness | Volume corrected for ICV, age at testing | Smaller volumes were found for bilateral frontal regions, as well as right MFG in the TBI group compared to controls (Cohen's f = 0.42, 0.37 and 0.35 respectively). Reported group effects on cortical thickness across regions of frontal lobe (pTRI, pORB, LOF, MOF, rostral rMFG, FP, SFG) and right temporal lobe (STG, MTG, ITG and FFG). |
| McCauley et al. (2010), USA | 1.5 T | Freesurfer (QDEC, vertex-wise) | Cortical Thickness | Age at testing | TBI showed significantly thinner cortex than controls bilaterally for anterior prefrontal (superior, middle, inferior, and medial cortices), temporal lobes and parahippocampal gyri, posterior cingulate, and parietal and precuneus regions. |
| Chronic stage (1–5 years post injury) | |||||
| Konigs et al. (2017), Netherlands | 3 T | SIENAX and FIRST (ANOVA, Global-brain, ROI) | Volume | Head size | Main effect of severity on the volume of total brain WM, but not GM. Mild and Moderate/Severe groups had significantly smaller WM volumes than controls (Cohen's d = −0.74 and − 0.80 respectively). No significant differences were found for the tested subcortical structures. |
| Drijkoningen et al. (2017), Belgium | 3 T | Freesurfer (ANOVA, Global-brain, ROI) | Volume | ICV | Total subcortical GM (not total cortical volume) was smaller in the TBI group compared to controls. No significant differences in cortical ROIs, but subcortically, thalamus, putamen, hippocampus and cerebellar cortex were significantly smaller in TBI. |
| Bigler et al. (2016), Canada & USA | 1.5 T | Freesurfer (QDEC, vertex-wise) | Cortical Thickness | Sex, Age | No significant effect of group on vertex-wise cortical thickness. Age was significantly related to decreasing cortical thickness, with distribution of age-related changes being similar for TBI and OI. |
| Drijkoningen et al. (2015), Belgium | 3 T | SPM8, SUIT toolbox, DARTEL, MRIcron (GLM, Global-brain, Voxel-wise) | Volume | ICV | No significant differences in total ICV. Reduced volume in TBI compared to OI for global infratentorial GM and WM. Cerebellar volume as a percentage of total ICV was significantly lower in TBI. A significant cluster of reduced WM volume in the infratentorial region for TBI compared to OI (but not for GM). |
| Bigler et al. (2013), USA | 1.5 T | Freesurfer and VBM (voxel-wise) | Volume | None reported | Smaller CC volumes were found for severe injury compared to controls in anterior, mid-anterior, central, mid-posterior and posterior regions and total CC as well as total brain, total GM, total WM, thalamus, basal ganglia, amygdala and hippocampus. Posterior and anterior CC also showed reductions compared to controls in moderate and mild-complicated injuries. Severe injury group also had greater total ventricular volume and ventricle-to-brain ratio than controls. VBM showed largest significant reductions for severe injury compared to controls in CC, ventral frontal, basal forebrain regions and lateral ventricles. |
| Dennis et al. (2013), USA | 1.5 T | Freesurfer (MANOVA, Network ROI summed for DMN, CEN, SN, MN and MNEN) | Volume | None reported | No significant differences in total ICV. Significant reductions in DMN, CEN, SN, MN and MNEN network volumes was found for severe TBI compared to OI and mild-moderate. Severe TBI group had significantly reduced volumes, compared to OIs, in PCC, HF, PPC, TH, I, A and STS. |
| Bigler et al. (2010), USA | 1.5 T | Freesurfer and ANALYZE (ANCOVA, ROI) | Volume | Age at testing | TBI had reduced volume compared to controls in amygdala, brain stem, globus pallidus and thalamus, regardless of method (Freesurfer and ANALYZE). Putamen only smaller in TBI group when using ANALYZE method. |
| Fearing et al. (2008) | 1.5 T | ANALYZE (MANCOVA and GLM, ROI) | Volume | Age at Scanning and ICV | TBI group showed reduced thalamic GM (but not WM) compared to controls (Cohen's d = 1.050), as well as total midbrain volume (Cohen's d = 1.91) and also its constituent parts, the tectum and tegmentum (d = 0.999 and 1.074 respectively). The pons, medulla and total brainstem did not significantly differ. |
| Merkley et al. (2008), USA | 1.5 T | Freesurfer (ANCOVA, ROI) | Cortical Thickness | Age and gender | Significantly reduced cortical thickness in TBI compared to controls was found for lSFG, rpOPER, rFP, bilateral rostral MFG, bilateral caudal MFG, lpreC, bilateral supramarginal, lMTG, bilateral ITG, lFFG, bilateral postC, bilateral SPL, bilateral IPL, and bilateral precuneus regions. |
| Spanos et al. (2007), USA | 1.5 T | ANALYZE (GLM, ROI) | Volume | ICV | TBI group showed reduced volumes compared to controls in cerebellar WM and GM (even after removing patients with focal cerebellar lesions. A significant interaction between groups was found, in which a significant positive correlation between DLPFC/cerebellum was found in the TD but not in the TBI group. |
| Wilde et al. (2007), USA | 1.5 T | ANALYZE (ANCOVA, ROI) | Volume | Age and ICV | The TBI group showed volumetric reductions in bilateral hippocampus, amygdala and globus pallidus regions (Cohen's d = 2.140, 0.801 & 0.775 respectively) compared to controls, but not putamen and caudate. |
| Wilde et al. (2006), USA | 1.5 T | Picture Archival System Software (ANOVA, ROI) | Volume | None | Showed the anterior-commissure volume was significantly smaller in the TBI group compared to controls. |
| Wilde et al. (2005), USA | 1.5 T | ANALYZE (MANCOVA, ANCOVA, global brain and regional) | Volume | Age at testing | TBI group showed significantly reduced global brain measures of total brain and GM volumes, as well as increased ventricle to brain ratio, ventricle volume, whole brain, temporal and frontal CSF compared to controls. Regional reductions in the TBI group were found in lateral frontal WM, as well as ventromedial frontal, superior media frontal and temporal GM/WM. |
| Late chronic stage (9+ years post injury) | |||||
| Beauchamp et al. (2011b), AUS | 1.5 T | FSL and ANALYZE (ANCOVA, Global brain and ROI) | Volume | Age at Scanning and ICV | A significant effect of group (TBI vs control) was found for total CSF, GM and WM volumes (Partial η2 = 0.54, 0.41 and 0.17 respectively). Controls had less CSF and greater total GM and left hippocampus volume than all severity groups. Only severe injuries had smaller WM than controls. Right amygdala significantly bigger in controls than mild and moderate injury. |
| Serra-Grabulosa et al. (2005) | 1.5 T | ANALYZE (t-test, ROI and global-brain) | Volume | None reported | The TBI group showed significant reductions in global WM (specifically frontal WM) volume and increases in CSF volume. No significant differences were found in total or frontal GM. Significant reductions were found in bilateral hippocampal volume in TBI compared to control. |
Note. GLM = general linear model, ICV = Intra-cranial volume, OI=Orthopaedic Injury, QDEC = Query Design Estimate Contrast, ROI = Region of interest, SES = socio-economic status, VBM = voxel-based morphometry.
At the early stage post-injury differences were found for total WM (Ryan et al., 2016b) and total GM (Ryan et al., 2017), but these findings were not reliably replicated across these studies. When comparing summed volume of ROIs comprising major brain networks (default mode network (DMN), central executive network (CEN), salience network (SN), cerebro-cerebellar mentalising network (CCMN) and mirror neuron empathy network (MNEN), cortico-striatal network (CSN) and social brain network (SBN); Ryan et al., 2016a, Ryan et al., 2016b, Ryan et al., 2016c; Ryan et al., 2016a, Ryan et al., 2016b; Ryan et al. (2017)) as well as bilateral frontal regions (Wilde et al., 2011) smaller volumes were observed in the TBI groups compared to controls.
At the chronic stage post-injury, decreases to total brain and total GM (Bigler et al., 2013; Wilde et al., 2005), total WM (Bigler et al., 2013; Konigs et al., 2017), and increases to ventricles and ventricle to brain ratio were found in the TBI group (Bigler et al., 2013; Wilde et al., 2005). Specifically, whilst regional differences were understudied, volume differences were found in frontal and temporal GM/WM (Wilde et al., 2005) as well as the DMN, CEN, SN, MNEN and CCMN networks (Dennis et al., 2013), replicating findings from the early stage post-injury. Large WM tracts were also impaired across both corpus callosum (CC), and the anterior commissure (Bigler et al., 2013; Wilde et al., 2006). Commonly, replicated findings suggest that the thalamus, amygdala, hippocampus, putamen, global pallidus and cerebellar regions were smaller in volume cross-sectionally compared to controls (Bigler et al., 2013; Bigler et al., 2010; Dennis et al., 2013; Drijkoningen et al., 2017; Drijkoningen et al., 2015; Fearing et al., 2008; Spanos et al., 2007; Wilde et al., 2007).
This period post-injury was specifically characterised by studies which had a mean time since injury between 2.53 years ±1.24 (Bigler et al., 2013) and 3.83 years ±3.25 (Drijkoningen et al., 2015). However, the studies in this band of enquiry showed much greater variability in the time between injury and MRI at an individual study level. For example, Drijkoningen et al. (2015) reported a mean time since injury of 3.83 years ±3.25 but the reported range was 0.3 to 10.8 years post injury. Similarly, Bigler et al. (2010) reported a mean time post injury of 3.1 years ±2.4, but the range was 1.0 to 10.1 years. Thus, not all participants reported in this band of chronic stage post-injury are within this period, due to this large within-study variability. Given this large dispersion of time between injury and MRI/testing within-studies, we suggest greater caution when interpreting these findings and suggest that they may not be specific to the reported time post-injury.
It is pertinent to note that, of the cross-sectional studies included in the current review, only nine studies reported the range of time between injury and MRI/testing across time bands, and thus variability of time between injury and MRI may be greater than that reported in this review. In addition, even in studies that did not report the range of time between injury and MRI, standard deviations of this injury/MRI interval are particularly high.
At the late chronic stage, total cerebrospinal fluid (CSF) volume was greater for TBI patients (Beauchamp et al., 2011b; Serra-Grabulosa et al., 2005), total GM was reduced (Beauchamp et al., 2011b) and these changes where independent of severity, these differences were significant for all TBI severity sub-groups. However, total WM was found to be significantly lower only for severe injury group compared to controls (Beauchamp et al., 2011b; Serra-Grabulosa et al., 2005). At the ROI level, studies reliably found hippocampal volume differences across studies with the injury group showing smaller volumes (Beauchamp et al., 2011b; Serra-Grabulosa et al., 2005).
Morphometric investigations of the brain post-TBI were not limited to the volume of cortical regions, but also the cortical thickness. There were fewer investigations of cortical thickness, but early post-injury studies showed regions of dorso-lateral prefrontal cortex (dlPFC; McCauley et al., 2010; Urban et al., 2017; Wilde et al., 2011) and other prefrontal regions (McCauley et al., 2010; Wilde et al., 2011) as well as superior temporal sulcus (STS; McCauley et al., 2010; Wilde et al., 2011), cingulate regions (McCauley et al., 2010) and regions of the inferior parietal lobule (iPL; Urban et al., 2017) to be significantly thinner in the TBI group compared to controls. However, these differences were not replicated at a later timepoint post injury (Bigler et al., 2016) This is not to say that these differences have ‘recovered’ over time (due to the cross-sectional nature of this evidence) but more likely due to differences in methodology and samples.
The evidence presented from these cross-sectional studies suggests that frontal, temporal and parietal regions areas are commonly (and persistently over time) impacted following a pTBI (Wilde et al., 2005). However, it is important to note that the regions identified by individual studies span multiple regions of the cortex and subcortical regions, suggesting in fact that the effects of pTBI can be seen diffusely across the brain. This is specifically highlighted in studies investigating summed ROI volumes across distributed brain networks (Dennis et al., 2013; Ryan et al., 2016a, Ryan et al., 2016b, Ryan et al., 2016c; Ryan et al., 2016a, Ryan et al., 2016b; Ryan et al., 2017).
However, some studies used innovative methodologies to investigate the diffuse nature of morphometric brain changes post-injury. Spanos et al. (2007) took an innovative approach to investigate volumes of the cerebro-cerebellar network (dlPFC, thalamus, pons and cerebellum) by estimating correlations between volumes of these structures. Significant correlations were found between volumes of the thalamus/dlPFC and the pons/cerebellum in both groups. A significant interaction between groups was found, in which a significant positive relationship between dlPFC/cerebellum was found in the TD but not in the TBI group. Drijkoningen et al. (2017) investigated the statistical relationship between regional subcortical-atrophy. Volume deviation score was calculated with a linear regression of subcortical volumes against intracranial volume (ICV) in the control group, with the linear model providing a predicted volume for regions given an ICV. Thus, the deviation score for any given patient was actual volume minus predicted volume. Correlations were assessed between the volume deviation scores across the TBI group. Moderate to very strong positive correlations were found for these relationships, with significant correlations found between deviation scores for multiple, subcortical regions. This interrelation between deviation scores suggests a diffuse pathology that affects wider subcortical volume, rather than specific areas (Drijkoningen et al., 2017).
3.3. Longitudinal studies
Whilst there were significantly fewer studies eligible for inclusion that incorporated a longitudinal design compared to those who utilised a cross-sectional design, these longitudinal studies here showed that there were widespread differences in both volume and cortical thickness. Similarly small sample sizes were seen in the longitudinal studies as the cross-sectional studies with the average sample size for eligible studies being 20.83 (SD = 8.03, range = 10–36). A narrow distribution of age at scanning was seen (initial timepoint: meanpooled = 13.913, 1range of means = 12.9 years–16.0 years), with no studies looking at the very extremes of childhood. However, it is important to note that this does not refer to the age at injury, but the age at MRI scanning. This is because all six longitudinal studies did not report the mean age at which the injury occurred. Table 3a describes the sample demographics of each study, see Table 3b for the results of the study.
Table 3a.
Study demographics for all longitudinal studies included in the review.
| Reference | Sample and age (age at scanning; years, M ± SD) | Age at injury (years, M ± SD) | Longitudinal Timepoints (days/months/years, M ± SD) | Comparative Group and age at scan (years, M ± SD) | Quality Rating |
|---|---|---|---|---|---|
| Dennis et al. (2017b), USA | 11 TBI-slow IHTT, Timepoint 1: 14.1 years ±1.9, 3F, 8 M, Timepoint 2: 15.0 years ±2.0, 10 TBI-normal IHTT, Timepoint 1: 16.0 years ±2.6, 2F, 8 M, Timepoint 2: 17.0 years ±2.8 |
Not reported | Timepoint 1, TBI-slow IHTT 50.6 days ± 5.9, TBI-normal IHTT 52.5 days ± 9.7, Timepoint 2, 12 approximately 12 months post-timepoint 1 (Not reported) |
26 Healthy Controls, Timepoint 1: 14.5 years ±3.0, 11F, 15 M, Timepoint 2: 15.6 years ±3.0 |
Medium (1.33) |
| Wu et al. (2017), USA | 10 Sports concussion mTBI, Timepoint 1: 14.58 years ±1.5, 4F, 6 M, Timepoint 2: Not reported |
Not Reported | Timepoint 1, <96 h post injury (range 21-116 h), Timepoint 2, 3 months post injury (range 84-143 days) |
12 sports-related OI, 14.06 years ±1.63, 3F, 9 M (only 9 included for morphometric analysis at T1 and 12 at T2), 12 TD controls (no age or gender reported, only received single MRI) |
Medium (1.25) |
| Dennis et al. (2016), USA | 36 (18 completed longitudinal testing) Moderate-Severe TBI, Timepoint 1: 14.1 years ±2.7, 10F, 26 M, Timepoint 2: 15.9 years ±2.6, 5F, 13 M (some participants were tested at only timepoint 1, others at only timepoint 2) |
Not reported | Timepoint 1, post-acute phase (1–6 months post-injury), Timepoint 2, chronic phase (13–19 months post injury) |
35 (22 completed longitudinal testing) TD controls, Timepoint 1: 14.8 years ±2.8, 12F, 23 M, Timepoint 2: 16.2 years ±3.2, 7F, 15 M (matched for age, sex, and educational level) |
Medium (1.17) |
| Mayer et al. (2015), USA | 15 (11 completed longitudinal testing) Mild TBI, Timepoint 1: 13.47 years ±2.20, 2F, 13 M, Timepoint 2: Not reported |
Not reported | Timepoint 1, within 21 days post injury (TBI 15.87 days ± 4.93), Timepoint 2, 4 months post injury (TBI 127.82 days ± 14.60) |
15 (12 completed longitudinal testing) TD controls, Timepoint 1: 13.40 years ±1.84, 3F, 12 M (age and education matched), Timepoint 2: Not reported |
High (1.58) |
| Wilde et al. (2012b), USA | 13 Severe TBI, 4 Moderate TBI, 3 Complicated Mild TBI, Timepoint 1: 13.6 years ±2.9 (range 8.2–17.5), Timepoint 2: 14.8 years ±2.9 (range 9.3–18.7), 9F, 11 M |
Not reported | Timepoint 1, 3 months post injury (TBI 4.0 months ± 1.0, OI 4.7 months ± 2.6), Timepoint 2, 18 months post injury (TBI 18.5 months ± 3.6, OI 18.4 months ± 4.2) |
21 OI controls, Timepoint 1: 12.3 years ±2.5 (range 7.4–16.7), Timepoint 2: 13.2 years ±2.6 (range 8.8–18.0), 6F, 15 M |
Medium (1.33) |
| Wu et al. (2010), USA | 3 Complicated Mild TBI, 4 Moderate TBI, 16 Severe TBI, Timepoint 1: 12.9 years ±3.2 (range 7.8–17.2), 8F, 15 M, Timepoint 2: Not reported |
12.9 years ±3.2 | Timepoint 1, 3 months post injury (TBI 4.0 months ± 0.9, range 2.5–5.3, OI 4.2 months ±1.0, range 2.7–7.1), Timepoint 2, 18 months post injury (TBI 18.9 months ±1.5, range 16.7–22.6, OI 18.8 months ± 1.3, range 16.6–20.9) |
25 OI controls, Timepoint 1: 11.8 years ±2.7 (range 7.1–16.3), 7F, 18 M, Timepoint 2: Not reported |
Medium (1.50) |
Note. CT = computed tomography, HTT = Inter-hemispheric transfer time, OI=Orthopaedic Injury.
Table 3b.
Study findings for all longitudinal studies included in the review.
| Reference | Magnet Strength | Methodology (software, statistical approach, anatomical-level) | Measure of interest | Variables controlled | Findings |
|---|---|---|---|---|---|
| Dennis et al. (2017b), USA | 3 T | Tensor based morphometry (linear regression, voxel-wise) | Volume | Age at scanning, sex, scanner, and ICV | Longitudinal regional volume changes differed significantly across a number of clusters between TBI-slow, TBI-normal and controls. Over time, TD children showed significant volume increases, but TBI-slow group showed mostly decreases across regions of splenium, CC, capsule and claustrum, posterior thalamic radiation and hypothalamus. The TBI-normal group had significantly greater reductions in including SFG, parietal operculum, PCC, thalamus, MFG, putamen, MTG, postC, internal OG, SFG and insula compared to controls and increases in internal capsule. TBI-slow showed greater volume reduction whereas TBI-normal showed longitudinal increase in internal capsule, thalamus and superior corona radiata. TBI-slow group had significantly greater atrophy than TBI-normal group in regions of SFG, inferior OG, SPL, cingulate, MFG, cuneus, PCUN and parietal operculum. |
| Wu et al. (2017), USA | Not reported | Freesurfer (Between and paired t-test, ROI) | Volume | ICV | No cross-sectional or longitudinal differences in volume between TBI, and OI/TD groups. |
| Dennis et al. (2016), USA | 3 T | Tensor based morphometry (linear regression, voxel-wise) | Volume | Age at scanning, sex, scanner, and ICV | Longitudinal effects not statistically assessed. At timepoint 1 significantly greater volume for the lateral ventricles in TBI (indicative of CSF expansion). Lower volumes found compared to controls in left LING, bilateral PCG, right FFG, right STG, left thalamus, left PCUN, left SFG, left OG, right PCG, cingulum, and parahippocampal gyrus. At timepoint 2 significantly increased ventricle size for the TBI group and smaller volumes for the TBI group compared to controls bilateral LING, right MTG, bilateral OrbG, right FFG, ACC and mid-cingulate cortex, left SPL, and left preC. However, greater volumes in TBI group in left IFG, and the bilateral posterior thalamic radiations, right superior longitudinal fasciculus, right OG, right AG, and right SPL. |
| Mayer et al. (2015), USA | 3 T | Freesurfer longitudinal pipeline (GLM, MANOVA, Vertex, ROI) | Volume and Cortical Thickness | None reported | No significant group differences in vertex-wise cortical thickness or volume of hippocampus and thalamus at timepoint 1. No significant effect of group on subcortical volume change. TBI group showed greater atrophy over time in the left SFG and MFG, left MTG, left postC running into IPL, left IPL, left cuneus, left MOG, right SFG and MFG. |
| Wilde et al. (2012b), USA | 1.5 T | Freesurfer longitudinal pipeline (GLM, Vertex) | Cortical Thickness | None reported | At timepoint 1, smaller cortical thickness in TBI group compared to controls in bilateral rostral, MFG, SFG, lateral and medial OFC, anterior cingulate, and FP and unilaterally in the right pORB, right pTRI and right pOPER and at timepoint 2, bilateral rostral MFG, caudal MFG, FFG and lingual regions, and unilateral left SFG, preC, PCUN, isthmus cingulate, SPL and IPL, right pTRI, pORB, and lateral OFC. Longitudinally TBI group showed significant thinning in many cortical areas, with sparing of this effect seen in bilateral TP, and medial aspects of the frontal lobes, cingulate and left FFG. Significant longitudinal thinning in TBI versus OI group in SPL and right paracentral regions, but increase in medial OFC, bilateral cingulate, and right lateral OFC. |
| Wu et al. (2010), USA | 1.5 T | Freesurfer longitudinal pipeline (GLM, t-test difference score, ROI) | Volume | ICV | At timepoint 1, TBI showed smaller midanterior CC compared to OI. Total CC volume significantly smaller in TBI group at timepoint 2 (but not timepoint 1) and anterior, midanterior, central and mid posterior CC. Longitudinally, the total, anterior, midanterior, midposterior, and posterior regions of the CC reduced in volume for the TBI group compared to slight increases in volume for OI group. |
Note. GLM = general linear model, ICV = Intra-cranial volume, IHTT = Inter-hemispheric transfer time, OI=Orthopaedic Injury, ROI = Region of interest,
Differences in volume between timepoint one and two consistently changed as a function of group (patient vs control) across common regions of dlPFC (Dennis et al., 2017b; Mayer et al., 2015), STS (Dennis et al., 2017b; Dennis et al., 2016; Mayer et al., 2015), posterior parietal cortex (PPC) extending into iPL, cingulate regions (Dennis et al., 2016; Mayer et al., 2015; Wilde et al., 2012b), and hypothalamic, thalamic and CC regions (Dennis et al., 2017b; Wu et al., 2010). In these regions patients were more likely to show reductions or atrophy greater than that of the control group over the same time period, indicating that the rate of change in volume/cortical thickness differs between groups. However, whilst Dennis et al. (2016) and Wilde et al. (2012b) found significant differences between patients and controls in morphometry at both timepoint one and two, Wu et al. (2010) found differences at only timepoint two.
Interestingly, Dennis et al. (2017b) used a longitudinal design (upon the same data as Dennis et al. (2016)) to investigate two sub-groups of the original moderate/severe injury group. Patients were divided based upon inter-hemispheric transfer time (IHTT); those that were slower than normal (TBI-slow) and those with normal IHTT (TBI-normal). Longitudinal regional volume changes differed significantly across a number of regional-clusters for pairwise comparisons of TBI-slow, TBI-normal and controls. When comparing TBI-slow and TD control groups, over time TD children showed significant increases in volume in regions, whereas the TBI-slow group mostly showed decreases. This was across mostly WM regions of splenium, CC (two clusters), external/extreme capsule and claustrum, posterior thalamic radiation and hypothalamus. The TBI-normal group had significantly greater reductions in a number of GM regions compared to controls, including superior frontal gyrus (SFG, four clusters), parietal operculum, PCC (three clusters), thalamus, middle frontal gyrus (MFG), putamen, middle temporal gyrus (MTG), post central gyrus (postC), internal- occipital gyrus (OG), SFG and insula. However, the TBI-normal group had two clusters of greater longitudinal volume change compared to controls in the internal capsule. When comparing the two TBI subgroups, TBI-slow showed more longitudinal reduction whereas the TBI-normal showed longitudinal increase in mostly WM tissue regions of internal capsule, thalamus and superior corona radiata. However, the TBI-slow group had significantly less longitudinal growth/greater atrophy than the TBI-normal group in mostly GM regions of SFG (four clusters), inferior- OG, superior parietal lobule (SPL), cingulate (two clusters), MFG, cuneus, precuneus (PCUN) and parietal operculum. Whilst the direction of causality remains unclear, this suggests potential relationships between both structural and functional changes.
Some studies utilise statistical methods controlling for effects such as total intracranial volume (Dennis et al., 2017b; Dennis et al., 2016; Wu et al., 2017; Wu et al., 2010) or age at scanning (Dennis et al., 2017b; Dennis et al., 2016) as proxies for the stage of brain development, or reported using age-matched samples (Dennis et al., 2016; Mayer et al., 2015). Theoretically this would remove variance in morphometry due to the age-related development of the cortex, and group differences that survive removal of this covariance would be where the changes in morphology post-TBI are exceeding or fall short of typical development. However, in the current literature, when controlling for these proxies of development, the reported effects are not consistent across studies, with some studies still finding an interaction between group and timepoint on morphometry (Dennis et al., 2017b; Wu et al., 2010) and others not (Wu et al., 2017). Although it is interesting to note that Wu et al. (2017) investigated a cohort of mild TBI due to sports concussion. This potential lack of consensus amongst studies limits assessment of whether or not the effects of injury are truly beyond that of expected developmental differences over time and warrants further study.
3.4. Linking morphometry to cognition in TBI
Of the eligible papers, 16 investigated the associations between morphometry after a TBI and cognitive/neuropsychological outcomes across multiple domains. Some studies investigated outcome measures that were not directly linked to cognitive ability (e.g. postural control (Drijkoningen et al., 2017; Drijkoningen et al., 2015)). Although we accept that these outcome measures are important and may be related to variation in cognition (such as postural control), we only review those outcomes that are direct measures of cognition (such as IQ). The results of these studies are summarised in Table B.1 and are divided into the cognitive domains assessed. This table shows clearly the disparity in methods, measures and regions tested, thus highlighting the difficulty with which any significant qualitative synthesis can be achieved.
There were many ways in which studies designed analyses to probe brain-behaviour relationships post injury, and these are described in the design column of table 4. The majority of studies used a correlational design, and did not model group differences, but instead looked at whole sample (across patients and controls) or just correlations within the TBI group. Other studies took a cross-sectional approach but varied in how vigorously they probed the cross-sectional differences between groups. In Table B.1, cross-sectional (comparative) refers to studies which statistically investigated brain-behaviour relationships within both TBI and control groups but only qualitatively compared these relationships between the two groups, whereas cross-sectional (statistical) refers to those studies that statistically modelled differences in these brain-behaviour relationships between groups (for example modelling the main effect of group in a GLM of volume by performance relationship). Of the studies that used a cross-sectional design to probe these links between morphometry and cognition, the majority used the comparative approach.
The most common domain that was assessed was working memory, including a number of validated normed (i.e. WISC-III digit span test) and non-normed tests (i.e. Sternberg Item recognition tests (SIRT)). Reduced performance in the TBI group was seen repeatedly in relation to reduced volumes of parietal regions and cortical thickness of parietal and frontal regions (Merkley et al., 2008; Urban et al., 2017; Wilde et al., 2011). However, it is unclear if there are any meaningful differences in actual performance between patients and controls in working memory performance across the studies included in this review. Studies found significant reductions in performance for patients (Konigs et al., 2017), limited interaction effects of group and performance on certain task variables (Urban et al., 2017; Wilde et al., 2011) or did not report performance differences at all (Fearing et al., 2008; Merkley et al., 2008). Thus, without meaningful differences in performance it is difficult to realise the potential utility of these brain-behaviour relationships.
Multiple studies used a battery of tests to assess the relationship between cognitive (understanding false beliefs), affective (interpreting emotive communication) and conative (understanding social communication which influences others thinking i.e. irony) aspects of ToM morphometry after TBI (Dennis et al., 2013; Ryan et al., 2017; Yeates et al., 2014). Cognitive, conative and affective ToM abilities were all positively associated with total GM volume and negatively associated with ventricle to brain ratio (Yeates et al., 2014). Specifically cognitive ToM was related to total volume of the CCMN and affective to the SN (Ryan et al., 2017) Conative ToM was predicted by a model of DMN, CEN and MNEN volume (Dennis et al., 2013) and total MNEN volume (Ryan et al., 2017). Of the decomposed regional volumes of these networks only posterior cingulate/retrosplenial cortex and hippocampal formation remained significant following multiple comparison corrections (Dennis et al., 2013). VBM only found significant clusters of brain-behaviour relationship in the OI not the TBI group (Yeates et al., 2014).
Significant brain-behaviour relationships between morphometry and cognition post-injury were also found for other domains of executive functioning (Wilde et al., 2012b), anticipating social consequences (Cook et al., 2013), social problem solving (Hanten et al., 2011), and analogous reasoning (Krawczyk et al., 2010). Across two studies, Dennis and colleagues (Dennis et al., 2017b; Dennis et al., 2016) investigated the potential brain-behaviour relationships using a summary score of overall cognitive function (comprising a wide number of domains of processing speed, working memory, verbal learning, short term memory and attention switching), finding significant relationships both at a cross sectional and longitudinal basis, in the same sample. Domains of processing speed (Wu et al., 2010), IQ or verbal learning (Konigs et al., 2017) showed no significant relationships with morphometry. However, there were only a limited number of studies that measured each of these cognitive outcomes. As many of these studies had limited sample sizes and studies with significant findings utilised mass univariate approaches (i.e. voxel/vertex-wise analysis), there is a heightened risk of Type 1 errors even when controlling for multiple comparisons. Therefore, it is important to look at convergence of results across multiple studies to determine whether findings are reliable or not.
4. Discussion
The current review has found some consistency in the differences and changes to the brain following a TBI during childhood, with most findings reporting reduction of volume and cortical thickness at a whole brain and regional level compared to TD peers' between and across timepoints. This consistency across studies was found despite the considerable heterogeneity in the resulting neuropathology following a TBI (Dennis et al., 2017a), and the additionally complexity introduced by the fact that the injury occurs within the context of developing paediatric brain.
Overall, cross-sectional studies largely replicated the idea that frontal, temporal and parietal regions are particularly vulnerable following a pTBI (Wilde et al., 2005), likely due to the unique biomechanics of injury within the paediatric brain (Pinto et al., 2012). However, regions of significant differences identified by individual studies can also be seen across the brain, suggesting a diffuse effect of injury on post-pTBI morphometry.
We synthesised the data from the reviewed cross-sectional studies into ‘bands’ post-injury to make longitudinal inference in regard to the time since injury. It is important to note that these bands were derived based upon the ‘natural’ grouping of studies in the literature (see Fig. 2) and thus clinical relevance of these bands may be limited. This is especially true of the early-stage post-injury, given the very dynamic nature of evolving and resolving pathology. Differences in imaging methodology and participant cohorts did not allow for an alternative sub-grouping within this first year, however, some patterns still emerge. The cross-sectional evidence presented suggests that TBI is related to atrophy of the brain post-injury and that some regions are more vulnerable to these effects. The regions affected, whilst broadly similar, still vary across these post-injury bands. These findings indicate that cross-sectional studies can provide information about the morphometric differences related to a given condition (Madan, 2017), in this case pTBI by highlighting, for example, regions at high potential risk of atrophy (Irimia et al., 2017). Nevertheless, these studies are limited as they provide only a snapshot of the highly dynamic process of lesion and pathology development (Bigler, 2016). It is not possible to disentangle whether differences across time periods could be attributed to either true longitudinal differences or variability in samples and/or methodologies (Kraemer et al., 2000; Vijayakumar et al., 2017). Hence, as we cannot imply a longitudinal process from the comparison of these cross-sectional studies, we may conclude that in fact these spatial differences arise as a function of the variability in injury; no two individuals, or even two patient populations, experiences the same biomechanics of injury, genetic context, and experience-dependant plasticity (Saatman et al., 2008). The key evidence presented here is that differences occur at each of the three bands post injury, from acutely to as far as 9–10 years post injury (Beauchamp et al., 2011b). This suggests that there is a non-transient effect of paediatric traumatic brain injury, which neither recovers nor is compensated for over time.
The wide within-study variability of time between injury and MRI assessment affects interpretation of these cross-sectional data. The study with the greatest variability is Drijkoningen et al. (2015), with the range of time between injury and follow-up in their TBI cohort was 0.3 to 10.8 years post injury. Although this means that direct comparison between studies is not possible, it does not preclude studies from investigating time since injury as a covariate of analyses, an approach that no study included in this review took. Only Urban et al. (2017) investigated similar effects by looking at the correlation of time since injury on cortical thickness measures in the patient group, finding no significant relationship. This absence of evidence for an atrophic process differing as a function of time since injury would seem to disagree with a continuing, longitudinal injury process. However, it is important to consider that this univariate relationship does not consider other factors (such as age at time of injury) and would provide far more convincing evidence if conducted in a longitudinal cohort. Thus, at this point in time it is not possible to draw any conclusions about the influence of time since injury on brain morphometry on the basis of the cross-sectional data alone.
The longitudinal studies identified in the current systematic review point towards a divergence of the usual/expected developmental trajectory of the brain post-injury. Studies showed that change over time differed between groups (TBI vs Control) with patients more likely to show reductions or atrophy greater than that of the control group over the same time period. Given these data, and the presence of chronic cross-sectional differences between controls and patients highlighted previously (Beauchamp et al., 2011b), it is unlikely that the maturational processes which occur to the brain during childhood are able to ‘overwrite’ the original damage post-injury as proposed by Bigler et al. (2010), or even that brain development after a pTBI ‘catches up’ with that of healthy peers. However, the current literature is limited in understanding at an individual level where, how much and in which individuals these long-term changes occur, and how these relate to individual-level neuropsychological performance post injury.
The timing of both the initial brain injury and the resultant assessments that evaluate its effects, are known to be important factors in understanding the impact of TBI and subsequent neuropsychological sequelae in children (Anderson et al., 2011). Some research suggests that there are critical periods in development where the effects of injury are most severe (Anderson et al., 2011), potentially due to vulnerability to injury pathology that is specific to certain stages of brain development (Anderson et al., 2011; Goldstrohm and Arffa, 2005; McCrory et al., 2004; Urban et al., 2017; Wilde et al., 2006). This is also likely to go on to effect functional outcomes; if there is structural damage to still-developing brain networks which typically subsume given cognitive functions, then this may result in difficulties making “age-appropriate gains” (Ryan et al., 2016c, p. 27) in the acquisition of these skills (Anderson et al., 2009; Ryan et al., 2015). There was, however, a limited number of studies in the current review which investigated the effects of age at injury on morphometric differences/variables. Three studies reported analyses that examined the effect of age at injury on morphometry (Bigler et al., 2016; Max et al., 2012; Urban et al., 2017). Urban et al. (2017) found no significant correlations between cortical thickness and age at or time since injury, whilst (Max et al., 2012) found that structural volumes of regions did not differ as a function of age across both controls and TBI patients. Bigler et al. (2016) found a significant relationship between age and cortical thickness but this relationship did not statistically differ between groups (although they do not report if this is age at injury or age at MRI, it is likely to be age at scan). None of the longitudinal studies investigated morphometric changes differed as a function of age at injury. If we assume that there are critical periods of development when there is specific vulnerability to the pathology of injury, then TBI at these critical periods may result in changes to morphometric measures that are greater than if the injury occurs at other stages of development. Further to this, without thorough investigation of patient-control differences across the range of time post-injury it is difficult to assess the emergence of differences in the post-TBI developmental trajectory. That is to say, the exact timings of when this developmental ‘divergence’ is unknown, based on the present state of the literature.
Although age at injury is a salient variable when trying to understand the impact of TBI on brain development and later functional outcomes, the review demonstrates a paucity of studies in some age groups. At key stages of postnatal cortical development - in preschool age groups and late adolescence - the consequences of TBI on the morphometry of the brain are understudied. This is of particular concern given that these are both periods of non-linear cortical change (Mills et al., 2016; Raznahan et al., 2011) in which developing brain networks are crucial for neurodevelopment. In order to understand the specific consequences and subsequently make treatment or rehabilitation recommendations for cognitive and behavioural impairments, a better understanding of age-related effects is needed. Thus, future studies should sample these age-bands.
A fundamental challenge for the field is to tease apart the various factors that interact with one another to determine brain morphology, such as the interaction between age at injury and the age at MRI scan. This is further complicated by the fact that these variables are unlikely to be independent, especially due to current practices of recruiting patients at an a-priori defined period post injury (i.e. acute, chronic). In such studies, the age at scanning will be systematically related to the age at injury (by the amount of the post-injury period). Future longitudinal studies (and even cross-sectional designs) may therefore be advised to take an accelerated longitudinal design approach to time since injury. By choosing a prospective study design which recruits at varying times post-injury (from acute to chronic stages) it will enable more effective statistical modelling of the independent trajectories that are determined by age at which an injury has occurred and the time since the injury, by giving suitable range of sampling of each of these variables.
One of the greatest challenges to the field is to understand how the whole-system level pathology to the brain gives rise to changes in functional behaviour (Bigler, 2016). The current review specifically investigated how gross brain atrophy in children with TBI may be associated with differences in post-injury cognition from TD controls. However, the lack of consistency in methods, measures and brain partitions used across the included literature makes synthesis of findings across studies difficult. The most commonly investigated association was between brain morphology and working memory. Specifically, regions of parietal and frontal lobe morphometry not only related to working memory measures (Merkley et al., 2008; Urban et al., 2017; Wilde et al., 2011), but also contributed to the difference in performance between controls and patients (McCauley et al., 2010). Longitudinal investigations of cognitive change over time also suggest that possible ‘divergence’ of morphometric maturation may be associated with differing development of and performance on a number of cognitive domains for the TBI group (Dennis et al., 2017b; Dennis et al., 2016). However, it is important to note that, due to our inclusion criteria, we only looked at studies with a control group to assess morphometric change after injury. Papers that examined at brain-cognition relationships in solely a patient group were not included in the initial search.
The interrogation of any association between morphometry and cognition in children with TBI varies across studies. Individual differences in morphometry were typically correlated with individual differences in neurocognitive performance. Some studies did this solely in the TBI group (Konigs et al., 2017; Ryan et al., 2017; Wilde et al., 2012b; Wu et al., 2010) and not in the TD control group. Thus, on the basis of their reports, it was not possible to not separate out developmentally-appropriate brain behaviour relationships from those that are truly atypical. For example, if cognitive ability ‘X’ scales linearly/non-linearly as a function of the size of region ‘Y’ (or network ‘Z’) during development, then any brain-behaviour relationships between region ‘Y’/network ‘Z’ and cognitive tasks assessing ‘X’ seen in a TBI population could potentially represent normative development, rather than informing us how damage and/or atrophy is potentially disrupting the development and retention of cognitive skills. Few papers in the current review approached this question using a cross-sectional approach, and even fewer statistically modelled the effect of group in these brain-behaviour relationships (i.e. through GLM using group as a between-subjects factor, (Dennis et al., 2013; Fearing et al., 2008; McCauley et al., 2010)). It is important to recognize that these differing approaches answer very different hypotheses on how the injured brain relates to cognitive development. It is our opinion that, in order to make clinically useful predictions about functional outcome based on morphometry measures of the brain, then it is important to see if the brain-behaviour relationships differ post-injury from those seen in typical development. If this is not the case, then it would be just as prudent to predict cognitive performance in the TBI group using morphometric models derived from healthy participants.
Synthesis of a large body of literature is important for understanding the nature of morphometric changes post-pTBI. However, there are methodological considerations within the field that must be considered both in the interpretation of this synthesis and in future studies. A key issue is the presence of macroscopic lesions on MR images as well as more subtle pathology. These include lesions due to WM deformation and shear, Wallerian degeneration, compromised vascular integrity, hemosiderin deposition and encephalomalacia, which are highly heterogeneous between individuals (Bigler et al., 2013; Bigler et al., 2016). In a study of a pTBI sample (used by multiple papers in the current review (Ryan et al., 2016a, Ryan et al., 2016b, Ryan et al., 2016c; Ryan et al., 2016a, Ryan et al., 2016b; Ryan et al., 2017)) the presence of a lesion on MRI (T1w, T2w or FLAIR) was detected in 54% of cases (Beauchamp et al., 2011a). This represents ~56% (n = 20) of the cases for which the researchers had access to MRI, CT and susceptibility weighted imaging (n = 36), and is therefore likely a slight overestimation. Despite the prevalence of lesions on MRI scans included in papers reporting global and regional morphometry following pTBI, only four studies discussed methodological approaches to deal with the presence of lesions. Spanos et al. (2007) replicated findings of cerebellar differences even when removing patients with focal cerebellum lesions, whilst Serra-Grabulosa et al. (2005) listed focal lesions as an exclusion criterion for their sample selection and still found cross-sectional differences between non-lesioned TBI cases and controls. Bigler et al. (2013) stated that, due to extreme structural damage in two patients, Freesurfer was unable to reconstruct the brain surfaces and thus these patients were excluded from analyses. The most proactive approach to controlling for the effect of lesion was that of Drijkoningen et al. (2017) who excluded regions where the presence of a focal lesion (>0.5 cm3) had resulted in distortion of the segmentation or parcellation by Freesurfer, resulting in the exclusion of seven regions across two participants (although it is pertinent to note that only 1.8% of all ROI data across the whole TBI sample was excluded in this way). However, the remaining studies did not explicitly state how lesions were addressed in their quantitative neuroimaging pipelines or even if any lesions were present in their sample at all.
The presence of lesions may influence image processing pipelines, and therefore the resultant morphometric findings. This might lead to under- or over-reporting of TBI-control differences, depending on the approach adopted. For example, disruptions to voxel intensities (due to edema for example) can lead to inappropriate solutions to cost-function algorithms (such as those in spatial normalization), causing observable distortion around the lesion (Brett et al., 2001; Goh et al., 2014; Irimia et al., 2012). Gross anatomical lesions can also result in brain segmentation and surface reconstruction failures (Irimia et al., 2014; Merkley et al., 2008; Wang et al., 2012a, Wang et al., 2012b). Anatomy can also be mislabelled by probabilistic-labelling when pathological lesions lead to gross and/or focal deformation of tissue, producing morphometric measures for ROIs which are not accurate (Dennis et al., 2016; Goh et al., 2014; Irimia et al., 2014; Irimia et al., 2012). Other methodologies, such as Freesurfer, are also semi-automated, and thus require manual intervention to ‘correct’ potential inaccuracies such as this. However, the degree to which manual intervention is conducted is solely at the discretion of the researcher and the details of which are often not transparently reported (Vijayakumar et al., 2017). None of the morphometric studies in the current systematic review reported how lesions were approached within this framework of manual editing, and there are no clear recommendations in software documentation as to how to approach such pathology.
The methods used to estimate morphometric estimates of the brain may not be robust in the presence of the lesions characteristic of TBI, and there is a lack of validation of these methods in TBI cohorts (Goh et al., 2014; Irimia et al., 2011; Irimia et al., 2014). This is especially true given the fact that many of these methodologies operate on detection of tissue boundaries within an MRI via changes in image contrast. In the presence of a TBI, tissue contrast of an MRI is suggested to be different to controls (Palacios et al., 2013). Even though some software allows (limited) integration of lesion masks into processing (ANTS allows users to perform cost-function masking during registration using a lesion mask), studies did not outline how the processing pipeline had been tested or optimised for use with MRI where there are traumatic lesions present. These methodological concerns raise questions about the credibility of the individual studies reported here, but also creates a critical question for our field; in order to accurately identify and report data on brain changes following pTBI it is important that our quantitative methodologies include pathological brains. Although excluding cases is an appropriate approach, and sometimes the only option available when registration failures occur, these cases warrant inclusion in large, representative datasets. Future work needs to assess how lesions may impact the processing of neuroimaging data, however, due to the fact there is no one ‘universal’ TBI lesion (Bigler, 2016), this is unlikely to be a trivial endeavour.
The current review specifically focused on structural changes to the brain as measured with T1w structural MRI. Structural changes post pTBI have also been recognized using diffusion weighted imaging (DWI) and related WM-tract modelling (for an extensive review of this literature see Dennis et al. (2017a)). The two methods provide unique information about differing injury mechanisms. For instance, fractional anisotropy of the diffusion signal can infer microstructural properties of WM following diffuse axonal injury (Dennis et al., 2017a). GM measures of structure outlined in this review, such as cortical thickness or volume, aim to assess the potential atrophic effects of the cascade of mechanisms that occur post-injury (Bigler, 2013). Whilst indexing different injury mechanisms, these neuroimaging methodologies provide complementary information for the basis of understanding the brain post pTBI. For instance, multimodal imaging can enhance the segmentation of pathological lesions in pTBI (Irimia et al., 2011) with each modality detecting specific properties of the lesion (Goh et al., 2016). Future research should therefore echo approaches of studies such as Konigs et al. (2017), by combining multiple modalities of imaging to better understand the brain post pTBI.
5. Concluding remarks
In the adult TBI literature, Cole et al. (2015) propose a model for changes to the ‘brain age’ of a patient after TBI. They prescribe that TBI does in fact cause a long-term chronic disease process, and these interact with the normative process of aging of the brain. Thus, the resultant state of the brain can be expressed in terms of additive effects, the sudden departure of the brain from the ‘healthy’ brain state for an individual of that age, and interaction effects, which potentially accelerate the aging process (particularly atrophy) due to the interaction of this process with the cascade of pathologies following injury. The studies shown in this review seem to paint a similar picture, but with the idea of ‘healthy aging’ replaced instead with ‘normative development’. Our findings of the both volumetric and cortical thickness differences form controls in the initial stages of early injury highlight this potential ‘additive effect’ where the injury has caused sudden change to the morphometry to the brain. The current review also highlights the longitudinal effect of injury on development, supporting such a model of ‘interactive effects' in paediatric TBI.
Overall the current systematic review draws the following conclusions from the existing literature on morphometric changes to the brain post pTBI; a) differences are apparent cross-sectionally at both acute and late-chronic timepoints post-injury, thus suggesting a non-transient effect of injury and b) morphometric change over time is altered in TBI groups compared to patients, but it is currently unclear if this is an effect of disrupted development or a continuing ‘neurodegenerative’ effect of injury.
The current review also highlights challenges to the field in regard to within-study sample heterogeneity, limited investigations of the extreme tails of childhood, and the potential effect of lesions on analyses. In addition, further work is needed to effectively relate these morphometric measures to cognitive measures of post-injury functioning to firmly establish the role of TBI-related brain changes in long-term functional outcomes.
Funding
This work was supported by a European Research Council (ERC) - Consolidator Grant (ERC-CoG) to AW [grant number 682734].
Footnotes
This value does not consider the overlap of sample/datasets
Appendix A. Appendices
A.1. Appendix A
Study characteristics visualisation.
Visualisation of dispersion of studies based upon sample characteristics of age at injury and injury-scan interval was achieved with the ggplot2 package in R (Wickham, 2009). Level of measurement across these two variables was standardized as years for both age at injury and injury-scan interval. Those studies using different levels of measurement (months and/or days) were converted (divided by 12 and 365 respectively). For studies reporting only ranges, the middle value was used.
Both mean values and standard deviations were used for visualisation. For studies that reported mean and standard deviation of these variables separately across injury severities, pooled mean and standard deviation were calculated. These were calculated in line with guidelines from the Cochrane handbook (Higgins and Green, 2011, Table 7.7.a) using the following formulae (Eq. (A.1), (A.2));
| (A.1) |
| (A.2) |
where Nx is the sample size of the subgroup, Mx is the value and SDx is the standard deviation of that mean. It is important to note that the pooled SD gives an approximation which is known to be a slight underestimation of the true SD however, for the purposes of visualisation, this is unlikely to be an issue.
All data used in the visualisation of studies are listed in the table below. It is important acknowledge that the use of multiple methods of imputation may slightly misrepresent the true data for studies. However, imputations and inferences made are fully transparent and are listed in the appendix (Table A.1), whilst the data actually reported in each paper can be seen in Table 2. Despite these caveats, Fig. 2 provides a useful visualisation with which to grasp the extent of the current research in the field.
Table A.1.
Imputed data used for visualisation of cross sectional studies.
| Reference | Age at injury |
Injury – MRI interval |
Patient sample size (n) | Data-set | ||
|---|---|---|---|---|---|---|
| Mean (years) | SD | Mean (years) | SD | |||
| Beauchamp et al. (2011b) | 6.58 a | 3.19 a | 10.40 a | 1.45 a | 49 | NA |
| Dennis et al. (2013) | 7.80 | 2.00 | 2.60 | 1.20 | 82 | 4 |
| Yeates et al. (2014) | 7.83 | 1.94 | 3.13 b,c | NA | 82 | 4 |
| Bigler (2013) | 7.92 a, e | 1.90 a, e | 2.53 a, e | 1.24 a, e | 72 | 4 |
| Bigler (2016) | 7.92 f | NA | 2.70 | NA | 72 | 4 |
| Serra-Grabulosa et al. (2005) | 8.18 | 3.65 | 9.68 | 1.88 | 16 | NA |
| Drijkoningen et al. (2015) | 9.30 b | NA | 3.83 c | 3.25 c | 18 | NA |
| Bigler et al. (2010) | 9.75 | 3.00 | 3.10 | 2.40 | 16 | 3 |
| Wilde et al. (2005) | 9.75 | 3.00 | 3.10 | 2.40 | 16 | 3 |
| Fearing et al. (2008) | 9.75 | 3.00 | 3.10 | 2.40 | 16 | 3 |
| Wilde et al. (2006) | 9.75 | 3.00 | 3.10 | 2.40 | 16 | 3 |
| Merkley et al. (2008) | 9.75 | 3.00 | 3.10 | 2.40 | 16 | 3 g |
| Wilde et al. (2007) | 9.75 f | NA | 3.00 c | 2.42 c | 16 | 3 |
| Spanos et al. (2007) | 9.75 f | NA | 3.10 | 2.40 | 16 | 3 |
| Ryan et al. (2016a) | 10.37 a | 2.51 a | 0.12 d | 0.08 d | 103 | 5 |
| Ryan et al. (2016b) | 10.44 a | 2.48 a | 0.11 a,d,e | 0.06 a, d | 76 | 5 |
| McCauley et al. (2010) | 12.00 b | NA | 0.34 d | 0.08 d | 40 | 1 |
| Wilde et al. (2011) | 12.00 f | NA | 0.01 d | 0.00 d | 40 | 1 |
| Max et al. (2012) | 13.40 | 3.00 | 0.25 | NA | 44 | NA |
| Hanten et al. (2011) | 13.43 | 2.35 | 3.23 c | 0.87 c | 15 | 2 |
| Cook et al. (2013) | 13.43 | 2.35 | 3.23 c | 0.87 c | 15 | 2 |
| Krawczyk et al. (2010) | 13.86 h | NA | 2.65 | 0.76 | 12 | NA |
| Juranek et al. (2012) | 11.84 h | NA | 0.24 a,d | 0.11 a,d | 21 | NA |
| Konigs et al. (2017) | 7.38 a | 2.13 a | 2.89 a | 1.23 a | 37 | NA |
| Drijkoningen et al. (2017) | 10.08 c | 3.40 c | 3.67 c | 3.40 c | 19 | NA |
| Urban et al. (2017) | 11.87 h | NA | 0.33 d | 0.01 d | 13 | NA |
| Ryan et al. (2017) | 10.31 a | 2.50 a | 0.12 f | NA | 112 | 5 |
Note.
Pooled mean and SD from sub groups.
Not available, middle value from reported range used for visualisation.
Converted from months.
Converted from days.
Demographics refer to all participants in paper, not just those used for morphometry analyses.
Inferred from other papers utilising dataset.
Inferred from overlapping demographics with other papers from similar authors.
Mean age imputed as the mean age at testing minus mean injury-MRI interval.
Table B.1.
Characteristics for all studies investigating relationship between cognition and morphometry included in the review by domain of cognitive functioning.
| Cognitive Domain | Reference | Measures Administered | Between-group performance | Design | Statistical Approach | Brain regions tested | Findings |
|---|---|---|---|---|---|---|---|
| IQ | Konigs et al. (2017) | WISC-III short form FS-IQ | FS-IQ lower in Mild RF+ TBI and Moderate/severe TBI compared to controls. | Correlational | Pearsons correlations (only investigated in TBI group) | WM volume of ‘affected’ tracts | No significant relationships found between test and volume of WM regions |
| Executive Functioning | Wilde et al., 2012b | BRIEF Behavioural regulation and emotional control indexes (at the 18 month timepoint) | Children with TBI were rated significantly more highly for both subscales than the OI group, suggesting greater behavioural problems for the patient group at 18 months post-injury. | Correlational | Vertex-wise correlations (only investigated in TBI group) | Vertex-wise longitudinal cortical thickness change | Emotional control index showed significant correlation with longitudinal cortical thickness change in right MFG and right anterior cingulate gyrus. The behavioural regulation index showed similar significant correlations but instead with the medial aspect of the left frontal lobe. |
| Processing Speed | Wu et al. (2010) | Arrow-flanker task (baseline condition) | No differences were found between OI and TBI groups for processing speed at 3 or 18 months. However, the OI group saw a significant improvement with timepoint (from 3 to 18 months) but the TBI group did not | Cross-sectional (comparative) | Pearsons partial correlations (age at injury and SCI | Total corpus callosum and sub-regions of corpus callosum | No significant relationship between processing speed and corpus callosum sub region volume at 3 or 18 months post injury for either group. |
| Working Memory | Konigs et al. (2017) | WISC-III Digit Span test | Digit span scores lower Mild RF+ TBI and Moderate/severe TBI compared to controls. | Correlational | Pearsons correlations (only investigated in TBI group) | WM volume of ‘affected’ tracts | No significant relationships found between test and volume of WM regions |
| Urban et al. (2017) | N-back task and dual n-back task (with motor-task component) | Accuracy on n-back tasks in both conditions was not different between groups, however for reaction times there was an interaction of group and single vs dual task condition, with the mTBI group being slower for the dual task condition. | Cross-sectional (comparative) | Pearsons correlations (in both groups) | DLPFC and parietal cortices | In controls, better accuracy during single task condition 0-back, was associated with increased left DLPFC thickness and faster reaction times for single task 1-back was related to thicker anterior and posterior IPL. In patients, thicker DLPFC was related to poorer accuracy for 1-back single task condition. However, during the dual condition, thinner left DLPFC resulted in slower RT for all three n-back conditions. Also, thinner anterior IPL was associated with slower performance in 2-back dual-task condition. | |
| Wilde et al. (2011) | SIRT | Only significant group difference (covarying for age) was found on the interaction of interference and on accuracy and reaction time, with the OI group showing a more negative effect of interference than the TBI group. No group differences in errors | Cross-sectional (comparative) | Pearsons correlations (in both groups) | Frontal and parietal lobes, middle frontal gyrus and cingulate gyrus | Significant negative correlations between right and left cingulate volumes as well as left parietal lobe volume with the non-interference condition reaction times in the TBI group, where smaller volume was associated with a longer RT. These relationships were not replicated, or new relationships found, in the OI group. Cortical thickness of bilateral caudal MFG, left SFG, SPG, and cuneas regions and right rostral MFG, preC, PCC, and PCUN regions was positively correlated with task errors in the OI group, whereas in the TBI group thickness of left parietal and inferior temporal regions and the right frontal, paracentral, rostral MFG and SPG regions was related to task errors. This difference in brain-cognition relationships was despite no differences in errors being found. | |
| Merkley et al. (2008) | BRIEF working memory scale | Not reported | Correlational | Pearsons correlations (unclear whether TBI group or whole sample) | Not reported | Significant correlations (no direction given) were found between working memory subscale and cortical thickness of bilateral inferior temporal, superior and inferior parietal as well as thickness of left FFG. | |
| Fearing et al. (2008) | SIRT | Not reported | Cross-sectional (statistical) | GLM (correcting for age and TIV) across groups | Total midbrain, total brainstem, total thalamus | Significant relation between decreased baseline (memory testing set of 1) reaction time and total brainstem volume. There was a significant interaction effect of group on the relationship between higher memory load (memory testing set of 6) reaction time and total midbrain, but total brainstem volume was marginally outside the alpha limit. Post-hoc tests for the total midbrain showed that only TBI children showed a significant relationship with higher memory load reaction time. This relationship persisted when total lesion volume was also controlled for. No relationships were found for Thalamic volumes. | |
| Memory | McCauley et al. (2010) | Event-based prospective memory task | OI group significantly outperformed the TBI group on overall performance | Cross-sectional (statistical) | QDEC general linear model (controlling for age) across groups | Vertex-wise | Thinning of bilateral regions in middle and IFG, MTG and ITG, PARH and cingulate gyri contributed to group differences in performance |
| Overall Functioning (composite score) | Dennis et al. (2017b) | Composite score of WISC-IV processing speed index, WISC-IV working memory index, Trials 1–5 CVLT-C/II and Trails 4 DKEFS trail-making test | Not reported | Cross-sectional (comparative) | Voxel-wise linear regression (TBI and OI group investigated separately) of volume change against cognitive performance change | Voxel-wise analysis | Voxel-wise linear regression showed no relationship between longitudinal volume change and changes in cognition in the control group. In the TBI group (both IHTT slow and normal) there were a considerable number of diffuse clusters where morphometric change related to differences in the cognitive summary score. More generally, clusters which were positively associated with cognitive change (where greater volume was associated with better performance) were found across GM and WM tissues (n = 18 clusters), whereas clusters where reduced volume was related to increased cognition were largely found in only GM regions (n = 33 clusters). |
| Dennis et al. (2016) | Composite score of WISC-IV processing speed index, WISC-IV working memory index, Trials 1–5 CVLT-C/II and Trails 4 DKEFS trail-making test | Not reported | Cross-sectional (comparative) | Voxel-wise linear regression (TBI and OI group investigated separately) | Voxel-wise analysis | At timepoint 1, across all participants, there were significant regions of positive correlation between cognitive summary score and volume (bilateralITG, OG, FFG and left STG) and multiple regions of negative correlation (lateral ventricles, left OG, left MTG and right cingulate gyrus. Correlations specific to the TBI-only analysis found specific regions of positive correlation between volume and performance (bilateral SFG, bilateral FFG, right OG, right SPL, right PCUN, right preC, left ITG and MFG) with less negative correlations found (lateral ventricles, the left OG, and left transverse temporal gyrus). At timepoint 2, positive correlations across all participants were found in bilateral postC, bilateral insula, right middle cerebellar peduncle, and left ITG, with TBI specific correlations being found in right middle cerebellar peduncle, right OrbG, and bilateral FFG. Negative correlations were also found in lateral ventricles, left entorhinal cortex, left STG and IFG and specific TBI relationships found in bilateral MFG, right hippocampus, right STG, left amygdala, left fornix, left ITG, left supramarginal gyrus, left STG and IFG. | |
| Theory of Mind (ToM) | Ryan et al. (2017) | Jack and Jill task, Emotional and emotive faces task, Ironic criticism and empathic praise task (cognitive, affective and conative ToM) | No significant effect of group on Jack and Jill cognitive ToM, but for affective and conative ToM there was a main effect of severity group; for affective ToM the mild complicated group performed significantly worse than controls and severe injury, for conative ToM mild complicated TBI performed worse than control, mild and moderately injured groups. | Correlational | Multivariate regression (covarying for age, ICV, pre-injury ABAS, sex, SES, ToM control trial performance, and injury severity) Only investigated in TBI group. | CCMN, SN, MNEN, CEN and DMN network volumes (summed from ROIS) | For volumes of the networks hypothesized to be important for the different aspects of ToM, each regression model was significant. For cognitive ToM, the CCMN network volume was the only significant regressor, where reduced volume was associated with worse performance. Similar patterns were found for affective ToM and the SN, as well as conative ToM and the MNEN. |
| Yeates et al. (2014) | Jack and Jill task, Emotional and emotive faces task, Ironic criticism and empathic praise task (cognitive, affective and conative ToM) | Not reported | Cross-sectional (comparative) | Pearsons correlations controlling for age and group membership across all participants, only TBI and only controls, VBM | Global WM and GM volumes and voxel-wise | Conative ToM across groups was positively correlated with GM and WM volumes and negatively correlated with VBR when controlling for group. Conative ToM was positively correlated with GM in both groups but WM volume only in the TBI group. Cognitive and affective ToM was correlated positively with GM volume and negatively with VBR respectively. VBM identified significant clusters associated with ToM but only in the OI group, not TBI patients. | |
| Dennis et al. (2013) | Jack and Jill task, Emotional and emotive faces task, Ironic criticism and empathic praise task (cognitive, affective and conative ToM) | Main effect of group on ToM performance, post-hoc tests showing that the OI group performed significantly better than severe TBI. | Cross-sectional (statistical) | MANOVA with group membership (TBI vs OI) as a between subjects and networks as within-subjects factor | CCMN, SN, MNEN, CEN and DMN network volumes (summed from ROIs) | Regression models were non-significant for cognitive or affective ToM but were significant for conative ToM. Individual predictors of the DMN, CEN and MNEN network were not individually significant, even though the overall model was. When these network volumes were decomposed, 8 out of 12 regions were significantly related to conative ToM outcome, with greater volume related to greater performance. After multiple correction, only posterior cingulate/retrosplenial cortex and hippocampal formation survived. | |
| Miscellaneous | Konigs et al. (2017) | RAVLT | Only encoding (not retrieval or consolidation subscores) was lower for Mild RF+ TBI and Moderate/severe TBI compared to controls | Correlational | Pearsons correlations (only investigated in TBI group) | WM volume of ‘affected’ tracts | No significant relationships found between test and volume of WM regions |
| Cook et al. (2013) | Anticipating consequences VR-task | The TBI group performed significantly worse on predicting long term outcomes compared to controls, but not short term consequences | Cross-sectional (Statistical) | QDEC general linear model (controlling for age) across groups | Vertex-wise | Between-group differences in performance of the overall measure were found to be significantly related to the CT of the medial PFC/FP region and bilateral PCUN. Stronger brain-behaviour relationships were found for the control group. | |
| Hanten et al., 2011) | Social problem solving VR-task | Adolescents with TBI performed significantly poorer on the summary score of his task, across all processing load conditions, compared to controls | Cross-sectional (Statistical) | QDEC general linear model (controlling for age) across groups | Vertex-wise | There was a significant group difference in relationship between cortical thickness and performance measured by the task summary score in the right orbitomedial frontal cortex and cuneus. This showed a positive relationship (greater thickness related to greater performance) for the control group only. For the ‘defining problem’ step there was a significant group difference in relationship between cortical thickness and performance with decreased cortical thickness in temporal areas related to better performance. There were also group differences for the ‘evaluate outcome’ step, with better performance related to decreased cortical thickness in the bilateral medial prefrontal regions. | |
| Krawczyk et al. (2010) | Picture analogy task | TD controls performed significantly better at reasoning analogous roles in scenes than the TBI patient group. | Cross-sectional (Statistical) | QDEC general linear model across groups | Vertex-wise | The strongest correlations were found in the control group, and inverse relationships between cortical thickness and accuracy on analogical reasoning tasks in anterior PFC, bilateral anterior and posterior lateral PFC, bilateral superior and inferior temporal gyri, and medial PFC. Relationships in the TBI group were less clear, but inverse relationships were seen in left medial OFC, and left SFG. Accuracy on trials with a distractor showed similar inverse relationships with clusters in the left STG and left MTG, right IFG, and left PCC but additionally the anterior left dorsal PFC and right OFC in the TBI group. |
Note. WISC=Wechsler Intelligence Scale for Children, FS-IQ = Full scale IQ, WASI=Wechsler abbreviated scale of Intelligence-, BRIEF=Behaviour rating inventory of executive functioning, CVLT-C/II = California verbal learning test, VR = Virtual reality, RAVLT = Rey auditory verbal learning test, DKEFS = Delis-Kaplan Executive Function System, SCI=Social composite index, SIRT = Sternberg item recognition task, ICV = Total intracranial volume, SES=Socio-economic status, ABAS = Adaptive Behaviour Assessment System, VBM = Voxel based morphometry.
References
- Adelson P.D., Kochanek P.M. Head injury in children. J. Child Neurol. 1998;13(1):2–15. doi: 10.1177/088307389801300102. [DOI] [PubMed] [Google Scholar]
- Anderson V., Spencer-Smith M., Leventer R., Coleman L., Anderson P., Williams J.…Jacobs R. Childhood brain insult: can age at insult help us predict outcome? Brain. 2009;132(Pt 1):45–56. doi: 10.1093/brain/awn293. [DOI] [PubMed] [Google Scholar]
- Anderson V., Spencer-Smith M., Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134(Pt 8):2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- Babikian T., Asarnow R. Neurocognitive outcomes and recovery after paediatric TBI: meta-analytic review of the literature. Neuropsychology. 2009;23(3):283–296. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalle D., Edwards A.D., O'Muircheartaigh J. Annual research review: not just a small adult brain: understanding later neurodevelopment through imaging the neonatal brain. J. Child Psychol. Psychiatry. 2018;59(4):350–371. doi: 10.1111/jcpp.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp M.H., Ditchfield M., Babl F.E., Kean M., Catroppa C., Yeates K.O., Anderson V. Detecting traumatic brain lesions in children: CT versus MRI versus susceptibility weighted imaging (SWI) J. Neurotrauma. 2011;28(6):915–927. doi: 10.1089/neu.2010.1712. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.H., Ditchfield M., Maller J.J., Catroppa C., Godfrey C., Rosenfeld J.V.…Anderson V.A. Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int. J. Dev. Neurosci. 2011;29(2):137–143. doi: 10.1016/j.ijdevneu.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Quantitative magnetic resonance imaging in traumatic brain injury. J. Head Trauma Rehabil. 2001;16(2):117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. 2007;21(5):515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Traumatic brain injury, neuroimaging, and neurodegeneration. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D. Systems biology, neuroimaging, neuropsychology, Neuroconnectivity and traumatic brain injury. Front. Syst. Neurosci. 2016;10 doi: 10.3389/fnsys.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D., Abildskov T.J., Wilde E.A., McCauley S.R., Li X., Merkley T.L.…Levin H.S. Diffuse damage in paediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. Neuroimage. 2010;50(3):1017–1026. doi: 10.1016/j.neuroimage.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Abildskov T.J., Petrie J., Farrer T.J., Dennis M., Simic N.…Owen Yeates K. Heterogeneity of brain lesions in paediatric traumatic brain injury. Neuropsychology. 2013;27(4):438–451. doi: 10.1037/a0032837. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Zielinski B.A., Goodrich-Hunsaker N., Black G.M., Huff B.S., Christiansen Z.…Yeates K.O. The relation of focal lesions to cortical thickness in paediatric traumatic brain injury. J. Child Neurol. 2016;31(11):1302–1311. doi: 10.1177/0883073816654143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Leff A.P., Rorden C., Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Cole J.H., Leech R., Sharp D.J., Alzheimer's Disease Neuroimaging, I Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015;77(4):571–581. doi: 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L.G., Hanten G., Orsten K.D., Chapman S.B., Li X., Wilde E.A.…Levin H.S. Effects of moderate to severe traumatic brain injury on anticipating consequences of actions in adolescents: a preliminary study. J. Int. Neuropsychol. Soc. 2013;19(5):508–517. doi: 10.1017/S1355617712001452. [DOI] [PubMed] [Google Scholar]
- Crowe L.M., Catroppa C., Anderson V. Sequelae in children: developmental consequences. Handb. Clin. Neurol. 2015;128:661–677. doi: 10.1016/B978-0-444-63521-1.00041-8. [DOI] [PubMed] [Google Scholar]
- Cullen D.K., Vernekar V.N., LaPlaca M.C. Trauma-induced plasmalemma disruptions in three-dimensional neural cultures are dependent on strain modality and rate. J. Neurotrauma. 2011;28(11):2219–2233. doi: 10.1089/neu.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Simic N., Bigler E.D., Abildskov T., Agostino A., Taylor H.G.…Yeates K.O. Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury. Dev Cogn Neurosci. 2013;5:25–39. doi: 10.1016/j.dcn.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E.L., Hua X., Villalon-Reina J., Moran L.M., Kernan C., Babikian T.…Asarnow R.F. Tensor-based Morphometry reveals volumetric deficits in moderate=severe paediatric traumatic brain injury. J. Neurotrauma. 2016;33(9):840–852. doi: 10.1089/neu.2015.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E.L., Babikian T., Giza C.C., Thompson P.M., Asarnow R.F. Diffusion MRI in paediatric brain injury. Childs Nerv. Syst. 2017;33(10):1683–1692. doi: 10.1007/s00381-017-3522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E.L., Faskowitz J., Rashid F., Babikian T., Mink R., Babbitt C.…Asarnow R.F. Diverging volumetric trajectories following paediatric traumatic brain injury. Neuroimage Clin. 2017;15:125–135. doi: 10.1016/j.nicl.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drijkoningen D., Leunissen I., Caeyenberghs K., Hoogkamer W., Sunaert S., Duysens J., Swinnen S.P. Regional volumes in brain stem and cerebellum are associated with postural impairments in young brain-injured patients. Hum. Brain Mapp. 2015;36(12):4897–4909. doi: 10.1002/hbm.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drijkoningen D., Chalavi S., Sunaert S., Duysens J., Swinnen S.P., Caeyenberghs K. Regional Gray matter volume loss is associated with gait impairments in young brain-injured individuals. J. Neurotrauma. 2017;34(5):1022–1034. doi: 10.1089/neu.2016.4500. [DOI] [PubMed] [Google Scholar]
- Dubois J., Benders M., Borradori-Tolsa C., Cachia A., Lazeyras F., Ha-Vinh Leuchter R.…Huppi P.S. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131(Pt 8):2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearing M.A., Bigler E.D., Wilde E.A., Johnson J.L., Hunter J.V., Li X.Q.…Levin H.S. Morphometric MRI findings in the thalamus and brainstem in children after moderate to severe traumatic brain injury. J. Child Neurol. 2008;23(7):729–737. doi: 10.1177/0883073808314159. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Prastawa M.W., Looney C.B., Vetsa Y.S., Knickmeyer R.C.…Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S.Y., Irimia A., Torgerson C.M., Horn J.D. Neuroinformatics challenges to the structural, connectomic, functional and electrophysiological multimodal imaging of human traumatic brain injury. Front Neuroinform. 2014;8(19) doi: 10.3389/fninf.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S.Y.M., Irimia A., Vespa P.M., van Horn J.D. Medical Imaging 2016: Pacs and Imaging Informatics: Next Generation and Innovations. 2016. Patient-tailored multimodal neuroimaging, visualization and quantification of human intra-cerebral hemorrhage; p. 9789. (doi: Artn 97890310.1117/12.2216150) [Google Scholar]
- Goldstrohm S.L., Arffa S. Preschool children with mild to moderate traumatic brain injury: an exploration of immediate and post-acute morbidity. Arch. Clin. Neuropsychol. 2005;20(6):675–695. doi: 10.1016/j.acn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hanten G., Cook L., Orsten K., Chapman S.B., Li X., Wilde E.A.…Levin H.S. Effects of traumatic brain injury on a virtual reality social problem solving task and relations to cortical thickness in adolescence. Neuropsychologia. 2011;49(3):486–497. doi: 10.1016/j.neuropsychologia.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Gautam P., Spielberg J.M., Dahl R.E., Sowell E.R. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Green S. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J., Green S., editors. 2011. www.handbook.cochrane.org doi:Available from. [Google Scholar]
- Irimia A., Chambers M.C., Alger J.R., Filippou M., Prastawa M.W., Wang B.…Van Horn J.D. Comparison of acute and chronic traumatic brain injury using semi-automatic multimodal segmentation of MR volumes. J. Neurotrauma. 2011;28(11):2287–2306. doi: 10.1089/neu.2011.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Wang B., Aylward S.R., Prastawa M.W., Pace D.F., Gerig G.…Van Horn J.D. Neuroimaging of structural pathology and connectomics in traumatic brain injury: toward personalized outcome prediction. Neuroimage Clin. 2012;1(1):1–17. doi: 10.1016/j.nicl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Goh S.Y., Torgerson C.M., Vespa P., Van Horn J.D. Structural and connectomic neuroimaging for the personalized study of longitudinal alterations in cortical shape, thickness and connectivity after traumatic brain injury. J. Neurosurg. Sci. 2014;58(3):129–144. [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Goh S.Y.M., Wade A.C., Patel K., Vespa P.M., van Horn J.D. Traumatic brain injury severity, Neuropathophysiology, and clinical outcome: insights from multimodal neuroimaging. Front. Neurol. 2017;8 doi: 10.3389/fneur.2017.00530. (doi: Artn 53010.3389/Fneur.2017.00530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J., Johnson C.P., Prasad M.R., Kramer L.A., Saunders A., Filipek P.A.…Ewing-Cobbs L. Mean diffusivity in the amygdala correlates with anxiety in paediatric TBI. Brain Imaging Behav. 2012;6(1):36–48. doi: 10.1007/s11682-011-9140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley M.L., Sinopoli K.J., Davis K.D., Mikulis D.J., Wennberg R., Tartaglia M.C.…Tator C.H. Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K.…Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek P.M., Clark R.S., Ruppel R.A., Adelson P.D., Bell M.J., Whalen M.J.…Jenkins L.W. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: lessons learned from the bedside. Pediatr. Crit. Care Med. 2000;1(1):4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Konigs M., Pouwels P.J., Ernest van Heurn L.W., Bakx R., Jeroen Vermeulen R., Carel Goslings J.…Oosterlaan J. Relevance of neuroimaging for neurocognitive and behavioral outcome after paediatric traumatic brain injury. Brain Imaging Behav. 2017:1–15. doi: 10.1007/s11682-017-9673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H.C., Yesavage J.A., Taylor J.L., Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am. J. Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Krawczyk D.C., Hanten G., Wilde E.A., Li X., Schnelle K.P., Merkley T.L.…Levin H.S. Deficits in analogical reasoning in adolescents with traumatic brain injury. Front. Hum. Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H.S., Hanten G., Roberson G., Li X., Ewing-Cobbs L., Dennis M.…Swank P. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. J. Neurosurg. Pediatr. 2008;1(6):461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Madan C.R. Advances in studying brain morphology: the benefits of open-access data. Front. Hum. Neurosci. 2017;11:405. doi: 10.3389/fnhum.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel B.E., DeWitt D.S. Traumatic brain injury: a disease process, not an event. J. Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- Max J.E., Wilde E.A., Bigler E.D., Thompson W.K., MacLeod M., Vasquez A.C.…Levin H.S. Neuroimaging correlates of novel psychiatric disorders after paediatric traumatic brain injury. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(11):1208–1217. doi: 10.1016/j.jaac.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell W.L. Traumatic brain injury in the neonate, child and adolescent human: an overview of pathology. Int. J. Dev. Neurosci. 2012;30(3):167–183. doi: 10.1016/j.ijdevneu.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Maxwell W.L., MacKinnon M.A., Stewart J.E., Graham D.I. Stereology of cerebral cortex after traumatic brain injury matched to the Glasgow outcome score. Brain. 2010;133:139–160. doi: 10.1093/brain/awp264. [DOI] [PubMed] [Google Scholar]
- Mayer A.R., Hanlon F.M., Ling J.M. Gray matter abnormalities in paediatric mild traumatic brain injury. J. Neurotrauma. 2015;32(10):723–730. doi: 10.1089/neu.2014.3534. [DOI] [PubMed] [Google Scholar]
- McCauley S.R., Wilde E.A., Merkley T.L., Schnelle K.P., Bigler E.D., Hunter J.V.…Levin H.S. Patterns of cortical thinning in relation to event-based prospective memory performance three months after moderate to severe traumatic brain injury in children. Dev. Neuropsychol. 2010;35(3):318–332. doi: 10.1080/87565641003696866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P., Collie A., Anderson V., Davis G. Can we manage sport related concussion in children the same as in adults? Br. J. Sports Med. 2004;38(5):516–519. doi: 10.1136/bjsm.2004.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A., Grace R.C., Horwood L.J., Fergusson D.M., Ridder E.M., Macfarlane M.R. Prevalence of traumatic brain injury among children, adolescents and young adults: prospective evidence from a birth cohort. Brain Inj. 2008;22(2):175–181. doi: 10.1080/02699050801888824. [DOI] [PubMed] [Google Scholar]
- Merkley T.L., Bigler E.D., Wilde E.A., McCauley S.R., Hunter J.V., Levin H.S. Diffuse changes in cortical thickness in paediatric moderate-to-severe traumatic brain injury. J. Neurotrauma. 2008;25(11):1343–1345. doi: 10.1089/neu.2008.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Herting M.M., Meuwese R., Blakemore S.J., Crone E.A.…Tamnes C.K. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Li G., Wang L., Shi F., Lin W., Gilmore J.H., Shen D. Longitudinal development of cortical thickness, folding, and fiber density networks in the first 2 years of life. Hum. Brain Mapp. 2014;35(8):3726–3737. doi: 10.1002/hbm.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios E.M., Sala-Llonch R., Junque C., Roig T., Tormos J.M., Bargallo N., Vendrell P. White matter/Gray matter contrast changes in chronic and diffuse traumatic brain injury. J. Neurotrauma. 2013;30(23):1991–1994. doi: 10.1089/neu.2012.2836. [DOI] [PubMed] [Google Scholar]
- Pinto P.S., Poretti A., Meoded A., Tekes A., Huisman T.A. The unique features of traumatic brain injury in children. Review of the characteristics of the paediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings–part 1. J. Neuroimaging. 2012;22(2):e1–e17. doi: 10.1111/j.1552-6569.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- Polinder S., Haagsma J.A., van Klaveren D., Steyerberg E.W., van Beeck E.F. Health-related quality of life after TBI: a systematic review of study design, instruments, measurement properties, and outcome. Popul. Health Metrics. 2015;13(4) doi: 10.1186/s12963-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D.…Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D.E. Review of longitudinal studies of MRI brain volumetry in patients with traumatic brain injury. Brain Inj. 2011;25(13–14):1271–1278. doi: 10.3109/02699052.2011.624568. [DOI] [PubMed] [Google Scholar]
- Ryan N.P., Catroppa C., Cooper J.M., Beare R., Ditchfield M., Coleman L.…Anderson V.A. The emergence of age-dependent social cognitive deficits after generalized insult to the developing brain: a longitudinal prospective analysis using susceptibility-weighted imaging. Hum. Brain Mapp. 2015;36(5):1677–1691. doi: 10.1002/hbm.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan N.P., Beauchamp M.H., Beare R., Coleman L., Ditchfield M., Kean M.…Anderson V.A. Uncovering cortico-striatal correlates of cognitive fatigue in paediatric acquired brain disorder: evidence from traumatic brain injury. Cortex. 2016;83:222–230. doi: 10.1016/j.cortex.2016.07.020. [DOI] [PubMed] [Google Scholar]
- Ryan N.P., Catroppa C., Beare R., Silk T.J., Crossley L., Beauchamp M.H.…Anderson V.A. Theory of mind mediates the prospective relationship between abnormal social brain network morphology and chronic behavior problems after paediatric traumatic brain injury. Soc. Cogn. Affect. Neurosci. 2016;11(4):683–692. doi: 10.1093/scan/nsw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan N.P., van Bijnen L., Catroppa C., Beauchamp M.H., Crossley L., Hearps S., Anderson V. Longitudinal outcome and recovery of social problems after paediatric traumatic brain injury (TBI): contribution of brain insult and family environment. Int. J. Dev. Neurosci. 2016;49:23–30. doi: 10.1016/j.ijdevneu.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Ryan N.P., Catroppa C., Beare R., Silk T.J., Hearps S.J., Beauchamp M.H.…Anderson V.A. Uncovering the neuroanatomical correlates of cognitive, affective, and conative theory of mind in paediatric traumatic brain injury: a neural systems perspective. Soc. Cogn. Affect. Neurosci. 2017 doi: 10.1093/scan/nsx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., Manley G.T.…Advisory Panel M. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25(7):719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Grabulosa J.M., Junque C., Verger K., Salgado-Pineda P., Maneru C., Mercader J.M. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2005;76(1):129–131. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N.…Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J. Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- Spanos G.K., Wilde E.A., Bigler E.D., Cleavinger H.B., Fearing M.A., Levin H.S.…Hunter J.V. Cerebellar atrophy after moderate-to-severe paediatric traumatic brain injury. AJNR Am. J. Neuroradiol. 2007;28(3):537–542. [PMC free article] [PubMed] [Google Scholar]
- Staal J.A., Vickers J.C. Selective vulnerability of non-myelinated axons to stretch injury in an in vitro co-culture system. J. Neurotrauma. 2011;28(5):841–847. doi: 10.1089/neu.2010.1658. [DOI] [PubMed] [Google Scholar]
- Tomson Reuters . 2013. Endnote. [Google Scholar]
- Urban K.J., Riggs L., Wells G.D., Keightley M., Chen J.K., Ptito A.…Sinopoli K.J. Cortical thickness changes and their relationship to dual-task performance following mild traumatic brain injury in youth. J. Neurotrauma. 2017;34(4):816–823. doi: 10.1089/neu.2016.4502. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Mills K.L., Alexander-Bloch A., Tamnes C.K., Whittle S. Structural brain development: a review of methodological approaches and best practices. Dev. Cogn. Neurosci. 2017 doi: 10.1016/j.dcn.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Prastawa M., Awate S.P., Irimia A., Chambers M.C., Vespa P.M.…Gerig G. 2012 9th Ieee International Symposium on Biomedical Imaging (Isbi) 2012. Segmentation of serial Mri of Tbi patients using personalized atlas construction and topological change estimation; pp. 1152–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Prastawa M., Irimia A., Chambers M.C., Vespa P.M., van Horn J.D., Gerig G. Medical Imaging 2012: Image Processing. 2012. A patient-specific segmentation framework for longitudinal MR images of traumatic brain injury; p. 8314. (doi: Artn 83140210.1117/12.911043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker K.J., Vertes P.E., Romero-Garcia R., Vasa F., Moutoussis M., Prabhu G.…Consortium N. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc. Natl. Acad. Sci. U. S. A. 2016;113(32):9105–9110. doi: 10.1073/pnas.1601745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; New York: 2009. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Wilde E.A., Hunter J.V., Newsome M.R., Scheibel R.S., Bigler E.D., Johnson J.L.…Levin H.S. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2005;22(3):333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Bigler E.D., Haider J.M., Chu Z.L., Levin H.S., Li X.Q., Hunter J.V. Vulnerability of the anterior commissure in moderate to severe paediatric traumatic brain injury. J. Child Neurol. 2006;21(9):769–776. doi: 10.1177/08830738060210090201. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Bigler E.D., Hunter J.V., Fearing M.A., Scheibel R.S., Newsome M.R.…Levin H.S. Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Dev. Med. Child Neurol. 2007;49(4):294–299. doi: 10.1111/j.1469-8749.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Newsome M.R., Bigler E.D., Pertab J., Merkley T.L., Hanten G.…Levin H.S. Brain imaging correlates of verbal working memory in children following traumatic brain injury. Int. J. Psychophysiol. 2011;82(1):86–96. doi: 10.1016/j.ijpsycho.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A., Hunter J.V., Bigler E.D. Paediatric traumatic brain injury: neuroimaging and neurorehabilitation outcome. Neurorehabilitation. 2012;31(3):245–260. doi: 10.3233/NRE-2012-0794. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Merkley T.L., Bigler E.D., Max J.E., Schmidt A.T., Ayoub K.W.…Levin H.S. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int. J. Dev. Neurosci. 2012;30(3):267–276. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2006. Neurological Disorders: Public Health Challenges. [Google Scholar]
- Wu T.C., Wilde E.A., Bigler E.D., Li X., Merkley T.L., Yallampalli R.…Levin H.S. Longitudinal changes in the corpus callosum following paediatric traumatic brain injury. Dev. Neurosci. 2010;32(5–6):361–373. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Merkley T.L., Wilde E.A., Barnes A., Li X., Chu Z.D.…Levin H.S. A preliminary report of cerebral white matter microstructural changes associated with adolescent sports concussion acutely and subacutely using diffusion tensor imaging. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9752-5. [DOI] [PubMed] [Google Scholar]
- Yeates K.O., Bigler E.D., Abildskov T., Dennis M., Gerhardt C.A., Vannatta K.…Taylor H.G. Social competence in paediatric traumatic brain injury: from brain to behavior. Clin. Psychol. Sci. 2014;2(1):97–107. [Google Scholar]