Abstract
Background
Surgery in small cell lung cancer (SCLC) is limited to very early stages, but several reports suggest a potential broader role. Little is known of the influence of microenvironment on the biology of SCLC.
Methods
We assessed the clinical prognostic factors in a large series of resected SCLC patients. The prognostic value of Programmed cell Death Ligand-1 (PD-L1) expression in tumor cells and tumor infiltrating lymphocytes (TILs), and the percentage of CD3, CD20, CD45 and CD68 positive cells, were also investigated.
Results
205 SCLC cases were resected between 2005 and 2015 and the median follow-up was 29 months (range: 2-135 months). Median survival of all patients was 69 months, and 5-year survival rates were 63.8%, 65.5%, 34.9%, and 0% for pathological stages I, II, III, and IV, respectively. By multivariate analysis complete resection, cigarette index (CI), lymph node metastatic rate (LNR), percentage of CD3 positive cells and PD-L1 expression in tumor cells and TILs were independent prognostic factors. High PD-L1 expression was present in 3.2% and 33.5% of all tumor samples in tumor cells and TILs, respectively. High PD-L1 expression in tumor cells or TILs correlated with shorter survival, whereas high expression of CD3, CD20 and CD45 correlated with better survival.
Conclusions
Resected stage II SCLC patients have similar survival as stage I, suggesting that surgery could be extended to patients with hilar lymph node involvement. Survival was better in tumors with a higher percentage of T cells and B cells, whereas PD-L1 expression in tumor cells and TILs correlated with worse survival, which suggests a potential role of immunotherapy in resected SCLC.
Keywords: small cell lung cancer, prognosis, surgery, ratio of metastatic lymph nodes, PD-L1
Introduction
Lung cancer is the leading cause of cancer death in the world. In 2015, 733,300 cases were newly diagnosed with lung cancer in China and 610,200 died1. Small cell lung cancer (SCLC) accounts for 15-20% of all lung cancer cases2, is associated with smoking, and its incidence has been steadily decreasing in Europe and North America as a result of smoking control. However, its incidence has not decreased in China, where tobacco control has not been implemented to the same extent. Compared to NSCLC, SCLC is characterized by a shorter tumor doubling time, and higher rates of early distant metastases. Although chemotherapy combined with radiotherapy can improve survival in patients with limited disease, and potentially cure 20-25% of patients, local recurrence rate is still as high as 28%~47%. Despite its exquisite chemosensitivity, drug resistance invariably occurs, and long-term survival is less than 10%2. SCLC is clinically staged into limited disease and extensive disease, according to the Veterans Administration Lung Cancer Study Group (VALCSG) staging system revised by the International Association for Study of Lung Cancer (IASLC) in 1989. In 2009, IASLC enacted the 7th edition of UICC TNM Classification for Lung Cancer, and recommended using the TNM classification for SCLC staging. Chemotherapy combined with radiotherapy is the standard therapeutic approach for patients with limited disease stage SCLC (Stage I-III). A limited role for surgery has been recognized. The National Comprehensive Cancer Network guidelines and the latest American College of Chest Physicians guidelines limit the use of surgery to treat Stage I (T1-2N0M0)3,4 however, in the European Society for Medical Oncology guidelines, surgery is considered for both stage I and II (T1-2N0-1M0)5. Large retrospective population-based databases suggest a broader role of surgery and in selected cases it might actually be preferred to chemo-radiation6, 7.
Besides stage, ratio of metastatic lymph nodes (LNR), and examined lymph nodes (ELN) have been shown to be independent prognostic factors in resected non-small cell lung cancer (NSCLC)8, but no data are available in resected SCLC.
Immune escape mechanisms are important in tumor development and progression. Upregulation of Programmed Death Ligand −1 (PD-L1) on tumor cells and down regulation of the immune check-point Programmed Death 1 (PD-1) on the membrane of T cells, are crucial mechanisms by which tumors evade the immune system. Immune-checkpoints have recently been successfully targeted with antibodies, with activity demonstrated in a large number of tumors. PD-L1 expression is upregulated in many tumor types and expression of PD-L1 on tumor cells but also on TILs has been shown to be predictive of outcome in several studies9–11.
In NSCLC patients, meta-analyses showed that PD-L1 expression in tumors is associated with poor differentiation and shorter overall survival12. Expression of PD-L1 on tumor cells has been shown to be a predictor of response to anti-PD-1 and anti-PD-L1 antibodies9. Limited and conflicting data have been reported in SCLC7.
We conducted a retrospective analysis of a large series of resected SCLC treated at a single institution in China, and explored the PD-L1 expression in tumor cells and TILs, as well as other immune markers.
Materials and Methods
A total of 205 SCLC patients were surgically resected at the Tianjin Medical University Cancer Institute and Hospital, in Tianjin China, from January 2005 to January 2015.
Patients with a histologic diagnosis of SCLC confirmed by microscopic examination, and International Classification of Diseases for Oncology, Third Edition (ICD-0-3) codes 8041 to 8045 were included in this analysis. Each case was restaged in accordance with the 7th edition of UICC TNM Classification for Lung Cancer. Staging procedures before surgery consisted of chest CT-scan and brain CT scan and upper abdomen ultrasound. More recently PET-CT scan and brain MRI were introduced.
Two patients who died within 30 days from operation and patients with other active malignancies were excluded from this analysis. Updated survival was obtained by direct visits and/or by telephone.
We defined incomplete resections as (1) non-R0 resection (with positive bronchial resection margins either microscopically or macroscopically, R1/2 resection); (2) no lymph node dissection; (3) extranodal soft tissue invasion, and (4) involvement of blood vessels, nerves, or vital organs13.
The examined lymph node number (ELN) was the total number of dissected lymph nodes, and lymph node ratio (LNR) was the number of lymph nodes with metastasis divided by the total number of dissected lymph nodes.
Immunohistochemistry
After independent review of all pathological slides and assessment of FFPE blocks quality, 155 cases were adequate. The remaining 50 cases could not be used for the construction of a tumor tissue microarray (TMA) or whole slide section: 35 because of poorly preserved blocks, and 15 because of insufficient or highly necrotic tumor tissue. There were no significant differences between the clinical characteristics of the whole series (205 cases) and the series in which immunohistochemistry was feasible (155 cases) (Table S1). The survival analyses were based on the 205 cases database, unless immunohistochemistry results were considered (155 cases).
A TMA was constructed using the TMA Master (3DHISTECH, Budapest, Hungary) according to the manufacturer’s protocol and cores of each sample were in triplicate and 2 mm in diameter. Blocks were sectioned at a thickness of 4 pm. In 40 cases whole slides were also used. Briefly, sections were incubated in xylene, followed by ethanol, and then washed with distilled water. For antigen retrieval, sections were boiled in 10 mM sodium citrate buffer for 10 min at 121 °C. After rinsing with distilled water, sections were incubated in 3% peroxidase and washed with distilled water and then with buffer. For staining, sections were incubated with the primary antibody overnight. For antigen visualization, a peroxidase-labeled secondary antibody (EnVision/HRP system, Dako, Carpinteria, CA) was applied. Subsequently, the sections were rinsed in the buffer provided in the kit and immersed in 3,3’-diaminobenzidine (DAB) stain. All primary antibodies were Ready to Use (Pre-Diluted) antibodies from Dako, including PD-L1 (clone 22C3, Pharm Dx FDA Approved), CD3 (Polyclonal), CD45 (Leukocyte Common Antigen-LCA, Clone 2B11+PD7/26), CD20 (Clone L26) and CD68 (Clone KP1).
Two independent pathologists (BK and JC) reviewed the stained slides in a blinded fashion. The percentages of tumor cells or TILs with positive staining to PD-L1 (from 0 to 100%) were used to calculate mean PD-L1 expression scores. We selected two different cutoff values for PD-L1 staining in tumor cells: 1% (⩾ 1 % vs <1%) and 50% (⩾50% vs <50%). For PD-L1 expression in TILs, we selected the optimal value as the cutoff value using ROC curve. For all other staining results of continuous variables we selected the median value as the cutoff value. Kaplan-Meier method was used to build survival curves and the log-rank test was used to compare survival curves. The overall survival (OS) was defined as the time between the operation and the date of death or the latest follow up. The 1, 3, 5-year overall survival rates were calculated from the survivorship.
Statistical considerations
Statistically significant factors identified by univariate analysis were selected for the COX proportional hazard model for multivariate analysis. Pearson’s correlation analysis was performed on all statistically significant factors selected by univariate analysis, and the factors with a correlation coefficient larger than 0.7 were considered as highly correlated with each other, and would be adjusted to avoid multi-colinearity by using least absolute shrinkage and selection operator (LASSO), p values <0.05 were considered statistically significant. SPSS19.0 was used for statistical analysis of the data.
Results
A total of 205 surgery cases were analyzed. See Table 1 for patient characteristics. Most patients had a lobectomy and a radical dissection. Lymph node dissection was not performed in 10.2% of the patients, including 9 stage IV patients. A median of 19 lymph nodes were dissected per patient. Among them an average number of 2.4 were metastatic (by H&E stain). The median metastasis rate (number of lymph nodes involved in cases with positive lymph nodes divided by total number of resected lymph nodes) was 21.4%. In total, 1498 lymph node stations were dissected (7 per patient on average), including 698 stations from N1 regions among which 84 were metastatic, 1498 stations from N2 regions among which 108 were metastatic.
Table 1.
Patient characteristics (n=205)
| Factor | Number | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 164 | 80.0 |
| Female | 41 | 20.0 |
| Age, years | ||
| ⩽60 | 104 | 50.7 |
| >60 | 101 | 49.3 |
| Symptom duration | ||
| >30 days | 58 | 28.3 |
| ⩽30 days | 147 | 71.7 |
| Smoking index | ||
| <400 | 76 | 37.1 |
| ⩾400 | 129 | 62.9 |
| Lung | ||
| left | 102 | 49.6 |
| right | 103 | 50.4 |
| NSE | ||
| normal | 118 | 57.6 |
| elevated | 87 | 42.4 |
| CEA | ||
| normal | 147 | 71.7 |
| elevated | 58 | 28.3 |
| Cyfra 21–1 | ||
| normal | 163 | 79.5 |
| elevated | 42 | 20.5 |
| Surgery type | ||
| lobectomy | 151 | 73.7 |
| pneumonectomy | 20 | 9.8 |
| Wedge resection | 34 | 16.6 |
| Radical resection | ||
| Yes | 139 | 67.8 |
| No | 66 | 32.2 |
| Neoadjuvant chemotherapy | ||
| Yes | 23 | 11.2 |
| No | 182 | 88.8 |
| PCI | ||
| Yes | 18 | 8.8 |
| No | 187 | 91.2 |
| Histological type | ||
| Pure SCLC | 193 | 94.1 |
| combined | 12 | 5.9 |
| T stage | ||
| T0 | 3 | 1.5 |
| T1 | 119 | 58.0 |
| T2 | 79 | 38.5 |
| T3 | 4 | 2.0 |
| N stage | ||
| N0 | 102 | 55.4 |
| N1 | 22 | 12.0 |
| N2 | 58 | 31.5 |
| N3 | 2 | 1.1 |
| Pathological stage | ||
| I | 79 | 38.5 |
| II | 42 | 20.5 |
| III | 61 | 29.8 |
| IV | 9 | 4.4 |
| NA# | 14 | 6.8 |
| Lymph node dissection number | ||
| <19 | 90 | 49.5 |
| ⩾ 19* | 92 | 50.5 |
| Ratio of metastastic lymph nodes | ||
| 0 | 100 | 54.9 |
| >0, ⩽=0.214* | 43 | 23.6 |
| >0.214 | 39 | 21.4 |
| Postoperative radiotherapy | ||
| Yes | 46 | 22.4 |
| No | 159 | 77.6 |
| Adjuvant chemotherapy | ||
| Yes | 140 | 68.3 |
| No | 65 | 31.7 |
The median number was used as the cut-off value.
The pathological stage could not be estimated because of lack of information on the LN status.
Neoadjuvant and adjuvant chemotherapy was a platinum-based doublet in 97.1% of patients (cisplatin-etoposide in 78.6%). Adjuvant chemotherapy was given in 68.3% of patients and postoperative chest radiotherapy was with doses between 50Gy/25f and 60Gy/30f. Eighteen patients (8.8%), received prophylactic cranial irradiation (PCI), mostly as 30Gy/10f (61.1%).
Ten cases of combined small cell carcinoma are reported in detail elsewhere14.
In total, 68.2% of the patients received adjuvant chemotherapy, 67.1% (51/76) and 73.9% (34/46) of patients in stage I and stage II respectively. There was a borderline survival influence of chemotherapy for all resected SCLC patients (p=0.04). But there was no significant difference in OS for stage I (median OS for patients who did and did not receive chemo were 108 months and NR, p=0.237) and stage II (median OS for patients did and did not receive chemo were 120 months and 61 months respectively, p=0.346). For all p-stage, there was no difference between patients who did and did not receive adjuvant radiotherapy (p=0.945). For p-stage I, the median OS for patients who received or did not receive radiotherapy were NR and 108 months, respectively (p=0.506); for p-stage II, the median OS were 69 months and 120 months, respectively (p=0.467). There was no survival difference in stage FII patients who did/did not receive radiotherapy.
PD-L1 and other markers in tumor cells and TILs
The mean PD-L1 expression in tumor cells and TIFs was 3.0% and 3.3%, respectively. Only 5 cases (3.2%) showed PD-L1 positive tumor cells when 50% was used as the cutoff value, whereas 20 cases (12.9%) were positive with a PD-L1 expression cut-off value of 1%. There was no difference in the rate of PD-L1 expression positivity in the different pathological stages (p=0.628). Positive PD-L1 expression in TIFs was observed in 52 cases (33.5%). PD-L1 expression in tumor cells was highly correlated with PD-L1 expression in TIFs (Pearson test r=0.564, p<0.001) (Figure S1A).
The mean expression of CD3, CD20, CD45 and CD68 positive cells in whole slides or cores were 10.3%, 5.8%, 16.2% and 5.6%, respectively. Most cases showed a diffuse staining for CD3 positive cells, whereas CD20 positive cells appeared mainly as clusters of cells in between tumor cells (Figure S2). The expression of CD3, CD20, and CD45 positive cells were significantly correlated with each other (p<0.001). Compared with PD-L1 expression in tumor cells, PD-L1 expression in TILs had a stronger positive correlation with the expression of CD3, CD20 and CD45 (p<0.001) (Table 2). CD3 and CD45 expression had a weak negative correlation with the maximum tumor diameter (Spearman test rho=−0.234, p=0.003; rho=−0.200, p=0.014, respectively), and CD3 has also a weak negative correlation with pathological stage (Spearman test rho=−0.202, p=0.016)(Figure S1B–D).
Table 2.
Correlation Coefficient between All Overall Survival Related Clinic-Pathological Factors
| age | CD3 percentage |
CD20 percentage |
CD45 pencentage |
PDL1* | PDL1inTILs | smoking | surgery type |
LN dissection number |
Metastasis Rate |
Stage | complete resection |
adjuvant chemotherapy |
N stage | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age |
1 | 0.154 | 0.056 | 0.13 | 0.187 | 0.12 | 0.14 | −0.007 | −0.051 | −0.046 | −0.079 | −0.011 | −0.173 | −0.143 |
| CD3 percentage |
0.154 | 1 | 0.394 | 0.774 | 0.091 | 0.056 | 0.015 | −0.02 | −0.126 | −0.14 | −0.143 | −0.071 | 0.111 | −0.181 |
| CD20 percentage |
0.056 | 0.394 | 1 | 0.498 | −0.097 | 0.177 | −0.088 | 0.012 | −0.052 | −0.037 | 0.061 | 0.054 | −0.059 | 0.027 |
| CD45 percentage |
0.13 | 0.774 | 0.498 | 1 | 0.049 | 0.07 | −0.023 | −0.042 | −0.055 | −0.073 | −0.029 | 0.017 | 0.067 | −0.05 |
| PDL1* |
0.187 | 0.091 | −0.097 | 0.049 | 1 | 0.227 | 0.033 | −0.052 | −0.089 | 0.059 | 0.059 | −0.103 | 0.028 | 0.036 |
| PDL1inTILs |
0.12 | 0.056 | 0.177 | 0.07 | 0.227 | 1 | 0.039 | −0.101 | −0.148 | 0.012 | 0.184 | −0.035 | −0.074 | 0.203 |
| smoking |
0.14 | 0.015 | −0.088 | −0.023 | 0.033 | 0.039 | 1 | 0.043 | −0.026 | 0.022 | 0.064 | −0.003 | 0.134 | 0.036 |
| surgery type |
−0.007 | −0.02 | 0.012 | −0.042 | −0.052 | −0.101 | 0.043 | 1 | −0.246 | 0.072 | −0.013 | 0.503 | −0.024 | −0.026 |
| LN dissection number |
−0.051 | −0.126 | −0.052 | −0.055 | −0.089 | −0.148 | −0.026 | −0.246 | 1 | −0.005 | −0.025 | −0.157 | 0.037 | 0.002 |
| Metastasis Rate |
−0.046 | −0.14 | −0.037 | −0.073 | 0.059 | 0.012 | 0.022 | 0.072 | −0.005 | 1 | 0.745 | 0.217 | −0.034 | 0.777 |
| stage |
−0.079 | −0.143 | 0.061 | −0.029 | 0.059 | 0.184 | 0.064 | −0.013 | −0.025 | 0.745 | 1 | 0.187 | 0.048 | 0.909 |
| complete resection |
−0.011 | −0.071 | 0.054 | 0.017 | −0.103 | −0.035 | −0.003 | 0.503 | −0.157 | 0.217 | 0.187 | 1 | −0.021 | 0.215 |
| adjuvant chemotherapy |
−0.173 | 0.111 | −0.059 | 0.067 | 0.028 | −0.074 | 0.134 | −0.024 | 0.037 | −0.034 | 0.048 | −0.021 | 1 | 0.043 |
| N stage |
−0.143 | −0.181 | 0.027 | −0.05 | 0.036 | 0.203 | 0.036 | −0.026 | 0.002 | 0.777 | 0.909 | 0.215 | 0.043 | 1 |
All variates classification rules base on Table S1
PDL1 showing PDL1 expression in tumor cells (using 50% as the cutoff value)
Survival
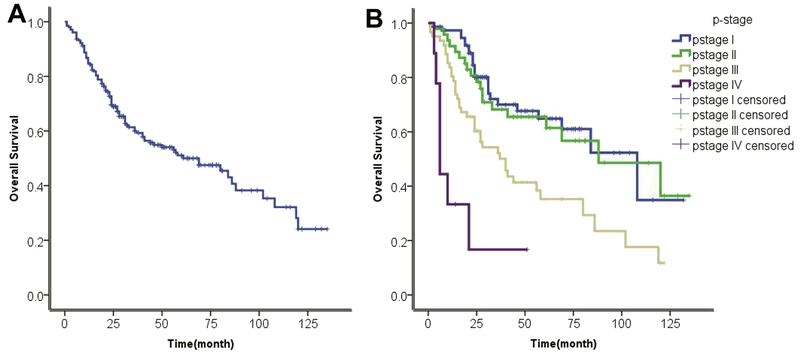
The median follow-up time was 29 months (range: 2-135 months). Median OS of all 205 patients was 69 months, and 1, 3 and 5 years survival rates were 84.8%, 60.0% and 51.1%, respectively, OS was significantly different between stage I and III and between stage II and III (median 108, 88, and 40 months respectively, p<0.05), whereas there was no significant difference between stage I and II. Stage I and II combined had a median survival of 88 months (95%CI 62.9-113.1)(Figure 1A–B).
Figure 1.
Overall survival curves for (A) all 205 resected SCLC patients; (B) by pathological stage (blue, stage I; green, stage II; brown, stage III; purple, stage IV).
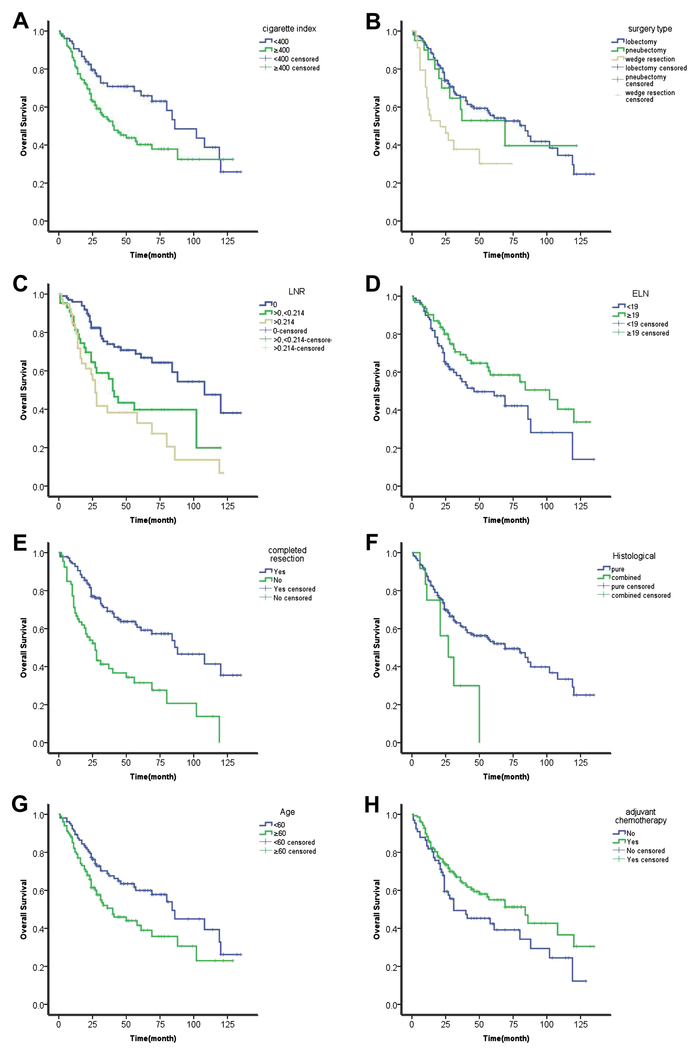
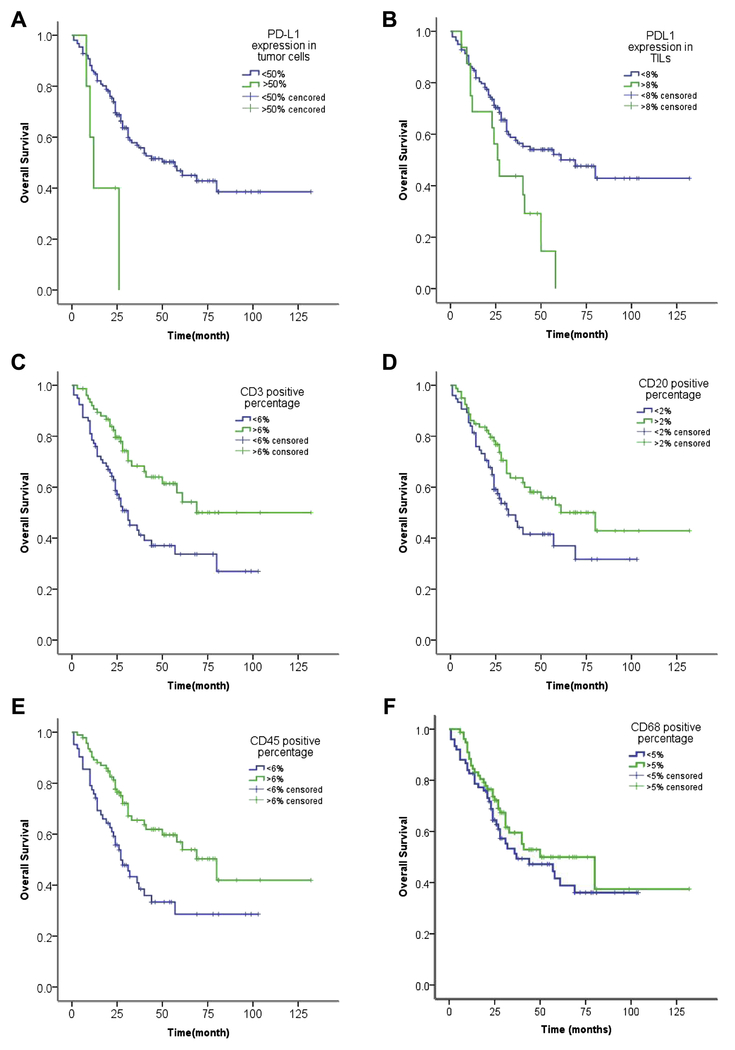
Younger age (<60 years old), cigarette index (CI) less than 400, ELN greater than 19, LNR less than 21.4%, lobectomy, R0 and complete resection, postoperative chemotherapy, and pathological stage I and II had a significantly longer overall survival than their respective counterparts, by univariate analysis (Table S2; Figure 2). Furthermore, higher PD-L1 expression in tumor cells, using both 1% and 50% cutoff values, and higher PD-L1 expression in TILs (≥8%) correlated with poorer OS. Median OS of cases with high PD-L1 expression in tumor cells (using 50% as cutoff value) was much shorter than that of cases with lower expression (12 months, 95%CI, 7.7-16.3 vs 57 months, 95%CI, 36.3-77.7; p=0.007). High PD-L1 expression in TILs was also associated with shorter survival (median OS 26 months 95%CI, 20.1-31.9 vs 69 months, 95%CI, 34.2-103.8; p=0.011). On the other hand, high expression of CD3, CD20 and CD45 was associated with longer OS (Table S3; Figure 3). CD68 positivity and tumor biomarker levels, including neuron-specific enolase (NSE), carcinoembryonic antigen (CEA) and fragments of cytokeratin-19 (Cyfra21-1) did not have prognostic value.
Figure 2.
Overall survival curves by: (A) cigarette index; (B) surgery type; (C) LNR; (D) ELN; (E) complete resection/R0 resection; (F) histology; (G) age; and (H) adjuvant chemotherapy. All p<0.05.
Figure 3.
Overall survival curves by (A) PD-L1 expression in tumor cells (using 50% as the cut-off value); (B) PD-L1 expression in TILs; (C) CD3 positive percentage; (D) CD20 positive percentage; (E) CD45 positive percentage; (F) CD68 positive percentage. All p<0.05 except CD68 (p=0.229).
Using COX proportional hazards model, PD-L1 expression in tumor cells and TILs, LNR, complete resection, CI and CD3 percentage were independent prognostic factors for OS (Table 3). Among all significant variables selected by univariate analysis, pathological stage was significantly correlated with LNR and N stage (Pearson’s test r=0.806, p<0.001) (Table 2). Because of high co-linearity between pathological stage, N status and LNR, pathological stage did not play a role in the Cox proportional hazards model. Since pathological stage is one of the most important independent prognostic factors in SCLC15, we kept it in the Cox hazard model with LASSO-COX statistic method. After this procedure, besides pathological stage, age, complete resection and cigarette index were independent factors for overall survival (Table S4; Figure S3).
Table 3.
Multivariate analysis of the correlation between clinical and pathological variables and OS.
| Factor | Hazard | 95%CI | p value |
|---|---|---|---|
| Complete Resection | |||
| R0/complete | 1.000 | ||
| R1/2/incomplete | 2.354 | 1.289-4.297 | 0.005 |
| Smoking index | |||
| <400 | 1.000 | ||
| ≥400 | 2.524 | 1.323-4.814 | 0.005 |
| CD3 percentage | |||
| ≤6% | 1.000 | ||
| >6% | 0.505 | 0.288-0.884 | 0.017 |
| PD-L1 expression in TILs | |||
| <8% | 1.000 | ||
| ≥8% | 2.182 | 1.061-4.486 | 0.034 |
| PD-L1 expression in tumor cells | |||
| <50% | 1.000 | ||
| ≥50% | 5.296 | 1.454-19.28 | 0.011 |
| LNR | 0.004 | ||
| LNR=0 | 1.000 | ||
| LNR>0,≤0.214 | 4.920 | 1.636-14.793 | 0.005 |
| LNR>0.214 | 6.005 | 1.880-19.178 | 0.002 |
LNR= Ratio of metastatic lymph nodes.
Discussion
Surgery has been banned in the vast majority of patients with SCLC based on dacades old randomized studies 16, 17. In recent years, retrospective analyses of large population databases have provided evidence for a potentially more important role of surgery in SCLC. The five-year survival for approximately 2500 resected SCLC patients was 51%, 25%, and 18% for clinical stages I, II, and IIIA, respectively, in the National Cancer Data Base (NCDB)6. Using an earlier NCDB cohort from 1992 to 2002, Gaspar et al7 demonstrated a similar benefit for surgery combined with nonsurgical treatment, compared with chemoradiation therapy alone in stage I and II patients. The improved survival of potentially resectable SCLC patients treated with surgery and chemotherapy appears similar to that of NSCLC patients18. The 5-year survival rates were 34.6-50.3% in patients undergoing lobectomy, compared to 9.9-14.9% in patients receiving non-surgical therapy in the National Cancer Registry, the Surveillance, Epidemiology and End-Results (SEER) database19, 20.
Our results are similar or better, with 5-year survival rates of 63.8%, 65.5% and 34.9% for pathological stage I, II and III, respectively, and suggest that surgery may also have potential benefit for stage II and some stage IIIA SCLC patients. In our study there was a relatively large number of stage III patients, which can be explained by the absence of mediastinoscopy and FDG-PET use in the preoperative staging until 2010. Since 2010, patients with clinical stage IIIA2 or stage IIIA3 by mediastinoscopy were excluded from surgery.
Compared with several large retrospective analyses based on SEER or NCDB databases, our series had more favorable overall survival results. Other series of resected stage IIB SCLC patients who underent surgery displayed 40% 5-yrs survival rate and 34-39 months median survival21. A number of small institutional studies have also demonstrated 5-year OS rates of 19% to 43% for stage III patients undergoing surgery as a component of multimodality therapy for SCLC22–24. A report from Mayo Clinic demonstrated a 71% 3-year survival for Stage III SCLC patients who underwent surgery25. Better OS data for stage III SCLC surgery patients was observed in Japanese studies21, suggesting a potential positive influence of Asian race on outcome.
Surgery is to be part of a combined modality approach, since surgery alone has inferior results26,27. In our study patients who underwent surgery followed by adjuvant chemotherapy had a significantly longer survival time than those who did not receive adjuvant chemotherapy (median 84 months vs 31 months, p=0.043; five-year survival rates 51.2% vs 43.8%, respectively).
The type of pulmonary resection is also an important prognostic factor19 and in our study the median OS for patients who received a lobectomy was 84 months, compared to 69 and 21 months, respectively for pneumonectomy or wedge resection (p<0.001).
The influence of the N status on prognosis is well recognized. In our study the median OS for patients with N stage N0, N1, and N2 was 120, 28 and 40 months, with five-year survival rates of 69.4%, 40.6% and 35.7%, respectively (p<0.001). This compares favorably with the results of the SEER database20.
PD-1, a member of the CD28 superfamily, is an important immunosuppressive molecule, mainly expressed in activated T cells and B cells and is a surface receptor of activated T cells. PD-L1, one of 2 ligands of PD-1, is present on the surface of various antigen presenting cells, stromal cells, and tumor cells. Activation of the PD-1 signaling pathway inhibits the T cell function, therefore inactivating the immune response. In some SCLC cell lines both PD-1 and PD-L1 molecules are co-expressed28.
Although there is plenty of literature on PD-L1 expression in NSCLC and other tumor types, data in SCLC are conflicting, with PD-L1 expression ranging from 0% to 78%29–32. This discrepancy can be due to a number of reasons, including the way that scores were calculated and use of different cutoff values 33. In our study we used 1% and 50% as cutoff values, which have been commonly used in NSCLC. Unlike NSCLC where PD-L1 positivity has been reported in 36%−56% of cases (using 5% as the cutoff value)34, 35, our results show much lower (12.9%) percentages in SCLC.
Miao et al.31 and Chang et al.30 reported PD-L1 positivity in 51.8% and 78% of the cases respectively in SCLC, using a scoring system based on intensity of staining and percentage of stained cells. The former study used the SP66 monoclonal antibody against PD-L1 and tumors were positive when over 5% of the tumor cells had PD-L1 expression. The latter study used a less well established rabbit polyclonal antibody that has been, and positive cases had at least 5% of the tumor cells stained with moderate or strong intensity. Two other studies found very low (5.8%)29 or zero expression32 in tumor cells. In a recent phase II study of pembrolizumab in extensive disease SCLC using the FDA approved 22C3 antibody to select patients who had at least 1% of PD-L1-positive tumor and associated inflammatory cells or stroma 36 only 31.7% of screened cases were positive. A lower degree of positivity for PD-L1 expression in SCLC than in NSCLC has also been noted with the antibody clone 28-8 used in clinical studies of nivolumab, where PD-L1 positivity in at least 1% of tumor cells was 13%−24% and in at least 5% of cells was 3%−6%37. The antibody 22C3 that we used is approved by FDA and used to select NSCLC patients for pembrolizumab treatment with cut-off values of 1% and 50%.
PD-L1 expression on tumor cells and TILs has been proposed as predictive biomarker for response to PD-1 and PD-L1 inhibitors in NSCLC. However the use of different antibodies and platforms has made comparisons of studies very challenging. In a study of 98 limited stage SCLC samples the antibodies SP142 and 28-8 gave 14.7-19.4% tumor cell positivity when ≥1% was the cutoff value, and results were similar between the 2 antibodies and in extensive stage cases38.
In our study, we showed that higher PD-L1 expression either in tumor cells or in TILs correlates with poorer survival. This finding is in agreement with the study by Chang et al.30 and in line with reports showing that high PD-L1 expression is associated with higher stage disease30,39. However, opposite results were reported by two other studies 40,41. Reasons for this discrepancy may be the use of different antibodies, cut-off points and an unusually high level of described positivity38 and small sample size.
We also showed that higher expression of CD3, CD20 and CD45, the classic surface biomarkers on TILs, correlated with a better survival, suggesting that tumor infiltration by TILs leads to stronger anti-tumor efficacy.
In conclusion, our study suggests that extending surgery to stage II SCLC patients may be beneficial, in addition to adjuvant chemotherapy. Although the PD-L1 expression in SCLC is lower than in NSCLC, a subset of SCLC has high PD-L1 expression and poorer survival. Recently, a large randomized study in extensive disease SCLC showed improved overall survival and progression-free survival of chemotherapy combined with the PD-L1 inhibitor atezolizumab, compared to chemotherapy alone 42. This study and phase II studies demonstrating activity of nivolumab (a PD-1 inhibitor) in refractory patients with SCLC, definitely support the use of immune checkpoint inhibitors in this disease. Our data warrant the investigation of these agents in the adjuvant setting in addition to chemotherapy.
Supplementary Material
Footnotes
All authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 2.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet (London, England) 2011;378:1741–1755. [DOI] [PubMed] [Google Scholar]
- 3.Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. Journal of the National Comprehensive Cancer Network : JNCCN 2013;11:78–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudin CM, Ismaila N, Hann CL, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:4106–4111. [DOI] [PubMed] [Google Scholar]
- 5.Fruh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 2013;24 Suppl 6:vi99–105. [DOI] [PubMed] [Google Scholar]
- 6.Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:316–323. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar LE, McNamara EJ, Gay EG, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clinical lung cancer 2012;13:115–122. [DOI] [PubMed] [Google Scholar]
- 8.Wang CL, Li Y, Yue DS, et al. Value of the metastatic lymph node ratio for predicting the prognosis of non-small-cell lung cancer patients. World journal of surgery 2012;36:455–462. [DOI] [PubMed] [Google Scholar]
- 9.Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Critical reviews in oncology/hematology 2016;100:88–98. [DOI] [PubMed] [Google Scholar]
- 10.Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. Journal of the National Cancer Institute 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bremnes RM, Busund LT, Kilvaer TL, et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016;11:789–800. [DOI] [PubMed] [Google Scholar]
- 12.Parra ER, Behrens C, Rodriguez-Canales J, et al. Image Analysis-based Assessment of PD-L1 and Tumor-Associated Immune Cells Density Supports Distinct Intratumoral Microenvironment Groups in Non-small Cell Lung Carcinoma Patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2016;22:6278–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung cancer (Amsterdam, Netherlands) 2005;49:25–33. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, McCutcheon JN, Kallakury B, et al. Combined Small Cell Carcinoma of the Lung: Is It a Single Entity? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2018;13:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata T, Nishiyama N, Nagano K, et al. Role of pulmonary resection in the diagnosis and treatment of limited-stage small cell lung cancer: revision of clinical diagnosis based on findings of resected specimen and its influence on survival. General thoracic and cardiovascular surgery 2012;60:43–52. [DOI] [PubMed] [Google Scholar]
- 16.Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320s–323s. [DOI] [PubMed] [Google Scholar]
- 17.Comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. First report to the Medical Research Council by the working-party on the evaluation of different methods of therapy in carcinoma of the bronchus. Lancet (London, England) 1966;2:979–986. [PubMed] [Google Scholar]
- 18.Luchtenborg M, Riaz SP, Lim E, et al. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998–2009. Thorax 2014;69:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2010;5:215–219. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer 2010;116:1350–1357. [DOI] [PubMed] [Google Scholar]
- 21.Takei H, Kondo H, Miyaoka E, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2014;9:1140–1145. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd FA, Ginsberg RJ, Feld R, et al. Surgical treatment for limited small-cell lung cancer. The University of Toronto Lung Oncology Group experience. The Journal of thoracic and cardiovascular surgery 1991;101:385–393. [PubMed] [Google Scholar]
- 23.Fujimori K, Yokoyama A, Kurita Y, et al. A pilot phase 2 study of surgical treatment after induction chemotherapy for resectable stage I to IIIA small cell lung cancer. Chest 1997;111:1089–1093. [DOI] [PubMed] [Google Scholar]
- 24.Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2008;3:1267–1271. [DOI] [PubMed] [Google Scholar]
- 25.Stish BJ, Hallemeier CL, Olivier KR, et al. Long-Term Outcomes and Patterns of Failure After Surgical Resection of Small-Cell Lung Cancer. Clinical lung cancer 2015;16:e67–73. [DOI] [PubMed] [Google Scholar]
- 26.Shields TW, Higgins GA Jr., Matthews MJ, et al. Surgical resection in the management of small cell carcinoma of the lung. The Journal of thoracic and cardiovascular surgery 1982;84:481–488. [PubMed] [Google Scholar]
- 27.Saji H, Marushima H, Nakamura H. Role of adjuvant therapy in early-stage small-cell lung cancer: comment on a population-based cohort study of patients with early-stage small-cell lung cancer. Journal of thoracic disease 2016;8:E1404–e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamane H, Isozaki H, Takeyama M, et al. Programmed cell death protein 1 and programmed death-ligand 1 are expressed on the surface of some small-cell lung cancer lines. American journal of cancer research 2015;5:1553–1557. [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuruoka K, Horinouchi H, Goto Y, et al. PD-L1 expression in neuroendocrine tumors of the lung. Lung cancer (Amsterdam, Netherlands) 2017;108:115–120. [DOI] [PubMed] [Google Scholar]
- 30.Chang YL, Yang CY, Huang YL, et al. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget 2017;8:18021–18030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao L, Lu Y, Xu Y, et al. PD-L1 and c-MET expression and survival in patients with small cell lung cancer. Oncotarget 2017;8:53978–53988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultheis AM, Scheel AH, Ozretic L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. European journal of cancer (Oxford, England : 1990) 2015;51:421–426. [DOI] [PubMed] [Google Scholar]
- 33.Kerr KM, Tsao MS, Nicholson AG, et al. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:985–989. [DOI] [PubMed] [Google Scholar]
- 34.D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. British journal of cancer 2015;112:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui S, Dong L, Qian J, et al. Classifying Non-Small Cell Lung Cancer by Status of Programmed Cell Death Ligand 1 and Tumor-Infiltrating Lymphocytes on Tumor Cells. Journal of Cancer 2018;9:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:3823–3829. [DOI] [PubMed] [Google Scholar]
- 37.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet Oncology 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 38.Yu H, Batenchuk C, Badzio A, et al. PD-L1 Expression by Two Complementary Diagnostic Assays and mRNA In Situ Hybridization in Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2017;12:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eerola AK, Soini Y, Paakko P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2000;6:1875–1881. [PubMed] [Google Scholar]
- 40.Toyokawa G, Takada K, Haratake N, et al. Favorable Disease-free Survival Associated with Programmed Death Ligand 1 Expression in Patients with Surgically Resected Small-cell Lung Cancer. Anticancer research 2016;36:4329–4336. [PubMed] [Google Scholar]
- 41.Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:426–430. [DOI] [PubMed] [Google Scholar]
- 42.Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. The New England journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.