Abstract
Many attempts have been made to repair articular cartilage defects, including mesenchymal stem cell (MSC)-based tissue engineering strategies. Although this approach shows promise, optimizing MSC sources and their delivery is challenging. This study was designed to test the feasibility of using MSCs found in the human arthroscopic flushing fluid (AFF) for cartilage regeneration, by incorporating them into a newly developed one-step rapid cross-linking hyper-branched polyPEGDA/HA hydrogel. AFF-MSCs were isolated from the original intra-articular flushing fluid of 10 patients prior to arthroscopic procedures. The hydrogel was fabricated with hyper-branched polyPEGDA and thiolated hyaluronic acid (HA). In vitro assays demonstrated that AFF-MSCs possessed the typical MSC morphology and phenotype, and maintained chondrogenic differentiation properties when encapsulated within the hydrogel. The AFF-MSC/hydrogel composite could significantly repair full-thickness cartilage defects generated in a rat model after 8 weeks of implantation; smooth cartilage was formed with evidence of hyaline cartilage formation. These data suggest that human AFF-MSCs are a novel and abundant MSC source that have high therapeutic value for cartilage regeneration.
Keywords: Arthroscopic flushing fluid, Mesenchymal stem cells, Hydrogel, Cartilage regeneration
1. Introduction
Articular cartilage (AC) is an avascular and aneural load-bearing tissue with minimal capacity to spontaneously heal and regenerate [1]. AC injuries are common [2] and their management poses a great clinical challenge [3]. Current therapies include arthroscopic lavage with or without debridement, microfracture with bone-marrow-stimulation technique [4], autologous chondrocyte implantation (ACI) [5] or matrix-induced autologous chondrocyte implantation (MACI) [6]. Despite these various treatment options, it remains very difficult to re-create intact hyaline cartilage with normal anatomy and function after injury [7].
Mesenchymal stem cell (MSC)-based therapy exploits MSC expansion potential, low immunogenicity and multipotency to repair cartilage defects [8]. MSC do, however, have a limited capacity for self-renewal and decreased stemness with donor age [9]. In addition, adult MSCs isolated from different tissues differ dramatically in chondrogenic potential [10,11] and innate tissue-specific MSC characteristics should be considered before application to cartilage regeneration [12]. For example, MSCs or progenitor cells derived from synovial joint tissues, including AC [13], synovium [14], and synovial fluid [15], were found to have superior chondrogenic ability over those derived from non-joint tissues, such as bone marrow and adipose tissue [16]. MSCs from synovial joint tissues are likely ideal for cartilage regeneration, but a simple and manipulable approach to obtain such stem or progenitor cells remains to be developed.
Arthroscopy is the most commonly performed orthopedic procedure [17,18] for diagnosis and for removal, repair and replacement of tissues; several million arthroscopies are performed globally each year [19]. During the procedure, ~5000 mL saline is used to wash the joint tissues inside the articular cavity. This flushing fluid, which can be collected prior to other arthroscopic procedures such as cartilage shaving or drilling, contains plenty of cells from synovium, synovial fluid, and superficial zone of AC. Hence, the basic hypothesis of this study was that the early intra-articular flushed fluid (AFF) contains MSCs (AFF-MSCs) that may have the innate properties and functions required to promote cartilage regeneration.
Hydrogel is commonly used as a scaffold or cell carrier in AC repair [20]. As the three-dimensional microenvironment provided by extracellular matrix (ECM) has a critical role in influencing cell behaviors and directing stem cell fates, many different hydrogel platforms have been developed for the delivery of stem cells in different clinical conditions [21]. However, a major challenge remains to design a hydrogel that can be tuned to the relevant microenvironment in terms of its mechanical properties and cell interactions, can undergo efficient gelation, and is biodegradable, and convenient to use [22]. We recently reported that hyper-branched polyPEGDA efficiently cross-links with a thiolated bio-macromolecule of hyaluronic acid (HA) to form stable hydrogels within a few minutes [23]. Due to the high ratio of pendant acrylate functional groups on the polymer chains, polyPEGDA offers the hydrogel system a wide range of tunability that can be optimized to regulate stem-cell behaviors and activities [22]. In addition, the hydrogel does not require UV illumination or chemicals for cross-linking, which may otherwise induce cytotoxicity and elicit an immune responses. So far, our hydrogel system has not yet been applied in cartilage repair. Hence, the working hypothesis of this study was that the multifunctional hyper-branched polyPEGDA could spontaneously and rapidly cross-link with thiolated HA under physiological conditions to achieve gelation for convenient in situ encapsulation of AFF-MSCs and support their survival, self-renewal, and differentiation.
To test our hypotheses, we isolated human MSCs from AFF of patients undergoing arthroscopic surgeries and assessed their abundance and regenerative properties. We then used the PEGDA/HA hydrogel system to encapsulate AFF-MSCs to maintain their viability for chondrogenesis. Finally, we validated the effectiveness of the AFF-MSC/hydrogel composite in repairing cartilage defects using a critical-size rat cartilage defect model. We found that AFF-MSCs are a novel and feasible source of MSCs suitable for cartilage repair.
2. Materials and methods
2.1. Isolation and functional validation of AFF-MSCs
With the approval of the University of Shenzhen and the Hospital Institutional Ethical Review Board of Peking University Shenzhen Hospital and the full informed consent of all patients, 10 patients (aged 45 to 65 years, mean 55.8 years) who were clinically diagnosed to undergo arthroscopic lavage and debridement of the knee joints (Supplementary Table S1) and one AFF sample was taken from each patient. In all cases, AFF-cells were harvested prior to any other procedure such as cartilage shaving and drilling, using the same procedure of centrifugation and culture. Briefly, the articular cavity was rinsed out using normal saline, and ~500 mL of the original AFF were collected under sterile conditions. the cell were pelleted by centrifugation at 1000g for 10 min, and the pellet was then re-suspended in Dulbecco’s modified Eagle’s medium (Thermo Scientific, USA) and supplemented with 20% fetal bovine serum (Thermo Scientific, USA) and 10,000 Units/mL of penicillin-streptomycin (Gibico, USA). Cell suspensions were plated on 100 mm dishes and maintained in a humidified incubator at 37 °C with 5% CO2. The culture medium was changed after 24 h to remove the non-adherent cells.
2.1.1. Colony forming assay
Samples 1–3 were used for colony forming assay. Briefly, 200 primary cells were initially plated and the medium was changed every 3 days. After 10 days of culture, attached cells were washed and fixed with 4% paraformaldehyde for 15 min, and then stained with 0.07% crystal violet (in ethanol) at 4 °C. The colonies were counted under a microscope (IX71, Olympus, Japan).
2.1.2. Flow cytometry for MSC surface marker expression
All 10 samples were examined for the expression of MSC surface markers by flow cytometry. The cells (1 × 106) at passage 5 were harvested by trypsin digestion, washed with PBS, blocked by 2% FBS at 4 °C for 0.5 h and then incubated individually with FITC-conjugated anti-human CD34 and CD45, PE-conjugated anti-human CD105, APC-conjugated CD73 and PerCP-Cy5.5-conjugated CD90 (Thermo Scientific, USA) at 4 °C for 1 h. The incubated cells were resuspended in 0.4 mL PBS and subjected to analyze with a FACSCalibur instrument (Becton Dickinson, USA). Data were processed using FlowJo software (Java Software).
2.1.3. CCK-8 proliferation assay
Cells from samples 1–4 and 6 at passage 5 were used for CCK-8 assay. Briefly, 150 μL of cell suspension at a concentration of 1 × 104/mL cells were seeded in a 96-well plate. At the designated time points, the cells were washed twice with PBS and 90 μL medium and 10 μL Cell Counting Kit-8 (Keygen Biothch, Nanjing, China) was added to each well. After 2 h of incubation, the absorbance at 450 nm was measured using a microplate reader (BioTek, Instruments, USA).
2.1.4. Tri-lineage differentiation assay
Cells from samples 4–6 were used for the tri-lineage differentiation assay. Briefly, cells at passage 5 were seeded at a density of 4 × 104 cells/well in 6-well plates and induced to differentiate by osteogenic, adipogenic and chondrogenic media (Supplementary Table S2).
For osteogenic differentiation, cells were cultured in a medium containing 10 nM dexamethasone, 10 mM β-glycerol phosphate and 50 μM ascorbate-2-phosphate. The culture medium was replaced every 2–3 days. After 21 days of induction, cells were fixed with 4% paraformaldehyde for 30 min and incubated with 0.1% Alizarin Red S solution for 30 min at room temperature.
For adipogenic differentiation, cells were incubated for 2 weeks in adipogenic induction medium containing 1 μM dexamethasone, 10 μM insulin, 0.5 mM isobutyl-methylxan-thine and 200 μM indomethacin, and then fixed with 4% paraformaldehyde for 30 min and stained with 0.36% Oil-red O solution.
For chondrogenic differentiation, cells were induced in the medium containing with 100 nM dexamethasone, 1% insulintransferrinsodium selenite (ITS), 150 μM ascorbate-2-phosphate, 100 μg/mL sodium pyruvate, 10ng/mL TGF-β1. After 14 days of induction, the cells were fixed and stained with 0.1% Safranin O solution.
2.2. Hyper-branched polymer synthesis and hydrogel fabrication
The hyper-branched acrylate functional polymer was synthesized by poly(ethylene glycol) diacrylate (PEGDA, Mn = 575 g mol−1, Sigma-Aldrich) via a reversible additionfragmentation chain transfer (RAFT) approach, as described previously [23]. Briefly, the PEGDA monomer (25 equiv.) was dissolved, and the previously synthetic AIBN (Sigma-Aldrich, 98%, 0.5 equiv.) and chain transfer agent (CTA, 1 equiv.) [24] were added to react at 60 °C in an oil bath. Two days after dialysis at 4 °C, the purified polymer product was harvested. the molecular weight and polydispersity index (PDI) of the polymer were determined according to our previous protocol [23], using gel permeation chromatography (GPC) and viscosity detectors. The pendant acrylate groups in the polymer were identified by 1H NMR analysis, and the Mark-Houwink constant (a) was used to define the relationship between the viscosity of polymer solution and molecular weights.
For hydrogel fabrication, hyper-branched polyPEGDA (HB-PEGDA) was dissolved in PBS pH 7.4. Then, a commercially available thiolated HA (Vornia) was added, and gelation occurred at room temperature by Michael-type addition. The hydrogels were fabricated with HB-PEGDA at concentrations of 2.5%, 5% and 10%, and 1% HA. On the basis of our previous report [23], rheological assessment, swelling behaviours, and mechanical properties were completed to determine the polymeric hydrogel characterization. Subsequent in vitro cell survival and in vivo cell transplantation assay used HB-PEGDA at a final concentration of 5% (w/v), thiolated HA at a final concentration of 1% (w/v), and 1 × 106/mL MSCs.
2.3. The growth characteristics of AFF-MSCs in hydrogel
Samples 7–10 were used particularly for characterizing the survival and growth properties of AFF-MSCs in our hygdrogel. About 100 μL mixture of AFF-MSCs and hydrogel was pipetted to a 96-well plate. After about 6 min for complete gelation, 100 μL culture medium was added for in vitro 3-D culture. Cell viability and proliferation were detected using LIVE/DEAD® and Quant-iT™ PicoGreen® dsDNA assay kits (Thermo Scientific). For quantitative cell viability analysis, live and dead cells were counted using the ImagePro® Plus (Media Cybernetics, USA) software. For proliferation analysis, after adding the detection reagent (PicoGreen®) to samples in the 96-well plate, the fluorescence values were read at 480 nm excitation and 520 nm emission using a spectrofluorometer. In addition, to test the chondrogenic differentiation potential AFF-MSCs in hydrogel, cells were incubated in chondrogenic differentiation medium or basic medium for 14 days, and then total RNA was extracted and reverse transcribed using commercial kits (Invitrogen). The gene expression of collagen type II (Col-II), aggrecan (ACAN), and Sox9 were analyzed by RT-qPCR (Bio-Rad CFX96™). The primer sequences were synthesized by Invitrogen (Supplementary Table S3). The copy numbers were normalized to GAPDH, and the fold difference was calculated using the ΔΔCt method.
2.4. The animal model for cartilage defects and cell implantation
All the animal procedures were approved by the Experimental Animal Center, Shenzhen University. Thirty male Sprague-Dawley (SD, CLEA Japan Inc., Tokyo) rats (~250g) were administered routine anesthesia (pentobarbital sodium salt, 30 mg/kg body weight, Sigma-Aldrich®). The mice were divided into three groups: control (PBS; n = 10), hydrogel alone (n=10), and AFF-MSC/hydrogel group (n = 10). After adequate skin preparation and sterilization, a medial para-patellar incision was made to expose the articular surface in the right knee of each rat. Full-thickness cartilage defects (2.0 mm in diameter, 1.0 mm in depth) were created in the center of the trochlear groove. After the defects were made, a 50 μL of mixture containing 5% (w/v) polyPEGDA, 1% (w/v) HA and 1 × 106 cells/mL AFF-MSCs (from sample 10) was immediately injected into the cartilage defect site until AFF-MSC/hydrogel composite formed (within 2 min at room temperature). The control groups were instead administered PBS or hydrogel only. The animals were allowed to bear the whole weight and have free mobilization in the cage post-operatively. General health status was monitored by a veterinarian. The right knee of each mouse per group was then harvested on week 4 (n = 5) or week 8 (n = 5) after surgery for subsequent analysis.
2.5. Macroscopic and SD-OCT evaluation of cartilage defects
Knee samples were harvested and the AC at the defect area was assessed using the International Cartilage Repair Society (ICRS) macroscopic score, which includes three categories: degree of defect repair, integration to board zone, and macroscopic appearance [25].
Prior to the histological process, knee tissue samples were placed on a platform of the spectral domain optical coherence tomography (SD-OCT) device (Fig. S1), and the focal plane of the object lens, which was set at the sample surface, was placed below the zero-delay line of the SD-OCT system. The light source was a super luminescent diode (S-840-B-I-20) with a central wave length of 835 nm and a band width (FWHM) of 45 nm, and the output power was 12 mW. The spectrometer’s resolution was set at 0.04818 nm, and individual samples were scanned in an area of 3 mm × 3 mm (length × width) and detected in a depth of ~1.58 mm. The OCT images of the trochlear groove median sagittal plane were continuously collected by the Matlab program (Amira software, Thermo Fisher Scientific, USA) and analyze using Image-Pro® software (Media Cybernetics, Inc., USA).
2.6. Histology and immunohistochemistry
Following the SD-OCT evaluation, the knee samples were treated for histological analysis, and serial sections (5 μm) were cut in the vertical direction at the cartilage defect sites for Hematoxylin and Eosin (H&E), Toluidine blue, Safranin O and Fast green, and Masson staining. The repaired tissue was graded using a modified O’Driscoll histology scoring system [26].
For immunohistochemical (IHC) evaluation, the slides were incubated with primary antibodies anti-collagen II (ab34712, Abcam, USA, 1:100 dilution) at 4 °C overnight. After washing, the slides were incubated with biotinylated goat anti-rat antibody (Vector Laboratories, BA-9400) for 30 min, followed by VECTAS-TAIN Elite ABC HRP Kit (Vector Laboratories, PK-6100) for 30 min. Positive staining was detected by ImmPACT DAB Peroxidase (HRP) Substrate (Vector Laboratories, Inc.,USA).
2.7. Statistical analysis
All quantitative data were expressed as mean ± SEM. Statistical analysis was performed using SPSS software (version 20.0; IBM, USA). For experiments comparing multiple groups of data, one-way analysis of variance (ANOVA) and two-way ANOVA were conducted, followed by Tukey’s post-hoc test. Statistical significance was accepted when P < 0.05.
3. Results
3.1. AFF-MSCs possess typical properties of MSCs
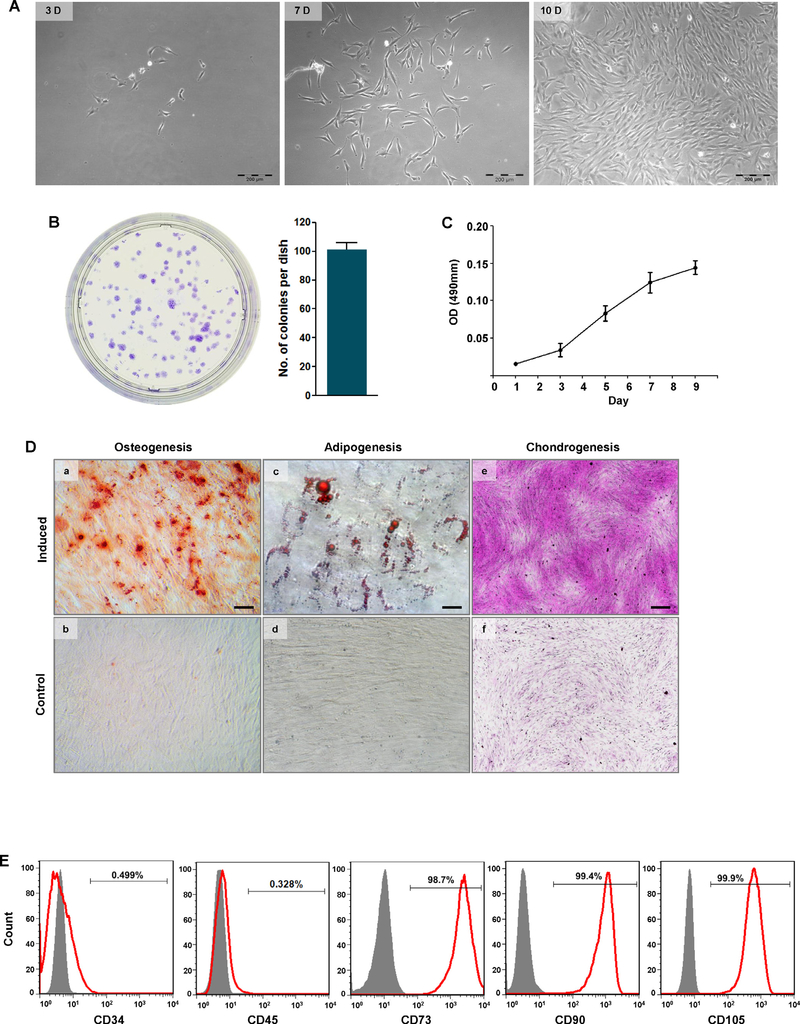
All 10 analyzed AFF samples were visibly in individual cells, after removal of obvious debris using filters with a 40 μM pore size. The primary cells isolated from AFF were cultured in growth medium, and remained quiescent for 3–4 days before attaching to the culture dish and forming colonies. By day 10, the cell morphologies in the colonies appeared fibroblast-like or cobblestone-like (Fig. 1A), and the colonies varied in diameter ranging from 1 to 5 mm (Fig. 1B). The number of colony formation units from each of the 10 samples did not markedly differ. Cell from all samples were able to continue to proliferate well over 5 passages. And in Fig. 1C, it showed that these cells at passage 5 could continue growing for 9th day.
Fig. 1.
Isolation and characterization of AFF-MSCs. (A) Morphology of primary AFF-MSCs at 3, 7 and 10 days. Scale bars, 200 μm. (B) Colony formation identified by crystal violet staining at 10days and the colony number per dish (n = 3). (C) Growth curve of AFF-MSCs at passage 5. (D) Osteogenic-, adipogenic- and chondrogenic-induced differentiation of AFF-MSCs and the corresponding labelling with Alizarin red S (scale bar, 200 μm), Oil red O (scale bar, 20 μm) and Safranin O (scale bar, 200 μm), respectively. (E) MSC surface marker expression, including the positive markers CD73, CD90 and CD105 and the negative markers CD34 and CD45. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
After 21 days of osteogenic induction, calcium deposits were highly visible with Alizarin red S staining (Fig. 1Da) compared to control cells cultured in basic media (Fig. 1Db). During adipogenic differentiation, small oil droplets started to emerge at day 7, and numerous lipid droplets with varying sizes were observed with Oil red O staining at day 14 (Fig. 1Dc), while no obvious lipid droplets were seen in the control cells without induction (Fig. 1Dd). After chondrogenic induction, strong production of proteoglycans in the induced cells was observed with Safranin O staining at day 14 (Fig. 1De); in contrast, control cells cultured in basic medium were stained negatively (Fig. 1Df).
Flow cytometry indicated that more than 95% of the cells were positive for CD73, CD90 and CD105, whereas less than 1% of the cells were positive for CD34 and CD45 (Fig. 1E).
3.2. Optimization of HB-PEGDA/HA hydrogel
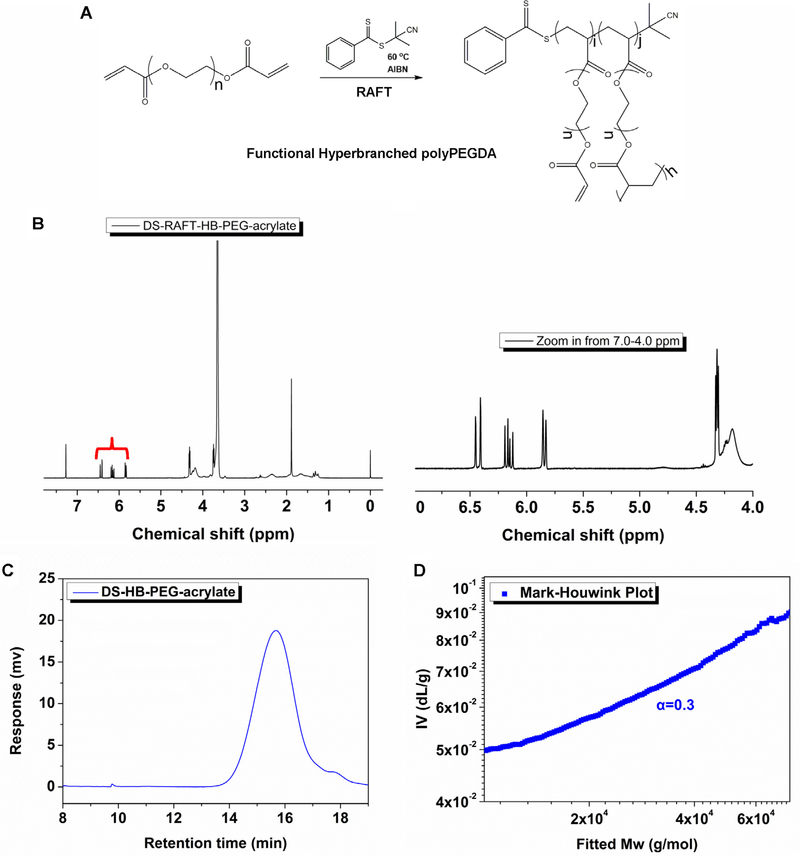
The schematic of hyper-branched polyPEGDA synthesis was shown in Fig. 2A. Using 1H NMR analysis, we identified the pendant acrylate groups in the polymer as three characteristic chemical shifts of 6.4–5.8 ppm (Fig. 2B). The molecular weight (Mn) of the polymer was ~12.0 kDa, and GPC traces showed a symmetrical peak with a relatively narrow polydispersity index (PDI) of 1.4 (Fig. 2C). Additionally, the Mark-Houwink exponent (α value) was ~0.3, demonstrating that the polyPEGDA homopolymers possessed branched structures (Fig. 2D). Further characterization of the HB-PEGDA/HA hydrogel showed that higher concentration of the polymer accelerated the rate of gelation (Fig. 3A), with the gelation point being about 1 min for the 10% (w/v) polymer, 1.5 min for the 5% (w/v) polymer and 2 min for the 2.5% (w/v) polymer at 25 °C. The swelling profile of the hydrogel indicated that all the hydrogels formed, regardless of polymer concentration, reached a steady state and maintained their weight, except for the HA-only group (Fig. 3B). Additionally, we found that higher polymer concentrations enhanced the mechanical strength of the hydrogel: under the same conditions (frequency = 10 rad/s), the storage modulus was 135.19 ± 4.55 Pa for the 2.5% (w/v) polymer, 146.25 ± 7.83 Pa for the 5% (w/v) polymer and 185.63 ± 3.54 Pa for the 10% (w/v) polymer (Fig. 3C and D).
Fig. 2.
Polymer synthesis and characterization. (A) Hyper-branched acrylate functional polymer synthesized by homo-polymerization of polyPEGDA via reversible additionfragmentation chain transfer (RAFT). (B) 1H NMR for hyper-branched polyPEGDA in CDCl3. The spectrum clearly shows the double bonds within the structure at the chemical shifts between 6.4 and 5.8 ppm. (C) Mark-Houwink value α is 0.3, indicating the hyper-branched structure of the HB-PEGDA polymer. (D) GPC curve of polyPEGDA by RAFT polymerization from the RI detector, Mw = 12 kDa, PDI = 1.4.
Fig. 3.
HB-PEGDA/HA hydrogel properties. (A) Real-time chemical cross-linking rheological measurements at different HB-PEGDA concentrations of 2.5%, 5% and 10% (with 1% HA at 25 °C). Insets show the same data on a log10 scale of G′ and G″. (B) All hydrogels with a different polymer concentration reached a steady state and maintained their weight for the swelling test at 37 °C (n = 3). (C) The storage modulus (G′) of the hydrogel with different HB-PEGDA concentrations at 25 °C (D) The average G′ and loss modulus (G″) over the frequency ranging from 0.1 to 10 rad s−1 with a constant strain of 0.05 at 25 °C (r = 1:1). A higher polymer concentration enhanced the mechanical properties of the hydrogel (n = 3). *P < 0.05.
3.3. Hydrogel supports the survival and differentiation of encapsulated AFF-MSCs
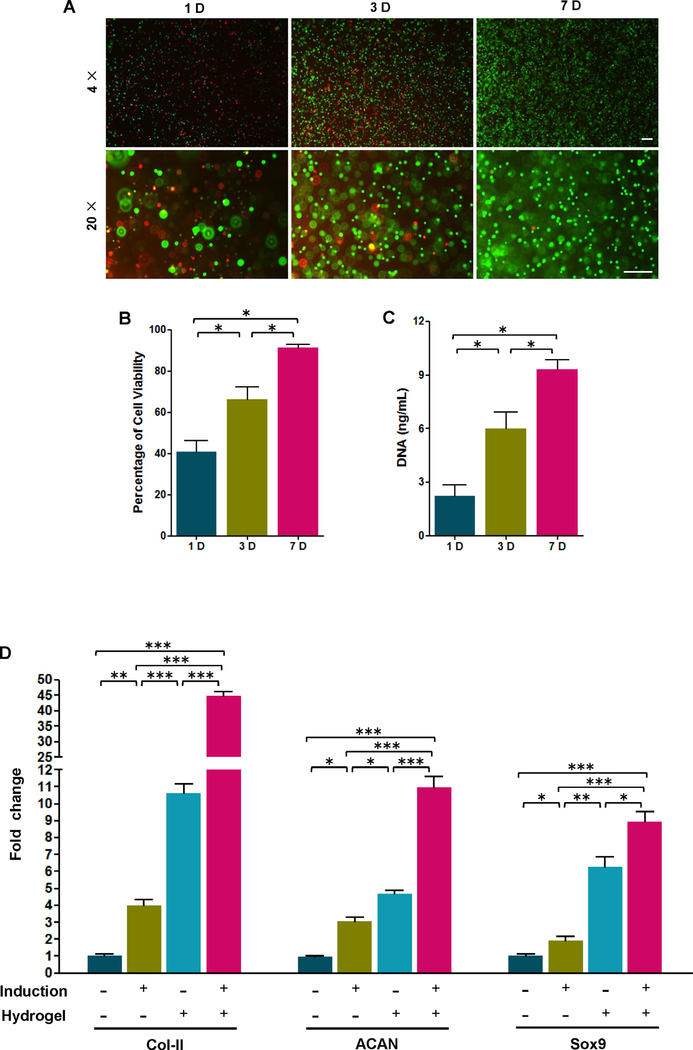
By cell viability assay, we found that the viable AFF-MSCs encapsulated in hydrogel occupied 41.7 ±2.96% at the first day. The percentage significantly increased by day 3 to reach 65.3 ± 3.02%, and reached 91.3 ± 1.02% by day 7 (Fig. 4B). In addition, the total amount of DNA significantly increased from day 1 to day 7, suggesting that the AFF-MSCs in hydrogel had a high proliferative capacity (Fig. 4C). Finally, the AFF-MSCs showed significantly increased Col-II, ACAN and Sox9 expression upon encapsulated in the hydrogel compared to control cells cultured as normal in dishes. The gene expression peaked when the AFF-MSC/hydrogel composite was incubated with extra chondrogenic-induction medium (Fig. 4D), demonstrating that the hydrogel could assist AFF-MSC chondrogenic differentiation. These data indicated that the hydrogel had the high biocompatibility and supporting role to AFF-MSCs.
Fig. 4.
Viability, proliferation and chondrogenic differentiation of AFF-MSCs in hydrogel. (A) LIVE/DEAD® assay of AFF-MSCs encapsulated in the hydrogel. Calcein AM (green) stain for live cells and ethidium homodimer-1 (red) for dead cells. (B) Percentage of live cells to the total cell number calculated from LIVE/DEAD® staining micrographs (n = 3). (C) Cell proliferation assay by testing total DNA amount in the hydrogel (n = 3). (D) Gene expression analysis of Col-II, ACAN, and Sox9 for AFF-MSC alone and AFF-MSC/Hydrogel with or without induction treatment (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. AFF-MSC/hydrogel composites successfully repair the cartilage defects
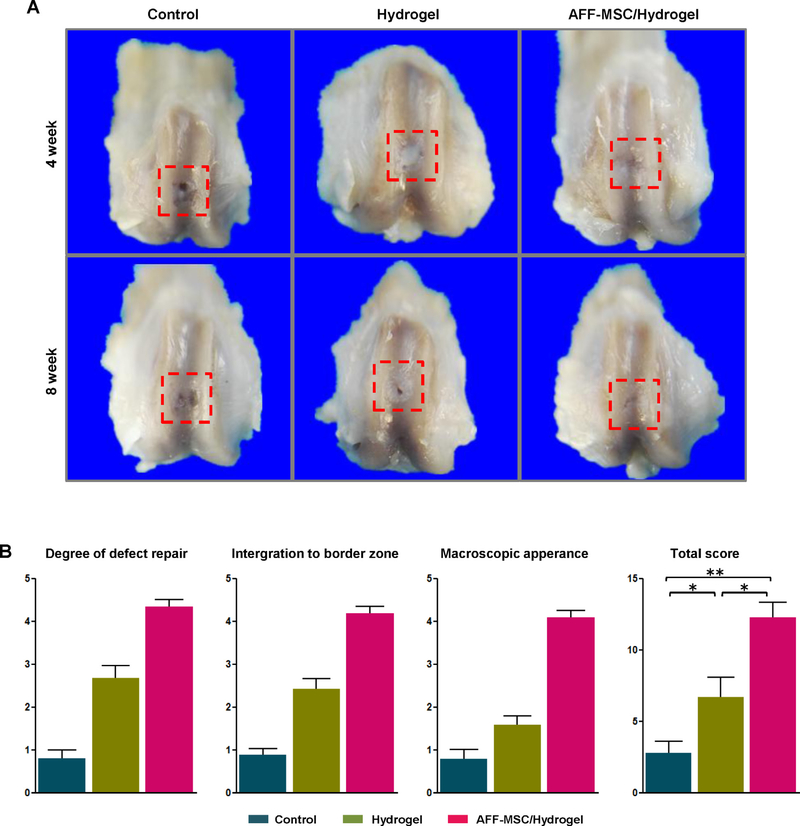
In order to assess the regenerative properties of AFF-MSC, we implanted the AFF-MSC/hydrogel composite into a rat cartilage defects model. On week 4 and 8 post-implantation, we observed at the macroscopic level that the cartilage defects in the AFF-MSC/hydrogel group were markedly more filled compared to the control groups; the most marked difference was seen at 8 weeks (Fig. 5A). Indeed, macroscopic ICRS evaluation at 8 weeks indicated that the total score for the AFF-MSC/hydrogel group (12.68 ±2.11) was significantly higher than scores for the control (2.52 ± 0.84) and hydrogel alone groups (6.73 ± 1.68) (Fig. 5B).
Fig. 5.
Macroscopic appearance and ICRS quantitative score for the cartilage defect repair. (A) Macroscopic appearance of samples harvested at 4 and 8 weeks after surgery. (B) ICRS score system for gross evaluation at 8 weeks after surgery (n = 3). *P < 0.05, **P < 0.01.
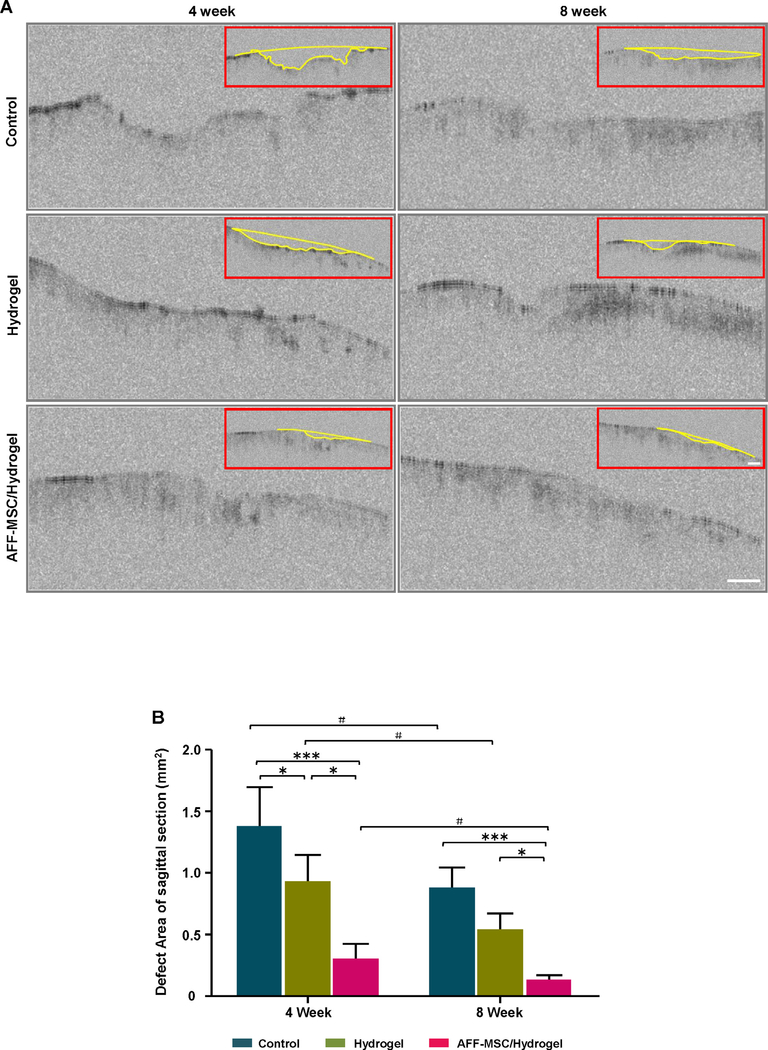
OCT images demonstrated that the cartilage surface in the AFF-MSC/hydrogel group was obviously more even and smooth than that of control groups (Fig. 6A). The quantitative analysis showed that the defect area significantly reduced, reaching the range of 0.148 ± 0.074 mm2 in the AFF-MSC/hydrogel group by 8 weeks, whereas the defects remained at 0.885 ± 0.156 mm2 and 0.551 ± 0.124 mm2 in the control and hydrogel alone groups, respectively (Fig. 6B).
Fig. 6.
SD-OCT assessment for the cartilage defect area. (A) OCT images of the tissue samples were collected at 4 and 8 weeks after treatment. (B) The defect area in the median sagittal plane was marked with a yellow line, and then was quantified using the ImagePro® software (n = 3). * represents a significantly different compared to the other groups at the same time point; # indicates that the result is significantly different compared to the same group at a different time point. #P < 0.05, *P < 0.05, ***P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
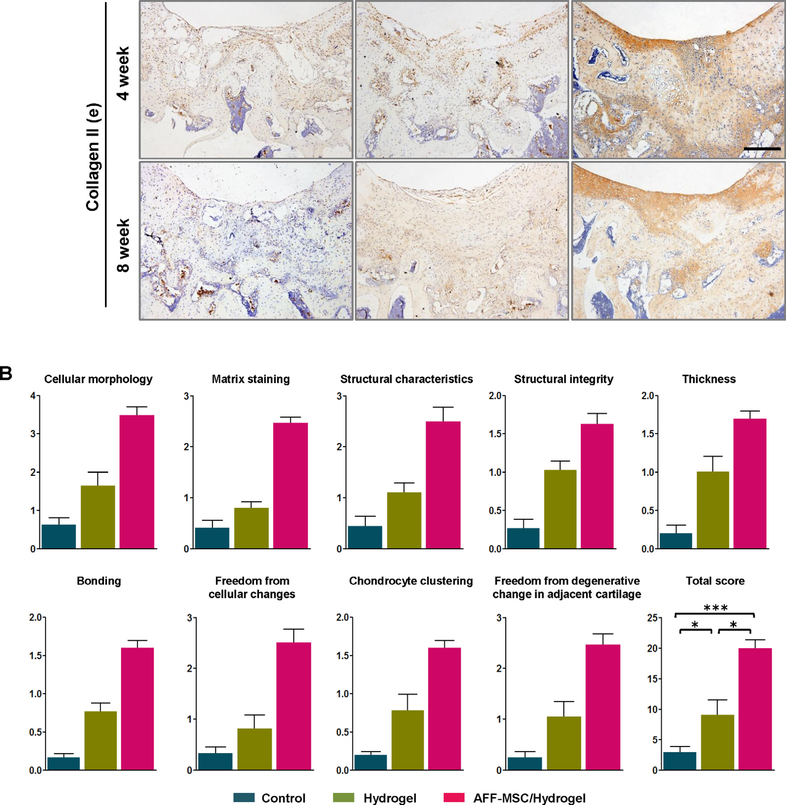
By histological analysis, we found that there was an unfilled and disorganized structure to the defect in the control groups, however, cartilage repair and regeneration was confirmed in the AFF-MSC/hydrogel groups at weeks 4 and 8 by H&E (Fig. 7Aa), Toluidine blue (Fig. 7Ab), and Safranin O and Fast green staining (Fig. 7Ac). The defect seemed to have been refilled in the hydrogel alone group, but the filled tissue probably was identified by Masson staining as an overgrowth of fibrous tissue (Fig. 7Ad). Histological scoring further demonstrated that the total score for the AFF-MSC/hydrogel group (20.05 ± 1.73) was significantly higher than the control (2.98 ± 0.49) and hydrogel alone (9.11 ± 1.36) groups (Fig. 7B). Furthermore, IHC analysis of collagen type II expression supported that the formation of hyaline cartilage in AFF-MSC/hydrogel group was higher than the control and hydrogel alone groups by week 8 (Fig. 7Ae). These data indicated that the AFF-MSC/hydrogel group had higher tissue repair and regenerative capacity.
Fig. 7.
Histologic analysis and quantitative evaluation of the cartilage defect repair. (A) Hematoxylin and eosin, Toluidine blue, Safranin O and Fast green, Masson staining, and immunohistochemistry staining for collagen type II at 4 and 8 weeks. Scale bar: 1 mm (a); 500 μm (b–e). (B)The modified O’Driscoll histology scoring of cartilage defect repair at 8 weeks after surgery (n = 3).*P< 0.05, ***P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The numbers of patients undergoing arthroscopic procedures annually have reached the level of multi-millions globally, and AFF is normally discarded as medical waste. In this study, we report that AFF-derived MSCs could be a vital source of MSCs. Furthermore, based on our recently developed protocol of combining hyper-branched polyPEGDA with HA to achieve rapid gelation [22,23], we adapted a one-step hydrogel for encapsulating MSCs and demonstrated the therapeutic value of the AFF-MSC/hydrogel composite in promoting cartilage repair in a rat model.
This is the first study to systematically demonstrate that AFF medical waste contains a large number of MSCs that can be harvested through simple adherence to cell culture vessels. Several previous studies have reported that synovial fluid is a source of stem cells, but only a few millilitres of fluid can be aspirated from the joint cavity, and stem-cell yields and quality vary greatly by donor [27–29]. Such variation may be attributed to the uneven distribution of cell components in a rather small volume of synovial fluid. Joint conditions, such as local injuries, inflammation and degeneration, may be other contributing factors to this variation [30–32]. In our approach, we collected 500 mL AFF prior to any other arthroscopic procedure, thus avoiding a cartilage shaving that can produce a mass of cartilage particles and drilling that can incur bone-marrow leakage. The cell components of the AFF included those from the complete synovial fluid, synovial membrane, superficial zone of AC, meniscus, and even the surrounding adipose and ligament tissues, which is more similar to the constituents of the innate articular cavity. The relatively easy clinically access to the AFF and simple sample collection procedures through centrifugation without enzyme digestion are unique advantages to AFF-MSCs. Importantly, the length of time needed to expand these stem cells to a quantity sufficient for therapeutic use is relatively short, thus significantly facilitating their utility in subsequent clinical procedures.
Our in vitro assays showed that AFF-MSCs possess typical MSCs features, including an excellent capacity for colony formation, expansion in culture, multi-lineage differentiation and expression of specific MSC surface markers.
Previous studies have indicated that MSCs can actively respond to injury and osteoarthritis (OA) and that their properties may be altered in mid-late stages of OA [33]. Therefore, we selectively collected samples from patients diagnosed with only early-mid-phase knee OA or with a fresh accessory tissue injury of the knee joint, including meniscus, cruciate ligament, and collateral ligament injury (Supplementary Table S1). Consequently, the cells collected from all samples included in this study exhibited comparable proliferative and differential capacity, with at least 2 × 108 cells obtained by passage 5. The quantity of cells may be sufficient for some clinical applications, as several recent clinical trials have reported that ~1.5 × 108 bone marrow MSCs [34] and ~1 × 108 adipose derived MSCs [35] can effectively relieve pain and enhance joint functions in patients with OA. Our preliminary assay showed that AFF-MSCs from a single sample could actually be expanded over 10 passages and reach the cell number of 1010–1011 MSCs while still maintaining MSC properties, as verified by differentiation potential and transcriptome analysis (unpublished).
MSCs from synovial tissues and fluid are preferential for cartilage regeneration compared to MSCs isolated from the bone marrow or other tissue sources [36,37]. The main reason underlying this might be that synovial-derived MSCs include cells originating from AC as tissue endogenous stem and progenitor cells, which preferentially differentiate into cells of the chondrogenic lineage [33]. Indeed, an early study using genomic, transcriptomic, and proteomic approaches showed that synovium or synovial fluid-derived MSCs have a higher cartilage repair capacity [38]. In line with these previous studies, we also found that chondrogenesis and the cartilage regenerative capacity of AFF-MSCs is highly efficient. Furthermore, the human AFF-MSCs transplanted into our rat model had a strong capacity to promote cartilage regeneration. This effect occurred despite the xenogenic nature of our experimental system, which could have suboptimal therapeutic effects. It is worth of pointing out that the distributional and functional fates of implanted cells should be traced and monitored in future studies in order to better understand the therapeutic mechanisms MSC therapy. We propose that human AFF-MSCs will likely exert a much better therapeutic effect than that observed in our rat model if transplanted into human cartilage defects.
HA hydrogels have remarkable properties, such as an ability to regulate the stemness and differentiation of stem cells [39,40], improve tissue adhesive properties and appropriate biodegradation rate [41]. The PEGDA hydrogel has been used in animal models and clinical trials, and has a proved capacity to mediate cartilage regeneration [42]. However, there are considerable limitations restraining their clinical utility, such as a requirement of chemical or enzymatic cross-linkers and catalysts [43], and a UV- or photo-cross-linking strategy for gelation, which is not only achieve in practice but also can cause cytotoxicity [44,45]. Moreover, due to the limited number of functional groups of HA molecules, it can be difficult for HA hydrogels to achieve fine control of their cross-linking or hydrogel characteristics. Our study used the novel hyper-branched structure of the HB-PEGDA with a highly number of functional acrylate groups as a tissue-adhesive hydrogel precursor. This precursor provided more binding positions for the HA thiol groups, thus permitting tunability of hydrogel characteristics—namely, increased structural stability and reduced gelation time. Consequently, the HB-PEGDA/HA gelation system can regulate stem-cell behaviours and mediate cutaneous healing [22,23], and promote cartilage regeneration. We thus consider that HB-PEGDA/HA is a desirable stem-cell delivery scaffold. Finally, the HB-PEGDA/HA hydrogel is composed of medically approved materials that would give rise to few safety concerns.
A subjective, reliable, sensitive and quantitative technique is required to detect early changes in the cartilage surface. SD-OCT, also known as an “optical biopsy”, is a newly developed, noninvasive and non-destructive optical imaging technology [46]. Compared with X-ray, ultrasound and magnetic resonance imaging, SD-OCT permits higher resolution and higher sensitivity, real-time imaging [47,48], and has already been used successfully in clinic to monitor cartilage surface [49]. Here, we also proved the value of this technique in qualitatively and quantitatively evaluating cartilage repair and regeneration in our rat model.
In summary, this study tested the feasibility of AFF-MSCs encapsulated within HB-PEGDA/HA hydrogel to repair full-thickness cartilage defects. In clinical settings, AFF-MSCs will likely have utility in treating degenerative pathologies, such as OA. Intraarticular injection, with or without a hydrogel, would be an appropriate approach, but a more detailed in vivo validation of AFF-MSCs using OA models is now needed. While AFF-MSCs may be convenient for autologous use in most cases, they may need to be characterized at the single-cell and molecular levels, depending on the disease and injury types of the donors, before they are ready for allogenic use as a standardized shelf product. Although a cell density of 1 × 106 cells/mL seeded into the hydrogels [50,51], was sufficient for chondrogenesis in vitro and cartilage repair in vivo, this number still needs to be optimized for human transplantation therapy. Finally, to take full advantage of AFF-MSCs in future clinical studies and applications, it would be worth carefully comparing AFF-MSCs with MSCs from other sources, in terms of their expandability in vitro, exosome profile, molecular regulation, differentiation potentials and ability to regenerate joint tissue defects.
5. Conclusion
This study explored a new approach to regenerate cartilage based on human MSC isolated from AFF. We fabricated a new, one-step rapid cross-linking hydrogel system (HB-PEGDA/HA) for cell delivery. We found that our AFF-MSC/hydrogel underwent efficient chondrogenesis in vitro and had a strong capacity to promote cartilage repair in vivo. These data suggest that the AFF-MSC/hydrogel may have potential uses in cell therapy and tissue engineer of the AC in the future.
Supplementary Material
Statement of Significance.
Many attempts have been made to repair the defects of articular cartilage, including mesenchymal stem cell (MSC)-based tissue engineering strategies. Optimizing MSC sources and their delivery approaches still remain clinically challenging. Recent studies determined that MSCs derived from synovium and synovial fluid exhibited superior chondrogenic potential. However, no feasible methods to harvest these human tissues and cells have been impeding them for clinical application. Hereby, we explored a simple and easy accessible approach to obtain a new stem cell source from arthroscopic flushing fluid (AFF-MSCs), which probably contains plenty of MSCs from synovium and synovial fluid. Further experiments demonstrated that encapsulation of these stem cells with one-step rapid cross-linked polyPEGDA/HA hydrogel held very encouraging potential for cartilage regeneration.
Acknowledgements
This work was supported by grants from Natural Science Foundation of China (81472126), the Shenzhen Science and Technology Innovation Committee (JCYJ20150324141711672 and JCYJ2016 0226192924528 to GQZ, JCYJ20150626090344603 and CKCY20160 82917372416 to JL, JCYJ20160331114205502, JCYJ20160301111 338144 to DC,JCYJ20150529143500954 to YCH)and the Guangdong Province Science and Technology Project (2017A010105026 to XTZ). J Li was also partially supported by China Postdoctoral Science Foundation (2016M602522). This work was also funded (to WW) by the Science Foundation Ireland (SFI) Principal Investigator Award (13/IA/1962), the Investigator Award (12/IP/1688) and the Health Research Board (HRA-POR-2013-412). We sincerely thank Mr Jie Du, Ms Peilin Hu and Yuhong Liang for their technical assistance in cell culture and animal experiments and also appreciate Dr Weimin Zhu for collecting some of the clinical AFF samples in our pilot experiments. We are also grateful to Ms Jessica Tamanini for English language editing.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.actbio.2018.08.029.
References
- [1].Hunziker EB, Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects, Osteoarthritis Cartilage 10 (2002) 432–463. [DOI] [PubMed] [Google Scholar]
- [2].Marcacci M, Filardo G, Kon E, Treatment of cartilage lesions: what works and why?, Injury 44 (Suppl 1) (2013) S11–S15 [DOI] [PubMed] [Google Scholar]
- [3].Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA, Repair and tissue engineering techniques for articular cartilage, Nat. Rev. Rheumatol 11 (2015) 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bae DK, Yoon KH, Song SJ, Cartilage healing after microfracture in osteoarthritic knees, Arthroscopy: J. Arthroscopic Related Surg.: Offic. Public. Arthroscopy Assoc. North Am. Int. Arthroscopy Assoc 22 (2006) 367–374. [DOI] [PubMed] [Google Scholar]
- [5].Behery OA, Harris JD, Karnes JM, Siston RA, Flanigan DC, Factors influencing the outcome of autologous chondrocyte implantation: a systematic review, J. Knee Surg 26 (2013) 203–211. [DOI] [PubMed] [Google Scholar]
- [6].Marlovits S, Aldrian S, Wondrasch B, Zak L, Albrecht C, Welsch G, Trattnig S, Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects, Am. J. Sports Med 40 (2012) 2273–2280. [DOI] [PubMed] [Google Scholar]
- [7].Huey DJ, Hu JC, Athanasiou KA, Unlike bone, cartilage regeneration remains elusive, Science 338 (2012) 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH, Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study, Am. J. Sports Med 38 (2010) 1110–1116. [DOI] [PubMed] [Google Scholar]
- [9].Li J, Pei M, Cell senescence: a challenge in cartilage engineering and regeneration, Tissue Eng. Part B, Rev 18 (2012) 270–287. [DOI] [PubMed] [Google Scholar]
- [10].Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ, Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy, Stem Cell Res. Ther 6 (2015) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Islam A, Hansen AK, Mennan C, Martinez-Zubiaurre I, Mesenchymal stromal cells from human umbilical cords display poor chondrogenic potential in scaffold-free three dimensional cultures, Eur. Cells Mater 31 (2016) 407–424. [DOI] [PubMed] [Google Scholar]
- [12].Huang YZ, Xie HQ, Silini A, Parolini O, Zhang Y, Deng L, Huang YC, Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: current status and future perspectives, Stem Cell Rev. 13 (2017) 575–586. [DOI] [PubMed] [Google Scholar]
- [13].Frisbie DD, McCarthy HE, Archer CW, Barrett MF, McIlwraith CW, Evaluation of articular cartilage progenitor cells for the repair of articular defects in an equine model, J. Bone Joint Surg. Am 97 (2015) 484–493. [DOI] [PubMed] [Google Scholar]
- [14].Hori J, Deie M, Kobayashi T, Yasunaga Y, Kawamata S, Ochi M, Articular cartilage repair using an intra-articular magnet and synovium-derived cells, J. Orthopaedic Res.: Offic. Public. Orthopaedic Res. Soc 29 (2011) 531–538. [DOI] [PubMed] [Google Scholar]
- [15].Tang HC, Chen WC, Chiang CW, Chen LY, Chang YC, Chen CH, Differentiation effects of platelet-rich plasma concentrations on synovial fluid mesenchymal stem cells from pigs cultivated in alginate complex hydrogel, Int. J. Mol. Sci 16 (2015) 18507–18521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ando W, Kutcher JJ, Krawetz R, Sen A, Nakamura N, Frank CB, Hart DA, Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788. [DOI] [PubMed] [Google Scholar]
- [17].Owings MF, Kozak LJ, Ambulatory and inpatient procedures in the United States, 1996. Vital and health statistics Series 13, Data from the National Health Survey. (1998) 1–119. [PubMed] [Google Scholar]
- [18].Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM, Wray NP, A controlled trial of arthroscopic surgery for osteoarthritis of the knee, New Engl. J. Med 347 (2002) 81–88. [DOI] [PubMed] [Google Scholar]
- [19].Alleyne KR, Galloway MT, Management of osteochondral injuries of the knee, Clin. Sports Med 20 (2001) 343–364. [DOI] [PubMed] [Google Scholar]
- [20].Yang J, Zhang YS, Yue K, Khademhosseini A, Cell-laden hydrogels for osteochondral and cartilage tissue engineering, Acta Biomater. 57 (2017) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chuah YJ, Peck Y, Lau JE, Hee HT, Wang DA, Hydrogel based cartilaginous tissue regeneration: recent insights and technologies, Biomater. Sci 5 (2017) 613–631. [DOI] [PubMed] [Google Scholar]
- [22].Dong Y, Sigen A, Rodrigues M, Li X, Kwon SH, Kosaric N, Khong S, Gao Y, Wang W, Gurtner GC, Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing, Adv. Funct. Mater 27 (2017). 1606619. [Google Scholar]
- [23].Dong YX, Qin Y, Dubaa M,Killion J, Gao YS, Zhao TY, Zhou DZ, Duscher D, Geever L, Gurtner GC, Wang WX, A rapid crosslinking injectable hydrogel for stem cell delivery, from multifunctional hyperbranched polymers via RAFT homopolymerization of PEGDA, Polym. Chem 6 (2015) 6182–6192. [Google Scholar]
- [24].Benaglia M, Rizzardo Z, Alberti A, Guerra M, Searching for more effective agents and conditions for the RAFT Polymerization of MMA: influence of dithioester substituents, solvent, and temperature, Macromolecules 38 (2005) 3129–3140. [Google Scholar]
- [25].Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S, A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II, Am. J. Sports Med 38 (2010) 880–890. [DOI] [PubMed] [Google Scholar]
- [26].Orth P, Madry H, Complex and elementary histological scoring systems for articular cartilage repair, Histol. Histopathol 30 (2015) 911–919. [DOI] [PubMed] [Google Scholar]
- [27].Hatakeyama A, Uchida S, Utsunomiya H, Tsukamoto M, Nakashima H, Nakamura E, Pascual-Garrido C, Sekiya I, Sakai A, Isolation and characterization of synovial mesenchymal stem cell derived from hip joints: a comparative analysis with a matched control knee group, Stem Cells Int. 2017 (2017) 9312329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim YS, Lee HJ, Yeo JE, Kim YI, Choi YJ, Koh YG, Isolation and characterization of human mesenchymal stem cells derived from synovial fluid in patients with osteochondral lesion of the talus, Am. J. Sports Med 43 (2015) 399–406. [DOI] [PubMed] [Google Scholar]
- [29].de Sousa EB, Casado PL, Moura Neto V, Duarte ME, Aguiar DP, Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic perspectives, Stem Cell Res. Ther 5 (2014) 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, Chapman T, Emery P, Hatton P, McGonagle D, Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level, Arthritis Rheumatism 58 (2008) 1731–1740. [DOI] [PubMed] [Google Scholar]
- [31].Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D, Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis, Arthritis Rheumatism 50 (2004) 817–827. [DOI] [PubMed] [Google Scholar]
- [32].Katagiri K, Matsukura Y, Muneta T, Ozeki N, Mizuno M, Katano H, Sekiya I, Fibrous synovium releases higher numbers of mesenchymal stem cells than adipose synovium in a suspended synovium culture model, Arthroscopy: J. Arthroscopic Related Surg.: Offic. Public. Arthroscopy Assoc. N. Am. Int. Arthroscopy Assoc 33 (2017) 800–810. [DOI] [PubMed] [Google Scholar]
- [33].Jiang Y, Tuan RS, Origin and function of cartilage stem/progenitor cells in osteoarthritis, Nat. Rev. Rheumatol 11 (2015) 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vangsness CT Jr., Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M, Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study, J. Bone Joint Surg. Am 96 (2014) 90–98. [DOI] [PubMed] [Google Scholar]
- [35].Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H,Shin JS, Shin IS, Ra JC, Oh S, Yoon KS, Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial, Stem Cells 32 (2014) 1254–1266. [DOI] [PubMed] [Google Scholar]
- [36].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell 126 (2006) 677–689. [DOI] [PubMed] [Google Scholar]
- [37].Mak J, Jablonski CL, Leonard CA,Dunn JF, Raharjo E,Matyas JR,Biernaskie J, Krawetz RJ, Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model, Sci. Rep 6 (2016) 23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C, Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo, Arthritis Rheumatism 63 (2011) 1289–1300. [DOI] [PubMed] [Google Scholar]
- [39].Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G, Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells, PNAS 104 (2007) 11298–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chung C, Burdick JA, Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis, Tissue Eng. Part A 15 (2009) 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Highley CB, Prestwich GD, Burdick JA, Recent advances in hyaluronic acid hydrogels for biomedical applications, Curr. Opin. Biotechnol 40 (2016) 35–40. [DOI] [PubMed] [Google Scholar]
- [42].Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, Coburn J, Hui AY, Marcus N, Gold GE, Elisseeff JH, Human cartilage repair with a photoreactive adhesive-hydrogel composite, Sci. Trans. Med 5 (2013). 167ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yoon DS, Lee Y, Ryu HA, Jang Y, Lee KM, Choi Y, Choi WJ, Lee M, Park KM, Park KD, Lee JW, Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing, Acta Biomater. 38 (2016) 59–68. [DOI] [PubMed] [Google Scholar]
- [44].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A, Cell-laden microengineered gelatin methacrylate hydrogels, Biomaterials 31 (2010) 5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero-Martin JM, Khademhosseini A, Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels, Adv. Funct. Mater 22 (2012) 2027–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fujimoto JG, Brezinski ME, Tearney GJ, Boppart SA, Bouma B, Hee MR,Southern JF, Swanson EA, Optical biopsy and imaging using optical coherence tomography, Nat. Med 1 (1995) 970–972. [DOI] [PubMed] [Google Scholar]
- [47].Reif R, Wang RK, Label-free imaging of blood vessel morphology with capillary resolution using optical microangiography, Quant. Imaging Med. Surg 2 (2012) 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].White B, Pierce M, Nassif N, Cense B, Park B, Tearney G, Bouma B, Chen T, de Boer J, In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical coherence tomography, Opt. Express 11 (2003) 3490–3497. [DOI] [PubMed] [Google Scholar]
- [49].te Moller NC, Brommer H,Liukkonen J, Viren T, Timonen M, Puhakka PH,Jurvelin JS, van Weeren PR, Toyras J, Arthroscopic optical coherence tomography provides detailed information on articular cartilage lesions in horses, Vet. J 197 (2013) 589–595. [DOI] [PubMed] [Google Scholar]
- [50].Lee KB, Hui JH, Song IC, Ardany L, Lee EH, Injectable mesenchymal stem cell therapy for large cartilage defects-a porcine model, Stem Cells 25 (2007) 2964–2971. [DOI] [PubMed] [Google Scholar]
- [51].Murphy JM, Fink DJ, Hunziker EB, Barry FP, Stem cell therapy in a caprine model of osteoarthritis, Arthritis Rheumatism 48 (2003) 3464–3474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.