Abstract
Background:
Severe congenital diaphragmatic hernia (CDH) requiring extracorporeal membrane oxygenation (ECMO) is associated with high mortality. Timing of CDH repair relative to ECMO therapy remains controversial. Our hypothesis was that survival would significantly differ between those who underwent repair during ECMO and those who underwent repair after ECMO therapy.
Methods:
We examined de-identified data from the CDH Study Group (CDHSG) registry from 1995–2005 on patients who underwent repair and ECMO therapy (n=636). We used Cox regression analysis to assess differences in survival between those repaired during and after ECMO.
Results:
Five covariates were significantly associated with mortality: timing of repair relative to ECMO (P=0.03), defect side (P=0.01), ECMO run length (P<0.01), need for patch repair (P=0.03), birth weight (P<0.01) and Apgar score at five minutes (P=0.03). Birth year, inborn vs. transfer status, diaphragmatic agenesis, age at repair and presence of cardiac or chromosomal abnormalities were not associated with survival. Repair after ECMO therapy was associated with increased survival relative to repair on ECMO (hazard ratio=1.407, P=0.03).
Conclusion:
These data suggest that CDH repair after ECMO therapy is associated with improved survival compared to repair on ECMO, despite controlling for factors associated with the severity of CDH.
Keywords: Congenital Diaphragmatic Hernia, extracorporeal membrane oxygenation
Severe congenital diaphragmatic hernia (CDH) is a life-threatening condition that often requires treatment with extracorporeal membrane oxygenation (ECMO) [1–3]. The optimal timing of repair of CDH in relation to treatment with ECMO remains controversial. Repair on ECMO allows for the removal of the compressive effects of the herniated viscera on the heart and lungs, as well as allowing for cardiopulmonary support during the repair [3, 4]. The primary disadvantage to repair on ECMO is the increased risk of bleeding due to the anticoagulation necessary for circuit function [5, 6]. One retrospective study at two institutions showed higher survival when repair was performed after ECMO [7]. We sought to compare outcomes when CDH repair was performed on ECMO compared to cases in which it was performed after ECMO.
The approach to surgical management of CDH in patients requiring ECMO varies widely between institutions, including repair within 48 hours of initiation of ECMO [8, 9] or waiting until the patient is nearly ready to be decannulated before attempting repair [10–12]. Our data set was drawn from all reporting institutions contributing to the CDHSG, regardless of which of the above surgical approaches was employed.
METHODS:
Registry Data
We examined a limited, de-identified data set of 1,108 patients from the registry of the Congenital Diaphragmatic Hernia Study Group (CDHSG) from 1995–2005. As the data was deidentified, this project was exempted from continuous institutional review board oversight. The data in this registry is provided from over 50 centers around the world and includes information on multiple aspects of the clinical course of patients with CDH [13]. The cases included in our dataset were those patients who underwent both repair of CDH and ECMO therapy. Therefore our dataset excluded less severe cases in which ECMO was not required (2,830 cases), as well as very severe cases of CDH in which all patients died prior to repair (204 cases). A total of 4,146 CDH cases were entered in the CDHSG registry over this twenty-year period; the 1,108 cases that were extracted for our analysis represent 26% of that total.
Since we sought to examine the association between timing of repair and mortality in the context of a patient with CDH on ECMO who still requires repair, we excluded some patients from our statistical analysis (Fig. 1). From our initial data set of 1,108 patients, we chose to exclude patients who had repair of CDH but later required ECMO, as their clinical course did not require a decision on whether to repair on ECMO or after decannulation (n=140). We also chose to exclude those patients who were repaired after 2 runs of ECMO for the same reason (n=7). We then performed one round of regression analysis; of the 963 cases remaining in our data set, 709 had all the requisite registry information to be included.
Figure 1.
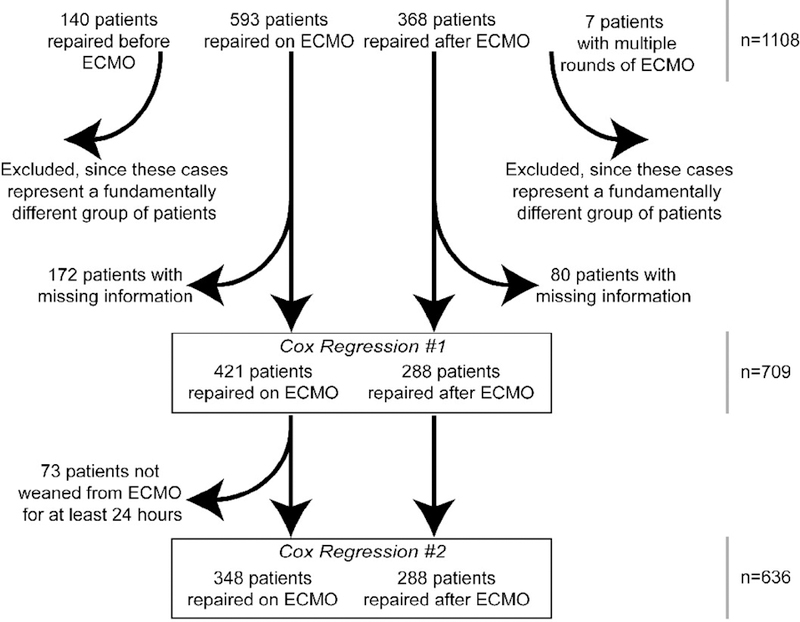
Process of Inclusion of Data from the Initial Sample of 1,108 Patients from the Congenital Diaphragmatic Hernia Study Group Registry
We then attempted to control for the fact that all the patients repaired after ECMO had, by definition, been weaned off ECMO, while this was not necessarily true for the group of patients repaired on ECMO. This would represent a potential bias toward increased disease severity among those repaired on ECMO. Therefore, we excluded all patients who underwent repair on ECMO but could not be successfully weaned from ECMO, which we defined as those dying within 24 hours of cessation of ECMO (n=73). This gave us a final data set of those patients who had surgical repair of CDH, received ECMO treatment, and were successfully weaned from ECMO for at least 24 hours (n=636), which we then entered into the second round of regression analysis.
Cox Regression Analysis
To address the relationship between timing of repair on ECMO and survival, we used a Cox regression procedure, also known as proportional hazards analysis [14]. Cox regression is a form of survival analysis that identifies the degree to which different factors contribute to the odds of an event (in this case, death) over time. The regression equation takes the form:
where h(t) is the expected hazard, exp is the exponential function (ex), and each βx term represents a coefficient generated by the regression analysis.
For this study, the endpoint was defined as days of life at either death or discharge home. The first step was calculating Kaplan-Meier survival curves to judge the appropriateness of the Cox regression model (Fig. 2). Second, each considered covariate was examined for any possible correlations with other covariates that might bias the results. Finally, the covariates were added into a Cox regression model; then using a backward-likelihood-ratio procedure, the covariates that were not significantly associated with survival at the level of 0.10 were removed. We then generated survival curves for those repaired on ECMO and after ECMO with all other covariates held constant (Fig. 3). We repeated this final step for the data set once the 73 patients who could not be weaned from ECMO were removed (Fig. 4). Tests of statistical significance were run on each coefficient in the regression equation to assess for a significant association between that covariate and survival.
Figure 2.
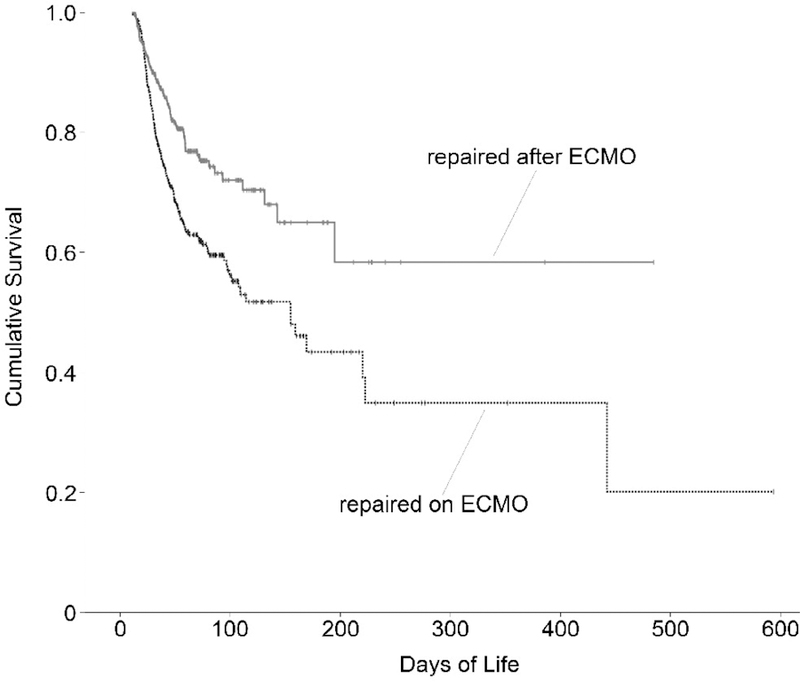
Kaplan-Meier Survival Curves for Patients Undergoing Congenital Diaphragmatic Hernia Repair on ECMO and After ECMO Therapy
Figure 3.
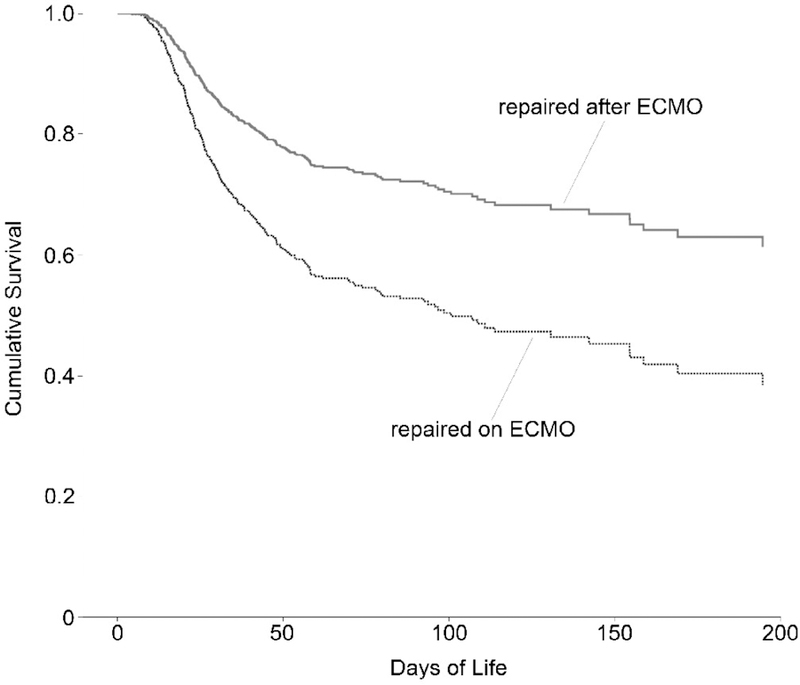
Proportional Hazards Survival Model Separated by Timing of Congenital Diaphragmatic Hernia Repair Relative to ECMO Treatment, Limited to 200 days (Total n=709)
Figure 4.
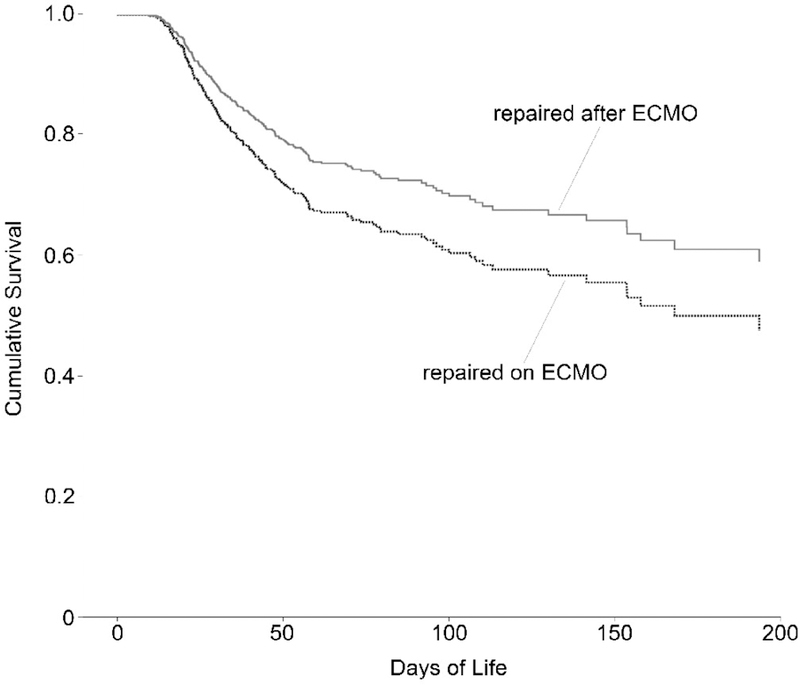
Proportional Hazards Survival Model Separated by Timing of Congenital Diaphragmatic Hernia Repair Relative to ECMO Treatment, Limited to 200 Days, After Removal of Cases who were Repaired on ECMO but Unable to be Weaned from ECMO for 24 Hours (Total n=636)
Other outcomes
We examined oxygen requirements of survivors by comparing ventilator-free days (number of the first 60 days of life when the patient was not mechanically ventilated) between the two groups, as well as oxygen use at discharge. All statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, Ill.) with statistical significance set at 0.05.
RESULTS:
The overall results of our Cox regression analysis were similar for the data set including those patients who underwent surgery but did not survive their ECMO run and the data set (n=709) and the data set excluding those patients (n=636). We examined baseline statistics of each data set, and report them for the final dataset (n=636) in Table 1. Kaplan-Meier survival functions for each group (those who underwent repair on ECMO and after ECMO) are shown in Figure 2. Since the Kaplan-Meier curves appeared to diverge, it was judged to be appropriate to pursue Cox regression analysis. In examining the bivariate correlations of individual covariates, none were correlated to a degree that would preclude their use as covariates. The maximum correlation coefficient between any two covariates was 0.18.
Table 1.
Baseline Statistics for 635 Patients with Congenital Diaphragmatic Hernia in the CDH Study Group Registry
| Repaired on ECMO | Repaired after ECMO | total | test statistic* | |
|---|---|---|---|---|
| n | 348 | 288 | 636 | |
| Birth data | ||||
| Mean birth weight (kg) | 3.10 ± 0.52 | 3.14 ± 0.52 | 3.11 ± .052 | t = −1.05 (P=0.30, df=612) |
| Mean Apgar score at 5 minutes | 6.00 ± 2.05 | 6.20 ± 2.12 | 6.15 ± 2.09 | t = −1.17 (P=0.24, df=601) |
| Demographics | ||||
| Significant cardiac defects (%) | 15 (4.3) | 15 (5.2) | 37 (5.2) | χ2 = 0.29 (P=0.59, df=1) |
| Significant genetic abnormalities (%) | 9 (2.6) | 5 (1.7) | 14 (2.0) | χ2 = 0.52 (P=−0.47, df=1) |
| Outborn (%) | 220 (63.2) | 176 (61.3) | 443 (61.9) | χ2 = 0.24 (P=0.62, df=1) |
| Right-sided CDH (%) | 72 (20.7) | 74 (25.8) | 160 (22.4) | χ2 = 2.31 (P=0.13, df=1) |
| Agenesis of diaphragm (%) | 139 (39.9) | 99 (34.5) | 257 (35.9) | χ2 = 1.92 (P=0.16, df=1) |
| ECMO & Surgery | ||||
| Mean length of ECMO run (days) | 12.7 ± 6.5 | 8.4 ± 4.9 | 10.6 ± 6.1 | t = 9.4 (P<0.01, df=629) |
| Mean age at CDH repair (days) | 8.9 ± 5.6 | 15.3 ± 8.6 | 10.8 ± 7.9 | t = −11.0 (P<0.01, df=471) |
| Patch closure of diaphragm (%) | 287 (82.5) | 221 (77.0) | 560 (78.2) | χ2 = 2.94 (P=0.09, df=1) |
equal variances not assumed
We then analyzed our covariates with two Cox regression procedures, which did not yield significantly different results. Since the second Cox regression is a more conservative analysis, we report those results in Table 2. Likelihood-ratio analysis showed that five covariates were not found to be significantly associated with mortality and their removal did not significantly affect the regression model (year of birth, inborn vs. transfer status, presence of cardiac anomalies, presence of chromosomal anomalies, and days of life at repair; alpha for removal of a covariate was 0.10). The five remaining variables were found to contribute significantly to the regression model: CDH repair on ECMO compared to repair after ECMO therapy (HR=1.41, P=0.03), birth weight (HR=0.64, P<0.01), Apgar score at five minutes (HR=0.93, P=0.03), left-sided CDH compared to right-sided CDH (HR=1.66, P=0.01), ECMO run length (HR=1.04, P<0.01), and primary repair compared to patch repair (HR=0.59, P=0.03).
Table 2.
Covariates Used in Cox Regression Analysis of Survival Among 635 Patients from the CDH Study Group Registry
| 95% CI | |||||
|---|---|---|---|---|---|
| Covariates found to be significantly associated with mortality (included in regression model) | hazard ratio | lower | upper | Wald χ2 (df=1) | P |
| Repair on ECMO vs. repair after ECMO | 1.41 | 1.03 | 1.92 | 4.60 | 0.03 |
| Birth weight | 0.64 | 0.49 | 0.84 | 10.49 | 0.00 |
| Apgar score at five minutes | 0.93 | 0.87 | 0.99 | 4.67 | 0.03 |
| Left-sided defect vs. right-sided | 1.66 | 1.14 | 2.41 | 7.08 | 0.01 |
| ECMO run length | 1.04 | 1.02 | 1.06 | 11.62 | 0.00 |
| Primary repair vs. patch repair | 0.59 | 0.37 | 0.94 | 4.89 | 0.03 |
| Covariates not found to be significantly associated with mortality (excluded from regression)* | Wald χ2 (df=1) | P | |||
| Year of birth | <0.01 | 0.96 | |||
| Inborn vs. outborn status | 0.28 | 0.59 | |||
| diaphragmatic agenesis | 0.25 | 0.62 | |||
| Presence of major cardiac abnormalities | 0.50 | 0.48 | |||
| Presence of chromosomal abnormalities | 0.81 | 0.37 | |||
| Days of life at repair | 1.34 | 0.25 | |||
(no hazard ratio calculated since these variables are not part of the regression model)
Separate survival curves were generated for those who underwent CDH repair during ECMO and after ECMO therapy (Figures 3 and 4). These are the predicted survival curves when all the covariates included in the regression are held at their mean values for this data set. As mentioned above, there is a statistically significant difference in the proportional hazard of death between those who had CDH repair after ECMO versus those repaired on ECMO (HR=1.41, 95% CI 1.03–1.92, P=0.03) relative to after-ECMO repair.
Among the survivors, there was no significant difference found between the two groups in terms of ventilator-free days at 60 days (t=0.66, p=0.51) or other measures of short-term morbidity including days of life at discharge and the need for supplemental oxygen or tube feeds at discharge (Table 3).
Table 3.
Other Outcomes for 488 Patients in the CDH Study Group Registry who Survived to Discharge
| Outcomes | Repaired on ECMO | Repaired after ECMO | total | test statistic* |
|---|---|---|---|---|
| n | 348 | 288 | 636 | |
| Survived (%) | 206 (48.2) | 222 (77.1) | 488 (60.6) | χ2=23.3 (P<0.01, df=1) |
| Days of life at extubation in surviors | 37.7 ± 58.1 | 32.7 ± 21.4 | 34.3 ± 41.5 | t=1.09 (P=0.28, df=235) |
| Ventilator-free days (out of 60) in survivors | 4.6 ± 5.9 | 5.4 ± 6.2 | 5.3 ± 6.3 | t=−1.34 (P=0.18, df=373) |
| Days of life at discharge (alive) | 79.3 ± 61.8 | 72.2 ± 56.5 | 74.1 ± 57.8 | t=1.241 (P=0.22, df=413) |
| Required supplemental oxygen at discharge (%) | 111 (56.6) | 107 (50.2) | 243 (52.4) | χ2=1.68 (P=0.20, df=1) |
| Required tube feeds at discharge (%) | 66 (53.2) | 50 (43.5) | 137 (47.9) | χ2=2.27 (P=0.13, df=1) |
equal variances not assumed
DISCUSSION:
Although survival in congenital diaphragmatic hernia (CDH) has improved in select centers [10, 15–17], overall survival remains approximately 68% [18]. The patients with highest risk for death are those who require extracorporeal membrane oxygenation (ECMO), with survival rates ranging from 50 to 65% [19, 20]. Although preoperative stabilization and delayed surgery has proven beneficial for CDH patients not requiring ECMO [21, 22], the optimal timing of CDH repair in patients requiring ECMO remains controversial.
Our analysis of data from the CDHSG registry shows increased survival among patients who undergo repair of CDH after ECMO therapy relative to those who undergo repair on ECMO, when holding constant the side of the defect, Apgar score at 5 minutes, birth weight, ECMO run length, and type of repair. The hazard ratio associated with repair on ECMO relative to repair after ECMO is 1.41. In other words, the odds of dying over a given time period for a patient repaired on ECMO is 1.41 times the odds of dying for a patient repaired after ECMO therapy. We found no significant association between year of birth, inborn versus transfer status, presence of cardiac anomalies, presence of chromosomal anomalies or days of life at repair and mortality. In analyzing outcomes among survivors, we did not find a significant difference in ventilator-free days of the first sixty days of life between the two groups.
The approach to surgical management of CDH in patients requiring ECMO varies widely between institutions. One institutional approach involves early repair on ECMO (repair within 48 hours of initiation of ECMO) with the idea of harnessing the advantages of ECMO and leaving more time for pulmonary recovery [8, 9]. Other institutions wait to repair CDH until approaching the end of the ECMO run (thus allowing more time for stabilization in anticipation of the repair), then weaning ECMO [10–12]. In a modification of this last approach, some institutions attempt to wean ECMO before repair, but repair CDH on ECMO if the patient cannot be weaned after about two weeks of ECMO.
Previous studies of timing of CDH repair in patients requiring ECMO include a review of CDHSG data that showed a lower rate of survival for those patients who were repaired on ECMO [1, 23]. One study of 36 patients with CDH requiring ECMO found improved survival among those repaired after ECMO therapy relative to those repaired on it [7].
We speculate that one reason for the survival benefit of postponing repair until ECMO has been weaned may be due to decreased bleeding risk associated with repair after ECMO therapy. Data from the registry of the Extracorporeal Life Support Organization (ELSO) showed a higher incidence of some types of bleeding complications in patients repaired on ECMO, as well as a significantly lower survival rate in that group [24]. A five-center review of 42 patients repaired on ECMO showed a similarly high incidence of hemorrhage and a low survival rate in those cases [25]. While aminocaproic acid has been shown to decrease the incidence of bleeding complications in CDH patients requiring ECMO [26–28], the use of such adjunct therapies is not captured in our data. New strategies and techniques to minimize bleeding may significantly decrease the risks of operating on ECMO and affect future analyses. Our data does not allow us to address the issue of bleeding directly, and therefore further investigation is necessary to elucidate the actual causes of the increased proportional hazard of mortality observed in this data set.
The strengths of our analysis include a large sample size from a high volume of registry data. Cox regression, or proportional hazards analysis, is a robust form of statistical analysis that allowed us to address the associations of each covariate with mortality. We were able to control for several factors that would normally obscure comparisons of patients based on their timing of CDH repair. In effect, Cox regression allowed us to assess survival while holding other factors constant within a group. Although we were able to control for several relevant factors, we were still limited to the use of proxies rather than any measurements that have been proven to directly quantify CDH severity. For example, the degree of pulmonary hypoplasia and pulmonary hypertension may have varied between the two groups, since this information is not directly captured in registry data. More specific indices of pulmonary hypoplasia (including lung-to-head ratio and lung volume calculated by fetal MRI) may prove better measures of CDH severity in future analyses.
We are unable to draw conclusions about the timing of CDH repair within the on-ECMO and after-ECMO groups. We had originally wanted to address the practice of repairing ECMO early in the course of ECMO (i.e. less than 48 hours after initiation) in addition to our between-groups comparison. This early-repair approach, which is theorized to allow for removal of compressive effects on the heart and allowing lung expansion and recovery during the remainder of the ECMO course, was only performed in 30 of the 636 patients in our data set. It would be interesting to compare survival associated with repair during the initial part of the ECMO run versus repair done later on in the ECMO run, but we do not have sufficient data in the CDHSG registry to do so. This avenue of research would also require information on institutional policies regarding the timing of repair during ECMO, institutional volume, expertise, and information on the extent to which individual patients’ treatment followed or deviated from institutional norms. Therefore we restricted our analysis to a comparison of all those repaired on ECMO versus all those repaired after ECMO therapy. We also acknowledge that patients differ in indications for ECMO and for surgery on ECMO, but feel that among patients with severe enough CDH to require ECMO, the range of specific indications is narrow enough to allow for retrospective analysis. We partially controlled for this by including “days of life at repair” as one of our covariates, but like defect size it was not significantly associated with survival.
There were a number of ancillary findings, some of which validate prior studies. We found a strong association between both Apgar score at 5 minutes and birth weight with survival. The CDHSG previously performed a logistic regression analysis that examined a number of factors. This analysis found Apgar score at five minutes and birth weight to be the only factors significantly associated with survival of CDH [29].
In contrast to previous studies, our analysis did not find evidence of a significant relationship between the presence of either cardiac or chromosomal abnormalities and survival. This was likely due to the relatively small number of patients with these conditions in our data set. Both analysis of data from the CDHSG and a meta-analysis of previous studies have shown an association between “major abnormalities” and mortality [30, 31]. Our data set excludes those patients who died before repair could be performed, a population more likely to have cardiac abnormalities, and this may be partly responsible for the lack of this association in our data.
We are unable to comment on any association between the size of the diaphragmatic defect and survival, although an association between defect size and mortality has been demonstrated in CDHSG registry data in the past [32]. This is most likely because our data is limited to those patients requiring ECMO and undergoing surgery, so we excluded a large number of patients with very small defect sizes (because they did not require ECMO) that contributed to those findings. We did include the presence of diaphragmatic agenesis as a covariate in our regression procedure, and therefore would have controlled for it in our regression analysis if it had proven to be significantly associated with mortality in our data. We also do not have long-term followup data that would allow us to comment on the morbidity associated with ECMO for CDH, a significant issue in the initial decision to initiate ECMO that remains unresolved [1, 33, 34].
In conclusion, these data suggest that CDH repair after ECMO therapy is associated with improved survival compared to repair on ECMO, despite controlling for factors associated with the severity of CDH. The potential selection bias in this study is reflective of the inherent nature of registry-based analysis but has been minimized by the Cox model. The limitations of our study discussed above prohibit us from saying with certainty that the timing of surgery relative to ECMO entirely explains the difference in mortality between those who underwent repair on ECMO compared to those who underwent repair after it. A randomized controlled trial has long been called for to help answer the question of optimal timing of CDH repair in patients who require ECMO. Pending such evidence, the strongest claim possible is that after controlling for severity between groups to the furthest extent that available registry data allows, CDH repair after ECMO therapy is associated with improved survival compared to repair on ECMO.
Acknowledgments
Grant information:
This research was supported by University of Michgan CTSA grant # UL1RR024986.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Khan AM, Lally KP: The Role of Extracorporeal Membrane Oxygenation in the Management of Infants with Congenital Diaphragmatic Hernia. Semin Perinatol 2005;29:118–122. [DOI] [PubMed] [Google Scholar]
- 2.The Congenital Diaphragmatic Hernia Study Group: Does extracorporeal membrane oxygenation improve survival in neonates with congenital diaphragmatic hernia? J Pediatr Surg 1999;34:720–724. [DOI] [PubMed] [Google Scholar]
- 3.Harrington KP, Goldman AP: The role of extracorporeal membrane oxygenation in congenital diaphragmatic hernia. Semin Pediatr Surg 2005;14:72–76. [DOI] [PubMed] [Google Scholar]
- 4.Connors RH, Tracy T, Bailey PV, et al. : Congenital diaphragmatic hernia repair on ECMO. J Pediatr Surg 1990;25:1043–1046. [DOI] [PubMed] [Google Scholar]
- 5.Austin MT, Lovvorn HN, Feurer ID, et al. : Congenital diaphragmatic hernia repair on extracorporeal life support: a decade of lessons learned. Am Surg 2004;70:389–395. [PubMed] [Google Scholar]
- 6.Wilson JM, Bower LK, Lund DP: Evolution of the technique of congenital diaphragmatic hernia repair on ECMO. J Pediatr Surg 1994;29:1109–1112. [DOI] [PubMed] [Google Scholar]
- 7.Sigalet DL, Tierney A, Adolph V, et al. : Timing of repair of congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation support. J Pediatr Surg 1995;30:1183–1187. [DOI] [PubMed] [Google Scholar]
- 8.Okuyama H, Kubota A, Oue T, et al. : Inhaled nitric oxide with early surgery improves the outcome of antenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surgery 2002;37:1188–1190. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JM, Lund DP, Lillehei CW, et al. : Congenital diaphragmatic hernia--A tale of two cities: The Boston experience. J Pediatr Surg 1997;32:401–405. [DOI] [PubMed] [Google Scholar]
- 10.Boloker J, Bateman DA, Wung J-T, et al. : Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg 2002;37:357–366. [DOI] [PubMed] [Google Scholar]
- 11.Rothenbach P, Lange P, Powell D: The use of extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia. Semin Perinatol 2005;29:40–44. [DOI] [PubMed] [Google Scholar]
- 12.Rozmiarek AJ, Qureshi FG, Cassidy L, et al. : Factors influencing survival in newborns with congenital diaphragmatic hernia: the relative role of timing of surgery. J Pediatr Surg 2004;39:821–824. [DOI] [PubMed] [Google Scholar]
- 13.Tsao K, Lally KP: The Congenital Diaphragmatic Hernia Study Group: a voluntary international registry. Semin Pediatr Surg 2008;17:90–97. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR: Regression Models and Life-Tables. J Royal Statistical Society 1972;34:187–220. [Google Scholar]
- 15.Downard CD, Jaksic T, Garza JJ, et al. : Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg 2003;38:729–732. [DOI] [PubMed] [Google Scholar]
- 16.Javid PJ, Jaksic T, Skarsgard ED, et al. : Survival rate in congenital diaphragmatic hernia: the experience of the Canadian neonatal network. J Pediatr Surg 2004;39:657–660. [DOI] [PubMed] [Google Scholar]
- 17.Kays DW, Langham MR Jr., Ledbetter DJ, et al. : Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg 1999;230:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Congenital Diaphragmatic Hernia Study Group: Report from the Congenital Diaphragmatic Hernia Study Group Published online at http://cdhsg.net/CDHSG_Report.doc, accessed 5/2008.
- 19.Morini F, Goldman A, Pierro A: Extracorporeal Membrane Oxygenation in Infants with Congenital Diaphragmatic Hernia: A Systematic Review of the Evidence. Eur J Pediatr Surg 2006;16:385–391. [DOI] [PubMed] [Google Scholar]
- 20.Reickert CA, Hirschl RB, Atkinson JB, et al. : Congenital diaphragmatic hernia survival and use of extracorporeal life support at selected level III nurseries with multimodality support. Surgery 1998;123:305–310. [PubMed] [Google Scholar]
- 21.Langer JC, Filler RM, Bohn DJ, et al. : Timing of surgery for congenital diaphragmatic hernia: Is emergency operation necessary? J Pediatr Surg 1998;23:731–734. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama DK, Motoyama EK, Tagge EM: Effect of preoperative stabilization on respiratory system compliance and outcome in newborn infants with congenital diaphragmatic hernia. J Pediatr 1991;118:793–799. [DOI] [PubMed] [Google Scholar]
- 23.Clark RH, Hardin WD, Hirschl RB, et al. : Current surgical management of congenital diaphragmatic hernia: A report from The Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg 1998;33:1004–1009. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez WD, Cheu HW: Hemorrhagic complications and repair of congenital diaphragmatic hernias: Does timing of the repair make a difference? Data from the Extracorporeal Life Support Organization. J Pediatr Surg 1994;29:1002–1006. [DOI] [PubMed] [Google Scholar]
- 25.Lally KP, Paranka MS, Roden J, et al. : Congenital diaphragmatic hernia. Stabilization and repair on ECMO. Ann Surg 1992;216:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz JR, Cofer BR, Warner BW, et al. : A multicenter trial of 6-aminocaproic acid (Amicar) in the prevention of bleeding in infants on ECMO. J Pediatr Surg 1998;33:1610–1613. [DOI] [PubMed] [Google Scholar]
- 27.Downard CD, Betit P, Chang RW, et al. : Impact of Amicar on hemorrhagic complications of ECMO: a ten-year review. J Pediatr Surg 2003;38:1212–1216. [DOI] [PubMed] [Google Scholar]
- 28.Wilson JM, Bower LK, Fackler JC, et al. : Aminocaproic acid decreases the incidence of intracranial hemorrhage and other hemorrhagic complications of ECMO. J Pediatr Surg 1993;28:536–541. [DOI] [PubMed] [Google Scholar]
- 29.The Congenital Diaphragmatic Hernia Study Group: Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg 2001;36:141–145. [DOI] [PubMed] [Google Scholar]
- 30.Graziano JN: Cardiac anomalies in patients with congenital diaphragmatic hernia and their prognosis: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg 2005;40:1045–1050. [DOI] [PubMed] [Google Scholar]
- 31.Skari H, Bjornland K, Haugen G, et al. : Congenital diaphragmatic hernia: A meta-analysis of mortality factors. J Pediatr Surg 2000;35:1187–1197. [DOI] [PubMed] [Google Scholar]
- 32.The Congenital Diaphragmatic Hernia Study Group: Defect Size Determines Survival in Infants With Congenital Diaphragmatic Hernia. Pediatrics 2007;120:651–657. [DOI] [PubMed] [Google Scholar]
- 33.Friedman S, Chen C, Chapman JS, et al. : Neurodevelopmental outcomes of congenital diaphragmatic hernia survivors followed in a multidisciplinary clinic at ages 1 and 3. J Pediatr Surg 2008;43:1035–1043. [DOI] [PubMed] [Google Scholar]
- 34.Davis PJ, Firmin RK, Manktelow B, et al. : Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr 2004;144:309–315. [DOI] [PubMed] [Google Scholar]


