Significance
Multiple myeloma (MM) is one of the most common hematological malignancies. Developing an effective treatment strategy for refractory/relapsed (R/R) MM represents a major challenge. In this trial, 17 R/R MM patients, who had previously experienced multiple lines of treatments, received a chimeric antigen receptor modified T (CAR T) cell preparation targeting two epitopes of BCMA, and a remarkable overall response of 88.2% was achieved. This work confirms the feasibility and importance of CAR T cell therapy in the treatment of patients with R/R MM. Meanwhile, the adverse events encountered in this study are analyzed, providing a reference for other studies. We also suggest that CAR T treatment can eventually be combined with other effective therapeutics to treat newly diagnosed MM for achieving a better result in future trials.
Keywords: multiple myeloma, chimeric antigen receptor modified T cells, biepitope, BCMA, cytokine release syndrome
Abstract
Relapsed and refractory (R/R) multiple myeloma (MM) patients have very poor prognosis. Chimeric antigen receptor modified T (CAR T) cells is an emerging approach in treating hematopoietic malignancies. Here we conducted the clinical trial of a biepitope-targeting CAR T against B cell maturation antigen (BCMA) (LCAR-B38M) in 17 R/R MM cases. CAR T cells were i.v. infused after lymphodepleting chemotherapy. Two delivery methods, three infusions versus one infusion of the total CAR T dose, were tested in, respectively, 8 and 9 cases. No response differences were noted among the two delivery subgroups. Together, after CAR T cell infusion, 10 cases experienced a mild cytokine release syndrome (CRS), 6 had severe but manageable CRS, and 1 died of a very severe toxic reaction. The abundance of BCMA and cytogenetic marker del(17p) and the elevation of IL-6 were the key indicators for severe CRS. Among 17 cases, the overall response rate was 88.2%, with 13 achieving stringent complete response (sCR) and 2 reaching very good partial response (VGPR), while 1 was a nonresponder. With a median follow-up of 417 days, 8 patients remained in sCR or VGPR, whereas 6 relapsed after sCR and 1 had progressive disease (PD) after VGPR. CAR T cells were high in most cases with stable response but low in 6 out of 7 relapse/PD cases. Notably, positive anti-CAR antibody constituted a high-risk factor for relapse/PD, and patients who received prior autologous hematopoietic stem cell transplantation had more durable response. Thus, biepitopic CAR T against BCMA represents a promising therapy for R/R MM, while most adverse effects are clinically manageable.
Multiple myeloma (MM) is a common hematological malignancy (1). With the emergence of more advanced therapeutics such as proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs), the survival of patients has been greatly improved. However, up to now, myeloma is still incurable in most cases, and the treatment of patients with relapse or refractory disease status represents a major challenge (2–4). In high-risk myeloma patients, including patients with double-hit myeloma or extramedullary infiltration, the survival rate is very low because of the lower efficiency of conventional treatments (5–8). Therefore, there is a need for the development of new therapeutic strategies with different mechanisms of action.
Recently, chimeric antigen receptor modified T (CAR T) cell therapy has brought new hopes (9). The effectiveness of anti-CD19 CAR T cells against lymphoproliferative diseases such as chronic lymphocytic leukemia (CLL) (10), B-precursor acute lymphoblastic leukemia (B-ALL) (11, 12), and B cell lymphoma (13, 14) has yielded encouraging therapeutic effects.
B cell maturation antigen (BCMA) is required for the survival of long-living plasma cells, and is commonly expressed at high levels in malignant myeloma (15, 16). It is therefore regarded as a potential therapeutic target in MM patients. A recent trial with anti-BCMA CAR T in a series of 24 relapsed and refractory (R/R) MM cases yielded an overall response rate (ORR) of 58.3%, and an 81% ORR was obtained among the 16 cases who received a high dose of CAR T cells (17, 18), providing evidence for the role that anti-BCMA CAR T cells may play in the treatment of myeloma.
An investigator-sponsored trial of anti-BCMA CAR T has also been initiated since 2016 at four different sites in China: Second Affiliated Hospital of Xi’an Jiao Tong University (XJTU), Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine (RJ), First Affiliated Hospital of Nanjing Medical University in Jiangsu (JS), and Changzheng Hospital affiliated with Shanghai Second Military Medical University (CZ). A specially designed CAR T cell preparation simultaneously targeting two epitopes of BCMA (LCAR-B38M) (Fig. 1A) was used in the trial, which was described in two preliminary reports (19, 20). Very recently, Zhao et al. (21, 22) from the XJTU site, one of the four sites, reported a remarkable response rate of 88% in 57 R/R MMs using a protocol of a three-infusion delivery of CAR T cells with cyclophosphamide as conditioning regimen. However, a number of issues using LCAR-B38M remained to be addressed. For example, the kinetics of LCAR-B38M after infusions in patients should be examined for a better understanding of the relationship between CAR T level and clinical outcome. Clinically, the adverse effects and their contributing factors should be analyzed so that appropriate management could be developed. Moreover, according to the experiences from CD19 CAR T studies in acute lymphoblastic leukemia (23), treatment protocol could be further improved to enhance the cost effectiveness and convenience for patient care, such as the CAR T delivery methods.
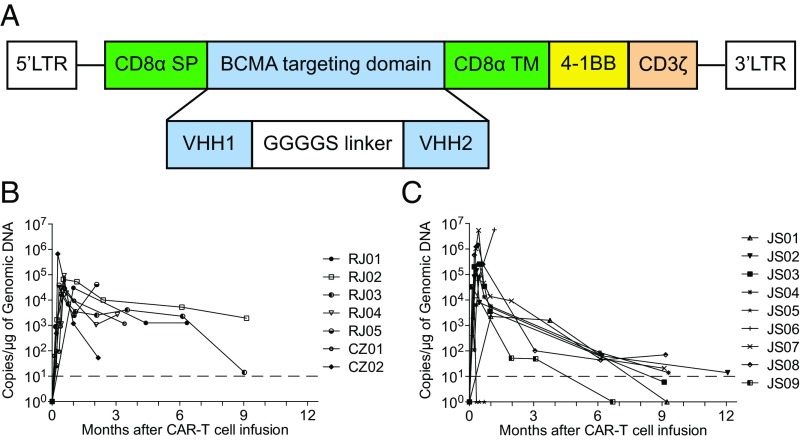
Fig. 1.
LCAR-B38M CAR T cells. (A) Diagram of the anti-BCMA CAR (LCAR-B38M) is shown. LCAR-B38M was composed of a human CD8 alpha signal peptide (CD8α SP), BCMA-targeting domain consisting of two different VHHs (single-domain antibody, clones VHH1 and VHH2), human CD8 alpha hinge and transmembrane domain (CD8α hinge + TM), human 4-1BB cytoplasmic domain, and a human CD3 zeta cytoplasmic domain (CD3ζ) (see Materials and Methods for details). (B and C) Measurements of LCAR-B38M gene-modified T cells assessed by means of qPCR assay in peripheral blood of patients treated with cyclophosphamide/fludarabine combination conditioning and three-infusion CAR T delivery (B) or cyclophosphamide conditioning and one-infusion CAR T delivery (C). The dashed line indicates the lower threshold value of the copy number.
In the present work, we present the results of LCAR-B38M in 17 R/R MM patients enrolled from the other three clinical centers as a group relatively independent from the aforementioned study by Zhao et al. Overall, a very high ORR of 88.2% [13 stringent complete responses (sCRs) and 2 very good partial responses (VGPRs)] was achieved in our patient series, and 7 sCR cases and 1 VGPR case sustained ongoing responses 11 mo after CAR T therapy. On the other hand, adverse events such as cytokine release syndrome (CRS) and tumor lysis syndrome (TLS) were encountered in 17 and 3 cases, respectively, requiring rapid and adequate handling. We also compare the efficacy and adverse effects of two distinct CAR T delivery protocols so that convenience could be offered to patient care without the compromise of therapeutic benefits. In addition, the possible factors associated with relapse or disease progression after initial response to CAR T are presented.
Results
Clinical and Hematological Data of the Patient Cohort.
Seventeen R/R MM cases were enrolled in the RJ, JS, and CZ sites from April to November 22, 2017. The clinical and hematological characteristics are summarized in Table 1. According to M-protein determination, the Ig or light-chain (LC) subtypes were IgG, λ in four cases; IgG, κ in four cases; IgA, κ in six cases; IgD, λ in one case; and λ light chain in two cases. Anemia, bone lesions, abnormal free light chain (FLC) ratio, and a high level of β2-microglobulin were commonly observed in patients at entry time, and half of the patients had elevated lactate dehydrogenase (LDH). Twelve out of 17 patients had received at least three lines of prior therapies including chemotherapy (CT), IMiDs, and PIs, while five received CT with either IMiDs or PIs. In addition, eight patients had also received autologous hematopoietic stem cell transplantation (auto-HSCT). BCMA was positive on plasma blasts in all cases (33.7 to 99%). Five patients exhibited extramedullary lesions.
Table 1.
Clinical and hematological data and dosage of LCAR-B38M cells in 17 relapsed/refractory multiple myeloma patients
| ID | Sex/age | Subtype | Lesion | Lines of prior therapy | Auto-HSCT | PI* | IMiD† | Clonal BM plasma cells, % | BCMA of plasma cells, % | Serum M protein, g/L | β2-MG, mg/L | LDH, IU/L | HB, g/L | Bone lesions‡ | FLC ratio | FISH | CAR+ T infused, ×106/kg | Peak value of CAR+ T, copy number/μg DNA |
| RJ01 | F/61 | IgG, λ | BM | 5 | Yes | Bortezomib | Lenalidomide | 26.2 | 33.7 | 27.4 | 7.0 | 297 | 69 | No | 0.040 | gain(1q) | 1.40 | 30562 |
| RJ02 | M/57 | IgD, λ | BM, EM§ | 6 | No | Bortezomib | Lenalidomide | 22.1 | 93.2 | 11.5 | 2.4 | 391 | 76 | Yes | 0.040 | gain(1q), del(13q), del(17p), IGH rearrangement¶ | 1.05 | 65925 |
| RJ03 | M/55 | IgG, κ | BM, EM§ | 4 | No | Bortezomib | Lenalidomide | 3.0 | 98.5 | 16.5 | 3.0 | 463 | 91 | Yes | 1.180 | gain(1q) | 1.05 | 16660 |
| RJ04 | M/68 | IgG, κ | BM | 3 | No | Carfilzomib | No | 6.0 | 99.0 | 14.8 | 3.8 | 184 | 81 | No | 24.620 | gain(1q), t (4; 14) | 0.29 | 92918 |
| RJ05 | F/56 | IgG, λ | BM | 3 | No | No | Lenalidomide | 4.5 | 95.0 | 40.2 | 3.7 | 171 | 89 | Yes | 0.050 | gain(1q) | 0.58 | 38428 |
| JS01 | M/67 | IgA, κ | BM, EM§ | 4 | No | Bortezomib | Thalidomid | 0.1 | 88.2 | 11.9 | 4.4 | 153 | 75 | Yes | 4.590 | negative | 0.21 | 2282 |
| JS02 | M/73 | λ | BM | 6 | No | Bortezomib | Lenalidomide/Thalidomid | 4.0 | 99.0 | Negative | 8.0 | 185 | 64 | Yes | 0.003 | del(13q) | 0.28 | 139568 |
| JS03 | M/52 | λ | BM | 3 | Yes | Bortezomib | No | 31.4 | 59.0 | Negative | 5.6 | 397 | 75 | Yes | 0.001 | gain(1q) | 0.57 | 255331 |
| JS04 | F/53 | IgG, κ | BM | 5 | Yes | Bortezomib | Thalidomid | 0.2 | 78.0 | 26.4 | 3.8 | 111 | 115 | Yes | 6.000 | gain(1q), del(13q), t (4; 14) | 0.46 | 83675 |
| JS05 | M/63 | IgA, κ | BM, EM§ | 3 | No | Bortezomib | Lenalidomide/Thalidomid | 0.2 | 78.0 | Trace | 4.2 | 2217 | 88 | Yes | 55.100 | NA | 0.35 | 6306 |
| JS06 | M/56 | IgA, κ | BM, EM§ | 5 | Yes | Bortezomib/Carfilzomib/Ixazomib | Lenalidomide/Pomalidomide | Negative | 95.4 | 7.3 | 3.7 | 194 | 122 | Yes | 15.200 | gain(1q), del(13q) | 1.52 | 225897 |
| JS07 | F/63 | IgA, κ | BM | 11 | Yes | Bortezomib | Lenalidomide | 15.3 | 99.0 | 13.3 | 4.5 | 158 | 83 | No | 4.400 | gain(1q), del(13q) | 1.47 | 5396510 |
| JS08 | M/55 | IgA, κ | BM | 4 | Yes | Bortezomib | Lenalidomide | 30.0 | 99.0 | 17.0 | 3.8 | 128 | 87 | No | 135.100 | gain(1q), t (4; 14) | 0.76 | 1446038 |
| JS09 | M/35 | IgA, κ | BM | 4 | No | Bortezomib | Thalidomid | 80.0 | 98.0 | 15.2 | 3.8 | 244 | 61 | Yes | 2532.200 | Negative | 0.49 | 32830 |
| CZ01 | M/35 | IgG, κ | BM | 3 | Yes | No | Thalidomid | 69.0 | 73.4 | 21.7 | 3.8 | 150 | 60 | Yes | 4.750 | IGH rearrangement¶ | 0.33 | 30861 |
| CZ02 | F/47 | IgG, λ | BM | 6 | Yes | Bortezomib | Lenalidomide/Thalidomid | 22.0 | 95.6 | 64.5 | 2.1 | 280 | 119 | Yes | 82.220 | del(17p) | 0.69 | 664403 |
| CZ03 | F/40 | IgG, λ | BM | 3 | No | Bortezomib | No | 45.0 | 97.7 | 56.0 | 3.2 | 143 | 84 | Yes | NA | gain(1q), del(13q), del(17p), t (4; 14) | 0.35 | NA |
Auto-HSCT, autologous hematopoietic stem cell transplantation; β2-MG, β2-microglobulin; BM, bone marrow; FLC, free light chain; HB, hemoglobin; LDH, lactate dehydrogenase; NA, not available.
Proteasome inhibitors (Bortezomib/Carfilzomib/Ixazomib).
Immunomodulatory drugs (Lenalidomide/Thalidomid/Pomalidomide).
Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or PET-CT.
Five patients exhibited extramedullary (EM) lesions. RJ02 and JS05 bore plasmacytoma on the forehead; RJ03 presented with extramedullary involvement in the lower jaw, chest skin, and liver; JS01 carried the infiltration lesion on the pleura; and JS06 had tumor infiltration in the pleura and peritoneum.
IGH rearrangement: split FISH signal of IGH without juxtaposing to the common known partner genes (FGFR3, MAF, MAFB, CCND1, CCND3).
Fluorescence in situ hybridization (FISH) detected the high-risk cytogenetic abnormalities t (4; 14) and del(17p) in 6 cases, and the unfavorable prognosis markers gain(1q) and del(13q), respectively, in 11 and 6 cases. The simultaneous presence of these markers was noted in some cases, including the coexistence of all four markers in case CZ03. Split FISH signal of IGH without known partner gene involvement was detected in two cases.
Detection of Blood CAR T Cells After Infusion.
Five days after conditioning, LCAR-B38M was i.v. infused. CAR T cells usually began to rise on the second day and reached a peak at 6 to 30 d. Similar CAR T profiles were found by qPCR and FACS (Fig. 1 B and C and SI Appendix, Fig. S1). The peak values were not dependent on the initial dose of LCAR-B38M administered (Fig. 1 B and C and Table 1). Engineered cells persisted in most of the patients after infusion, with the longest sustaining time up to 9 mo (Fig. 1 B and C).
Efficacy of LCAR-B38M.
Clinical overall response and durability of the initial response after LCAR-B38M.
Two protocols for conditioning and CAR T delivery were used in this work, with the dose ranges of CAR T similar between the two subgroups. When 8 RJ/CZ cases treated by cyclophosphamide/fludarabine combination conditioning and three-infusion CAR T delivery were compared with 9 JS cases by cyclophosphamide conditioning and one-infusion CAR T delivery, similar ORR (7/8 versus 8/9 cases), CRS rates (8/8 vs. 9/9 cases), as well as peak values of CAR copy number (P = 0.313) were noted. Therefore, the data of all 17 cases were combined for analysis. At 1 mo after CAR T treatment, 15 patients achieved a response, 1 had no obvious response (Fig. 2A), while another 1 unfortunately had an early death due to severe CRS/TLS. When the maximum therapeutic effects were analyzed in the 15 patients with response, 13 achieved sCR and 2 reached VGPR (Fig. 2A). Notably, although CR was achieved at an early time in some cases (Fig. 2 B and C), efficacy was improving in other patients as time went on (Fig. 2 D and E).
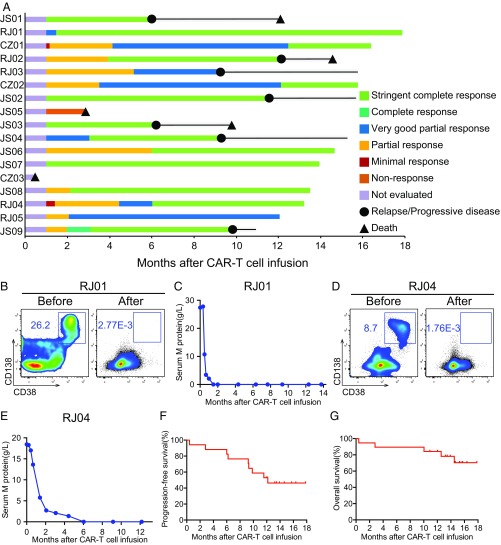
Fig. 2.
Clinical overall response and survival in R/R MM to CAR T. (A) Duration of response to LCAR-B38M and post-infusion survival in 17 cases. (B and C) Disease clearance upon LCAR-B38M in a representative case (RJ01). (B) Flow cytometry detection of BM MRD before and 2 mo after CAR T. (C) The change of plasma M-protein concentration in RJ01 after CAR T infusion. The latest assessment was on day 413 post CAR T. (D and E) Response to LCAR-B38M in another representative case (RJ04). (D) Flow cytometry detection of BM MRD before and 1 mo after CAR T. (E) The change of plasma M-protein concentration, with the latest assessment at day 365 post CAR T. (F) The curve shows the time to progression after infusion of LCAR-B38M. Tick marks indicate the time of data censoring at the last follow-up. (G) The curve shows overall survival data censored at the time of the last follow-up.
During a follow-up of 12 to 535 d (median 417 d) for 17 patients, as of October 20, 2018, 8 cases (47.1%), including 7 sCR and 1 VGPR, remained in ongoing response status, all of them having sustained response over 11 mo post CAR T. Relapse occurred in 6 patients after sCR while progressive disease (PD) appeared in 1 case after VGPR (Fig. 2A), and these events took place from 5 to 11 mo after response (Fig. 2A). The Kaplan–Meier curve showed progression-free survival (PFS) rates of 82.4% at 6 mo and 52.9% at 12 mo (Fig. 2F). The 1-y overall survival (OS) rate was 82.3% (Fig. 2G).
In patients with extramedullary involvements, CAR T therapy exerted therapeutic effect on the plasmacytoma, although it generally took a longer time to relieve extramedullary lesions than to eliminate intramedullary ones (Figs. 2A and 3 A and B and SI Appendix, Fig. S2 A and B). For instance, patient RJ02 exhibited extramedullary disease on the forehead, and the initial response to CAR T was PR with negative minimal residual disease (MRD) in the bone marrow (BM). Four months after CAR T, M protein and extramedullary mass were eliminated to obtain an sCR (Figs. 2A and 3A). Patient RJ03 had plasmacytomas on the skin, lower jaw, and liver before CAR T therapy. After quickly achieving negative BM MRD, these masses gradually disappeared as time went on (Figs. 2A and 3B).
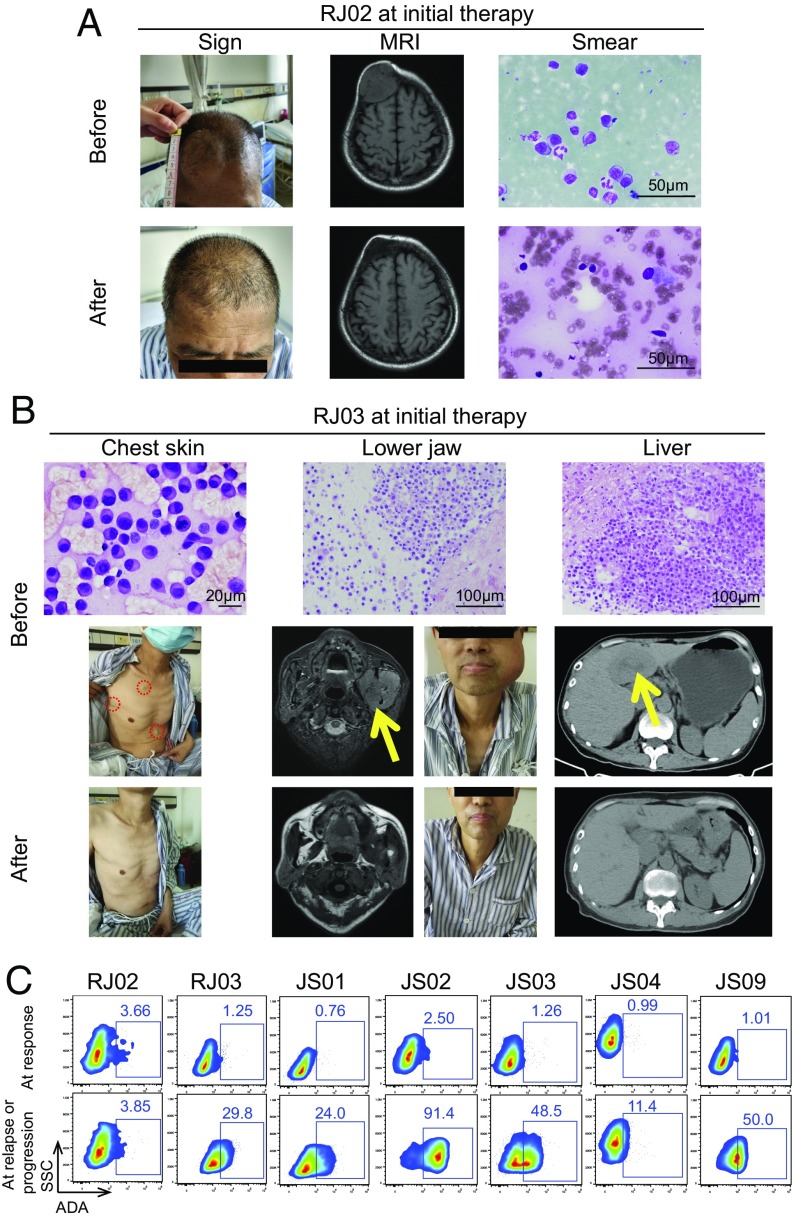
Fig. 3.
Response of extramedullary lesions. (A) Patient RJ02 presented with plasmacytoma on his forehead at half a year after the onset of the disease. The tumor enlarged gradually and lasted for nearly 3 y although he had been treated with three lines of anti-MM drugs. The cranial MRI and mass puncture cytological examination confirmed myeloma cells in the tumor. Four months after CAR T infusion, the plasmacytoma was obviously reduced, and neither occupying lesion nor plasma cells were observed in the original site. (B) Patient RJ03 had multiple extramedullary infiltration lesions, including skin, lower jaw, and liver, all of which were examined by aspiration or pathological section. At day 19 post CAR T infusion, the masses on the skin disappeared. At day 30, the skull MRI showed that the tumor in the lower jaw was relieved. The occupying lesion in the liver disappeared at 6 mo after CAR T cell therapy. Red dashed circles and yellow arrows indicate the tumor lesions. (C) Flow cytometry analysis shows the intensity of anti-BCMA CAR antibody (ADA) in seven relapse/PD patients’ sera, which were collected at the two time points (at response and at relapse or progression) for each case. ADA positivity was defined as a positive ratio of more than 5%.
Risk factors for relapse, PD, or no response.
We analyzed all the possible factors associated with relapse or PD after an initial response upon effect of LCAR-B38M, as well as nonresponse (NR) in 16 cases, not including the 1 with early death (CZ03) (Fig. 2A and Table 1). Before relapse/PD, BM MRD in those patients turned out to be undetectable 1 or 2 mo after CAR T infusion, and remained negative within the time of response (SI Appendix, Fig. S2C), while clonal plasma cells with expression of BCMA reappeared in the relapse/PD stage (SI Appendix, Fig. S2C). No correlations were observed in terms of age, gender, cytogenetic markers, conditioning scheme, CAR+ cell infusion dosage, delivery methods, and initial CR or VGPR (P > 0.05). However, patients who previously had auto-HSCT seemed more likely to obtain a sustained response than those who had not (P = 0.046, six of eight patients with sustained response versus two of the other eight patients with relapse/PD/NR).
Notably, four out of five patients with extramedullary lesions had worse outcomes, including relapse in RJ02 and JS01, PD in RJ03, and NR in JS05. For RJ02, RJ03, and JS01, extramedullary tumors at relapse/PD all appeared at new sites along with intramedullary progression. The extramedullary disease samples before CAR T therapy showed a high proportion of Ki-67 cells (SI Appendix, Fig. S2D), suggesting a much stronger proliferative potential of tumors.
We also analyzed the residual CAR T cells in the seven relapsed or PD patients. Importantly, the amount of CAR T cells in peripheral blood was deeply reduced in six patients except RJ02 (copy number near to or lower than 10) (Fig. 1 B and C). In addition, we found an anti-CAR T antibody (ADA) in these six patients (RJ03, JS01, JS02, JS03, JS04, and JS09) before or at relapse/PD (Fig. 3C). However, only one patient (JS07) among those with ongoing response had a high positive ratio of ADA. Therefore, ADA constituted another high risk for relapse/PD after CAR T therapy (P = 0.005, 6/7 relapse/PD versus 1/8 sustained response).
Since the tumor cells collected at relapse or progression still expressed BCMA, two patients (RJ02 and RJ03), who carried MM cells with 82.5 and 92.9% BCMA expression levels, respectively (SI Appendix, Fig. S2C), received another anti-MM CAR T as salvage. In RJ03, 1 mo after the treatment, although symptoms were reduced, clonal plasma cells in the BM reappeared and the serum M protein remained high. In RJ02, severe toxicity occurred during reinfusion of CAR T. The patient experienced acute pulmonary edema, thrombocytopenia, hepatic dysfunction, and high fever. Meanwhile, serum cytokines IL-2R, IL-6, IL-8, IL-10, and TNF-α remarkably increased, particularly IL-6, which was 631-fold higher than baseline level. There was no evidence of bacterial, mycotic, or viral infection in samples from the pharynx, sputum, feces, and blood. Because of increased pulmonary vascular permeability and severe thrombocytopenia, fatal pulmonary hemorrhage occurred and the patient died of respiratory failure.
Adverse Effects and Their Management.
Characterization of adverse effects.
The most common adverse effect was CRS, clinical manifestations being accompanied by dramatically up-regulated serum cytokine profiles. All patients had high fever, which usually occurred at 7 to 14 d post CAR T infusion. Four cases with fever at 0 to 4 d responded to antibiotics (one from RJ and three from JS) (Fig. 4A). Next, 52.9% of patients showed liver dysfunction above grade 1, mainly manifested by the elevation of aspartate aminotransferase (Fig. 4B) usually occurring at day 10 to 17 post CAR T. Other CRS-associated syndromes are listed as follows in order of incidence rate: hypotension (5/17), hypoxemia (4/17), prolonged activated partial thromboplastin time (APTT) (2/17), systemic edema (1/17), and renal impairment (1/17) (Table 2). Moreover, the peak value time of C-reactive protein (CRP) and ferritin in peripheral blood correlated well with the period for patients to develop CRS (SI Appendix, Fig. S3). In terms of severity of CRS, 10 cases had grade 1 to 2 CRS, 6 experienced grade 3 CRS, while 1 had a grade 5 reaction. Analysis showed that CRS grades were associated with the abundance of BCMA on the clonal plasma cells (P = 0.035), and were closely related to the existence of the high-risk cytogenetic marker del(17p) (P = 0.029). Other factors, including patient characteristics before CAR T therapy and CAR+ cell dosage infused, did not exert impact on CRS severity (Table 3). Of note, the case with grade 5 CRS (CZ03) had four poor cytogenetic abnormalities.
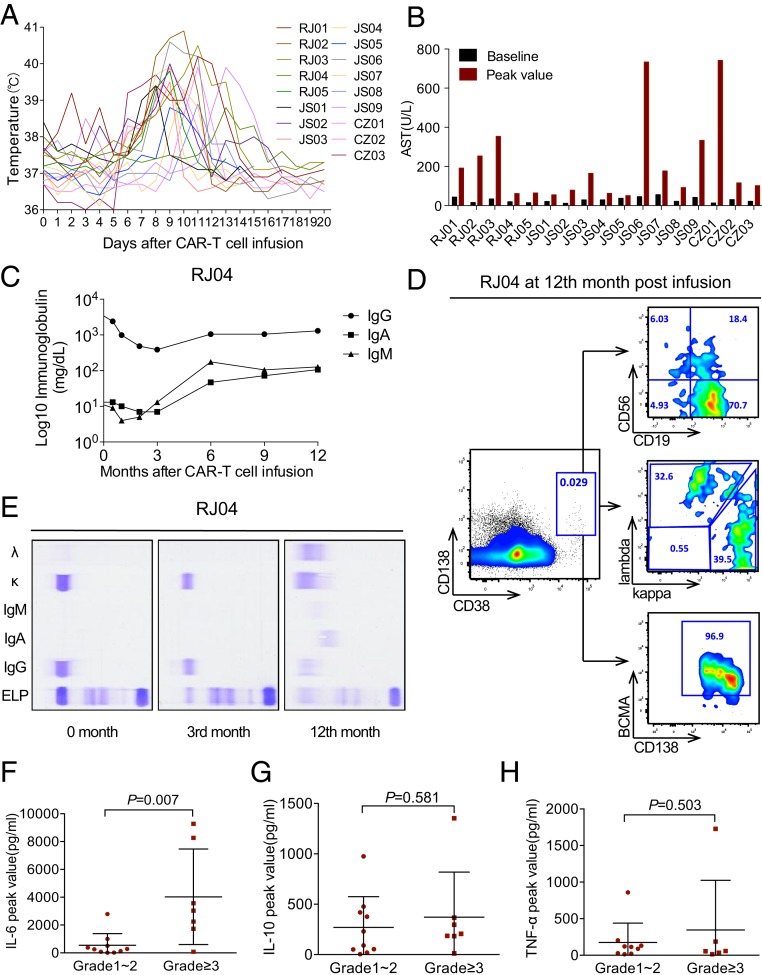
Fig. 4.
Evaluations of adverse events of CAR T in R/R MM. (A) The profile shows the maximum temperature of the 17 patients for each day from day 0 to 20 after LCAR-B38M. (B) The columns exhibit the baseline and peak values of aspartate aminotransferase (AST) levels in the 17 patients. (C) The chart shows the changes of serum IgG, IgA, and IgM levels in patient RJ04 after CAR T therapy. (D) Flow cytometry detection of RJ04 BM at 1 y post CAR T infusion. The plots gated by CD38+CD138+ represent BM plasma cells, which expressed CD19, balanced kappa and lambda LC on the cell surface membrane, and BCMA. It is noteworthy that the immunophenotype of the previous residual myeloma cells in this case was CD38+CD138+CD56+CD19− with restrictive expression of the cytoplasmic kappa light chain. (E) Serum immunofixation electrophoresis analyses of IgG, IgA, IgM, and kappa and lambda LC before and 3 and 12 mo after CAR T in RJ04. (F) The chart shows the peak levels of IL-6 in the first 30 d after infusion of LCAR-B38M cells in patients with grade 1 or 2 CRS (n = 10) compared with patients with grade 3 CRS and above (n = 7). (G) The graphical representation exhibits the peak levels of IL-10 within 1 mo after CAR T therapy in patients with grade 1 or 2 CRS (n = 10) compared with patients with grade 3 CRS and above (n = 7). (H) The chart displays the peak levels of TNF-α during the first 30 d post infusion of CAR T cells in patients with grade 1 or 2 CRS (n = 9) compared with patients with grade 3 CRS and above (n = 6).
Table 2.
Adverse effects of LCAR-B38M and their management
| ID | Cytopenia (AE grading) | CRS (AE grading) | TLS | CRS grading | Use of IL-6R inhibitor |
| RJ01 | WBC↓ (4), NEU↓ (4), PLT↓ (4) | Fever (3), AST↑ (2) | No | 2 | Yes |
| RJ02 | WBC↓ (3), NEU↓ (3), PLT↓ (1) | Fever (4), hypotension (2), ALT↑ (1), AST↑ (3) | No | 3 | Yes |
| RJ03 | PLT↓ (2) | Fever (3), hypotension (2), AST↑ (3) | Yes | 3 | Yes |
| RJ04 | WBC↓ (3), NEU↓ (1) | Fever (1), hypotension (2), hypoxia (3), edema (2), AST↑ (1) | No | 3 | Yes |
| RJ05 | WBC↓ (3), NEU↓ (2) | Fever (2), hypoxia (2), AST↑ (1) | No | 2 | Yes |
| JS01 | WBC↓ (2), NEU↓ (2) | Fever (3), ALT↑ (1) | No | 1 | Yes |
| JS02 | WBC↓ (4), NEU↓ (4) | Fever (4), AST↑ (1) | No | 1 | No |
| JS03 | WBC↓ (4), NEU↓ (4), PLT↓ (4) | Fever (2), ALT↑ (2), AST↑ (2) | No | 2 | No |
| JS04 | WBC↓ (2), NEU↓ (2) | Fever (2), ALT↑ (1), AST↑ (1) | No | 1 | No |
| JS05 | WBC↓ (2), NEU↓ (2) | Fever (2), AST↑ (1) | No | 1 | No |
| JS06 | WBC↓ (3), NEU↓ (3) | Fever (3), hypotension (3), ALT↑ (1), AST↑ (3) | Yes | 3 | Yes |
| JS07 | WBC↓ (4), NEU↓ (4) | Fever (3), AST↑ (2) | No | 2 | No |
| JS08 | No | Fever (3), ALT↑ (1), AST↑ (1) | No | 1 | No |
| JS09 | WBC↓ (3), NEU↓ (3) | Fever (3), AST↑ (3) | No | 3 | Yes |
| CZ01 | No | Fever (2), hypoxia (2), ALT↑ (1), AST↑ (1) | No | 2 | No |
| CZ02 | WBC↓ (4), NEU↓ (4), PLT↓ (4) | Fever (2), APTT↑ (2), AST↑ (3) | No | 3 | No |
| CZ03 | No | Fever (2), hypotension (5), hypoxia (5), bilirubin↑ (3), renal insufficiency (5), APTT↑ (1), AST↑ (1) | Yes | 5 | Yes |
AE, adverse events (the grading of AE was according to the CTCAE 4.03); ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CRS, cytokine release syndrome; NEU, neutrophil count; PLT, platelet count; TLS, tumor lysis syndrome; WBC, white blood cell; ↓, decrease; ↑, increase.
Table 3.
Factors possibly associated with severe CRS
| Characteristic | Grade 1 or 2 | ≥Grade 3 | P value |
| Case, n | 10 | 7 | |
| Clonal BM plasma cells | |||
| Mean value, % | 18.09 | 25.44 | 0.579* |
| ≥10%, n | 5 | 4 | 0.772† |
| <10%, n | 5 | 3 | |
| Serum M protein | |||
| Mean value, g/L | 15.79 | 26.56 | 0.301* |
| ≥30 g/L, n | 1 | 2 | 0.323† |
| <30 g/L, n | 9 | 5 | |
| Lactate dehydrogenase | |||
| Mean value, U/L | 396.70 | 271.29 | 0.562* |
| ≥192 U/L, n | 3 | 5 | 0.092† |
| <192 U/L, n | 7 | 2 | |
| Extramedullary lesions | |||
| Present, n | 2 | 3 | 0.309† |
| Absent, n | 8 | 4 | |
| Hemoglobin | |||
| Mean value, g/L | 80.50 | 90.57 | 0.330* |
| ≥90 g/L, n | 1 | 3 | 0.116† |
| <90 g/L, n | 9 | 4 | |
| del(17p) | |||
| Yes, n | 0 | 3 | 0.029† |
| No, n | 9 | 4 | |
| BCMA abundance | |||
| Mean value, % | 80.23 | 96.77 | 0.035* |
| Bone lesions | |||
| Yes, n | 7 | 6 | 0.452† |
| No, n | 3 | 1 | |
| CAR+ cell dose | |||
| Mean value, ×106/kg | 0.64 | 0.78 | 0.549* |
| ≥0.7 × 106/kg, n | 3 | 3 | 0.585† |
| <0.7 × 106/kg, n | 7 | 4 |
P value is calculated by t test.
P value is calculated by χ2 test.
TLS was observed in three patients (RJ03, JS06, and CZ03), who also presented with laboratory metabolic abnormalities such as elevation of LDH. Clinically, patient RJ03 had an unpredicted rupture of a lower jaw plasmacytoma, which injured a tumor-supporting artery and caused a hemorrhagic shock at day 15 after CAR T infusion. Wound stuffing, fluid supplement, and red blood cell transfusion were quickly given and the patient recovered. JS06 presented with fever at day 6 after CAR T therapy, and exhibited a severe breathing problem attributed to tumor lysis on the pleura. Although the patient received Tocilizumab treatment twice, CRS progressed to grade 3, with dyspnea, hypoxemia (FiO2 > 40%), and arthralgia at day 11. Cytokine analysis showed IL-6 was 3,000-fold and TNF-α was more than 400-fold of baseline. The third shot of Tocilizumab and the anti–TNF-α drug Etanercept were successively applied. The patient’s hypoxemia and dyspnea were resolved and the fever declined. CZ03, who developed grade 5 CRS, also had an increase of LDH, uric acid, urea nitrogen, hyperkalemia, and metabolic acidosis on day 11 post infusion. The patient died of the concurrence of serious TLS and CRS despite administration of hemodialysis, Tocilizumab, Etanercept, and other supportive care. CZ03 was the only case in this series who died of CAR T-related toxic reaction.
Fourteen patients demonstrated cytopenia. In RJ01 and CZ02, severe cytopenia lasted for over 2 mo post CAR T but, in other patients, blood cells recovered within 1 mo (Table 2). Neither white blood cell decrease nor platelet reduction was correlated with prior auto-HSCT and CRS grade (P > 0.05).
Apart from the acute side effects, very low levels of IgG, IgA, and IgM were found in all patients 1 mo after LCAR-B38M infusion, lasting for at least 3 mo. During the follow-up, four cases experienced upper respiratory tract infection, three encountered pulmonary infections, one suffered from severe herpes zoster virus infection, and one case experienced severe oral mucosa infection. However, in two cases (RJ04 and CZ02) with long ongoing response, Igs, including IgG, IgA, and IgM, with apparently normal κ/λ light chain ratio, were restored to normal levels at 1 y post CAR T infusion. Furthermore, plasma cells were detected in the BM of these two cases (see Fig. 4 C–E for representative results of RJ04).
Analysis of serum cytokine profiles.
After LCAR-B38M infusion, IL-6, IL-10, and TNF-α were monitored in real time. We found that, consistent with the clinical CRS period, the three cytokines tended to increase. When CRS above grade 3 was defined as a serious event, the severity of CRS was only significantly associated with a higher level of IL-6 (P = 0.007) (Fig. 4 F–H).
Treatments of adverse events.
Among 10 cases with mild CRS, symptomatic care was performed in 7 cases, while anti–IL-6 receptor (anti–IL-6R) treatment with Tocilizumab was needed in the remaining 3 cases to treat complications which were not relieved by supporting care. In contrast, 6 patients with severe CRS required Tocilizumab at a dose of 4 to 8 mg/kg for up to 5 consecutive days to control toxic reactions. CRS symptoms usually disappeared within 7 d after this specific treatment. One patient experienced very severe hepatic dysfunction but was relieved by effective medication without receiving Tocilizumab, although the CRS was defined as grade 3. In case JS06, TNF-α inhibitor was applied when Tocilizumab was ineffective and serum TNF-α level was high. Special supportive measures were also used when other complications of CRS or TLS occurred, including the use of hepatoprotectants for severe liver dysfunction, vasopressor for hypotension, mechanical ventilation for hypoxemia, fresh plasma and human fibrinogen for coagulopathy, and hemodialysis for acute renal failure. The treatment experiences of three representative cases (RJ03, JS06, and CZ03) showed the importance of close monitoring and adequate therapeutic decision making in emergency situations.
For the prevention of infectious diseases due to hypoimmunoglobulinemia, a γ-globulin preparation at a dose of 400 mg/kg body weight every month was used for 3 mo as a routine in all 15 patients with therapeutic response. No major infections were noted under this protective measure over the follow-up time.
Discussion
Since our study was an exploratory one, we designed the study with two infusion methods. The administration of three infusions was based on the references of CD19 CAR T treatment in CLL/ALL (23, 24). The rationale for the one-infusion method was also consistent with the further development of CD19 CAR T delivery protocol: While earlier studies used split infusions, later-registration clinical studies used single infusions with the same effects (NCT02348216, NCT02435849). Similar to the infusion methods, two different lymphodepleting therapies were adopted. The rationale for such a design was to study the effect of different conditioning regimens in the CAR T treatment. The results from our study provide evidence that no obvious efficacy and toxicity differences are observed between the subgroups of the three-infusion and one-infusion delivery approaches. Obviously, the one-infusion delivery of the total CAR T dose should be more convenient for patients and care providers. On the other hand, cyclophosphamide/fludarabine (Cy-Flu) combination conditioning seemed to yield similar kinetics of CAR T cells compared with cyclophosphamide single-drug conditioning. Therefore, the single CAR T infusion regimen will be applied in our future patient management, while Cy-Flu conditioning is preferred according to the literature (13, 25) in other lymphoproliferative diseases.
In this work, we obtained a very high ORR (88.2%; 76.5% sCR and 11.8% VGPR) among 17 R/R MM cases with LCAR-B38M. The 1-y PFS rate was 52.9%, while the 1-y OS rate reached 82.3%. Compared with the data released by other BCMA-directed CAR T trials, the efficacy in our study seemed to be better. It is worth noting that in the design of LCAR-B38M, the antigen recognition moiety is composed of two heavy chains of camel antibody against the two epitopes of BCMA. This structure may increase the specificity of antigen recognition and possibly also the affinity of antigen binding of CAR T, leading to a stronger anti-MM effect. Furthermore, the in vivo expansion patterns of LCAR-B38M in peripheral blood were similar to the previously reported anti-BCMA and anti-CD19 CAR T cells for MM (17, 26), with a peak value followed by a gradual decrease parallel to the clearance of MM lesions.
Contrary to the situation of CAR T therapy against B-ALL, where a complete remission can be achieved as an initial response (11, 12), the dynamics of the clinical benefits of MM patients from LCAR-B38M was quite unique. In a number of patients the initial therapeutic effect was a PR, followed by VGPR, and then CR. This time course might be explained by either the difficulty for CAR T cells to penetrate into the BM/extramedullary plasmacytoma, or the relatively long half-life of M protein. It is worth noting that although LCAR-B38M generated significant therapeutic benefits in R/R MM, a fraction of patients eventually had relapse/PD while ADA emergence was found to be the major risk factor. The epitope of the BCMA CAR should be an inducer of ADA. Notably, among our 15 patients with available data, 7 were ADA-positive while the remaining 8 cases were all ADA-negative. There was no difference in peak values of CAR copy numbers between ADA-positive and ADA-negative groups within the first month of treatment. However, residual CAR T cells decreased remarkably in all those carrying ADA, while those in ADA-negative patients remained at a relatively high level. This situation suggests that the antibody-producing cells might be another reason for ADA emergence. Therefore, in the future, on the one hand, the epitope of anti-BCMA CAR can be further optimized to reduce the immunogenicity, and on the other hand, anti-plasma cell therapies including distinct CAR T, BiTE antibody constructs, or immunoregulatory drugs should be taken into consideration to remove ADA-secreting cells. At present, ADA should be routinely examined to help investigators make an early judgment of possible relapse/progression. Moreover, since experiences with anti-CD19 CAR T in the treatment of R/R B-ALL showed a high recurrence rate after initial CR, the current strategy is to incorporate CAR T into the consolidation therapy in newly diagnosed B-ALL (27). It is thus reasonable to suggest that in the future, LCAR-B38M can be used for newly diagnosed MM, particularly in patients with unfavorable prognosis. Meanwhile, previous auto-HSCT seemed to be beneficial for R/R MM patients to obtain a long-lasting response after CAR T therapy, which might be due to a relatively lower burden of tumor stem cells in the BM. Hence, auto-HSCT should be encouraged for eligible patients before consolidation CAR T therapy.
In this trial, CRSs were the common adverse effects, possibly associated with BCMA-positive tumor burden and cytokine IL-6 profiles. The incidence of severe CRS (grade ≥3) was similar to that in another trial with BCMA CAR T (7/17 versus 6/16) (18). We noted that CRS occurred in our study later than that in some reports (18, 23, 25), which could be attributed to several reasons: First, the number of cells we infused was about 1/10 of that in other trials, so the immunological response might be a bit slower; and second, the costimulatory factor on our CAR vector is 4-1BB, whose effect may be more moderate relative to CD28. Besides, we cannot rule out another possibility: Compared with B-ALL, tumor cells of MM are less aggressive, and are very restricted in bone marrow and/or local plasmacytoma, rather than massively present in both bone marrow and peripheral blood. So the CAR T immunological reaction with plasma cells might be milder and later than that with B-ALL cells.
Most of the adverse events were manageable, owing to proper therapeutic measures with anti–IL-6 therapy and supportive care. For patients who carried very high risk factors and heavy tumor burden, such as CZ03, earlier application of IL-6R inhibitor should be considered. TNF-α inhibitor is worth utilizing in patients with refractory CRS, particularly when CRS is combined with TLS. A late-stage adverse event was the occurrence of low serum polyclonal Ig, that might reflect the anti-BCMA effect of LCAR-B38M against normal plasmacytes. Ig replacement therapy is necessary for this challenge until the restoration of normal plasma cell growth in the BM and of serum polyclonal Ig levels.
Materials and Methods
Patient Population.
The trial was a phase 1, open-labeled, multicenter study to evaluate the safety and efficacy of LCAR-B38M CAR T cells in R/R MM, which was registered with clinicaltrials.gov (NCT03090659) and chictr.org (ChiCTR-ONH-17012285), and approved by the institutional review boards of three participating hospitals including the Ruijin Hospital Ethics Committee, First Affiliated Hospital of Nanjing Medical University Ethics Committee, and Changzheng Hospital Ethics Committee. Informed consent was obtained from all patients for the treatment protocol.
Eligible subjects were 18 to 75 y of age with a documented diagnosis of MM according to International Myeloma Working Group (IMWG) diagnostic criteria (1). While entering into the trial, all subjects had documented disease progression during, or within 12 mo of, their most recent anti-MM drugs or auto-HSCT, as determined by routine blood examinations, BM MM cells, M protein (serum Ig and LC), serum FLC, 24-h urine protein, and body imaging by virtue of radiography, computed tomography, magnetic resonance (MR), and/or positron emission tomography-computed tomography (PET-CT). Interphase FISH was conducted on BM mononucleated cells according to the manufacturer’s instructions (Abbott Molecular). See details of inclusion and exclusion criteria in SI Appendix.
Preparation of LCAR-B38M CAR T Cells.
The novel design of the BCMA-targeting domain facilitated binding of two epitopes on BCMA. The expression of LCAR-B38M was driven and controlled by a human elongation factor 1 alpha (hEF1α) promoter. BCMA (NCBI NP_001183; UniProt Q02223) is a transmembrane protein 184 amino acids in length which consists of the extracellular domain (ECD; amino acids 1 to 54), transmembrane domain (TM; amino acids 55 to 77), and cytoplasmic domain (CD; amino acids 78 to 184). Three disulfide bonds (Cys–Cys) were located in the ECD of BCMA, which are at positions 8–21, 24–37, and 28–41. The secondary structure of the BCMA ECD sequentially consists of a beta strand (amino acids 12 to 15), turn (amino acids 16 to 19), beta strand (amino acids 20 to 23), helix (amino acids 24 to 27), beta strand (amino acids 30 to 32), helix (amino acids 35 to 37), turn (amino acids 38 to 40), and turn (amino acids 42 to 44). The BCMA epitope peptides (269EP001 to 269EP007) were designed as shown in SI Appendix, Table S1. VHH1 tends to bind the epitope located in 269EP005 from amino acids 24 to 36, which contains a secondary structure of helix (amino acids 24 to 27), beta strand (amino acids 30 to 32), and helix (amino acids 35 to 37). VHH2 tends to bind the epitope located in the first two beta strands. CAR T cells were manufactured according to a modified published method (23). See a detailed procedure in SI Appendix.
Conditioning, Dosage, and Administration of CAR T Cells.
A cyclophosphamide-based lymphodepleting chemotherapy was used as conditioning regimen. Cyclophosphamide 250 mg/m2 i.v. daily and fludarabine 25 mg/m2 i.v. daily for 3 d or cyclophosphamide 300 mg/m2 i.v. daily for 3 d was administered (SI Appendix, Table S2). LCAR-B38M cell i.v. infusion took place 5 d after the start of the conditioning regimen. The starting day of LCAR-B38M CAR T cell infusion is day 0. The dosage used in our study was determined by two criteria: first, the number of CAR T cells available in the manufactured product; and second, the tumor burden and general condition of the patient as judged by investigators. The mean dose was 0.70 × 106 CAR-positive viable T cells per kg (range 0.21 × 106 to 1.52 × 106 cells per kg; Table 1). The positive percentage of CAR T cells was 9.4 to 75.9% (mean 30.5%), and the CD4+/CD8+ ratio was 0.1 to 1.6 (SI Appendix, Table S2). Two different delivery methods were used: three infusions given at days 0, 3, and 6 in eight patients from RJ and CZ; and one infusion given at day 0 in the remaining nine cases of JS (SI Appendix, Table S2). See detailed treatment administration in SI Appendix.
Detection of CAR T Cells by Fluorescence-Activated Cell Sorter and Quantitative Real-Time PCR.
Fresh peripheral blood mononuclear cells or genomic DNA was isolated from patients’ whole blood for CAR T cell detection by means of flow cytometry or qPCR, respectively. See the detailed procedure in SI Appendix.
Detection of Anti-BCMA CAR Antibody.
A clonal CHO cell line constantly expressing the BCMA CAR construct was produced. After incubation with patients’ serum to capture ADA, the CHO cells were washed and suspended in FACS buffer. A PE-conjugated antibody against human IgG Fc (BioLegend) was then labeled on CHO cells to detect ADA by FACS (Attune NxT; Invitrogen). The intensity of ADA was reflected by the positive ratio of CHO cells.
Evaluation of Clinical Response.
Efficacy of LCAR-B38M was evaluated by criteria of response according to the IMWG consensus recommendations (28). Patients were closely followed with BM cytological or biopsy examination for plasma cells, minimal residual disease (MRD) detection by FACS of BM, pathological section of extramedullary plasmacytoma, serum protein electrophoresis, Ig and LC immunofixation of serum and urine, serum FLC, bone radiography or MR, or systemic PET-CT scanning.
Evaluation of Safety.
Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE v. 4.03), with the exception of CRS. Criteria previously reported by the CARTOX working group (29) were adopted for the grading of CRS. In this study, grade 3 or higher toxicities were defined as severe adverse effects, while grades 1 and 2 were defined as mild events. A number of serum biochemistry biomarkers including C-reactive protein, ferritin, and cytokines were used to evaluate adverse events. Cytokines indicative of response to CAR T cells were determined according to the protocol provided by the manufacturer (Quantikine ELISA; R&D Systems). The safety of the patient was monitored by individual institutional policies.
Statistical Analysis.
The data were analyzed with the software packages SAS 9.3 and GraphPad Prism 5.0. Survival statistics was estimated using the Kaplan–Meier method. Categorical variables were compared with t test or χ2 test as appropriate. P value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This study was funded by 111 Project (B17029), Shanghai Collaborative Innovation Program on Regenerative Medicine and Stem Cell Research (2019CXJQ01), Chinese National Key Basic Research Project 973 (2013CB966800), National Key Research and Development Program (2016YFC0902800), National Science Foundation of China (81670147, 81800099), Academic Leader Program of the Shanghai Science and Technology Committee (16XD1402000), Shanghai Rising-Star Program (17QA1402200, 19QA1407800), Shanghai Excellent Youth Medical Talents Training Program (2018YQ09), and National Science and Technology Major Project (2018ZX09101001, 2018ZX09301052002).
Footnotes
Conflict of interest statement: L.Z. and X.-H.(F.)F. are employees of Nanjing Legend Biotech. All other authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819745116/-/DCSupplemental.
References
- 1.Rajkumar SV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, et al. Medical Research Council Adult Leukaemia Working Party High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, et al. IFM 2009 Study Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–1320. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Facon T, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018;131:301–310. doi: 10.1182/blood-2017-07-795047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avet-Loiseau H, et al. Long-term analysis of the IFM 99 trials for myeloma: Cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol. 2012;30:1949–1952. doi: 10.1200/JCO.2011.36.5726. [DOI] [PubMed] [Google Scholar]
- 6.Walker BA, et al. Mutational spectrum, copy number changes, and outcome: Results of a sequencing study of patients with newly diagnosed myeloma. J Clin Oncol. 2015;33:3911–3920. doi: 10.1200/JCO.2014.59.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar SK, et al. International Myeloma Working Group Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia. 2012;26:149–157, and erratum (2012) 26:1153. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 9.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 10.Turtle CJ, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35:3010–3020. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner RA, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry TJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locke FL, et al. Phase 1 results of ZUMA-1: A multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25:285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster SJ, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor BP, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai YT, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. 2016;127:3225–3236. doi: 10.1182/blood-2016-01-691162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali SA, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brudno JN, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36:2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan F, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol. 2017;35(Suppl 18):LBA3001. [Google Scholar]
- 20.Mi J-Q, et al. Effective treatment of relapsed/refractory multiple myeloma including extramedullary involvement by BCMA-specific chimeric antigen receptor-modified T cells. Blood. 2017;130:3115. [Google Scholar]
- 21.Zhao W-H, et al. Updated analysis of a phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B-cell maturation antigen, in patients with relapsed/refractory multiple myeloma. Blood. 2018;132:955. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao WH, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11:141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay KA, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown PA, et al. NCCN guidelines insights: Acute lymphoblastic leukemia, version 1.2017. J Natl Compr Canc Netw. 2017;15:1091–1102. doi: 10.6004/jnccn.2017.0147. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 29.Neelapu SS, et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.