The TGF-β signaling pathway is involved in the development of many types of cancer. In two recent PNAS papers, Montsivais et al. (1) and Kriseman et al. (2) of the Matzuk laboratory investigate the role of the TGF-β signaling pathway in endometrial carcinoma by using gene knockout mouse models. They find that either uterine conditional inactivation of TGF-β receptor Alk5 (1) or uterine double-conditional inactivation of Smad2 and Smad3 (2), which are transcription factors downstream of the receptor, leads to the development of estrogen-dependent metastatic endometrial cancer. They suggest that the TGF-β signaling pathway may serve as an important tumor suppressor in the uterus. These findings significantly advance our understanding of endometrial carcinoma and open novel avenues for future therapeutic strategies.
We are particularly interested in the phenotype difference in these two mouse models: Mice with uterine double-conditional inactivation of Smad2 and Smad3 developed endometrial cancer spontaneously (2), whereas mice with uterine conditional inactivation of Alk5 developed endometrial cancer only if they were continuously stimulated by pregnancy (1). According to the University of California, Santa Cruz Genome Browser mouse genome mm9 assembly (3), Alk5, Smad2, Smad3, and progesterone receptor (Pgr) are located on chr4:47366094–47427796, chr18:76401355–76471401, chr9:63494574–63605801, and chr9:8899833–8968611, respectively. Because Smad3 and Pgr are on the same chromosome, uterine conditional inactivation of Smad3 using Pgr-Cre would only result in haploinsufficiency. Therefore, uterine conditional inactivation of Alk5 may cause complete, whereas uterine double-conditional inactivation of Smad2 and Smad3 may cause only partial, loss of the TGF-β signaling pathway. This may explain the phenotype difference between these two mouse models.
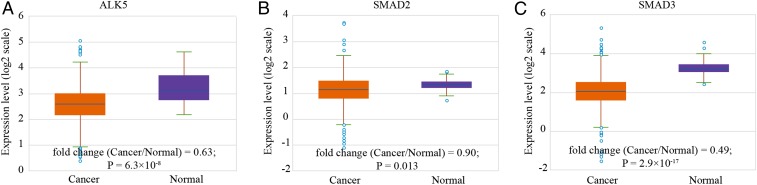
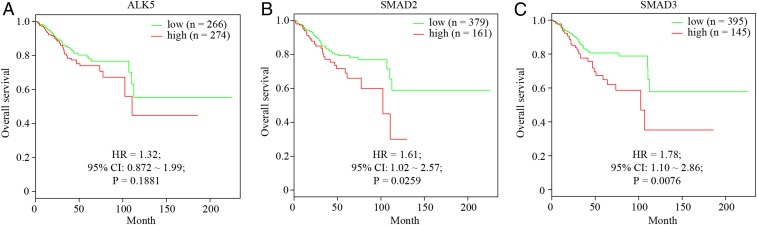
There are serval steps during tumorigenesis, from hyperplasia to dysplasia and eventually invasion. In theory, TGF-β can have a dual action in cancer (4): It may inhibit hyperplasia by inducing growth arrest as a tumor suppressor, and it may also enhance tumor cell migration by stimulating epithelial to mesenchymal transition as a tumor promoter. Taking the gene knockout results (1, 2) into consideration, we suspect that endometrial carcinoma may favor partial, but not complete, loss of the TGF-β signaling pathway. Additional evidence in support of this hypothesis was obtained by analyzing human samples of Uterine Corpus Endometrial Carcinoma (UCEC) from The Cancer Genome Atlas (TCGA) project (5). Expression levels of Alk5, Smad2, and Smad3 are significantly down-regulated in endometrial cancer tissues compared with the matched noncancer tissues (Fig. 1), indicative of a role as a tumor suppressor. Paradoxically, we found that although Alk5 does not reach the significant threshold, low expression levels of Smad2 and Smad3 are significantly associated with better prognosis and longer survival time in endometrial cancer patients (Fig. 2), which is in line with a role as a tumor promoter (6).
Fig. 1.
(A–C) Box plots showing the expression of ALK5 (A), SMAD2 (B), and SMAD3 (C) in the cohort of UCEC from TCGA database. RNA sequencing data were used to estimate gene expression levels as fragments per kilobase of transcript per million mapped fragments (FPKM).
Fig. 2.
(A–C) Kaplan–Meier curves showing the prognostic value of ALK5 (A), SMAD2 (B), and SMAD3 (C) in the cohort of UCEC from the TCGA database. The maximally separated Kaplan–Meier curve based on the log-rank test was chosen, and the cutoff to divide the patients into two groups of high and low was determined accordingly. A P value of <0.05 was considered to be of prognostic value. HR, hazard ratio.
In conclusion, by integrating mouse gene knockout results and human endometrial carcinoma gene expression data, we suspect that endometrial carcinoma may favor partial, but not complete, loss of the TGF-β signaling pathway. This pathway may have a dual action as a tumor suppressor and a tumor promoter. This hypothesis deserves further investigation.
Acknowledgments
This work was funded by the National Natural Science Foundation of China Grant 31771665 (to J.-L.L.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Monsivais D, Peng J, Kang Y, Matzuk MM. Activin-like kinase 5 (ALK5) inactivation in the mouse uterus results in metastatic endometrial carcinoma. Proc Natl Acad Sci USA. 2019;116:3883–3892. doi: 10.1073/pnas.1806838116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriseman M, et al. Uterine double-conditional inactivation of Smad2 and Smad3 in mice causes endometrial dysregulation, infertility, and uterine cancer. Proc Natl Acad Sci USA. 2019;116:3873–3882. doi: 10.1073/pnas.1806862116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haeussler M, et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 2019;47:D853–D858. doi: 10.1093/nar/gky1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colak S, Ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer. 2017;3:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Hutter C, Zenklusen JC. The Cancer Genome Atlas: Creating lasting value beyond its data. Cell. 2018;173:283–285. doi: 10.1016/j.cell.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Muinelo-Romay L, et al. High-risk endometrial carcinoma profiling identifies TGF-β1 as a key factor in the initiation of tumor invasion. Mol Cancer Ther. 2011;10:1357–1366. doi: 10.1158/1535-7163.MCT-10-1019. [DOI] [PubMed] [Google Scholar]