Significance
A healthy nutritional status is essential for proper brain development and for the maintenance of optimal cognitive function during adulthood and aging. The effects of ω-3 polyunsaturated fatty acids (ω-3 PUFAs) and vitamin supplementation or deficiency on neurodevelopment have been extensively studied in healthy infants and postweaning laboratory animals, but little is known about the influence of these micronutrients on the cognitive and neurochemical consequences of chronic stress during adolescence. We provide evidence that a diet supplemented with ω-3 PUFAs and vitamin A prevents deleterious cognitive impairment and shift of microbiota composition induced by social instability stress during adolescence, and that amelioration is maintained through adulthood, suggesting that a healthy diet may have long-lasting beneficial effects and help fight off neurodegenerative diseases.
Keywords: contextual fear conditioning, novel object recognition, gut microbiota, memory, SCFA
Abstract
Psychological stress during adolescence may cause enduring cognitive deficits and anxiety in both humans and animals, accompanied by rearrangement of numerous brain structures and functions. A healthy diet is essential for proper brain development and maintenance of optimal cognitive functions during adulthood. Furthermore, nutritional components profoundly affect the intestinal community of microbes that may affect gut-brain communication. We adopted a relatively mild stress protocol, social instability stress, which when repeatedly administered to juvenile rats modifies cognitive behaviors and plasticity markers in the brain. We then tested the preventive effect of a prolonged diet enriched with the ω-3 polyunsaturated fatty acids eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid and vitamin A. Our findings highlight the beneficial effects of this enriched diet on cognitive memory impairment induced by social instability stress, as stressed rats fed the enriched diet exhibited performance undistinguishable from that of nonstressed rats on both emotional and reference memory tests. Furthermore, in stressed rats, the decline in brain-derived neurotrophic factor expression in the hippocampus and shifts in the microbiota composition were normalized by the enriched diet. The detrimental behavioral and neurochemical effects of adolescent stress, as well as the protective effect of the enriched diet, were maintained throughout adulthood, long after the exposure to the stressful environment was terminated. Taken together, our results strongly suggest a beneficial role of nutritional components in ameliorating stress-related behaviors and associated neurochemical and microbiota changes, opening possible new venues in the field of nutritional neuropsychopharmacology.
In rodents, as in humans, adolescence is a time of developmental changes and reorganization in the brain and stress systems, marked by cognitive maturation and behavioral changes (1). Interactions with age-matched conspecifics during adolescence are important for appropriate rodent neurodevelopment, and any alterations to such adolescent social experiences can result in neurobehavioral measurements relevant to anxiety, depression, and substance abuse (2). Preclinical research has focused on earlier and later periods of development, with several studies demonstrating that early-life stress in both rodents and humans represents a neurodevelopmental risk with implications for subsequent cognitive abilities in adulthood (3, 4).
Given the paucity of data on the key factors contributing to the detrimental effects of adolescence stress, no effective strategies have been developed to prevent or cure these problems. In this respect, nutrition is one of the key lifestyle factors that contributes to mental health and has far-reaching consequences on cognitive functions that extend into later life (5, 6).
Among the nutritional components associated with optimal brain functioning, the ω-3 polyunsaturated fatty acids (ω-3 PUFAs) play critical roles in the development and function of the central nervous system (CNS). In animal studies, prenatal deficiency of brain ω-3 PUFAs is associated with enduring neuroanatomic and neurotransmitter alterations and neurocognitive deficits; elevated behavioral indices of anxiety, aggression, and depression (reviewed in ref. 7); and increased vulnerability to the effects of inflammatory events (8, 9). Recent studies have demonstrated the long-lasting beneficial cognitive effects of a diet supplemented with the ω-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on early stressful events, such as maternal separation (10, 11), or following ω-3 PUFA deficiency during the perinatal period (12, 13). Moreover, supplementation with ω-3 PUFAs has been shown to prevent the emotional and neuronal impairments induced by chronic social defeat in adult mice (14) and ameliorated memory performance in aged animals (15).
Another essential nutrient is vitamin A, which through its active metabolite retinoic acid plays a key role in cognitive functions in adult (16) and aged rats (17). Vitamin A deficiency increases hypothalamus-pituitary-adrenal axis activity, and vitamin A-deficient rats show a delayed and heightened corticosterone response to restraint stress (18). In contrast, vitamin A supplementation to adult rats was shown to prevent age-associated spatial memory decline (19).
A vast literature indicates that ω-3 PUFAs and retinoic acid signaling pathways may act together to modulate memory and synaptic plasticity, and indeed, a combined diet enriched with EPA-DHA and vitamin A demonstrated synergistic behavioral and neurochemical effects in middle-aged rats (20). However, the long-lasting effects of a prolonged diet enriched with these micronutrients on the detrimental consequences of adolescent stress remain largely unknown.
The gut microbiota has emerged as one of the key regulators of brain health across the lifespan, including in adolescence (21–23). Stress and other insults can seriously impact the composition of the microbiome (24), and dietary interventions have been shown to normalize such effects (10). Moreover, ω-3 PUFA deficiency and supplementation have been shown to differentially modulate microbiome composition (10, 13, 25).
In the present study, we hypothesized that a diet enriched in ω-3 PUFAs and vitamin A may prevent immediate and long-lasting behavioral deficits and neurochemical and intestinal microbiota changes induced by stress during adolescence. We used social instability stress, a well-validated animal model of social stress that produces long-lasting effects on cognitive and emotional responses that may persist for the entire lifespan (2, 26). We adopted a multilevel approach at two different age stages—immediately after completion of the social instability stress procedure and at adulthood—using a battery of behavioral tests assessing several domains potentially affected by chronic stress: cognition pertaining to emotional and recognition memory, anxiety-like responses, and anhedonia-like responses. We also measured the expression of brain-derived neurotrophic factor (BDNF), which promotes neuronal survival, regulates nerve cells differentiation, and may influence cognition (27), and of synaptophysin, a synaptic vesicle-associated protein involved in synaptic formation (28), in the hippocampus and frontal cortex of adult and adolescent rats. These brain regions are particularly vulnerable to the negative impact of stress, especially early in life (29). Finally, we investigated the short- and long-term impact of social instability stress and our dietetic intervention on caecal microbiota composition and short-chain fatty acid (SCFA) production. Our findings reveal that the ω-3 PUFA/vitamin A–enriched diet prevented adolescent stress-induced cognitive and microbiome changes.
Results
We adopted the social instability protocol (26) consisting of daily isolation followed by a change of cage partners from postnatal day (PND) 30 to PND 45 (Fig. 1A). In rodents, adolescence is defined by the time interval between weaning at PND 21 and the first signs of puberty, which in males coincides with preputial separation occurring around PND 42 ± 3 (1). To assess whether social instability stress causes acute and long-lasting deficits and whether an ω-3 PUFA/vitamin A–enriched diet (SI Appendix, Table S1) could ameliorate stress-induced deficits, we used a battery of measurements and behavioral tests performed starting on the day after completion of the stress protocol, PND 46 (in adolescents) or PND 70 (in adults).
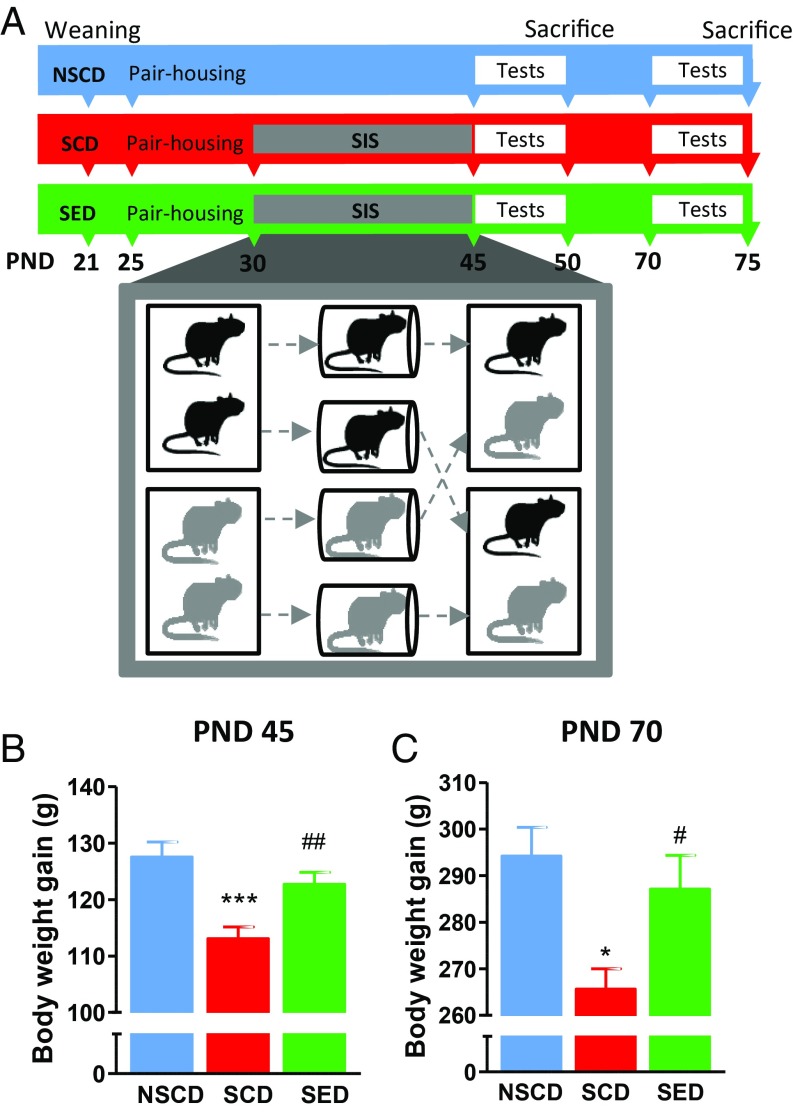
Fig. 1.
(A) Time line for the adolescent social instability stress experiment. Adolescent rats were randomly assigned to three experimental groups: NSCD, SCD, and SED. (B and C) Effects of stress and enriched diet on body weight at PND 45 on completion of the stress procedure (B) and at PND 70 (C). n = 18–24 rats/group. ***P < 0.001, *P < 0.05 vs. NSCD rats; ##P < 0.01, #P < 0.05 vs. NSCD by one-way ANOVA and the Bonferroni test.
Effects of Stress and the Enriched Diet on Body Weight and Food Consumption.
As shown in Fig. 1B, adolescent stressed rats gained less weight than nonstressed rats [F(2,61) = 9.950; P < 0.001], an effect that persisted until adulthood [F(2,59) = 5.262; P < 0.01] (Fig. 1C), as reported previously (30). This effect was counteracted by the ω-3 PUFA/vitamin A–enriched diet. At both ages, rats ate comparable amounts of food independent of stress and diet (SI Appendix, Fig. S1 A and B).
The Enriched Diet Prevented the Cognitive Impairments Induced by Social Instability Stress.
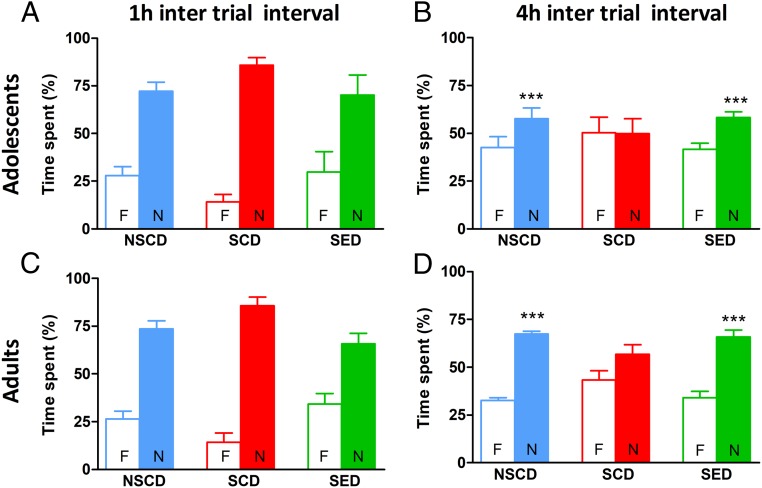
Two weeks of social instability stress had a negative impact on recognition memory that persisted into adulthood. For short-term memory (i.e., at 1 h after training), adolescent rats spent significantly more time exploring the novel object regardless of stress or diet [objects: F(1,34) = 62.88, P < 0.001; condition: F(2,34) = 0.0, P > 0.05; interaction: F(2,34) = 4.43, P > 0.05] (Fig. 2A). Regarding long-term memory (i.e., at 4 h after training), stressed rats fed the control diet (SCD rats) did not discriminate between the two objects (Fig. 2B). In the stressed rats fed with enriched diet (SED rats), the enriched diet fully prevented the stress-induced impairment of object discrimination [objects: F(1,30) = 59.11, P < 0.001; condition: F(2,30) = 0, P > 0.05; interaction: F(2,30) = 15.01, P < 0.01]. The cognitive impairment induced by the social instability stress persisted into adulthood and was prevented by the enriched diet administered since adolescence [objects: F(1,30) = 67.45, P < 0.001; conditions: F(2,30) = 0, P > 0.05; interaction: F(2,30) = 14.08, P < 0.001] (Fig. 2D).
Fig. 2.
The enriched diet prevented stress-induced cognitive impairment in the novel object recognition test. (A and C) Stress did not affect the performance of either adolescent or adult rats when the test was performed at 1 h after training. (B and D) Adolescent and adult stressed rats showed memory impairment when tested at 4 h after training, which was prevented by dietary supplementation with ω-3 PUFA/vitamin A. n = 6–8 rats/group. ***P < 0.001; **P < 0.01; *P < 0.05 vs. familiar object within each experimental group by two-way ANOVA and the Bonferroni test.
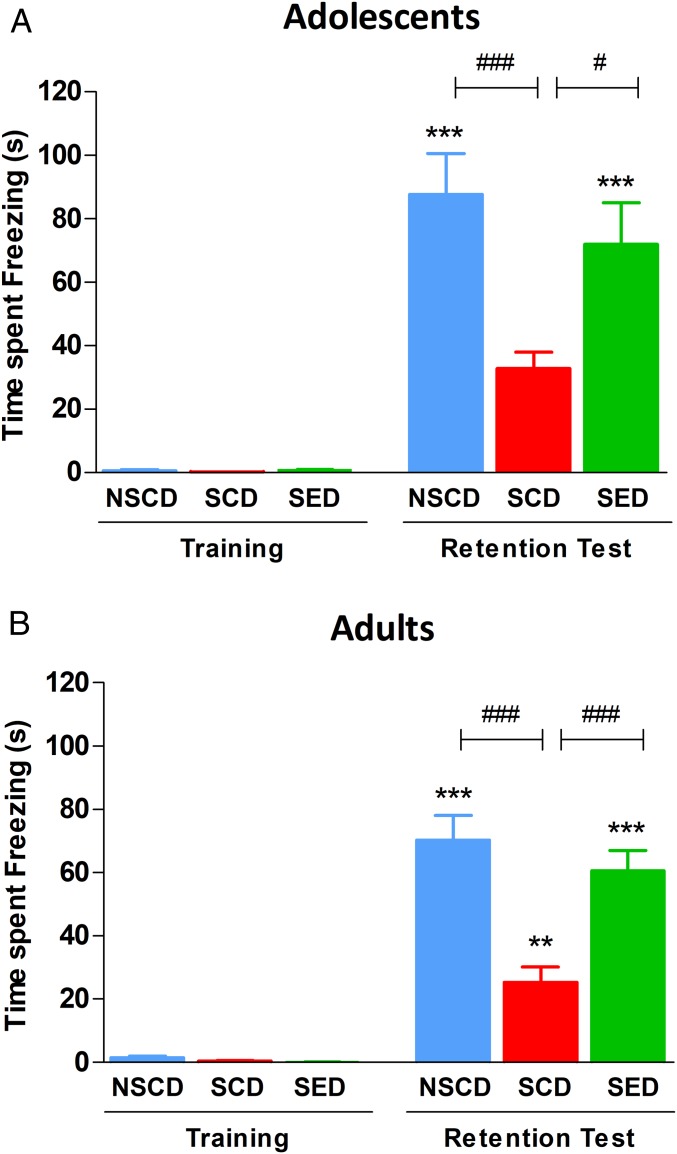
We also tested rats with an emotional arousing training experience that engages both contextual and emotional memory processing: the contextual fear conditioning paradigm. Freezing time obtained during a 3-min reexposure of rats to the conditioning apparatus at 24 h after acquisition served as an index of memory of the aversive experience. As shown in Fig. 3A, social instability stress did not affect the acquisition of fear memory irrespective of diet, whereas SCD adolescent rats froze less during context retrieval compared with non-stressed adolescent rats fed with control diet [NSCD, F(5,55) = 22.77, P < 0.001]. The enriched diet restored contextual fear memory expression, as SED rats spent significantly more time freezing at recall compared with SCD rats, which were indistinguishable from NSCD rats. The emotional memory deficit and beneficial effects of the enriched diet were long-lasting, as the SED rats froze significantly longer than the SCD rats [F(5,55) = 47.95, P < 0.001] (Fig. 3B)
Fig. 3.
The enriched diet prevented immediate (A) and long-term (B) stress-induced cognitive impairment in the contextual fear conditioning test. Rat freezing time did not differ at training regardless of treatment condition. When tested at 24 h after training, the SCD rats showed a lower freezing time than the NSCD rats, and the SED rats showed no stress-induced cognitive impairment. n = 9–10 rats/group. ***P < 0.001, **P < 0.01 vs. respective training; ###P < 0.001, #P < 0.05 vs. SCD by one-way ANOVA and the Bonferroni test.
The Enriched Diet Prevented Short- and Long-Term Effects of Social Stress on BDNF Expression.
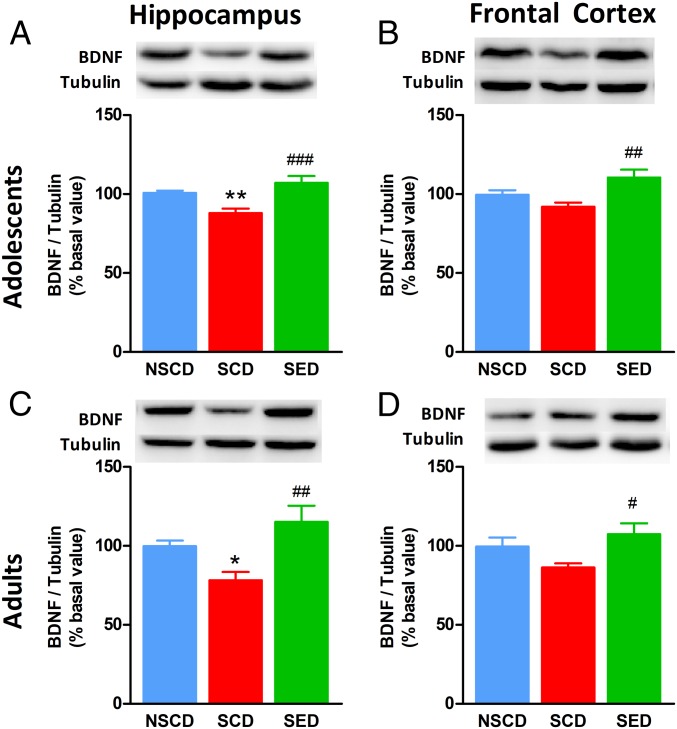
To investigate the effect of stress and diet on brain plasticity, we assessed the expression of BDNF in the brain. We found significant differences in BDNF protein levels in the hippocampus of adolescent rats [F(2,27) = 10.33, P < 0.001; Fig. 4A]. BDNF expression was decreased in SCD rats compared with NSCD rats, and this effect was prevented by the enriched diet. In the frontal cortex of adolescent rats, stress did not significantly modify BDNF expression, whereas the enriched diet increased BDNF levels [F(2,27) = 5.808, P < 0.01] (Fig. 4B). Interestingly, social instability stress led to a long-lasting decrease in hippocampal BDNF expression, while the enriched diet prevented the effects of social instability stress [F(2,28) = 6.896, P < 0.01] (Fig. 4C). BDNF levels in the frontal cortex were significantly increased following the enriched diet in adult rats as well [F(2,24) = 3.680, P < 0.05] (Fig. 4D).
Fig. 4.
The enriched diet restored BDNF expression in the brain of stressed rats. (A and B) Stress decreased BDNF levels in the hippocampus in both adolescent (A) and adult (B) rats. The enriched diet restored BDNF expression to control levels. (C and D) In the prefrontal cortex of stressed rats, the BDNF decrease did not reach statistical significance in either adolescence (C) or adulthood (D); nonetheless, the enriched diet augmented BDNF expression compared with stressed and control rats. (Insets) Representative immunoblots for each experimental group. n = 8–10 rats/group. **P < 0.01; *P < 0.05 vs. NSCD; ###P < 0.001, ##P < 0.01, #P < 0.05 vs. SCD by one-way ANOVA and Bonferroni’s test.
We also used Western blot analysis to detect synaptophysin (a glycoprotein associated with presynaptic vesicles) as a marker of synaptic density. Synaptophysin expression was not significantly affected by stress in both adolescent and adult rats (SI Appendix, Fig. S2), in agreement with a previous report (31). In adolescent rats, the enriched diet did not significantly affect synaptophysin expression in the hippocampus [F(2,28) = 2.121, P > 0.05] or in the cortex [F(2,29) = 2.727, P > 0.05]. In adult rats, however, we observed a significant diet-induced increase of synaptophysin expression in both the hippocampus [F(2,26) = 8.858, P < 0.001] and the frontal cortex [F(2,27) = 3.705, P < 0.05], which is consistent with the previous observation that long exposure to ω-3 PUFAs increases hippocampal synaptophysin expression (32).
Anhedonia-Like Behavior.
In agreement with recent data (33), we observed no differences between SCD and NSCD rats in terms of preference for the consumption of a sweetened solution when evaluated at different time points (SI Appendix, Fig. S3). Neither stress nor the enriched diet had an effect on anhedonia-like behavior, as the preference for a sucrose-sweetened drink was not affected by any of the experimental manipulations in either age group tested.
Locomotor Activity and Anxiety-Related Behaviors.
Locomotor activity, measured as the distance traveled and time spent moving in an open field, was comparable across the three cohorts in each age group, although adult rats were less active than adolescents (SI Appendix, Table S2). Neither stress nor diet affected the number of entries and time spent in the center or the periphery of the open field (SI Appendix, Table S2). Treatment conditions did not affect other exploratory behaviors indicative of a nondistressed state, such as climbing, rearing, and grooming. Accordingly, treatment conditions also had no effect on the number of entries and percentage of time spent by adolescent or adult rats in the open arms of the elevated plus maze (SI Appendix, Table S3).
The Enriched Diet Prevented Acute Stress-Induced Changes in the Caecal Microbiota Composition of Adolescent Rats.
We found that the microbiome was relatively homogeneous and thus characteristic of the animal species under study, with no significantly different patterns between and within groups (SI Appendix, Fig. S4A). However, the enriched diet increased alpha diversity (Chao1, a measure that reflects species richness) of the caecal microbiota in adolescent SED rats compared with both SCD rats (P < 0.05) and NSCD rats (P < 0.01) (SI Appendix, Fig. S4B). Consequently, significant differences were found in observed species (species count, SI Appendix, Fig. S4B) (P < 0.05 and P < 0.01, respectively), indicating that the dietary intervention increased microbiota richness in adolescence irrespective of stress exposure. This effect was restricted to adolescence, as there was no long-lasting effect of diet on diversity into adulthood.
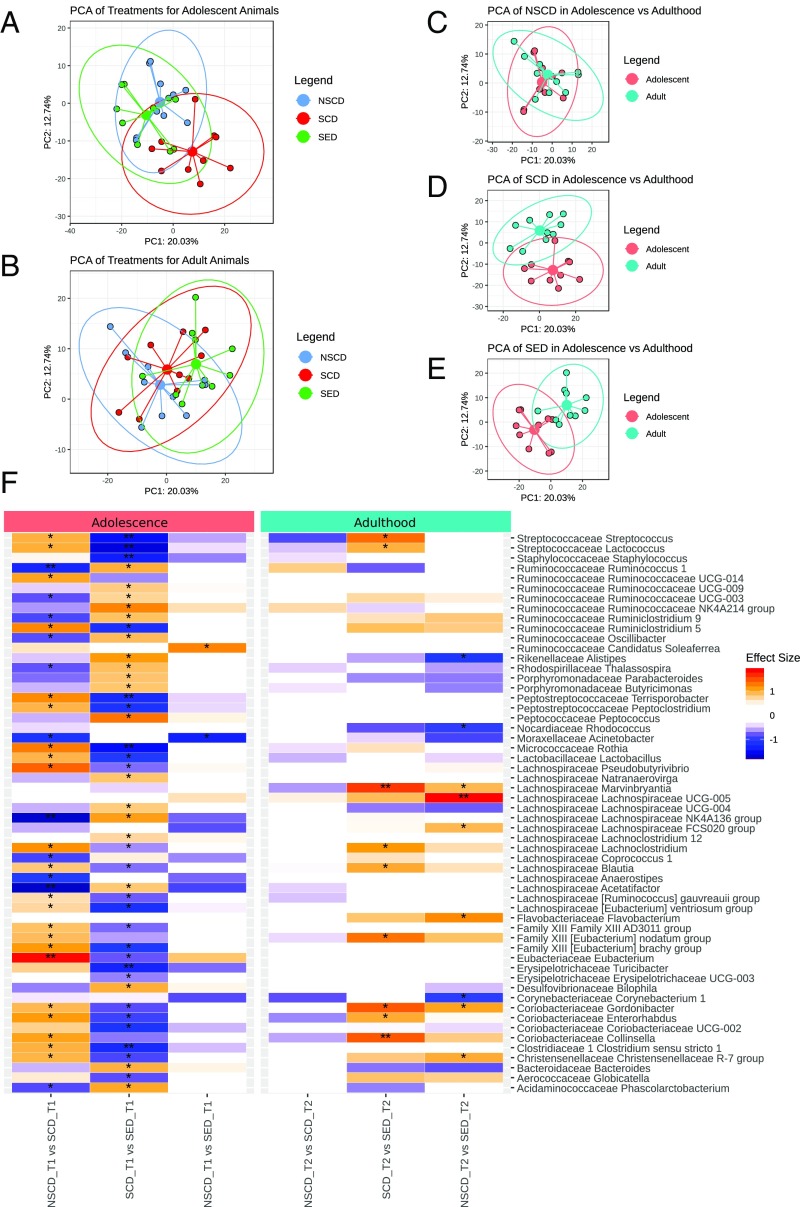
A principal component analysis (PCA) identified structural differences in the microbial community (beta diversity, i.e., difference in taxonomic profiles from different samples) between adolescent NSCD and SCD rats [P < 0.05, pairwise permutational multivariate ANOVA (PERMANOVA)] (Fig. 5A), indicating a shift of the microbiome composition following social instability stress that was almost entirely prevented when stressed rats were fed the ω-3 PUFA/vitamin A–enriched diet (P < 0.1, pairwise PERMANOVA) (Fig. 5A). No long-lasting impact of stress on the composition of the gut microbiota was identified in adult rats (Fig. 5B); however, adult SED rats exhibited a long-lasting shift in gut microbiota composition (P < 0.05, pairwise PERMANOVA) (Fig. 5B). Compared with adolescent rats, adult rats exhibited a shift in gut microbiota composition in all three treatment groups (P < 0.05, pairwise PERMANOVA) (Fig. 5 C–E).
Fig. 5.
Social instability stress shapes the gut microbiome in adolescence. (A and B) PCA plots displaying beta diversity of the gut microbiome in adolescent (A) and adult (B) rats. (C–E) PCA plots comparing beta diversity of the gut microbiome for each treatment between adulthood and adolescence. (F) Changes in gut microbiome composition (genera) in adolescence (T1) and adulthood (T2). Changes in genera in each age group are depicted as follows: (i) SCD vs. NSCD, (ii) SED vs. SCD, (iii) SED vs. NSCD. *P < 0.1, **P < 0.01, post hoc Benjamini–Hochberg test.
Social instability stress during adolescence significantly modulated a multitude of taxa that were normalized by the ω-3 PUFA/vitamin A–enriched diet (Fig. 5F). Among these, SCD rats had decreased relative abundance of genera of the Lachnospiraceae family (particularly Acetatifactor, Anaerostipes, and Lachnospiraceae NK4A136 group) and several family members in the Ruminococcaceae family (particularly Ruminococcus 1), whereas the relative abundances of the Eubacterium genus as well as Coriobacteriaceae family members were increased. These changes were largely counterbalanced by the ω-3 PUFA/vitamin A–enriched diet. Independent of social instability stress, the enriched diet had a long-lasting impact on only a few taxa into adulthood (Fig. 5F).
Social instability stress reduced the concentration of the SCFAs butyrate (P < 0.05), valerate (P < 0.05), and isobutyrate (P < 0.05) in adolescent rats (SI Appendix, Fig. S4C). In contrast, SED rats showed increases in isobutyrate (P < 0.001) and isovalerate (P < 0.05) that led to an overall increase in branched-chain fatty acids (P < 0.001). In adult rats, the enriched diet decreased butyrate concentrations compared with stressed controls (P < 0.05) (SI Appendix, Fig. S4C).
Discussion
Nutrition has a fundamental role in maintaining brain health and behavior at critical periods, especially in adolescence (34). In rodents, appropriate essential micronutrient supplementation protects against the cognitive decline associated with early life stress (35). Our study corroborates the idea that dietary intervention affects neurobehavioral development (13) by demonstrating that a diet supplemented with the ω-3 PUFAs and vitamin A prevented the deleterious cognitive decline induced by social instability stress during adolescence, with the amelioration maintained in adulthood. The rationale for using a combination of ω-3 PUFAs and vitamin A stems from recent findings demonstrating a beneficial synergistic effect of vitamin A and EPA/DHA on behavioral and neurobiological markers in aged rats (20). Multiple levels of interactions occur between ω-3 PUFAs and retinoid signaling, because retinoic acid (the active metabolite of vitamin A) and DHA may bind to common nuclear receptors (36). Furthermore, retinoic acid and ω-3 PUFAs have common intracellular signaling pathways, such as AKT and ERK1/2 (37, 38), which are known to be activated in several neuronal functions (39).
Social instability stress during adolescence is known to cause emotional and recognition memory impairments that persist into adulthood. These behavioral changes are closely associated with alterations in BDNF expression in the hippocampus and the frontal cortex. In the present study, dietary ω-3 PUFA/vitamin A exposure from adolescence to adulthood was sufficient to prevent such alterations, and the beneficial outcomes were maintained throughout adulthood.
The Enriched Diet Prevents Memory and Brain BDNF Decline Induced by Social Stress.
Social instability stress in adolescence exerts long-lasting effects on aversive and recognition memory, as shown in previous reports describing enduring deficits in contextual fear memory in response to adolescence stress (40). The hippocampus is one of the brain structures crucially involved in regulation of stress responses (41). We found that social instability impaired contextual fear memory, a predominantly hippocampus-dependent form of aversive memory (reviewed in ref. 42), in both adolescent and adult rats.
In addition, stressed adolescent rats showed long-lasting memory impairment in the novel object recognition test, an effect not previously observed. In fact, McCormick et al. (31) found that stressed rats had hippocampal-dependent spatial recognition impairment but no reference memory deficit when tested at 4 h after training.
We found a close correspondence between rats’ memory performance and BDNF expression in the hippocampus, as both effects were significantly decreased in stressed adolescent and adult rats, and the enriched diet prevented both effects. Our results are in agreement with recent observations that ω-3 PUFAs induce increased BDNF expression in rat hippocampus (43). Unequivocal evidence suggests a key role for BDNF in the initiation of fear memory consolidation. Importantly, BDNF enhances fear memory, and antibodies against BDNF impair fear memory when administered into the CA1 region of the hippocampus (reviewed in ref. 44). Thus, BDNF expression and activity in the hippocampus are required to ensure successful storage for associative memory persistence over days (45). Our behavioral results are in accordance with these observations, as social instability stress reduced hippocampal BDNF expression and impaired contextual fear memory. In this regard, we cannot exclude the possibility that in our study, BDNF modulation in the hippocampus affected object memory as well. Although canonically the novel object recognition task is assumed to be largely independent of the hippocampus, some findings have challenged this idea (46, 47), pointing to a temporal specificity for hippocampal involvement in object recognition memory (48, 49). Nonetheless, our results do not exclude the possibility that the cognitive deficit in stressed rats represents a global impairment in learning and memory function with compromised signaling molecules other than BDNF. In the frontal cortex, BDNF expression is required for fear memory consolidation and expression (reviewed in ref. 50). Social instability stress did not significantly affect BDNF expression levels in the cortex of adolescent and adult rats, although the enriched diet augmented BDNF levels, presumably contributing to maintenance of long-term memory.
The Enriched Diet Does Not Affect Behaviors Relevant to Anxiety and Anhedonia.
Social instability administered during adolescence is known to modify several social behaviors, as stressed rats spend less time in social interactions with other males, have reduced sexual performance, and exhibit longer latency to enter the center of an open arena (51)—all validated measures of anxiety-like behavior. Furthermore, the modified social repertoire is evident in adulthood even weeks after the stressful procedure (2). In our study, we found no difference between stressed and nonstressed rats in the latency to enter the center of the arena or the number of entries, or in behavioral differences in the elevated plus maze during adolescence or adulthood. One factor contributing to these discrepancies may be strain differences, which are known to be responsible for the anxiety profile (52). Indeed, the Wistar rats used in our experiments appeared more resilient than those commonly used for anxiety-like tests. Other behavioral signs of stress, such as modified grooming, rearing, or climbing (53, 54) were not affected by our protocol or by the dietary supplementation. Confirming recent data regarding the consumption of natural rewards (33), neither adolescent nor adult stressed rats manifested anhedonia-like behavior in the sucrose preference test.
Social Instability Stress During Adolescence Dramatically Altered the Gut Microbiome, Which Was Reversed by the Enriched Diet.
Increasing evidence shows that intestinal microbiota influences behaviors relevant to mood and cognitive functions. One proposed mechanism for such effects is the production of metabolites with central effects, among which SCFAs are the most important (55–57). ω-3 PUFAs are known to have a positive action on the intestinal microbiota, increasing the production of SCFAs (reviewed in ref. 58). Moreover, perturbations of the microbiota during adolescence have been shown to result in enduring social and cognitive deficits (59).
Psychological stress (ranging from restraint stress to maternal separation and overcrowding) has been shown to alter microbiota composition (60–64). In the present study, social instability stress induced striking changes in the gut microbiome composition of adolescent rats, which were partially reversed by the ω-3 PUFA/vitamin A–enriched diet. Adolescence stress resulted in a decreased abundance of several genera in the Ruminococcaceae and Lachnospiraceae families. The functional consequences of such changes are not clear at this stage, but it is worth noting that decreases in both Ruminococcaceae and Lachnospiraceae have been reported in patients with depressive disorders (65). In contrast, the Eubacterium genus was increased in our SCD rats. Eubacteriaceae were found to be increased in rats with experimental colitis also exposed to stress (66), conditions under which Lachnospiraceae were decreased (66). Coriobacteriaceae, particularly Enterorhabdus, were increased in our SCD rats, in line with previous studies associating Coriobacteriaceae with colonic health and inflammation (67). Nonetheless, the enriched diet was sufficient to prevent such modifications. However, despite the stress-induced changes and independent of diet, the intestinal microbiota during adulthood recovered the core microbiota composition characteristic of adult animals (68). Interestingly, no long-lasting impact of stress was identified in adult rats; however, the SED rats exhibited a long-lasting shift in beta diversity.
A limitation of the present study is that we cannot definitely prove a causal correlation between changes in microbiota and cognitive performance at this juncture. However, given that the microbiome has been implicated as a conduit for the positive effects of nutrition on host health, modulation of the microbiota is a plausible mechanism for how nutritional interventions can reduce the effects of stress (69), based on our finding that both diet and stress induced effects on both the microbiome and behavioral/neurochemical measures of cognitive functions. We also found that the enriched diet increased the production of branched SCFAs, whereas unbranched SCFAs were unchanged. Previous studies have shown that SCFA levels are strongly correlated with improvement in tests of anxiety- and depression-like behaviors (70), and that SCFAs can reverse the enduring effects of social stress in adulthood (71).
Stress, emotional instability, and impulsivity are all enhanced during adolescence, and inadequate nutrition may exacerbate such conditions. Moreover, there is growing understanding of the link between changes in gut microbial composition and brain health in adolescence (22, 23). Clinical studies have shown that young adults who endured environmental or psychosocial stressors during development or have low blood ω-3 PUFA levels are often diagnosed with psychiatric disorders or cognitive impairments (72–76). Our present study provides preclinical evidence suggesting that supplementation with ω-3 PUFAs and vitamin A is sufficient to prevent long-lasting cognitive disturbances and to modulate the microbiota composition that accompanies repeated, prolonged stressful stimuli during adolescence. The optimization of dietary components that affect brain development suggests the likelihood that humans may improve cognition throughout the lifespan.
Materials and Methods
Social Instability Stress.
Male Wistar rats arrived at our animal facility at PND 25 and were assigned at random to three experimental groups: a group of nonstressed animals fed with the control diet (NSCD), a second group subjected to social instability protocol and fed with the control diet (SCD), and a third group also submitted to stressful manipulation and fed with the enriched diet (SED). The social instability stress involves changing the social housing conditions of adolescent rats, as described previously (26). In brief, on each day from PND 30 to PND 45, the rats were isolated for 1 h in ventilated round plastic containers (10 cm in diameter), akin to restraint. After isolation, the rats were housed with a new partner undergoing the same procedure in a new cage. The stress regimen was implemented at various times during the light cycle to decrease the predictability of the event. After the last isolation on PND 45, the rats were returned to their original cage partners. The nonstressed rats were not disturbed except for regular cage maintenance and weighing.
The consequences of the social instability stress procedure were assessed during adolescence (PND 46–51) and during adulthood (PND 70–76) using a battery of tests comprehensive of several domains affected by chronic stress: cognition (novel object recognition and contextual fear conditioning), anhedonia-like behavior (sucrose preference), and anxiety-like behavior (elevated plus maze). Locomotor activity was measured in an open field arena. At 1 d after completion of the behavioral tests, brains and caecal contents were collected for neurochemical and metagenomics analyses, respectively. Different cohorts of animals were used at the two time points. The experimental timeline is depicted in Fig. 1A. Details of the behavioral tests, Western blot analysis, and metagenomics analysis are provided in SI Appendix.
Previous reports have indicated that while adult rats readily habituate to this procedure, adolescents show increased corticosterone release in response to repeated changes of cage partners. Consistently, the long-lasting cognitive and emotional alterations observed in adolescent rats subjected to the social instability procedure were not found in adults rats, suggesting that this model may capture adolescent-specific stress reactivity (26, 30). Experimental approval was granted by the Italian Ministry of Health (no. 114/2017-PR).
Diet Composition.
Diets were matched for macronutrient content; the specific compositions are provided in SI Appendix, Table S1. To prevent oxidation of PUFAs, diets were maintained in air-sealed bags at 4 °C in the dark. Food was changed and weighed every day.
Supplementary Material
Acknowledgments
We thank the Teagasc sequencing facility, Drs. Fiona Crispie, Laura Finnegan, and Paul Cotter. This research was supported through the Italian Ministry of Education, Universities, and Research/European Research Area-Healthy Diet for Healthy Life Project AMBROSIAC and the Science Foundation Ireland (Proposal 15/JP-HDHL/3270). G.P. was supported by the University of Florence (DR 175372-1210), the Umberto Veronesi Foundation, and the Brazilian National Council for Scientific and Technological Development (CNPq; 201511/2014-2). S.D.S. was supported by ERA-HDHL Project AMBROSIAC and by the Italian Ministry of Foreign Affairs and International Cooperation and the Brazilian Coordination of Improvement of Higher Education Personnel (CAPES Brazil; Finance Code 001). I.I. was supported by CAPES Brazil (Finance Code 001) and the CNPq.
Footnotes
Conflict of interest statement: J.F.C. and C. Stanton have received research funding from Dupont Nutrition Biosciences APS, Cremo SA, Alkermes Inc., 4D Pharma PLC, Mead Johnson Nutrition, Nutricia Danone, and Suntory Wellness and have spoken at meetings sponsored by food and pharmaceutical companies.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820832116/-/DCSupplemental.
References
- 1.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Burke AR, McCormick CM, Pellis SM, Lukkes JL. Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci Biobehav Rev. 2017;76:280–300. doi: 10.1016/j.neubiorev.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan GA, et al. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- 4.Oomen CA, et al. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology (Berl) 2011;214:249–260. doi: 10.1007/s00213-010-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laus MF, Vales LD, Costa TM, Almeida SS. Early postnatal protein-calorie malnutrition and cognition: A review of human and animal studies. Int J Environ Res Public Health. 2011;8:590–612. doi: 10.3390/ijerph8020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72:267–284. doi: 10.1111/nure.12102. [DOI] [PubMed] [Google Scholar]
- 7.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Delpech JC, et al. Dietary ω-3 PUFA deficiency increases vulnerability to inflammation-induced spatial memory impairment. Neuropsychopharmacology. 2015;40:2774–2787. doi: 10.1038/npp.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labrousse VF, et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav Immun. 2018;73:427–440. doi: 10.1016/j.bbi.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Pusceddu MM, et al. ω-3 PUFAs have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology. 2015;58:79–90. doi: 10.1016/j.psyneuen.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Mathieu G, et al. Dietary ω-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot Essent Fatty Acids. 2011;85:129–136. doi: 10.1016/j.plefa.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Lozada LE, Desai A, Kevala K, Lee JW, Kim HY. Perinatal brain docosahexaenoic acid concentration has a lasting impact on cognition in mice. J Nutr. 2017;147:1624–1630. doi: 10.3945/jn.117.254607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson RC, et al. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 14.Larrieu T, et al. Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl Psychiatry. 2014;4:e437, and erratum (2014) 4:e468. doi: 10.1038/tp.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis I, Potier B, Vancassel S, Heberden C, Lavialle M. Omega-3 fatty acids and brain resistance to ageing and stress: Body of evidence and possible mechanisms. Ageing Res Rev. 2013;12:579–594. doi: 10.1016/j.arr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res. 2010;54:489–495. doi: 10.1002/mnfr.200900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonhomme D, et al. Retinoic acid modulates intrahippocampal levels of corticosterone in middle-aged mice: Consequences on hippocampal plasticity and contextual memory. Front Aging Neurosci. 2014;6:6. doi: 10.3389/fnagi.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marissal-Arvy N, Hamiani R, Richard E, Moisan MP, Pallet V. Vitamin A regulates hypothalamic-pituitary-adrenal axis status in LOU/C rats. J Endocrinol. 2013;219:21–27. doi: 10.1530/JOE-13-0062. [DOI] [PubMed] [Google Scholar]
- 19.Touyarot K, et al. A mid-life vitamin A supplementation prevents age-related spatial memory deficits and hippocampal neurogenesis alterations through CRABP-I. PLoS One. 2013;8:e72101. doi: 10.1371/journal.pone.0072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Létondor A, et al. EPA/DHA and vitamin A supplementation improves spatial memory and alleviates the age-related decrease in hippocampal RXRγ and kinase expression in rats. Front Aging Neurosci. 2016;8:103. doi: 10.3389/fnagi.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinan TG, Cryan JF. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVey Neufeld KA, Luczynski P, Dinan TG, Cryan JF. Reframing the teenage wasteland: Adolescent microbiota-gut-brain axis. Can J Psychiatry. 2016;61:214–221. doi: 10.1177/0706743716635536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McVey Neufeld KA, Luczynski P, Seira Oriach C, Dinan TG, Cryan JF. What’s bugging your teen? The microbiota and adolescent mental health. Neurosci Biobehav Rev. 2016;70:300–312. doi: 10.1016/j.neubiorev.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Foster JA, Rinaman L, Cryan JF. Stress and the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson RC, et al. Deficiency of essential dietary ω-3 PUFA disrupts the caecal microbiome and metabolome in mice. Br J Nutr. 2017;118:959–970. doi: 10.1017/S0007114517002999. [DOI] [PubMed] [Google Scholar]
- 26.McCormick CM, Hodges TE, Simone JJ. Peer pressures: Social instability stress in adolescence and social deficits in adulthood in a rodent model. Dev Cogn Neurosci. 2015;11:2–11. doi: 10.1016/j.dcn.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchman AS, et al. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86:735–741. doi: 10.1212/WNL.0000000000002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarsa L, Goda Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2002;99:1012–1016. doi: 10.1073/pnas.022575999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Hodges TE, McCormick CM. Adolescent and adult male rats habituate to repeated isolation, but only adolescents sensitize to partner unfamiliarity. Horm Behav. 2015;69:16–30. doi: 10.1016/j.yhbeh.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 31.McCormick CM, et al. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces deficits in spatial location memory in adulthood. Hippocampus. 2012;22:1300–1312. doi: 10.1002/hipo.20966. [DOI] [PubMed] [Google Scholar]
- 32.Venna VR, et al. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology. 2009;34:199–211. doi: 10.1016/j.psyneuen.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Marcolin ML, Hodges TE, Baumbach JL, McCormick CM. Adolescent social stress and social context influence the intake of ethanol and sucrose in male rats soon and long after the stress exposures. Dev Psychobiol. 2019;61:81–95. doi: 10.1002/dev.21800. [DOI] [PubMed] [Google Scholar]
- 34.Hueston CM, Cryan JF, Nolan YM. Stress and adolescent hippocampal neurogenesis: Diet and exercise as cognitive modulators. Transl Psychiatry. 2017;7:e1081. doi: 10.1038/tp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naninck EF, et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- 36.de Urquiza AM, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 37.Masiá S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21:2391–2402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- 38.Rao JS, et al. Dietary ω-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 39.Tang S, Yasuda R. Imaging ERK and PKA activation in single dendritic spines during structural plasticity. Neuron. 2017;93:1315–1324.e3. doi: 10.1016/j.neuron.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrissey MD, Mathews IZ, McCormick CM. Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol Learn Mem. 2011;95:46–56. doi: 10.1016/j.nlm.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 42.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 43.Vines A, et al. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology. 2012;62:184–191. doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Izquierdo I, Furini CR, Myskiw JC. Fear memory. Physiol Rev. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- 45.Bekinschtein P, et al. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 46.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen SJ, et al. The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen SJ, Stackman RW., Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76:677–683. doi: 10.1016/j.neuropharm.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev Psychobiol. 2013;55:849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- 52.Ramos A, Berton O, Mormède P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 53.Kruk MR, et al. The hypothalamus: Cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci Biobehav Rev. 1998;23:163–177. doi: 10.1016/s0149-7634(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 54.Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun. 2016;7:11937. doi: 10.1038/ncomms11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. 2011;105:100–105. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bercik P, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18:E2645. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desbonnet L, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Bailey MT, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017;15:7. doi: 10.1186/s12916-016-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Palma G, Collins SM, Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes. 2014;5:419–429. doi: 10.4161/gmic.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Mahony SM, et al. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 66.Konturek P, et al. P109 Rifaximin and Saccharomyces boulardii increase stress resilience in TNBS-induced colitis. J Crohn’s Colitis. 2018;12(Suppl_1):S148. [Google Scholar]
- 67.Morgan XC, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flemer B, et al. Fecal microbiota variation across the lifespan of the healthy laboratory rat. Gut Microbes. 2017;8:428–439. doi: 10.1080/19490976.2017.1334033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandhu KV, et al. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223–244. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Burokas A, et al. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 71.van de Wouw M, et al. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Espejo EP, et al. Stress sensitization and adolescent depressive severity as a function of childhood adversity: A link to anxiety disorders. J Abnorm Child Psychol. 2007;35:287–299. doi: 10.1007/s10802-006-9090-3. [DOI] [PubMed] [Google Scholar]
- 73.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Joffre C, Nadjar A, Lebbadi M, Calon F, Laye S. n-3 LCPUFA improves cognition: The young, the old and the sick. Prostaglandins Leukot Essent Fatty Acids. 2014;91:1–20. doi: 10.1016/j.plefa.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Kuratko CN, Barrett EC, Nelson EB, Salem N., Jr The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: A review. Nutrients. 2013;5:2777–2810. doi: 10.3390/nu5072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ. Low blood long-chain omega-3 fatty acids in UK children are associated with poor cognitive performance and behavior: A cross-sectional analysis from the DOLAB study. PLoS One. 2013;8:e66697. doi: 10.1371/journal.pone.0066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.