Significance
Solution-processing metal oxide (MO) films at low temperature enables their integration in mechanically flexible and inexpensive substrates for unconventional optoelectronics. However, temperature reduction has not been accompanied by a corresponding reduction in film-processing time, which remains far greater than acceptable for efficient/continuous additive manufacturing as in typical fabrication lines. Here, we report a highly efficient cofuel-assisted combustion process, which involves introducing both a fluorinated fuel and a preannealing step, that achieves ultrafast reaction and metal–oxygen–metal (M-O-M) lattice condensation within 10–60 s for several MO semiconductors and aluminum oxide dielectric. The resulting MO transistors exhibit high carrier mobility, excellent bias stability, and good flexibility.
Keywords: thin-film transistor, solution process, ultrashort annealing time, combustion synthesis, blade coating
Abstract
Metal oxide (MO) semiconductor thin films prepared from solution typically require multiple hours of thermal annealing to achieve optimal lattice densification, efficient charge transport, and stable device operation, presenting a major barrier to roll-to-roll manufacturing. Here, we report a highly efficient, cofuel-assisted scalable combustion blade-coating (CBC) process for MO film growth, which involves introducing both a fluorinated fuel and a preannealing step to remove deleterious organic contaminants and promote complete combustion. Ultrafast reaction and metal–oxygen–metal (M-O-M) lattice condensation then occur within 10–60 s at 200–350 °C for representative MO semiconductor [indium oxide (In2O3), indium-zinc oxide (IZO), indium-gallium-zinc oxide (IGZO)] and dielectric [aluminum oxide (Al2O3)] films. Thus, wafer-scale CBC fabrication of IGZO-Al2O3 thin-film transistors (TFTs) (60-s annealing) with field-effect mobilities as high as ∼25 cm2 V−1 s−1 and negligible threshold voltage deterioration in a demanding 4,000-s bias stress test are realized. Combined with polymer dielectrics, the CBC-derived IGZO TFTs on polyimide substrates exhibit high flexibility when bent to a 3-mm radius, with performance bending stability over 1,000 cycles.
The recent commercialization of metal oxide (MO) thin-film transistors (TFTs) highlights the many attractions of amorphous MOs (a-MOs) over competing semiconductor technologies (1–9). Furthermore, and in contrast to current capital-intensive vapor deposition growth, solution-processed a-MO films promise to reduce manufacturing costs (10–14) as well as enable flexible optoelectronics on inexpensive plastic substrates (15–21). This possibility reflects the substantial lowering of MO film-processing temperatures from >450–600 °C to recently as low as 150–300 °C (11, 22–29). However, this dramatic temperature reduction has not been accompanied by a corresponding reduction in film-processing time, which remains far greater (presently 1–2 h minimum) than acceptable for efficient, continuous additive manufacture, as in typical FAB lines. A noticeable exception is capital-intensive flash lamp annealing (FLA) for millisecond fabrication of silicon shallow junctions, which has recently been adapted to MO film growth (30–32). However, the high energy-density radiation pulses instantly generate temperatures >1,000 °C at the film surface with very large temperature gradients downward. These can produce extreme nonuniformity in FLA films and very large thermal stresses that permanently damage underlying functional layers and plastic substrates (32–34).
In the past decade, several laboratories including this one have shown that incorporating combustive fuel-oxidizer redox couples in MO-precursor solutions yields dense, electronic-quality MO nanometer-thick films at far lower temperatures than conventional sol–gel processes (15, 35, 36). Nevertheless, the subsequent post–film-deposition annealing step in combustion can require up to 2 h and is essential for lattice densification and eliminating organic contaminants (37, 38). Here, we report that a liquid MO precursor combining a coordinating fuel, AcAcH, a fluorinated cofuel, FAcAcH (Fig. 1A), and the corresponding metal nitrates and, equally important, removing extraneous solvent from the precursor films before combustion onset, dramatically shorten the processing/annealing time to as little as 10–60 s. The scope of this approach is demonstrated by fabricating MO TFTs with the technologically important oxide semiconductors indium oxide (In2O3), indium-zinc oxide (IZO), and indium-gallium-zinc oxide (IGZO). Maximum electron mobilities (µmax) for spin-coated 300 °C/10 s-annealed MO film on 300-nm SiO2/Si substrates are 7.2 (In2O3), 4.7 (IZO), and 2.6 (IGZO) cm2 V−1 s−1. Furthermore, we demonstrate expeditious wafer-scale fabrication of MO TFTs by combustion blade-coating (CBC) both an IGZO channel layer and an alumina (Al2O3) gate dielectric layer. The CBC Al2O3 films exhibit a large areal capacitance of 386 nF cm−1 at 10 kHz (k = 6.8), an extremely low leakage current of 8.1 × 10−8 A cm−2 at 2 MV cm−1, and a high breakdown field (BF) of 7.2 MV cm−1. The field-effect mobilities of these CBC IGZO-Al2O3 TFTs (300 °C/60 s) are as high as 25.2 cm2 V−1 s−1 with negligible threshold voltage deterioration in a 4,000-s bias stress test. Finally, highly flexible solution-processed CBC IGZO TFTs with a top-gated polymer dielectric on polyimide (PI) films are reported.
Fig. 1.
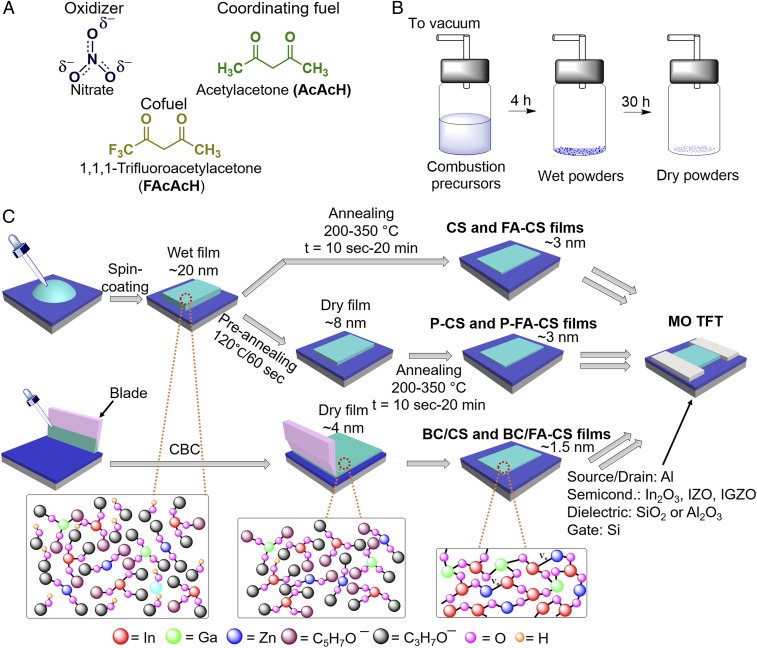
Chemical structures and synthetic pathways to MO films. (A) Chemical structures of acetylacetone and 1,1,1-trifluoroacetylacetone. (B) Synthetic pathway for wet and dry MO-precursor powders. (C) Film-fabrication processes with the schematic evolution of MO-precursor coordination chemistry and film thickness. TFT structures and materials used are also shown. Combustion synthesis (CS), fuel-assisted CS (FA-CS), preannealed CS (P-CS), and preannealed FA-CS (P-FA-CS) processes are done by spin-coating. BC/CS, blade coating/combustion synthesis; BC/FA-CS, blade coating/fuel-assisted combustion synthesis; CBC, combustion blade coating.
Results
MO-Precursor Powder Analysis and Thin-Film Growth.
To probe the importance of solvent removal from the MO-precursor powders/films before combustion, and that of the cofuel, we first investigated wet and dry IGZO powders as well as wet, dry, and combustion-derived IGZO films prepared with AcAcH and/or FAcAcH (see Fig. 1 and SI Appendix for details). The IGZO powder/film precursor solutions were prepared by 14 h of aging a mixture of In/Ga/Zn nitrates (1.4 mol, 1:0.1:0.3 atomic ratio) as the metal source/oxidizer, AcAcH as the fuel (1.4 mol), and FAcAcH as the cofuel (0.0 or 0.32 mol) in 2-methoxyethanol (2-ME) (SI Appendix, Fig. S1 and Table S1). Proton NMR (1H NMR) spectroscopy (SI Appendix, Figs. S2–S9) indicates that during the IGZO solution aging, the AcAcˉ coordinates to the metal ions, while less basic FAcAcˉ does not but nevertheless influences the metal–AcAc coordination (11). For powder preparation, the precursor solutions were dried in a vacuum for 5 and ∼30 h at 25 °C to obtain “wet” and “dry” samples, respectively (Fig. 1B). For film growth (Fig. 1C), the same solutions were spin-coated to afford wet films, while “preannealing” at 120 °C/60 s yielded dry MO-precursor films. Subsequent combustion of the wet or dry films by annealing at 200–300 °C for 10 s to 20 min afforded the corresponding MO films. Fig 1 B and C shows the process details and nomenclature.
Simultaneous thermogravimetry and differential scanning calorimetry (TGA/DSC) measurements were next used to investigate residual solvent and cofuel effects on the MO thermal and compositional evolution during combustion. TGA/DSC data (SI Appendix, Fig. S10) show that the combustion ignition temperature (Tc) for dry combustion synthesis (CS) IGZO and fuel-assisted CS (FA-CS) IGZO powders were 149.5 and 144.7 °C, respectively, versus 147.9 and 137.9 °C for the corresponding wet samples (SI Appendix, Table S2). The combustion heats (ΔHexo)/residual weights (RW) for the wet CS IGZO and FA-CS IGZO powder samples were 366.0 kJ g−1/33.4% and 429.6 kJ g−1/25.0%, respectively, compared with 640.6 kJ g−1/42.2% and 662.8 kJ g−1/37.1% for the corresponding dry powders. These results indicate that residual solvent significantly depresses the heat generated during combustion, a key factor in film densification and ultimate film charge-transport capacity. Furthermore, cofuel addition significantly increases the heat generated and accelerates precursor decomposition to volatile components.
Independent of the precursor composition and cofuel present, the wet films resulting from a single spin-coating step are ∼20 nm thick, as determined by ellipsometry. These films contain significant quantities of solvent, metal acetylacetonate complexes, and other organic species, as determined by 1H NMR spectroscopy (SI Appendix, Figs. S2–S9). Preannealing the wet films at 120 °C for 60 s removes solvent and significantly densifies them, with the film thickness reduced to ∼8 nm. Note that this step does not result in combustion or formation of significant metal–oxygen–metal (M-O-M) lattice condensation, as assessed by TGA/DSC and X-ray photoelectron spectroscopy (XPS) (vide infra). Subsequent CS of both wet and dry (prebaked) films at 200–300 °C for 10 s to 20 min then results in ∼3-nm-thick films. For MO film analysis and TFT fabrication, the spin-coating/annealing process was repeated four times (vide infra).
Oxide Thin-Film Characterization.
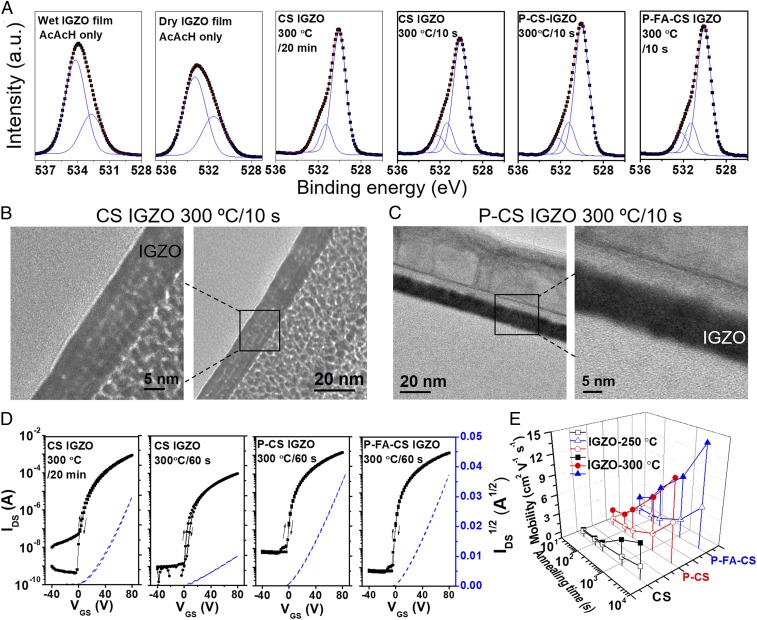
XPS was next applied to characterize the oxygen-bonding states and their evolution with film processing and fuel/cofuel composition (Fig. 2A). The O(1s) ionization can be deconvoluted into three peaks at 529.9, 531.2, and 532.3 eV, assigned to oxygen anions in an M-O-M lattice, M-OR bonds, and M-OH species, respectively (39). Data for all samples are summarized in SI Appendix, Table S3. From the XPS data, it is clear that both the wet and dry IGZO films (Fig. 2A, Left) do not contain significant densities of M-O-M species; however, the latter films have higher M-OR content (40.5% vs. 30.2%), consistent with more extensive acetylacetonate coordination and film densification on solvent removal. These data also confirm that negligible combustion occurs during the spin-coating/preannealing steps. Remarkably, comparing the XPS data for the wet film without cofuel heated at 300 °C for 20 min, which corresponds to the conventional combustion process for CS IGZO films (11, 15), to that obtained by annealing the dry film for 10 s in the present process to yield preannealing CS (P-CS) IGZO films, reveals that substantial/comparable M-O-M lattice formation occurs (74.5% vs. 75.0%, respectively) despite a >100× reduction in annealing time. Furthermore, using the fluorinated cofuel with preannealing (samples P-FA-CS IGZO) additionally densifies the IGZO films to a 75.9% M-O-M content. Top-view and cross-section transmission electron microscopy (TEM) of the IGZO films (Fig. 2 B and C and SI Appendix, Figs. S11–S12) fabricated by CS and P-CS further confirm the superior quality of the latter, which are denser and more uniform, in contrast to the multilayer morphology of the conventional CS sample. Finally, grazing incidence X-ray diffraction (GIXRD) film scans (SI Appendix, Fig. S13) indicate that the CS, P-CS, and P-FA-CS derived IGZO (300 °C/60 s) films remain amorphous.
Fig. 2.
Structural/morphological properties and device performance of IGZO films/TFTs. (A) O(1s) X-ray photoelectron spectra and their deconvolutions in spin-coated IGZO films subjected to the indicated processing stages: As-spun (wet), preannealed (dry), CS, P-CS, and P-FA-CS. (B and C) Cross-section TEM images of four-layer spin-coated IGZO films processed by CS (B) and P-CS (C) methods. (D) Transfer plots of 300 °C-processed IGZO TFTs on 300-nm SiO2/Si substrates processed by CS, P-CS, and P-FA-CS methods. (E) 3D plot of mobility-total annealing time-deposition process for IGZO TFTs on 300-nm SiO2/Si substrates.
IGZO TFTs.
Next, the performance of IGZO TFTs on 300-nm SiO2/Si dielectric/substrates [Al source-drain contacts; width/length (W/L) = 1,000/100 µm] fabricated using the different processing methods (CS, FA-CS, P-CS, P-FA-CS) and combustion temperatures/times are compared and contrasted. Fig. 2D and SI Appendix, Fig. S14 show representative TFT transfer and output characteristics. The device statistics are summarized in Fig. 2E and SI Appendix, Tables S1 and S4. The P-CS and P-FA-CS IGZO TFTs begin to function at 200 °C/20 min processing with average mobilities (µ) of 0.08 and 0.1 cm2 V−1 s−1, respectively, while all TFTs fabricated by the other methods are inactive. After 225 °C/20-min annealing, the µ of the IGZO TFTs increases from 0.17 cm2 V−1 s−1 (CS) to 0.78/0.92 cm2 V−1 s−1 (P-CS/P-FA-CS). These values are unprecedented for solution-processed IGZO TFTs at such a low temperature. As shown in Table 1, when the annealing temperature is further increased to 250 °C and then to 300 °C, the mobility increases dramatically even for the rather short annealing times (10–60 s). For example, the mobilities of the P-CS and P-FA-CS IGZO TFTs annealed at 300 °C for 10 s are 1.60 and 2.05 cm2 V−1 s−1, compared with only 0.19 cm2 V−1 s−1 for CS-derived IGZO TFTs. Annealing at the same temperature for 60 s further increases µ of the P-FA-CS devices to 5.44 cm2 V−1 s−1, even larger than that of CS IGZO film annealed for 20 min (4.2 cm2 V−1 s−1) and >10× greater than that of CS IGZO annealed for 60 s (0.41 cm2 V−1 s−1). Note that the control IGZO TFTs fabricated by sol–gel exhibit far lower performance, demonstrating that the present strategy is only applicable to combustion-derived formulations (SI Appendix, Fig. S14 and Table S5). Having the mobility data for IGZO TFTs fabricated at different temperatures and annealing times shows that there is a tradeoff between the annealing temperature and annealing time to achieve a specific mobility. For instance, SI Appendix, Fig. S14I shows the P-FA-CS IGZO TFTs with electron mobilities of 1 cm2 V−1 s−1 fabricated at different temperatures and annealing times for each layer, which follows an exponential relationship.
Table 1.
Average electron mobilities of IGZO, In2O3, and IZO TFTs on 300 nm SiO2/Si substrates processed by the indicated methods
| Method | Performance parameters | IGZO | In2O3 | IZO | ||||||
| 225 °C/20 min | 250 °C/60 s | 300 °C/10 s | 300 °C/60 s | 225 °C/20 min | 250 °C/60 s | 300 °C/10 s | 250 °C/60 s | 300 °C/10 s | ||
| CS | µ (cm2 V−1 s−1) | 0.17 ± 0.08 | 0.10 ± 0.05 | 0.19 ± 0.10 | 0.41 ± 0.10 | 0.21 ± 0.13 | 0.50 ± 0.06 | 1.25 ± 0.21 | 0.33 ± 0.05 | 0.31 ± 0.05 |
| VT (V) | 27.96 ± 10.20 | 31.37 ± 16.16 | 45.24 ± 10.16 | 10.50 ± 1.89 | 28.85 ± 5.32 | −18.5 ± 4.68 | −2.27 ± 1.82 | −20.75 ± 3.22 | −13.25 ± 2.70 | |
| Ion/Ioff | 106 | 105 | 105 | 106 | 104 | 103 | 103 | 105 | 105 | |
| FA-CS | µ (cm2 V−1 s−1) | 0.19 ± 0.11 | 0.08 ± 0.03 | 0.35 ± 0.14 | 1.62 ± 0.21 | 0.24 ± 0.13 | 0.32 ± 0.08 | 2.16 ± 0.33 | 0.28 ± 0.07 | 0.83 ± 0.24 |
| VT (V) | 26.70 ± 10.13 | 33.24 ± 17.25 | 21.17 ± 7.63 | 3.34 ± 1.75 | 26.70 ± 5.08 | 9.23 ± 2.85 | −12.08 ± 2.85 | −2.29 ± 1.72 | 12.4 ± 1.68 | |
| Ion/Ioff | 106 | 105 | 106 | 106 | 105 | 104 | 103 | 106 | 105 | |
| P-CS | µ (cm2 V−1 s−1) | 0.78 ± 0.20 | 0.29 ± 0.08 | 1.60 ± 0.22 | 3.67 ± 0.72 | 2.96 ± 0.50 | 2.94 ± 0.45 | 5.20 ± 0.62 | 2.32 ± 0.38 | 2.37 ± 0.45 |
| VT (V) | 21.59 ± 6.54 | 29.15 ± 8.86 | 11.12 ± 4.74 | 4.28 ± 1.02 | −19.90 ± 4.56 | 10.49 ± 3.41 | −23.98 ± 4.55 | −3.13 ± 2.50 | 12.01 ± 1.24 | |
| Ion/Ioff | 107 | 106 | 106 | 107 | 104 | 104 | 104 | 106 | 105 | |
| P-FA-CS | µ (cm2 V−1 s−1) | 0.92 ± 0.25 | 0.20 ± 0.09 | 2.05 ± 0.52 | 5.44 ± 1.22 | 3.82 ± 0.77 | 2.28 ± 0.33 | 6.52 ± 0.74 | 2.11 ± 0.32 | 3.78 ± 0.59 |
| VT (V) | 16.49 ± 3.56 | 20.8 ± 5.49 | 7.56 ± 2.43 | 3.61 ± 0.61 | −24.87 ± 3.85 | −26.97 ± 6.30 | −45.86 ± 8.85 | −22.15 ± 2.23 | 9.20 ± 2.58 | |
| Ion/Ioff | 107 | 106 | 106 | 107 | 103 | 104 | 103 | 106 | 105 | |
The results were measured from 3 batches having more than 30 devices.
Scope: Other MO Thin-Film Materials and Transistor-Structure Performance Correlations.
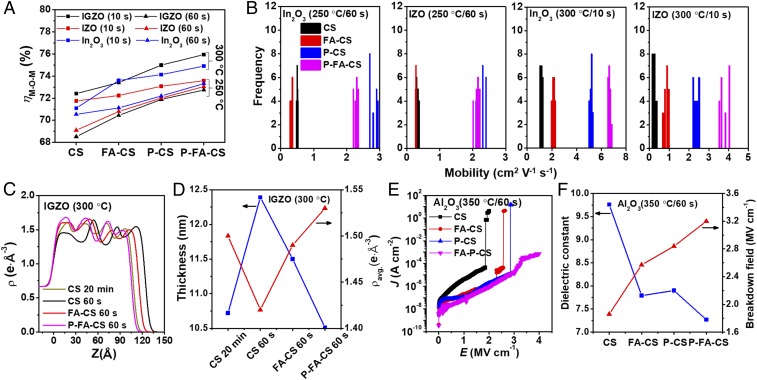
Considering the excellent results achieved for the P-FA-CS IGZO films discussed above, the scope of this study was expanded to other important MO semiconductors such as binary In2O3 and ternary IZO films. Comparative XPS analysis (Fig. 3A and SI Appendix, Fig. S15) indicates that, in all cases, using the P-CS and P-FA-CS processing enhances M-O-M content by ∼2–3%. For instance, 300 °C/10 s-processed In2O3 and IZO M-O-M content increases going from CS (71.5 and 71.9%, respectively) to P-CS (74.1 and 73.4%, respectively) to P-FA-CS (74.5 and 74.0%, respectively) processed films. Interestingly, although the carrier mobilities of the In2O3 and IZO TFTs are greater than those of corresponding IGZO TFTs (vide infra), the M-O-M ratios are smaller than those of the IGZO films, reflecting strong Ga oxygen “getter” effects (37). Next, the performance of In2O3 and IZO TFTs are compared in Fig. 3B, Tables 1, and SI Appendix, Figs. S16–S17 and Table S4. No TFT response is observed for 200 °C/20 min-processed CS In2O3 TFTs, while µ of the 225 °C/20 min-processed CS-In2O3 TFTs is 0.21 cm2 V−1 s−1. In contrast, P-CS In2O3 TFTs begin to function on annealing at 200 °C/20 min with µ = 0.075 cm2 V−1 s−1, increasing to ∼3 cm2 V−1 s−1 at 225 °C/20 min. On annealing at 250 °C for 60 s, µ of the In2O3 TFTs increases from CS (0.50 cm2 V−1 s−1) to P-CS (2.94 cm2 V−1 s−1). The µ of the P-CS In2O3 TFTs further increases to 9.60 cm2 V−1 s−1 after 250 °C/20-min annealing, vs. 2.06 cm2 V−1 s−1 for CS In2O3 TFTs. When the In2O3 films are annealed at 300 °C for only 10 s, high mobilities of 5.20 cm2 V−1 s−1 (P-CS) and 6.52 cm2 V−1 s−1 (P-FA-CS) are achieved, vs. 1.25 cm2 V−1 s−1 for CS In2O3 TFTs. For IZO TFTs, CS films and P-CS films annealed at 225 °C/20 min exhibit µ = 0.04 and 0.5 cm2 V−1 s−1, respectively. When processed at 250 °C/60 s, the P-CS films exhibit a µ = 2.34 cm2 V−1 s−1, 8.5× higher than that of the CS-IZO TFTs (0.33 cm2 V−1 s−1). For 300 °C/10-s annealing, the CS-IZO TFTs exhibit a µ = 0.31 cm2 V−1 s−1, considerably lower than those of P-CS, 2.37 cm2 V−1 s−1 and P-FA-CS, 3.78 cm2 V−1 s−1 processed devices.
Fig. 3.
Structural and electrical characterization of combustion-processed MO films. (A) Ratio of O(1s) X-ray photoelectron spectroscopic M-O-M peak area to total peak area for In2O3, IZO, and IGZO films subjected to the indicated processing. (B) Distribution of saturation mobilities of In2O3 and IZO TFTs fabricated by the indicated deposition processes. (C) Fitted XRR electron-density profiles of the indicated IGZO films. (D) Film thickness and average electron density of IGZO films processed by the indicated methods. (E) Leakage current-density versus electrical field (J-E) of Al2O3 dielectrics fabricated by the indicated methods. (F) Calculated dielectric constant (103 Hz) and BF for 350 °C-annealed CS, FA-CS, P-CS, and P-FA-CS Al2O3 dielectric films.
To better understand the origin of the impressive P-FA-CS IGZO TFT performance, X-ray reflectivity (XRR) measurements were performed on the IGZO channel layer (SI Appendix, Fig. S18) which, recall, is fabricated by four spin/preannealing/annealing steps. These measurements assess the film electron density (ρe) profiles, film thickness, and average electron density (ρavg) profiles (Fig. 3 C and D). The conventional CS IGZO film with 300 °C/20-min annealing of each layer yields ρavg = 1.5 e Å−3, similar to previous reports (10, 40). While the ρavg decreases to a greater extent for 300 °C/60 s-annealed CS IGZO films (1.42 e Å−3), it increases to 1.49 and 1.53 e Å−3 for FA-CS and P-FA-CS processed IGZO films. This result is unprecedented for 60 s-annealed films and is comparable to that of sputtered films (10). Interestingly, the XRR-derived film thicknesses also depend on the growth method. The 60 s-annealed P-FA-CS IGZO films exhibit a thickness (10.5 nm) that is slightly less than the 10.7 nm of the conventional CS IGZO films with 20-min annealing, supporting the XPS and TFT results that the preannealing step and cofuel-assisted combustion significantly enhance film densification and charge-transport capacity.
Extension to Oxide Dielectric Films.
The morphological and microstructural requirements for TFT dielectric layers are even more stringent than for semiconducting layers, to ensure low leakage currents, high breakdown voltages, high capacitances, and minimal bulk/interface trap densities. Thus, harsh processing conditions (>450 °C/1 h) and/or significant film thicknesses (≥100 nm) are typically required for high-performance solution-processed MO dielectric films (10, 39, 41–43). Here, we show that the present processing method yields high-quality Al2O3 dielectric films for low-voltage MO TFT operation. Thus, the Al2O3 precursors Al(NO3)3 and AcAcH/NH4OH in 2-ME with or without FAcAcH as a cofuel were spin-coated onto n++ Si substrates and subsequently preannealed at 120 °C for 60 s, then immediately annealed at 350 °C for 60 s. This process was repeated four times to achieve the desired film thickness. Fig. 3E details the insulating properties of the CS, FA-CS, P-CS, and P-FA-CS processed Al2O3 dielectrics (∼20 nm thick), indicating that the CS Al2O3 film leakage current density (4.1 × 10−6 A cm−1) at 1 MV cm−1 and the BF (1.9 MV cm−1) are inferior to those of P-CS Al2O3 (1.1 × 10−7 A cm−1 and 2.8 MV cm−1, respectively) and especially P-FA-CS Al2O3 (7.5 × 10−8 A cm−1 and 3.2 MV cm−1) films. SI Appendix, Fig. S19 provides the frequency- and voltage-dependent capacitance per unit area of the aforementioned dielectrics, and the corresponding dielectric constants are shown in Fig. 3F. The CS-Al2O3 films exhibit the largest dielectric constant (k ∼ 9.4), likely reflecting impurities and large densities of polar hydroxyl groups (39), as well as a pronounced fall in capacitance for frequencies >7 × 104 Hz. In contrast, the P-CS and P-FA-CS Al2O3 films exhibit frequency-stable capacitances of 317 nF cm−2 for P-CS and 286 nF cm−2 for P-FA-CS up to 4 × 105 and 5 × 105 Hz, respectively, and dielectric constants of 7.9 and 7.3, respectively. That the lower dielectric constant of these films is due to lowered impurities and fluoride incorporation (AlF3, k ∼ 2.2) is supported by XPS data, which indicate Al-F features at 685.6 eV (SI Appendix, Fig. S20) (44). From deconvolution, the estimated atomic F:Al ratios for the FA-CS and P-FA-CS Al2O3 films are 1:13.9 and 1:10.8, respectively. Note that no F incorporation is observed by XPS in the FAcAcH-assisted semiconductor MO films, even though the annealing temperature is 50–100 °C lower than that for the Al2O3 films (SI Appendix, Fig. S20). This result likely reflects the very strong Al-F bond energy (675 kJ mol−1) versus that of the other metal fluorides: In-F, 516 kJ mol−1; Ga-F, 584 kJ mol−1; and Zn-F, 364 kJ mol−1 (45). Regarding the oxygen environment (SI Appendix, Fig. S21), the deconvoluted XPS O(1s) spectra assign Al-O-Al at 531.1 ± 0.1 eV and O-associated hydroxyl groups at 532.3 ± 0.1 eV (46, 47). The Al-O-Al content increases from 59.5% (CS) to 77.5% (FA-CS) to 80.0% (P-CS) to 82.6% (P-FA-CS), again demonstrating that the cofuel/prebaking approach accelerates MO dielectric oxide lattice densification.
Printed MO Films and Devices.
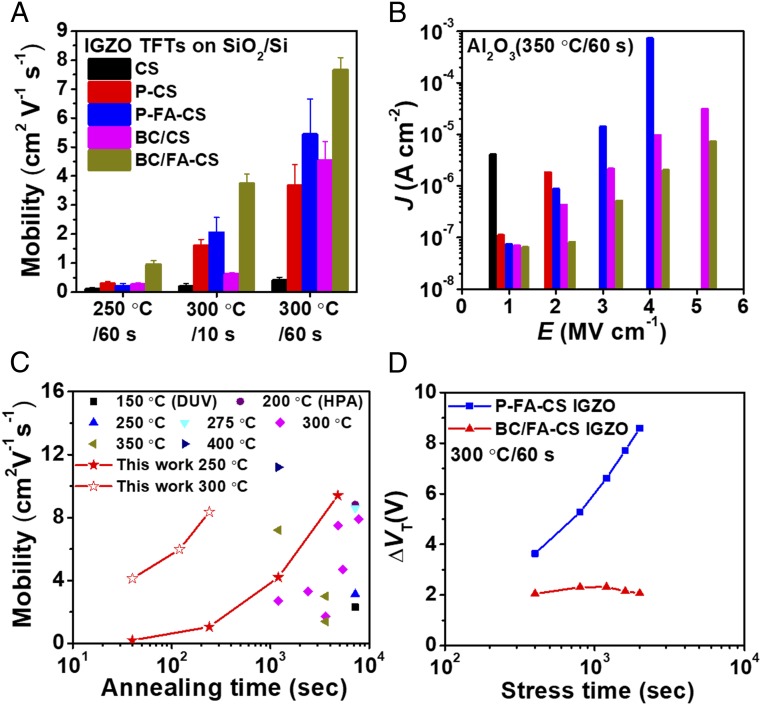
Blade coating (BC) was next applied to MO film growth using the present processing methodology as CBC. CBC has advantages over spin-coating, including concurrent preannealing during film deposition, minimal precursor solution wastage (reduced by ∼10×), and scalability (48). Critical deposition parameters including coating speed (5–20 mm s−1), gap height (100/300 µm), and substrate temperature (room temperature/70 °C) were optimized to balance metal ion-AcAcˉ/FAcAcˉ coordination, solvent evaporation, solution flow thermodynamics, and desired final film thickness (SI Appendix, Fig. S22 and Table S6). CBC of the IGZO semiconductor layer on Si/SiO2 substrates was first optimized, yielding the parameters: 10 mm s−1 coating speed, 100-µm gap height, 70 °C substrate temperature, and four successive coatings for ∼6-nm-thick films. Fig. 4A, Table 2, and SI Appendix, Fig. S23 summarize device metrics for the IGZO films processed by different methods. Thus, while the µ of spin-coated CS IGZO TFTs annealed at 250 °C/60 s is only 0.10 cm2 V−1 s−1 (vide supra), the BC/CS and BC/FA-CS devices exhibit mobilities of 0.26 and 0.96 cm2 V−1 s−1, respectively. By raising the temperature to 300 °C, the BC/FA-CS IGZO TFT mobilities reach 3.74 cm2 V−1 s−1 (10-s annealing) and 7.66 cm2 V−1 s−1 (60-s annealing), which are significantly larger than obtainable by spin-coating the IGZO layer (2.05 and 5.44 cm2 V−1 s−1, respectively). This result may reflect more efficient preannealing, which occurs immediately after dispensing the IGZO-precursor solution.
Fig. 4.
Comparison of IGZO TFT performance and stability. (A) IGZO TFT mobilities on 300-nm SiO2/Si with IGZO films processed by the indicated methods. (B) J-E curves of 350 °C/60 s Al2O3 dielectrics processed by the indicated methods. (C) Comparison with literature data for solution-processed IGZO TFT mobilities on 300-nm SiO2/Si vs. annealing time; references are provided in SI Appendix, Table S7. DUV, deep UV; HPA, high-pressure annealing. (D) Threshold voltage shift, ΔVT, of the indicated IGZO TFTs under PTBS (VGS = +20 V). Note “BC” indicates “blade-coating,” while all other devices were fabricated by spin-coating unless otherwise noted.
Table 2.
Performance metrics of IGZO TFTs on 300 nm SiO2/Si substrates processed by various combustion methods
| Processing method | Performance parameters | 250 °C/60 s | 300 °C/10 s | 300 °C/60 s |
| BC/CS | µ (cm2 V−1 s−1) | 0.26 ± 0.05 | 0.62 ± 0.05 | 4.53 ± 0.67 |
| VT (V) | 24.1 ± 4.79 | 4.55 ± 1.59 | 9.78 ± 0.99 | |
| Ion/Ioff | ∼106 | ∼106 | ∼106 | |
| BC/FA-CS | µ (cm2 V−1 s−1) | 0.96 ± 0.12 | 3.74 ± 0.23 | 7.66 ± 0.60 |
| VT (V) | 20.1 ± 6.71 | 22.54 ± 0.97 | 15.82 ± 0.42 | |
| Ion/Ioff | ∼107 | ∼107 | ∼106 |
Fig. 4C and SI Appendix, Table S6 summarize IGZO TFT performance on 300-nm SiO2/Si substrates (source/drain = Al; W/L = 1,000/100 µm) versus that of the recent literature report, clearly demonstrating the technological attractions of BC/FA-CS methodology. Positive gate-bias stress (PTBS) measurements were also carried out on the 60 s-annealed P-FA-CS and BC/FA-CS IGZO TFTs (Fig. 4D and SI Appendix, Fig. S24). These devices were subjected to a constant VGS of +20 V for up to 2,000 s (400-s intervals) in ambient, without encapsulation or back channel protection. The P-FA-CS IGZO devices exhibit ΔVT of ∼+9 V after 2,000 s, implying a large density of acceptor-like electron-trapping states (11, 17, 49). However, no obvious shift is observed for BC/FA-CS IGZO TFTs even after a gate-bias stress time of 2,000 s with ΔVT ∼+2 V.
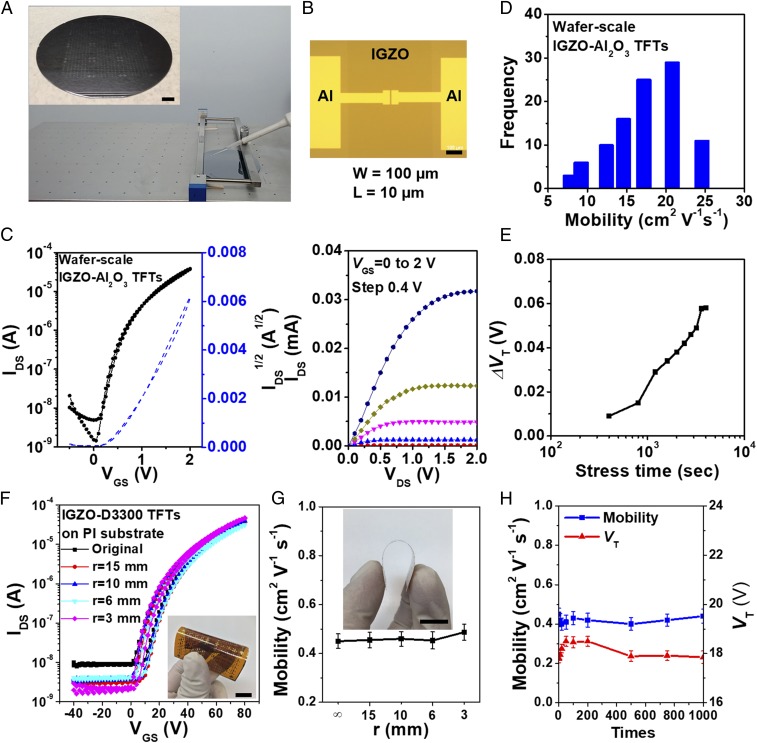
Next, BC Al2O3 dielectric films and BC IGZO/Al2O3 TFTs were fabricated to further demonstrate the technological advances provided here. BC/FA-CS Al2O3 fabrication process/parameters are identical to those of IGZO films, except that the gap height is 300 µm. Fig. 4B shows the variations in leakage current densities for 350 °C/60 s-annealed Al2O3 films at different electric field strengths, and SI Appendix, Fig. S25 shows the electrical and dielectric properties. Remarkably, the BC/FA-CS Al2O3 films, ∼15 nm thick after four sequential coatings, exhibit excellent dielectric characteristics, again with the performance of the processed films (J = 8.1 × 10−8 A cm−2 at 2.0 MV; BF = 7.2 MV cm−1) outperforming the BC/CS ones (J = 4.3 × 10−7 A cm−2 and BF = 6.5 MV cm−1). Both films exhibit frequency-stable capacitance up to 5 × 105 Hz, and their dielectric constants are 6.8 and 6.6, respectively. Fig. 5 A and B and Movie S1 show the BC/FA-CS IGZO/Al2O3 device fabrication process on a 4-inch-diameter Si substrate and the optical image of a low-voltage IGZO-Al2O3 TFT with patterned IGZO and S/D layers. These devices exhibit excellent TFT characteristics at low voltage (<2 V), negligible I-V hysteresis (Fig. 5C), and field-effect mobilities centered at ∼18.0 cm2 V−1 s−1 (maximum value = 25.2 cm2 V−1 s−1) with an SD of ±2.5 cm2 V−1 s−1 over 100 devices (Fig. 5D and SI Appendix, Fig. S26). These TFTs also exhibit excellent current on/off modulation, subthreshold swing, and threshold voltage (VT) values of >4 × 104, 0.15 ± 0.03 V per decade, and 0.55 ± 0.27 V, respectively, as well as a negligible ΔVT of +0.06 V after 4,000 s stress time (Fig. 5E and SI Appendix, Fig. S27).
Fig. 5.
Scalable CBC fabrication of high-performance IGZO TFTs on both high-k Al2O3 dielectrics and low-k polymer dielectrics. (A) Photo of the BC process; Inset shows blade-coated devices on a 4-inch Si wafer. (Scale bar, 1 cm.) (B) Optical image of a patterned low-voltage IGZO TFT. (Scale bar, 100 µm.) (C) Transfer (Left) and output (Right) characteristics of low-voltage CBC IGZO TFT on high-k Al2O3 dielectrics. The annealing time for both IGZO and Al2O3 layers is 60 s. (D and E) Distribution of saturation mobilities (D) and ΔVT vs. time (E) of CBC/FA-CS-derived IGZO TFTs on P-FA-CS Al2O3/Si substrates (VGS = +1.0 V). (F and G) Transfer curves (F) and mobility variation (G) of top-gate flexible CBC/IGZO TFTs on a PI substrate as a function of bending radius. (Inset) Photo of device under bending. (Scale bar, 1 cm.) (H) Mobility and threshold voltage stability for bending test cycles with a radius of 6 mm.
Finally, top-gate, top-contact IGZO TFTs were fabricated on a ∼25-cm2, 35-µm-thick PI substrate with a polymer dielectric (Activink D3300; Flexterra Corporation) and a CBC IGZO layer annealed for only 60 s at 300 °C (Fig. 5F). Fig. 5G shows the transfer plots of these devices at various bending radii about a curvature parallel to the channel length. These devices exhibit only a slight turn-on voltage (Von)/VT shift from +0.1/20.1 to 0.0/21.9 V as the bending radius is decreased from ∞ to 3 mm, along with a negligible mobility decline from 0.45 to 0.43 cm2 V−1 s−1. The lower mobility of these devices compared with that achieved with SiO2 reflects the difference in device architecture and the lower dielectric constant of polymer dielectric (50). Importantly, these TFTs exhibit excellent mechanical flexibility with negligible performance loss on bending at 6 mm for 1,000 times (Fig. 5H and SI Appendix, Fig. S28).
Conclusions
Low-temperature, wafer-scale fabrication of MO films via ultrafast combustion BC is reported. Using a fluorinated cofuel to promote metal coordination and impurity removal, combined with a precursor preannealing step to eliminate solvent, greatly enhances the efficiency of CS, which can be reduced to times as short as 10 s at temperatures as low as 200–300 °C. Thus, functional (µ > 1 cm2 V−1 s−1) MO TFTs are obtained at temperatures/times as low as 250 °C/60 s and/or 300 °C/10 s for In2O3, IZO, and IGZO. This approach is also extendable to a MO dielectric such as Al2O3. Additionally, ultrafast annealing of blade-coated IGZO and Al2O3 films yields excellent semiconductor and insulating characteristics and produces low-voltage wafer-scale MO TFTs with highly stable characteristics and ΔVT < 0.06 V under 4,000 s bias stress. Using a polymeric top-gated dielectric on PI film then yields highly flexible, solution-processed CBC/IGZO TFTs. We anticipate that these results will help advance low-cost, high-performance, roll-to-roll compatible large-area printed electronics as well as other technologies where high-quality MO films are essential components.
Methods
Precursor Preparation and Characterization.
All combustion precursor materials were purchased from Sigma-Aldrich and stored in a vacuum desiccator. First, measured quantities of the metal salts were dissolved in 5.0 mL of 2-ME. Thus, 75.2 mg of In(NO3)3 was used for the In2O3 precursor; 60.16 mg of In(NO3)3 and 9.47 mg of Zn(NO3)2 were used for the IZO precursor; 63.36 mg of In(NO3)3, 10.74 mg of Zn(NO3)2, and 5.38 mg of Ga(NO3)3 were used for the IGZO precursor; and 93.78 mg of Al(NO3)3 was used for the Al2O3 precursor. Next, 25 μL of acetylacetone and 11.25 μL of 14.5 M NH3(aq) were added in sequence to each metal nitrate precursor, except that 75 μL of acetylacetone and 33.75 μL of 14.5 M NH3(aq) were added to the In(NO3)3 precursor. The solutions were then stirred overnight (∼14 h) before film fabrication. For fuel-assisted combustion precursors, 1,1,1-trifluoro-2,4-pentanedione (10 wt% to the total weight of metal nitrates) was added to the above precursor solutions 1 h before spin-coating/BC. Wet and dried precursors were obtained by vacuum evaporation using Schlenk line for 5 and 30 h, respectively. Both DSC and TGA measurements were performed on a SDT Q60 instrument (TA Instruments, Inc.). The experiments were carried out on ∼1 mg of wet or dry samples with a heating rate of 10 °C min−1 under a 70 mL min−1 N2 flow. Vacuum-dried precursors in anhydrous dimethyl sulfoxide-d6 were used for 1H NMR spectroscopic analysis. Spectra were recorded on a Bruker Avance III 500-MHz spectrometer.
Metal Oxide Thin-Film and Device Fabrication.
All of the solutions were filtered through 0.2-μm syringe filters before fabrication. n++ silicon wafers with/without 300-nm SiO2 (WRS Materials) used as substrates were solvent-cleaned and then cleaned with an oxygen plasma for 5 min before use. For spin-coating fabrication, the In2O3, IZO, IGZO, and Al2O3 precursors were spin-coated on the substrates (n++ Si, 300 nm SiO2/Si) at 3,500 rpm for 30 s in a controlled atmosphere box [relative humidity (RH) ∼ 20%] and optionally preannealed at 120 °C for 60 s (RH ∼ 35%). Then, the resulting films were immediately placed on a 200–350 °C hotplate and annealed for 10 s to 20 min (RH ∼ 35%). This process was repeated four times to obtain the desired film thickness. For BC film fabrication, first, the substrate (n++ Si or 300 nm SiO2/n++ Si) was placed on the blade-coater (Erichsen Coatmaster 510) setup on a surface maintained at a temperature of 25–70 °C. Next, the blade was approached to the substrates maintaining a gap of 100–300 µm. After that, the precursor solution (10 µL for 1 inch × 1 inch square substrate, 80 µL for a 4-inch-diameter substrate) was injected at the interface between the substrate and the blade until a meniscus formed. The blade was horizontally transported at a constant velocity of 5–20 mm s−1, and the carried solution dried at a rate depending on the substrate temperature. Finally, the film was annealed at 250–350 °C for 10–60 s. This process was repeated four times to obtain the desired film thickness. Semiconductor layer patterning was achieved by spin-coating one layer of S1813 photoresist (5,000 rpm for 30 s, 1.2 μm) on the surface, which was thermally annealed at 115 °C for 1 min and then exposed to UV light (Heidelberg µPG 501 mask writer) for 26 ms. Next, the S1813 film was removed by soaking in MF319 for 30 s, and the films were cleaned with deionized (DI) water and dried with an N2 flow. The exposed oxide films were next removed in aqueous oxalic acid (10% wt/vol) for 30 s and rinsed with DI water. The remaining S1813 film was then removed with acetone. Source/drain electrode patterning was achieved by spin-coating/annealing/exposing/rinsing the S1813 photoresist on the IGZO layer. Al source and drain (S/D) electrodes (thickness = 40 nm) were then deposited by thermal evaporation, followed by soaking in acetone for 1 min. For flexible TFTs, PI film with thickness of 35 μm was used as substrate, after blade-coating the IGZO layer using the above-described procedure, Al S/D was patterned on it, and then polymer solution (Activink D3300) was spin-coated as dielectric. After UV curing under 1 J cm−2 and 120 °C/10-min annealing, the resulting polymer film (ε = 3.3) has a thickness 650 nm and an areal capacitance of 4.5 nF cm−2. The flexible devices were finished by evaporating 50 nm of Au as gate electrodes. The channel width and length for devices on the SiO2/Si substrate were defined as 1,000 and 100 μm, respectively, while those for wafer-scale IGZO-Al2O3 devices and flexible polymer-gated IGZO devices were 100 and 10 μm, respectively.
MO Thin-Film Characterization.
The surface characteristics of the resulting films were measured with a Bruker Dimensional Icon AFM system in the tapping mode. XPS was performed on a Thermo Scientific ESCALAB 250 Xi spectrometer, and the sample surfaces were etched before testing. O(1s) spectra were fitted using three Gaussian−Lorentzian convolution functions after subtracting a linear baseline. GIXRD and XRR were conducted with a Rigaku Smartlab workstation using CuKα (1.54-Å) radiation. Top-view TEM and cross-section TEM (CS-TEM) were collected using a JEOL JEM-ARM300F transmission electron microscope. The samples for top-view TEM images were obtained by spin-coating/annealing MO films on single-crystal NaCl, which was then dissolved in water and transferred onto Cu grids. The CS-TEM samples were prepared directly from actual TFT devices with standard focused ion-beam milling techniques (FEI Helios NanoLab 600). A ∼2-μm-thick platinum layer was deposited before the ion milling to protect samples from ion-beam damage. Electrical characterization of semiconductors was performed using MO TFT devices. Electrical characterization of dielectrics was performed using metal–insulator–metal devices. n++ Si wafers with 1.5-nm natural SiO2 layer were used as substrates, and then MO dielectrics were deposited by spin-coating or blade-coating on them. After that, top electrodes (Al, 40 nm) were thermally evaporated with size of 200 μm × 200 μm. Both the dielectrics and MO TFT measurements were performed under ambient conditions using an Agilent B1500A semiconductor parameter analyzer. The carrier mobility (µ) was evaluated in the saturation region. The areal capacitance for 300 nm SiO2/Si is taken as 11 nF cm−2 here. The areal capacitances of high-k Al2O3 and low-k polymer dielectrics (Cdiel) were calculated based on the equation 1/Ci = 1/Cdiel+1/CSiO2, assuming that the areal capacitance of native silicon oxide (1.5 nm) is 2,303.6 nF cm−2 (39). The Ci is the measured areal capacitance for dielectric films on 1.5 nm SiO2/Si wafer.
Supplementary Material
Acknowledgments
We thank the US–Israel Binational Science Foundation (Grant AGMT-2012250///02), the Northwestern University Materials Research Science and Engineering Center (MRSEC) (NSF Grant DMR-1720139), the Air Force Office of Scientific Research (Grant FA9550-18-1-0320), and Flexterra Corporation for support of this research. A.F. thanks the Shenzhen Peacock Plan Project (Grant KQTD20140630110339343) for support. This work was performed, in part, at the Center for Nanoscale Materials, a US Department of Energy Office of Science User Facility and supported by the US Department of Energy, Office of Science, under Contract DE-AC02-06CH11357. This work made use of the J. B. Cohen X-Ray Diffraction Facility, Northwestern University Micro/Nano Fabrication Facility, Electron Probe Instrumentation Center Facility, Keck Interdisciplinary Surface Science Facility, and Scanned Probe Imaging and Development Facility of the Northwestern University Atomic and Nanoscale Characterization Experimental Center, which received support from the Soft and Hybrid Nanotechnology Experimental Resource (NSF Grant NNCI-1542205); the MRSEC Program (NSF Grant DMR-1720139); the International Institute for Nanotechnology (IIN); the Keck Foundation; and the State of Illinois, through the IIN.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901492116/-/DCSupplemental.
References
- 1.Nomura K, et al. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature. 2004;432:488–492. doi: 10.1038/nature03090. [DOI] [PubMed] [Google Scholar]
- 2.Fortunato E, Barquinha P, Martins R. Oxide semiconductor thin-film transistors: A review of recent advances. Adv Mater. 2012;24:2945–2986. doi: 10.1002/adma.201103228. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, et al. High- k gate dielectrics for emerging flexible and stretchable electronics. Chem Rev. 2018;118:5690–5754. doi: 10.1021/acs.chemrev.8b00045. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Fu X, Liu J, Wang Z, Hu W. 25th anniversary article: Key points for high-mobility organic field-effect transistors. Adv Mater. 2013;25:6158–6183. doi: 10.1002/adma.201302514. [DOI] [PubMed] [Google Scholar]
- 5.Zschieschang U, et al. Electrical characteristics of field-effect transistors based on chemically synthesized graphene nanoribbons. Adv Electron Mater. 2015;1:1400010. [Google Scholar]
- 6.Kraft U, et al. Flexible low-voltage organic complementary circuits: Finding the optimum combination of semiconductors and monolayer gate dielectrics. Adv Mater. 2015;27:207–214. doi: 10.1002/adma.201403481. [DOI] [PubMed] [Google Scholar]
- 7.Klauk H. Organic thin-film transistors. Chem Soc Rev. 2010;39:2643–2666. doi: 10.1039/b909902f. [DOI] [PubMed] [Google Scholar]
- 8.Pierre A, et al. All-printed flexible organic transistors enabled by surface tension-guided blade coating. Adv Mater. 2014;26:5722–5727. doi: 10.1002/adma.201401520. [DOI] [PubMed] [Google Scholar]
- 9.Tee BCK, et al. A skin-inspired organic digital mechanoreceptor. Science. 2015;350:313–316. doi: 10.1126/science.aaa9306. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, et al. Spray-combustion synthesis: Efficient solution route to high-performance oxide transistors. Proc Natl Acad Sci USA. 2015;112:3217–3222. doi: 10.1073/pnas.1501548112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, et al. Carbohydrate-assisted combustion synthesis to realize high-performance oxide transistors. J Am Chem Soc. 2016;138:7067–7074. doi: 10.1021/jacs.6b02309. [DOI] [PubMed] [Google Scholar]
- 12.Liu GX, et al. High-performance fully amorphous bilayer metal-oxide thin film transistors using ultra-thin solution-processed ZrOx dielectric. Appl Phys Lett. 2014;105:113509. [Google Scholar]
- 13.Arias AC, MacKenzie JD, McCulloch I, Rivnay J, Salleo A. Materials and applications for large area electronics: Solution-based approaches. Chem Rev. 2010;110:3–24. doi: 10.1021/cr900150b. [DOI] [PubMed] [Google Scholar]
- 14.Khim D, et al. Simple bar-coating process for large-area, high-performance organic field-effect transistors and ambipolar complementary integrated circuits. Adv Mater. 2013;25:4302–4308. doi: 10.1002/adma.201205330. [DOI] [PubMed] [Google Scholar]
- 15.Kim MG, Kanatzidis MG, Facchetti A, Marks TJ. Low-temperature fabrication of high-performance metal oxide thin-film electronics via combustion processing. Nat Mater. 2011;10:382–388. doi: 10.1038/nmat3011. [DOI] [PubMed] [Google Scholar]
- 16.Banger KK, et al. Low-temperature, high-performance solution-processed metal oxide thin-film transistors formed by a ‘sol–gel on chip’ process. Nat Mater. 2011;10:45–50. doi: 10.1038/nmat2914. [DOI] [PubMed] [Google Scholar]
- 17.Kim YH, et al. Flexible metal-oxide devices made by room-temperature photochemical activation of sol-gel films. Nature. 2012;489:128–132. doi: 10.1038/nature11434. [DOI] [PubMed] [Google Scholar]
- 18.Rim YS, et al. Simultaneous modification of pyrolysis and densification for low-temperature solution-processed flexible oxide thin-film transistors. J Mater Chem. 2012;22:12491–12497. [Google Scholar]
- 19.Park YM, Daniel J, Heeney M, Salleo A. Room-temperature fabrication of ultrathin oxide gate dielectrics for low-voltage operation of organic field-effect transistors. Adv Mater. 2011;23:971–974. doi: 10.1002/adma.201003641. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Rim YS, Jiang C, Yang Y. Low-impurity high-performance solution-processed metal oxide semiconductors via a facile redox reaction. Chem Mater. 2015;27:4713–4718. [Google Scholar]
- 21.Martins R, et al. Complementary metal oxide semiconductor technology with and on paper. Adv Mater. 2011;23:4491–4496. doi: 10.1002/adma.201102232. [DOI] [PubMed] [Google Scholar]
- 22.Park S, et al. In-depth studies on rapid photochemical activation of various sol-gel metal oxide films for flexible transparent electronics. Adv Funct Mater. 2015;25:2807–2815. [Google Scholar]
- 23.Jo JW, et al. Highly stable and imperceptible electronics utilizing photoactivated heterogeneous sol-gel metal-oxide dielectrics and semiconductors. Adv Mater. 2015;27:1182–1188. doi: 10.1002/adma.201404296. [DOI] [PubMed] [Google Scholar]
- 24.Bang H-J, et al. Effect of high pressure hydrogen or deuterium anneal on polysilicon channel field effect transistors. J Nanosci Nanotechnol. 2016;16:10341–10345. [Google Scholar]
- 25.Oh S-M, Jo K-W, Cho W-J. High performance solution-deposited bilayer channel indium–zinc-oxide thin film transistors by low-temperature microwave annealing. Curr Appl Phys. 2015;15:S69–S74. [Google Scholar]
- 26.Kim W-G, et al. High-pressure gas activation for amorphous indium-gallium-zinc-oxide thin-film transistors at 100 °C. Sci Rep. 2016;6:23039. doi: 10.1038/srep23039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bashir A, et al. High-performance zinc oxide transistors and circuits fabricated by spray pyrolysis in ambient atmosphere. Adv Mater. 2009;21:2226–2231. [Google Scholar]
- 28.Lin YH, et al. High-performance ZnO transistors processed via an aqueous carbon-free metal oxide precursor route at temperatures between 80-180 °C. Adv Mater. 2013;25:4340–4346. doi: 10.1002/adma.201301622. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Ng TN. Reducing trap states in printed indium zinc oxide transistors by doping with benzyl viologen. Adv Electron Mater. 2018;4:1700631. [Google Scholar]
- 30.Yoo T-H, et al. Sub-second photo-annealing of solution-processed metal oxide thin-film transistors via irradiation of intensely pulsed white light. RSC Advances. 2014;4:19375. [Google Scholar]
- 31.Kang C-m, Kim H, Oh Y-W, Baek K-H, Do L-M. High-performance, solution-processed indium-oxide TFTs using rapid flash lamp annealing. IEEE Electron Device Lett. 2016;37:595–598. [Google Scholar]
- 32.Gebel T, et al. 14th IEEE International Conference on Advanced Thermal Processing of Semiconductors. IEEE; Piscataway, NJ: 2006. Millisecond annealing with flashlamps: Tool and process; pp. 47–55. [Google Scholar]
- 33.Acharya N, Timans PJ. Fundamental issues in millisecond annealing. In: Öztürk MC, Roozeboom F, editors. Electrochemical Society Proceedings. Electrochemical Society; Pennington, NJ: 2004. pp. 11–18. [Google Scholar]
- 34.Skorupa W, et al. Advanced thermal processing of semiconductor materials in the millisecond range. Vacuum. 2005;78:673–677. [Google Scholar]
- 35.Yu X, Marks TJ, Facchetti A. Metal oxides for optoelectronic applications. Nat Mater. 2016;15:383–396. doi: 10.1038/nmat4599. [DOI] [PubMed] [Google Scholar]
- 36.Kang YH, et al. Two-component solution processing of oxide semiconductors for thin-film transistors via self-combustion reaction. J Mater Chem C. 2014;2:4247–4256. [Google Scholar]
- 37.Hennek JW, et al. Oxygen “getter” effects on microstructure and carrier transport in low temperature combustion-processed a-InXZnO (X = Ga, Sc, Y, La) transistors. J Am Chem Soc. 2013;135:10729–10741. doi: 10.1021/ja403586x. [DOI] [PubMed] [Google Scholar]
- 38.Sanctis S, et al. Aqueous solution processing of combustible precursor compounds into amorphous indium gallium zinc oxide (IGZO) semiconductors for thin film transistor applications. Chem Asian J. 2018;13:3912–3919. doi: 10.1002/asia.201801371. [DOI] [PubMed] [Google Scholar]
- 39.Wang B, et al. Solution-processed all-oxide transparent high-performance transistors fabricated by spray-combustion synthesis. Adv Electron Mater. 2016;2:1500427. [Google Scholar]
- 40.Huang W, et al. Metal composition and polyethylenimine doping capacity effects on semiconducting metal oxide-polymer blend charge transport. J Am Chem Soc. 2018;140:5457–5473. doi: 10.1021/jacs.8b01252. [DOI] [PubMed] [Google Scholar]
- 41.Esro M, Vourlias G, Somerton C, Milne WI, Adamopoulos G. High-mobility ZnO thin film transistors based on solution-processed hafnium oxide gate dielectrics. Adv Funct Mater. 2015;25:134–141. [Google Scholar]
- 42.Rim YS, et al. Boost up mobility of solution-processed metal oxide thin-film transistors via confining structure on electron pathways. Adv Mater. 2014;26:4273–4278. doi: 10.1002/adma.201400529. [DOI] [PubMed] [Google Scholar]
- 43.Yang F, et al. Free-standing 2D hexagonal aluminum nitride dielectric crystals for high-performance organic field-effect transistors. Adv Mater. 2018;30:e1801891. doi: 10.1002/adma.201801891. [DOI] [PubMed] [Google Scholar]
- 44.Tressaud A, Labrugère C, Durand E. Functionalized Inorganic Fluorides: Synthesis, Characterization & Properties of Nanostructured Solids. John Wiley & Sons; Chichester, UK: 2010. Switchable hydrophobic-hydrophilic fluorinated layer for offset processing; pp. 571–582. [Google Scholar]
- 45.Speight JG. Lange’s Handbook of Chemistry. 16th Ed, Chap 2.9 McGraw-Hill Professional; New York: 2005. [Google Scholar]
- 46.van den Brand J, Sloof WG, Terryn H, de Wit JHW. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminium oxide layers. Surf Interface Anal. 2004;36:81–88. [Google Scholar]
- 47.Xu W, et al. Facile and environmentally friendly solution-processed aluminum oxide dielectric for low-temperature, high-performance oxide thin-film transistors. ACS Appl Mater Interfaces. 2015;7:5803–5810. doi: 10.1021/am508775c. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y-H, et al. Blade-coated sol-gel indium-gallium-zinc-oxide for inverted polymer solar cell. AIP Adv. 2016;6:115006. [Google Scholar]
- 49.Choi SH, Han MK. Effect of channel widths on negative shift of threshold voltage, including stress-induced hump phenomenon in InGaZnO thin-film transistors under high-gate and drain bias stress. Appl Phys Lett. 2012;100:043503. [Google Scholar]
- 50.Khim D, et al. Large enhancement of carrier transport in solution-processed field-effect transistors by fluorinated dielectric engineering. Adv Mater. 2016;28:518–526. doi: 10.1002/adma.201501967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.