Abstract
NMR spectroscopy and imaging (MRI) are two of the most important methods to study structure, function and dynamics from atom to organism scale. NMR approaches often suffer from an insufficient sensitivity, which, however, can be transiently boosted using hyperpolarization techniques. One of these techniques is parahydrogen-induced polarization which has been used to produce catalyst-free hyperpolarized propane gas with proton polarization that is three orders of magnitude greater than equilibrium thermal polarization at a 1.5 T field of a clinical MRI scanner. Here we show that more than 0.3 liters of hyperpolarized propane gas can be produced in 2 seconds. This production rate is more than an order of magnitude greater than that demonstrated previously, and the reported production rate is comparable to that employed for in-human MRI using HP noble gas (e.g. 129Xe) produced via Spin Exchange Optical Pumping (SEOP) hyperpolarization technique. We show that high polarization values can be retained despite the significant increase in the production rate of hyperpolarized propane. The enhanced signals of produced hyperpolarized propane gas were revealed by stopped-flow MRI visualization at 4.7 T. Achieving this high production rate enables the future use of this already compound (already approved for unlimited use in foods by the corresponding regulating agencies, e.g. FDA in the USA, and more broadly as E944 food additive) as a new inhalable contrast agent for diagnostic detection via MRI.
Graphical Abstract
NMR spectroscopy and imaging (MRI) are versatile tools widely employed to study structure, function and dynamics. NMR signal intensity is directly proportional to nuclear spin polarization P, which characterizes the degree of nuclear spin alignment with applied static magnetic field. In practice P is on the order of 10−5 to 10−6 under thermal equilibrium conditions of 298 K and magnetic field of several Tesla. For example, P is 1.0·10−5 for 1H and 2.6·10−6 for 13C nuclei at room temperature and 3 T, the magnetic field of a modern MRI scanner. Hyperpolarization techniques can transiently increase P up to unity, which leads to the temporary increase of magnetic resonance (MR) sensitivity by 4–5 orders of magnitude.1,2 The additional and largely unrealized benefit of hyperpolarization is the fundamental possibility to obtain higher signal-to-noise ratio at low (0.01–0.5 T) magnetic fields.3 The main drivers behind the development of hyperpolarization techniques are their biomedical applications.1,4–6 The inhalation or injection of hyperpolarized (HP) contrast agents enabled functional and metabolic imaging of lung disease, cancer, and others.7–12
Biomedical applications demand a sufficiently long lifetime of HP compounds.5,11,13 The decay of HP state is typically governed by the process of spin-lattice relaxation characterized by T1 constant. Because in a condensed phase low-gamma nuclei such as 13C and 129Xe typically have greater T1 than those of protons, the development of HP contrast agents have primarily employed these low-gamma nuclei.5,14,15 This approach has a significant translational challenge, because conventional clinical MRI scanners can only image protons, and therefore 13C/129Xe-based contrast agents require highly specialized hardware and software, which are available to selected research sites around the globe. The unique properties of the long-lived spin states (LLSS), sometimes also termed as long lived states (LLS) recently explored by Levitt and others allow preparing HP states with the exponential decay constant TS, which can significantly exceed T1 constant.16–19 As a result of this fundamental breakthrough, the interest in the use of HP protons sites have been rekindled,20–25 because the lifetime of HP state can be sufficiently long, and such HP states can be readily imaged on conventional clinical MRI scanners.26–29
HP propane gas can be readily prepared through the process of parahydrogen-induced polarization (PHIP)30–33 via parahydrogen (p-H2) addition to propylene over supported metal catalysts.34–37 Previously, we have demonstrated the existence of LLSS in propane gas at 0.05 T magnetic field with TS value approximately three times greater than the corresponding T1 value. TS exceeding 13 seconds has been demonstrated at high pressure, although TS is ~3 seconds at atmospheric pressure.38 Nevertheless, the extension of HP propane lifetime through LLSS increases the time window sufficiently for potential use as an inhalable contrast agent in a manner similar to that of HP noble gases, e.g. 129Xe and 3He.10
In this work, we show that clinically relevant quantity (~0.3 standard liters)39–44 of HP propane gas can be produced in approximately 2 seconds, i.e. sufficiently fast to retain its hyperpolarized state. We demonstrate the utility of large-scale preparation of HP propane via MR imaging at 4.7 T. High-resolution 2D MR imaging of HP propane is reported here.
MATERIALS AND METHODS
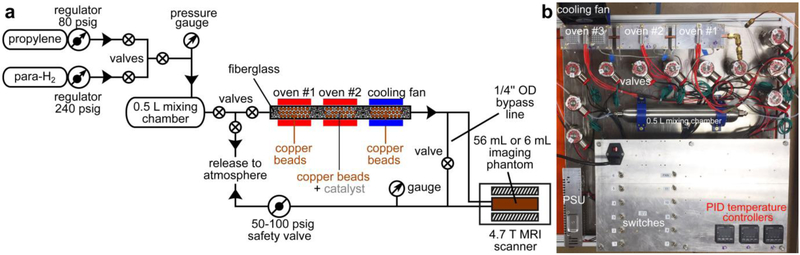
We have developed a clinical-scale propane polarizer prototype device (Figure 1) for reacting propylene with p-H2 gases over a heterogeneous catalyst to produce HP propane gas. The core of the polarizer is comprised of the 1/4 in. OD copper tubing, which is divided into three zones. The first zone is filled with Cu beads (10–40 mesh size, >99.90% purity, Sigma-Aldrich #311405, approximately 12 g filled in a 10-cm-long section) and is employed for preheating the reagents using oven #1 (see Figure 1). The second zone is filled with ~12 g of copper beads and a small quantity (60–280 mg) of fresh 1 wt% Rh/TiO2 catalyst. This type of Rh/TiO2 catalyst was tested in previous studies.36,38 The Cu particles provide a significantly more efficient dissipation of heat generated during the highly exothermic chemical reaction of hydrogenation, because the released heat from highly exothermic reaction is first absorbed by the Cu beads, and then dissipated through the copper reactor tube by the plates and radiators. The second zone is heated with oven #2 and used for production of HP propane gas by pairwise addition of p-H2 to propylene over the heterogeneous Rh catalyst.26 The third zone is filled with ~12 g of copper particles and employed for cooling the HP gas exiting the polarizer using a cooling fan (see Figure 1). The third zone is also additionally equipped with oven #3 which can be used to adjust the temperature of exiting gas. The zones of the reactor are separated by small quantities of glass wool. The reactor tube is sandwiched by ovens #1–3 that are comprised of aluminum plates with attached cartridge heaters and radiators. The temperature of all three zones is independently controlled by three PID controllers (Figure 1b and Figure 1c). In most of our experiments, the observed temperature fluctuations measured by the thermocouples attached to aluminum plates did not exceed 5 °C from the set point values. The output of the polarizer was connected via 1/16 in. OD (1/32 in. ID) Teflon tubing for low-flow conditions (2,000 standard cubic centimetres (sccm) or less) or 1/8 in. OD (1/16 in. ID) Teflon tubing for high-flow conditions to a 5 mm NMR tube via Y-connector as described previously45 or to an imaging phantom located inside the NMR spectrometer or the MRI scanner, respectively. The HP gas leaves the setup via the safety valve (Figure 1a).
Figure 1.
a) Schematic of the experimental setup for batch-mode production and MRI detection of hyperpolarized propane gas; b)) photograph of the constructed batch-mode propane hyperpolarizer.
RESULTS AND DISCUSSION
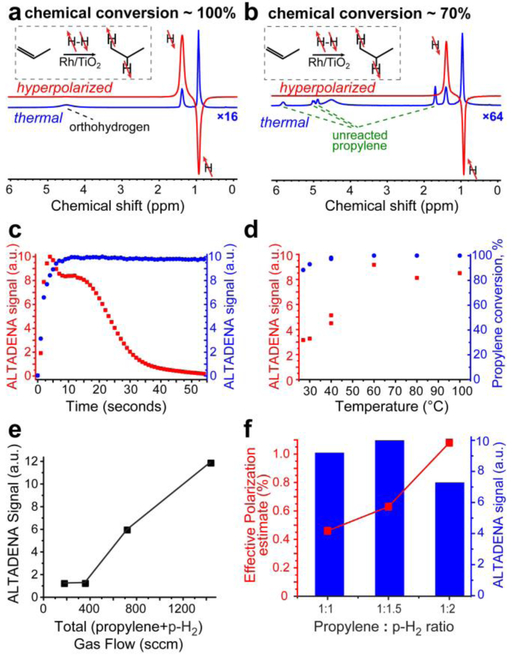
First, this polarizer was operated in a continuous flow regime by mixing the reagents in real time using two mass flow controllers (MFCs, one for propylene and the other one for ~81% p-H2). The details of such setup have been described previously46 and are also presented in the Supporting Information (SI, see Figure S2). The continuous flow operation allows recording NMR spectra of ALTADENA32 HP propane gas in real time (Figure 2a), although T1 relaxation losses are significant at very low flow conditions (e.g. 800 sccm or less, see Figure 2e). This mode of operation enabled a relatively low-flow regime (below 1,440 sccm total flow rate) to confirm that sufficient levels of polarization enhancement were indeed achieved at a lower production rate, Figure 2a (PH~0.58%) and Figure 2b (PH~1.8%). More importantly, this mode of operation allows for monitoring the dynamics of polarization production of HP propane. Figure 2c (blue trace) shows that the reactor performs robustly delivering constant level of polarization. Note the plateau is established in less than 10 seconds of gas flow.
Figure 2.
(a) ALTADENA 1H NMR spectrum of continuously flowing HP propane gas and the corresponding thermal 1H NMR spectrum acquired after interruption of gas flow (the thermal spectrum is multiplied by a factor of 16) under conditions of complete chemical conversion; catalyst mass = 280 mg, t = 130 °C, pressure = 90 psig, gas flow rates: 480 sccm for propylene and 960 sccm for p-H2; signal enhancement ~ 180, P1H ~ 0.58%. (b) ALTADENA 1H NMR spectrum of continuously flowing HP propane gas and the corresponding thermal 1H NMR spectrum acquired after interruption of gas flow (the thermal spectrum is multiplied by a factor of 64) under conditions of partial (~70%) chemical conversion; conditions: catalyst mass = 118 mg, t = 100 °C, pressure = 90 psig, gas flow rates 480 sccm for propylene and 960 sccm for p-H2. The catalyst was extensively treated with cyclopropane before this test (see main text for details); signal enhancement ~ 550, P1H = 1.8%. The insets in displays a) and b) show the reaction scheme of pairwise addition of parahydrogen to propylene to form hyperpolarized propane. (c) Dependence of ALTADENA signal of CH2 group of HP propane obtained in batch mode (red squares) and in continuous flow mode (blue circles) on time on stream. Conditions for batch mode: propylene/p-H2 ratio = 1 : 1.5, catalyst mass = 118 mg, t = 100 °C. Conditions for continuous flow mode: catalyst mass = 118 mg, t = 120 °C, pressure = 90 psig, gas flow rates 480 sccm for propylene and 960 sccm for p-H2. The plots are presented on different scales with the maximum ALTADENA signal in each set of experiments calibrated as 10 a.u. (d) Dependence of ALTADENA signal of CH2 group of HP propane (red squares) and propylene conversion (blue circles) on reaction temperature obtained in batch-mode propane hyperpolarization; conditions: propylene/p-H2 ratio = 1 : 1.5, catalyst mass = 118 mg, average gas flow rate ~4000 sccm. (e) The dependence of PHIP signal of HP propane on the gas flow in continuous-flow operation mode; conditions: propylene/p-H2 ratio = 1 : 2, catalyst mass = 118 mg, t = 100 °C, pressure = 90 psig, chemical conversion was ~100% in all cases. (f) The dependence of HP propane effective polarization estimates on the propane : p-H2 ratio using batch-mode production; conditions: catalyst mass = 118 mg, t = 100 °C. All data is acquired using 9.4 T NMR spectrometer. Note the display f) have dual axes of ALTADENA signal (in arbitrary units, a.u.), which has been employed to compute the effective polarization estimate via signal referencing to the thermally polarized signal.
The experiments at continuous flow conditions employing NMR detection at 9.4 T NMR spectrometer (Figure 2a) revealed nearly complete chemical conversion of the reaction mixtures at temperatures as low as 40 °C, Figure 2d, with the maximum signal enhancement observed in the 60–100 °C range. Operation at this relatively low temperature regime is welcome in the context of biomedical applications.
In some cases, the reactor was treated with cyclopropane gas stream,46 which boosted polarization values (see Figures S3 and S4 in the SI for details), which we hypothesize is due to passivation of active catalytic sites, because cyclopropane is less amenable to hydrogenation than propane, and therefore, may potentially be retained by the active catalytic sites. The excessive treatment with cyclopropane leads to decrease of propylene conversion to propane (from 100 to 70%), but the proton polarization levels are significantly increased to nearly 2%, Figure 2b.
Next, the polarizer was operated in a batch mode (see Figure S1 for experimental setup). In this case, the gases are mixed in the 0.5 L mixing chamber at 100 psig (7.8 atm) pressure resulting in a total volume of 3.9 standard liters. Next, the valves connecting the mixing chamber and oven #1 are opened, enabling the gas flow through the reactor (during the gas flow the pressure drops from 100 psig down to 50 psig, controlled by a safety valve located at the end of the lines). Note that operation in a batch mode results in a significantly higher and varying gas flow rate (the average flow rate is ~4000 sccm). In principle, high-flow regime (over 2000 sccm) would minimize the T1 relaxation losses of HP propane during transportation from the polarizer to the NMR spectrometer. However, the detection efficiency of HP gas in this case is lower, because high velocity of HP gas in the NMR tube results in a situation when only a small fraction of HP propane gas can be detected after radio-frequency (RF) pulse excitation as the RF-excited gas readily leaves the detection region of the NMR probe during signal acquisition. Nevertheless, it was still possible to detect HP propane gas, and Figure 2c (red trace) provides an example of the polarization dynamics monitoring using the batch mode of operation. In this operation mode, we noted an initial bump in the HP signal, which we attribute to a temporary NMR tube overpressurization. Next, the HP signal plateau is established, which follows by the decay of the HP signal due to the decreasing flow rate, which results in polarization losses during gas transfer from the reactor to the NMR detector.
The obtained NMR signals of HP propane exhibited an expected dependence on the propylene/p-H2 ratio in the reactants mixture (Figure 2f). On one hand, the use of higher fraction of p-H2 results in dilution of formed HP propane with the excess of p-H2 gas, thus lowering the observed NMR signal of HP propane. On the other hand, larger fraction of p-H2 also leads to higher NMR signal enhancement, as was shown in previous studies.36 The maximum ALTADENA signal of HP propane was obtained at 1 : 1.5 ratio of propylene and p-H2 in the reactants mixture. Unfortunately, it is difficult to reliably estimate polarization of HP propane obtained in the batch mode (using this setup) due to complicated dynamics of performance of polarizer setup (Figure 2c, red squares), in particular due to changing gas flow and probable temporary overpressurization. We expect propane polarization to be on the order of ~0.5–1% (and therefore, effective polarization level estimates are reported in Figure 2f), based on the results obtained in continuous flow mode, which were carried out using the maximum PHIP signals in dynamic profiles like the one presented in Figure 2c and NMR signals of stopped propane gas as PHIP and thermal reference signals, respectively. These computations of % polarization yielded ~0.5–1.1% effective polarization values (i.e. the values detected in the NMR spectrometer under continuous flow conditions) depending on reaction conditions (gas mixture composition and reaction temperature, Figure 2f). We note that these values may be ~1.2–1.5 times overestimated due to overpressurization (i.e. transient pressure bump) during gas flow. However, on the other hand, the reported effective polarization values may also be underestimated due to lower efficiency of NMR detection of rapidly flowing gas (versus stopped gas yielding thermally polarized reference signal). These two effects (under0 and over-estimation) likely partially cancel each other. Therefore, we expect that the actual polarization should be very close to (likely within 30%) the effective polarization estimate reported in Figure 2f.
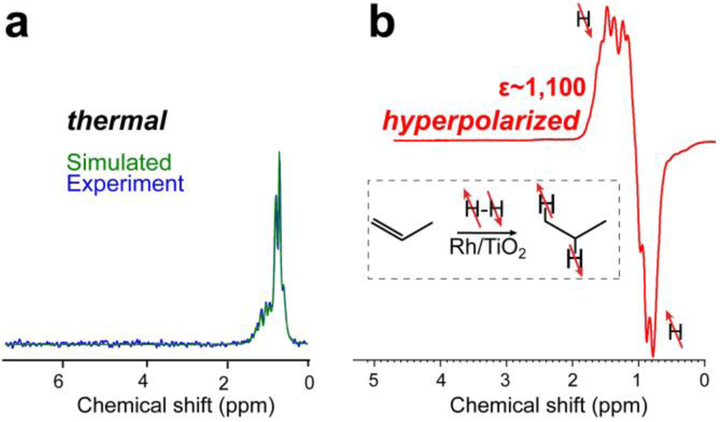
Next, the experimental setup was modified and 1/16 in. OD tubing connecting the reactor with the 5 mm NMR tube was replaced by 1/8 in. OD tubing with a four times greater cross-section to enable significantly higher flow rates of up to approximately 18 standard liters per minute (sLm) of the gas volume. The use of the bypass line (Figures 1, S1 and S2) serves two purposes in our setup. First, it allows to stop the gas flow through the NMR tube in the continuous flow mode as needed (e.g. to acquire thermal NMR spectra, Figure 2a and Figure 2b). Second, the bypass valve allows to significantly reduce the gas flow through the NMR tube in the batch mode operation (which otherwise generates very high gas velocities exceeding 2 sLm incompatible with quantitative NMR detection). Figure 3a shows the NMR spectra of thermally polarized propane after production at a high flow rate (~6.1 sLm) confirming the complete conversion of propylene to propane. No propylene NMR peaks were detected—note the simulation (green trace, Figure 3a) assigned all experimental spectral features (blue trace, Figure 3a) to the reaction product propane. Figure 3b shows NMR spectrum of HP propane obtained under flow conditions using batch-mode production. The spectra shown in Figure 3 were acquired using 1.4 T bench-top NMR spectrometer.
Figure 3.
Proton NMR spectroscopy using 1.4 T bench-top NMR spectrometer of a) thermally polarized reaction gas mixtures after passing through a reactor filled with 118 mg of Rh/TiO2 catalyst after cyclopropane treatment: note all NMR peaks are attributed to propane product; and b) HP propane after passing through a reactor filled with 120 mg of unaltered Rh/TiO2, signal enhancement ε ~ 1,100 corresponding to 0.5% polarization at 1.4 T. Other experimental conditions employed for spectra acquisition in displays a) and b) are t = 40 °C, 20% excess of p-H2 over propylene, ~6 sLm flow rate, ~4 atm total pressure.
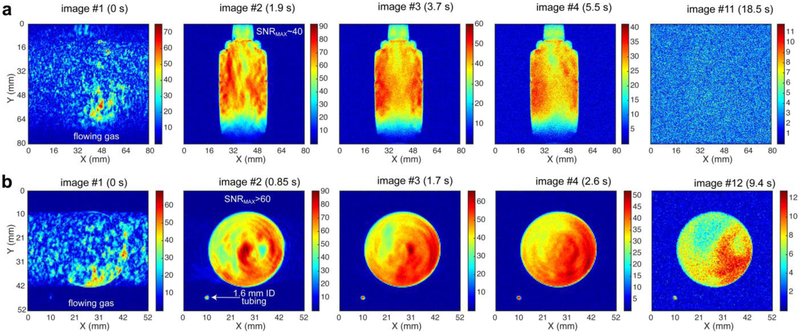
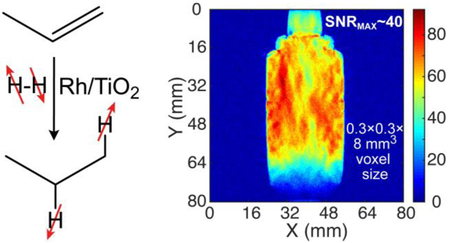
We demonstrate the utility of the batch-mode production approach of HP propane gas for high-resolution MR imaging on the example of HP propane production using the setup presented in Figure 1a at an average flow rate of ~18 sLm using a 1: 2 mixture of propylene with p-H2 yielding a mixture of HP propane:residual p-H2 of 1:1, Figure 4. Approximately 0.6 sL of final mixture was produced in about 2 seconds corresponding to production of 0.3 sL of HP propane. Approximately ⅔ of the HP gas mixture was collected in ~56 mL plastic vessel pressurized to ~7 atm of total pressure, corresponding to ~0.2 sL of HP propane retained in this vessel. MR imaging was started before the HP gas flow was stopped. As a result, the first image shown in each series of coronal (Figure 4a) and axial (Figure 4b) projections has significant distortions due to fast flowing gas. Once the gas is stopped, high resolution and high signal-to-noise ratio (SNR) images were acquired every 1.9 seconds (Figure 4a, SNRMAX of 40) or every 0.85 seconds (Figure 4b, SNRMAX > 40) respectively. The shape of the object is very well delineated in these images. Moreover, the 1.6 mm ID tubing filled with HP propane gas is also well delineated in the axial images (Figure 4b) No signal was detected after the decay of HP state (image #11, Figure 4a). The corresponding image of neat water phantom using thermal proton polarization revealed an image with maximum SNR value (SNRMAX) of ~128 (Figure S5).
Figure 4.
(a) 2D MRI of ~0.2 standard liters of HP propane gas in ~56 mL collection container after production of ~0.3-standard-liter (sL) batch of HP propane in ~2 seconds using an 1:2 mixture of propylene with p-H2. These 2D slices were acquired on a 4.7 T MRI instrument with the following imaging parameters: 2D gradient echo (GRE) images were acquired every ~1.85 sec/slice, 256×256 matrix with repetition time (TR) ~ 7.2 ms, slice thickness = 8 mm; field of view = 80×80 mm2, pixel size (spatial resolution) ~ 0.3×0.3 mm2, voxel size ~ 78 micro-liter; (b) similar 2D acquisition (axial projection): ~0.85 sec/slice, 128×128 matrix with TR ~ 6.6 ms, slice thickness = 12 mm. Field of view = 52×52 mm2, pixel size (spatial resolution) ~ 0.4×0.4 mm2, voxel size ~ 200 micro-liter. HP propane was produced using the following reaction conditions: batch-mode, propylene/p-H2 ratio = 1 : 2, catalyst mass = 280 mg, t = 100 °C.
CONCLUSION
We have demonstrated clinical-scale production of catalyst–free HP propane gas using PHIP technique of up to 0.3 sL in 2 seconds. Complete (~100%) chemical conversion of propylene substrate is achieved during its hydrogenation with parahydrogen over Rh/TiO2 catalyst. The heterogeneous catalyst is robust and can be repeatedly used numerous times. We have not seen a significant catalyst deactivation after producing more than 100 sL of HP propane using propylene and p-H2 as substrates. The produced HP propane dose is similar to that of HP 129Xe produced by Spin Exchange Optical Pumping (SEOP) technique,47 which has been employed for in-human MRI.10,48,49 For example, previous studies have employed 0.3–0.5 sL of HP 129Xe.39–44 Therefore, we expect that the presented approach can be potentially suitable for pulmonary imaging in large animals and humans. We note that although the nominal polarization values obtained here are on the order of 1–2% (versus more than 40% achieved by 129Xe SEOP50–55), HP propane molecule carries two HP nuclei versus one in HP noble gases. More importantly, protons have higher gyromagnetic ratio and higher detection frequency resulting in a significantly more sensitive detection: at least several-fold greater than that of HP 129Xe.56 Therefore, in vivo MRI imaging of HP propane even at these polarization levels may be potentially feasible. Furthermore, we hope that the future development of novel catalysts in heterogeneous PHIP57 may yield additional significant increase in attainable nuclear polarization in HP propane.
Since propane is already regulated, e.g. by FDA in the US with unlimited use in the food industry under good manufacturing practice (GMP), we envision a straightforward regulatory approval for HP propane use as an inhalable contrast agent. We note however that propane is a flammable gas, and this fact implies rather strict safety regulations for use with humans. High-resolution 2D MR images of stopped HP propane (~0.2 sL batch in ~56 mL container) gas were obtained at 4.7 T, the field at which the LLSS no longer exist in HP propane,26 and therefore the relaxation is governed by T1. We expect an approximately 3-fold greater lifetime of HP propane state in a low (<0.4 T) magnetic field, where the LLSS is retained, and the relaxation process is governed by TS,38 and therefore, a longer time window will be available to gas administration and imaging. Moreover, other developments in the preparation of longer-lived HP propane states and storage, e.g. through the use of cyclopropane as a precursor46 and longer lifetime in the dissolved phase,58 may potentially additionally significantly increase the time window for manipulation with a batch of produced HP propane.
Supplementary Material
ACKNOWLEDGMENT
O.G.S. and K.V.K. thank the Russian Science Foundation (grant #17-73-20030) for the support of experiments with HP propane production. The US team thanks the following funding support: NSF under grant CHE-1416432 and CHE-1836308, NIH 1R21EB020323, DOD CDMRP W81XWH-15-1-0271, and RFBR 17-54-33037 OHKO_a.
Footnotes
Supporting Information
The following files are available free of charge. Supporting figures of experimental setup and additional NMR spectra. (file type, PDF)
REFERENCES
- (1).Goodson BM; Whiting N; Coffey AM; Nikolaou P; Shi F; Gust BM; Gemeinhardt ME; Shchepin RV; Skinner JG; Birchall JR; et al. Hyperpolarization Methods for MRS. Emagres 2015, 4, 797–810. [Google Scholar]
- (2).Kovtunov KV; Pokochueva EV; Salnikov OG; Cousin S; Kurzbach D; Vuichoud B; Jannin S; Chekmenev EY; Goodson BM; Barskiy DA; et al. Hyperpolarized NMR: D-DNP, PHIP, and SABRE. Chem. Asian J 2018, 13, 1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Coffey AM; Truong ML; Chekmenev EY Low-Field MRI Can Be More Sensitive Than High-Field MRI. J. Magn. Reson 2013, 237, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hövener J-B; Pravdivtsev AN; Kidd B; Bowers CR; Glöggler S; Kovtunov KV; Plaumann M; Katz-Brull R; Buckenmaier K; Jerschow A; et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed 2018, 57, 11140–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kurhanewicz J; Vigneron DB; Brindle K; Chekmenev EY; Comment A; Cunningham CH; DeBerardinis RJ; Green GG; Leach MO; Rajan SS; et al. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research Neoplasia 2011, 13, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sriram R; Kurhanewicz J; Vigneron DB Emrstm1253. In Emagres; John Wiley & Sons, Ltd, 2014; pp 311–324. [Google Scholar]
- (7).Albert MS; Cates GD; Driehuys B; Happer W; Saam B; Springer CS; Wishnia A Biological Magnetic-Resonance-Imaging Using Laser Polarized Xe-129. Nature 1994, 370, 199–201. [DOI] [PubMed] [Google Scholar]
- (8).Barskiy DA; Coffey AM; Nikolaou P; Mikhaylov DM; Goodson BM; Branca RT; Lu GJ; Shapiro MG; Telkki V-V; Zhivonitko VV; et al. NMR Hyperpolarization Techniques of Gases. Chem. Eur. J 2017, 23, 725–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Goodson BM Nuclear Magnetic Resonance of Laser-Polarized Noble Gases in Molecules, Materials, and Organisms. J. Magn. Reson 2002, 155, 157–216. [DOI] [PubMed] [Google Scholar]
- (10).Mugler JP; Altes TA Hyperpolarized 129Xe MRI of the Human Lung. J. Magn. Reson. Imaging 2013, 37, 313–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nelson SJ; Kurhanewicz J; Vigneron DB; Larson PEZ; Harzstark AL; Ferrone M; van Criekinge M; Chang JW; Bok R; Park I; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized 1-C-13 Pyruvate. Sci. Transl. Med 2013, 5, 198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Branca RT; Cleveland ZI; Fubara B; Kumar CSSR; Maronpot RR; Leuschner C; Warren WS; Driehuys B Molecular MRI for Sensitive and Specific Detection of Lung Metastases. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 3693–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Nikolaou P; Goodson BM; Chekmenev EY NMR Hyperpolarization Techniques for Biomedicine. Chem. Eur. J 2015, 21, 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brindle KM Imaging Metabolism with Hyperpolarized 13C-Labeled Cell Substrates. J. Am. Chem. Soc 2015, 137, 6418–6427. [DOI] [PubMed] [Google Scholar]
- (15).Schroder L Xenon for NMR Biosensing - Inert but Alert. Phys. Medica 2013, 29, 3–16. [DOI] [PubMed] [Google Scholar]
- (16).Carravetta M; Johannessen OG; Levitt MH Beyond the T1 Limit: Singlet Nuclear Spin States in Low Magnetic Fields. Phys. Rev. Lett 2004, 92, 153003. [DOI] [PubMed] [Google Scholar]
- (17).Carravetta M; Levitt MH Long-Lived Nuclear Spin States in High-Field Solution NMR. J. Am. Chem. Soc 2004, 126, 6228–6229. [DOI] [PubMed] [Google Scholar]
- (18).Warren WS; Jenista E; Branca RT; Chen X Increasing Hyperpolarized Spin Lifetimes through True Singlet Eigenstates. Science 2009, 323, 1711–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Theis T; Ortiz GX; Logan AWJ; Claytor KE; Feng Y; Huhn WP; Blum V; Malcolmson SJ; Chekmenev EY; Wang Q; et al. Direct and Cost-Efficient Hyperpolarization of Long-Lived Nuclear Spin States on Universal 15N2-Diazirine Molecular Tags. Sci. Adv. 2016, 2, e1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Roy SS; Rayner P; Norcott P; Green GGR; Duckett SB Long-Lived States to Sustain Sabre Hyperpolarised Magnetisation. Phys. Chem. Chem. Phys 2016, 18, 24905–24911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Zhivonitko V; Kovtunov K; Chapovsky P; Koptyug I Nuclear Spin Isomers of Ethylene: Enrichment by Chemical Synthesis and Application for NMR Signal Enhancement. Angew. Chem. Int. Ed 2013, 52, 13251–13255. [DOI] [PubMed] [Google Scholar]
- (22).Franzoni MB; Buljubasich L; Spiess HW; Munnemann K Long-Lived H-1 Singlet Spin States Originating from Para-Hydrogen in Cs-Symmetric Molecules Stored for Minutes in High Magnetic Fields. J. Am. Chem. Soc 2012, 134, 10393–10396. [DOI] [PubMed] [Google Scholar]
- (23).Zhang YN; Soon PC; Jerschow A; Canary JW Long-Lived 1H Nuclear Spin Singlet in Dimethyl Maleate Revealed by Addition of Thiols. Angew. Chem. Int. Ed 2014, 53, 3396–3399. [DOI] [PubMed] [Google Scholar]
- (24).Zhang Y; Basu K; Canary JW; Jerschow A Singlet Lifetime Measurements in an All-Proton Chemically Equivalent Spin System by Hyperpolarization and Weak Spin Lock Transfers. Phys. Chem. Chem. Phys 2015, 17, 24370–24375. [DOI] [PubMed] [Google Scholar]
- (25).Zhang Y; Duan X; Soon PC; Sychrovský V; Canary JW; Jerschow A Limits in Proton Nuclear Singlet-State Lifetimes Measured with Para-Hydrogen-Induced Polarization. ChemPhysChem 2016, 17, 2967–2971. [DOI] [PubMed] [Google Scholar]
- (26).Kovtunov KV; Barskiy DA; Coffey AM; Truong ML; Salnikov OG; Khudorozhkov AK; Inozemtseva EA; Prosvirin IP; Bukhtiyarov VI; Waddell KW; et al. High-Resolution 3D Proton Hyperpolarized Gas MRI Enabled by Parahydrogen and Rh/TiO2 Heterogeneous Catalyst. Chem. Eur. J 2014, 20, 11636–11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kovtunov KV; Truong ML; Barskiy DA; Koptyug IV; Coffey AM; Waddell KW; Chekmenev EY Long-Lived Spin States for Low-Field Hyperpolarized Gas MRI. Chem. Eur. J 2014, 20, 14629–14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kovtunov KV; Truong ML; Barskiy DA; Salnikov OG; Bukhtiyarov VI; Coffey AM; Waddell KW; Koptyug IV; Chekmenev EY Propane-D6 Heterogeneously Hyperpolarized by Parahydrogen. J. Phys. Chem. C 2014, 118, 28234–28243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Graafen D; Franzoni MB; Schreiber LM; Spiess HW; Münnemann K Magnetic Resonance Imaging of 1H Long Lived States Derived from Parahydrogen Induced Polarization in a Clinical System. J. Magn. Reson 2016, 262, 68–72. [DOI] [PubMed] [Google Scholar]
- (30).Bowers CR; Weitekamp DP Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical-Reaction and Nuclear-Magnetic-Resonance. Phys. Rev. Lett 1986, 57, 2645–2648. [DOI] [PubMed] [Google Scholar]
- (31).Bowers CR; Weitekamp DP Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc 1987, 109, 5541–5542. [Google Scholar]
- (32).Pravica MG; Weitekamp DP Net NMR Alighnment by Adiabatic Transport of Parahydrogen Addition Products to High Magnetic Field. Chem. Phys. Lett 1988, 145, 255–258. [Google Scholar]
- (33).Eisenschmid TC; Kirss RU; Deutsch PP; Hommeltoft SI; Eisenberg R; Bargon J; Lawler RG; Balch AL Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc 1987, 109, 8089–8091. [Google Scholar]
- (34).Koptyug IV; Kovtunov KV; Burt SR; Anwar MS; Hilty C; Han SI; Pines A; Sagdeev RZ Para-Hydrogen-Induced Polarization in Heterogeneous Hydrogenation Reactions. J. Am. Chem. Soc 2007, 129, 5580–5586. [DOI] [PubMed] [Google Scholar]
- (35).Bouchard LS; Burt SR; Anwar MS; Kovtunov KV; Koptyug IV; Pines A NMR Imaging of Catalytic Hydrogenation in Microreactors with the Use of Para-Hydrogen. Science 2008, 319, 442–445. [DOI] [PubMed] [Google Scholar]
- (36).Barskiy DA; Kovtunov KV; Gerasimov EY; Phipps MA; Salnikov OG; Coffey AM; Kovtunova LM; Prosvirin IP; Bukhtiyarov VI; Koptyug IV; et al. 2D Mapping of NMR Signal Enhancement and Relaxation for Heterogeneously Hyperpolarized Propane Gas. J. Phys. Chem. C 2017, 121, 10038–10046. [Google Scholar]
- (37).Burueva DB; Kovtunova LM; Bukhtiyarov VI; Kovtunov KV; Koptyug IV Single-Site Heterogeneous Catalysts: From Synthesis to NMR Signal Enhancement. Chem. Eur. J 2019, 25, 1420–1431. [DOI] [PubMed] [Google Scholar]
- (38).Barskiy DA; Salnikov OG; Romanov AS; Feldman MA; Coffey AM; Kovtunov KV; Koptyug IV; Chekmenev EY NMR Spin-Lock Induced Crossing (SLIC) Dispersion and Long-Lived Spin States of Gaseous Propane at Low Magnetic Field (0.05 T). J. Magn. Reson 2017, 276, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Wild JM; Marshall H; Xu X; Norquay G; Parnell SR; Clemence M; Griffiths PD; Parra-Robles J Simultaneous Imaging of Lung Structure and Function with Triple-Nuclear Hybrid MR Imaging. Radiology 2013, 267, 251–255. [DOI] [PubMed] [Google Scholar]
- (40).Nikolaou P; Coffey AM; Walkup LL; Gust BM; Whiting N; Newton H; Barcus S; Muradyan I; Dabaghyan M; Moroz GD; et al. Near-Unity Nuclear Polarization with an ‘Open-Source’ 129Xe Hyperpolarizer for NMR and MRI. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Nikolaou P; Coffey AM; Walkup LL; Gust BM; Whiting NR; Newton H; Muradyan I; Dabaghyan M; Ranta K; Moroz G; et al. Xena: An Automated ‘Open-Source’ 129Xe Hyperpolarizer for Clinical Use. Magn. Reson. Imaging 2014, 32, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Norquay G; Parnell SR; Xu XJ; Parra-Robles J; Wild JM Optimized Production of Hyperpolarized Xe-129 at 2 Bars for in Vivo Lung Magnetic Resonance Imaging. J. Appl. Phys 2013, 113, 9. [Google Scholar]
- (43).Walkup LL; Woods JC Translational Applications of Hyperpolarized 3He and 129Xe. NMR Biomed. 2014, 27, 1429–1438. [DOI] [PubMed] [Google Scholar]
- (44).Walkup LL; Thomen RP; Akinyi TG; Watters E; Ruppert K; Clancy JP; Woods JC; Cleveland ZI Feasibility, Tolerability and Safety of Pediatric Hyperpolarized 129Xe Magnetic Resonance Imaging in Healthy Volunteers and Children with Cystic Fibrosis. Pediatr. Radiol. 2016, 46, 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Truong ML; Shi F; He P; Yuan B; Plunkett KN; Coffey AM; Shchepin RV; Barskiy DA; Kovtunov KV; Koptyug IV; et al. Irreversible Catalyst Activation Enables Hyperpolarization and Water Solubility for NMR Signal Amplification by Reversible Exchange. J. Phys. Chem. B 2014, 18 13882–13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Salnikov OG; Kovtunov KV; Nikolaou P; Kovtunova LM; Bukhtiyarov VI; Koptyug IV; Chekmenev EY Heterogeneous Parahydrogen Pairwise Addition to Cyclopropane. ChemPhysChem 2018, 19, 2621–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Walker TG; Happer W Spin-Exchange Optical Pumping of Noble-Gas Nuclei. Rev. Mod. Phys 1997, 69, 629–642. [Google Scholar]
- (48).Patz S; Hersman FW; Muradian I; Hrovat MI; Ruset IC; Ketel S; Jacobson F; Topulos GP; Hatabu H; Butler JP Hyperpolarized Xe-129 MRI: A Viable Functional Lung Imaging Modality? Eur. J. Radiol 2007, 64, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Kaushik SS; Cleveland ZI; Cofer GP; Metz G; Beaver D; Nouls J; Kraft M; Auffermann W; Wolber J; McAdams HP; et al. Diffusion-Weighted Hyperpolarized Xe-129 MRI in Healthy Volunteers and Subjects with Chronic Obstructive Pulmonary Disease. Magn. Reson. Med 2011, 65, 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zook AL; Adhyaru BB; Bowers CR High Capacity Production of > 65% Spin Polarized Xenon-129 for NMR Spectroscopy and Imaging. J. Magn. Reson 2002, 159, 175–182. [DOI] [PubMed] [Google Scholar]
- (51).Freeman MS; Emami K; Driehuys B Characterizing and Modeling the Efficiency Limits in Large-Scale Production of Hyperpolarized 129Xe. Phys. Rev. A 2014, 90, 023406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Nikolaou P; Coffey AM; Ranta K; Walkup LL; Gust B; Barlow MJ; Rosen MS; Goodson BM; Chekmenev EY Multi-Dimensional Mapping of Spin-Exchange Optical Pumping in Clinical-Scale Batch-Mode 129Xe Hyperpolarizers. J. Phys. Chem. B 2014, 118, 4809–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Nikolaou P; Coffey AM; Walkup LL; Gust B; LaPierre C; Koehnemann E; Barlow MJ; Rosen MS; Goodson BM; Chekmenev EYA 3D-Printed High Power Nuclear Spin Polarizer. J. Am. Chem. Soc 2014, 136 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Ruset IC; Ketel S; Hersman FW Optical Pumping System Design for Large Production of Hyperpolarized Xe-129. Phys. Rev. Lett 2006, 96, 053002. [DOI] [PubMed] [Google Scholar]
- (55).Norquay G; Collier GJ; Rao M; Stewart NJ; Wild JM 129Xe-Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Phys. Rev. Lett 2018, 121, 153201. [DOI] [PubMed] [Google Scholar]
- (56).Hoult DI; Richards RE The Signal-to-Noise Ratio of the Nuclear Magnetic Resonance Experiment. J. Magn. Reson 1976, 24, 71–85. [DOI] [PubMed] [Google Scholar]
- (57).Zhao EW; Maligal-Ganesh R; Xiao C; Goh T-W; Qi Z; Pei Y; Hagelin-Weaver HE; Huang W; Bowers CR Silica-Encapsulated Pt-Sn Intermetallic Nanoparticles: A Robust Catalytic Platform for Parahydrogen-Induced Polarization of Gases and Liquids. Angew. Chem. Int. Ed 2017, 56, 3925–3929. [DOI] [PubMed] [Google Scholar]
- (58).Burueva DB; Romanov AS; Salnikov OG; Zhivonitko VV; Chen Y-W; Barskiy DA; Chekmenev EY; Hwang DW-H; Kovtunov KV; Koptyug IV Extending the Lifetime of Hyperpolarized Propane Gas Via Reversible Dissolution. J. Phys. Chem. C 2017, 121, 4481–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.