Abstract
Objective
Women have >50% greater lifetime risk of dementia than men but the role of female-specific endocrine milieu is not well-understood. This study evaluates associations between indicators of estrogen exposure from women's reproductive period and dementia risk in a large diverse population.
Methods
We evaluated 15,754 female members (29.9% nonwhite) of Kaiser Permanente with clinical examinations and health survey data from 1964 to 1973 and were members as of January 1, 1996. In midlife (mean age 51.1 years), women reported age at menarche and menopause and hysterectomy status. Reproductive span was calculated as menopause age minus menarche age. Dementia diagnoses were abstracted from January 1, 1996 to September 30, 2017 medical records (mean age at start of dementia follow-up 76.5 years). Cox proportional hazard models evaluated associations between aspects of reproductive span and dementia risk adjusting for demographics and life course health indicators.
Results
Forty-two percent of women developed dementia. Compared to menarche at age 13.0 (mean menarche age), menarche at ≥16 was associated with 23% greater dementia risk (adjusted hazard ratio [HR] 1.23; 95% confidence interval [CI] 1.01–1.50) adjusting for demographics and life course health indicators. Natural menopause at age <47.4 (mean menopause age) was associated with 19% elevated dementia risk (HR 1.19; 95% CI 1.07–1.31). Reproductive spans <34.4 years (mean duration) were associated with 20% elevated dementia risk (HR 1.20; 95% CI 1.08–1.32). Hysterectomies were associated with 8% elevated dementia risk (HR 1.08; 95% CI 1.01–1.16).
Conclusion
In this large prospective cohort study, endocrine events signaling less estradiol exposure (i.e., later age at menarche, younger age at menopause, shorter reproductive span, and hysterectomies) were associated with elevated risk of dementia.
The lifetime risk of developing dementia at age 65 is more than 55% greater for women compared to men (24.6% vs 15.5%).1 Though women have increased life expectancy, evidence is accumulating that there are sex-specific biological underpinnings to this increased rate. Possible neuroprotective effects of estrogens, depending on life course stage,2,3 spur researchers to examine the association between reproductive factors and dementia. Indicators of reproductive history include age at menarche, age at menopause, menopause type (natural or hysterectomy), and reproductive span (i.e., the number of years between menarche and menopause). Findings to date show heterogeneity in the association between reproductive indicators and risk of dementia.4–7 For example, later age at menarche has been associated with elevated risk of Alzheimer disease (AD)5 (the most common form of dementia) yet other studies show no associated dementia risk.6,7 Similarly, earlier age at menopause has been associated with greater dementia risk6 but other studies show no association.7 Longer reproductive span has been associated with a marginally lower risk of AD8 in some studies but others show no association with dementia7 or AD.4 Inconsistencies in findings across these studies may be due to differences in study design, length of follow-up, age at follow-up, and differences in the adjustment of possible confounders. Overall, data are sparse from large prospective evaluations in cohorts with ethnic diversity.
In this study, we evaluate the prospective association between indicators of reproductive history and dementia in a large diverse sample of US women followed from midlife, also testing for possible nonlinear associations between reproductive factors and dementia. We hypothesized that indicators of reproductive history that signal shorter exposure to endogenous estrogen (later age at menarche, earlier age at menopause, shorter reproductive span, hysterectomies) are associated with elevated dementia risk.
Methods
Study population
This study follows female members of Kaiser Permanente Northern California (KPNC). KPNC is an integrated health care delivery system serving over 4 million members who are representative of the general population in the geographic catchment area, except for the extremes of the income distribution.9 From the 1960s–1990s, KPNC offered a series of optional checkups to members called multiphasic health checkups (MHC) that included health questionnaires and clinical measurements obtaining information on a wide range of health indicators and health-related behaviors. In general, MHC participants were older10 and less likely to smoke11 than the overall KPNC membership.
Standard protocol approvals, registrations, and patient consents
This study was approved by the KPNC internal review board and patient informed consent was waived since analyses were conducted on preexisting data.
Data availability
De-identified data are available to qualified investigators from the KPNC IRB Institutional Data Access/Ethics Committee for the purposes of replicating procedures and results.
Age at menarche and menopause, reproductive span, and hysterectomy
During MHC visits between 1964 and 1973, a series of questions captured information related to menarche and menopause. Age at menarche was captured by the open-ended question “How old were you when monthly menstrual periods began?” Responses were grouped into 5 categories decided a priori: ≤10, 11–12, 13, 14–15, and 16–17 years. Age at menopause was captured by the following question: “If you are no longer having menstrual (monthly) periods: At what age did you stop having periods?” Age at menopause was categorized into 5-year age groups with the exception of ages younger than 40, which were collapsed into ≤30 and 31–40 to avoid small counts. The final categories for age at menopause were as follows: ≤30, 31–40, 41–45, 46–50, and 51–55 years. The number of reproductive years was calculated by subtracting age at menarche from age at menopause and grouped into quintiles. The bottom quintile group encompassed a very large range (14–30 years), which was further divided into ≤20 years and 21–30 years. The final categories for reproductive span were as follows: ≤20, 21–30, 31–34, 35–36, 37–38, and 39–44 years. In addition, binary variables were created for age at menarche, age at menopause, and reproductive span using the mean as the threshold.
Hysterectomies were identified through a series of questions related to surgeries occurring at least 1 year before the MHC visit. Women reporting yes to “uterus (womb) removal” were coded as having undergone a hysterectomy and surgical menopause. Women missing information regarding hysterectomy status but reporting no surgeries were coded as not having hysterectomy.
Dementia diagnosis
Dementia diagnoses occurring between January 1, 1996 and September 30, 2017 were obtained from inpatient and outpatient electronic medical records using the following ICD-10 diagnosis codes: AD (ICD-9: 331.0; ICD-10: G30.x), vascular dementia (ICD-9: 290.4x; ICD-10: F01.5x), and other/nonspecific dementia (ICD-9: 290.0, 290.1x, 290.2x, 290.3, 294.1, 294.2x, and 294.8; ICD-10: F03.9x). This method of dementia ascertainment is consistent with previous studies in this population12–15 and with a previous study that had specificity of 95% and sensitivity of 77% for a similar set of ICD-9 codes compared with a consensus dementia diagnosis.16
Mortality
Information related to deaths was obtained through KPNC electronic medical records, California State Mortality File, and Social Security Death Records.
Covariates
Educational attainment and smoking status were captured in the 1964–1973 MHC questionnaires. Educational attainment was captured as the highest grade completed and was recoded as completed high school education or less vs greater than high school education. Self-reported smoking status was coded as current, past, or never smoker. Clinical measures of blood pressure, height, and weight were obtained during the 1964–1973 MHC visit. Midlife hypertension was defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90. Height and weight were combined to calculate midlife body mass index (BMI). Late-life stroke, diabetes, and heart failure occurring between January 1, 1996 and January 1, 1997 were identified in electronic medical records. KPNC records provided information on age and self-reported race and ethnicity, which were recoded as white, black, Asian, Hispanic, or “other racial/ethnic identity.” Missing indicator variables were created identifying missing information on each of the following covariates: education, midlife smoking, and midlife BMI.
Analytic cohort
A total of 37,226 women participated in the MHC during 1964–1973 between the ages of 45 and 55 years. Of these women, 6,967 died and 12,357 ended membership before the start of dementia follow-up in 1996. A total of 17,902 women were 45–55 years old when they participated in at least one MHC between 1964 and 1973 and were KPNC members as of January 1, 1996. Of these women, 1,890 (11%) were excluded from our sample because they did not provide an age at menarche and 146 women (<1%) were excluded because they provided an age at menarche of 18 years or greater. An additional 112 women (<1%) were excluded for not providing information regarding hysterectomy status. The remaining 15,754 women were included in analyses examining the association between hysterectomies and dementia risk. A total of 9,617 women did not provide information regarding age at menopause. Of the women who did not report age at menopause, 77.6% of them were younger than 50 at the time of the MHC visit. A total of 6,137 women provided information on age at menarche, age at menopause, and hysterectomy status. After excluding 2,090 women who reported a hysterectomy, the remaining 4,047 women were included in analyses examining timing of menarche and menopause and duration of reproductive span.
Among the 37,226 women who participated in the MHC during 1964–1973 between the ages of 45 and 55 years, those who were excluded from the study due to death, loss to follow-up, or missing information on age at menarche and hysterectomy status were older at their MHC visit (48.6 vs 48.2 years, p < 0.0001), less likely to be white (72% vs 70%, p < 0.0001), and more likely to have greater than high school education (57% vs 52%; p < 0.0001) than women included in our hysterectomy analyses.
Method of analysis
We examined the distribution of age at menarche, age at menopause, the length of reproductive span, hysterectomy, demographics, and health indicators in mid- and late-life among the 6,137 women who provided information on age at menarche and menopause and hysterectomy status by dementia status at end of follow-up.
A series of Cox proportional hazards models (age in years as the timescale) separately estimated the associations between age at menarche, age at menopause, and duration of reproductive span and dementia risk among 4,047 women who provided information regarding age at menarche and age at menopause and did not experience a hysterectomy. Exposures were operationalized as binary variables (below the mean vs the mean and above) as well as categorical variables. To examine a possible U-shaped relationship between age at menarche and dementia risk, the mean age at menarche served as the reference group. To examine possible nonlinear effects of age at menopause and reproductive span, each menopause age category or duration of reproductive span category was included in the regression with the highest category serving as the reference group. Covariates were sequentially added in groups following the life course trajectory: (1) demographics (age [as timescale], race/ethnicity, education); (2) midlife health indicators (hypertension, BMI, smoking status); and (3) late-life health indicators (stroke, diabetes, and heart failure). Individuals were followed until dementia diagnosis, death, the start of a membership gap lasting more than 90 days, or the end of the study period on September 30, 2017.
The association between hysterectomy and dementia risk was examined through a series of Cox proportional hazards models estimated among the larger sample of 15,754 women who provided information regarding hysterectomy status (i.e., not excluding those without information on age at menopause).
Some women in the sample may not have gone through menopause at the time of their MHC visit since the median age at natural menopause is not until ages 50 to 52 among white women in industrialized countries.17 Therefore, we replicated models related to menopause and reproductive span among a subsample of women ages 50–55 at time of the MHC (n = 3,191). Additional models examined the association between menopause before age 48.5 (mean menopause age for women between 50 and 55 at MHC), reproductive span of less than 35.5 years (mean duration of reproductive span for women between 50 and 55 at MHC), and dementia risk. Cox proportional hazards models were also implemented to examine the association between hysterectomies and dementia risk among 6,240 women who were 50–55 years old at the time of MHC and provided information regarding age at menarche and hysterectomy status.
Possible differences in dementia risk associated with hysterectomies and less than the mean of the exposure of interest (i.e., menarche before age 13.0, menopause before age 47.4, and reproductive spans shorter than 34.4 years) by race/ethnicity were explored using interaction terms. In additional analyses conducted within each racial/ethnic group, race- and ethnicity-specific mean values for age at menarche, age at menopause, and reproductive span were calculated for women 40–55 years of age. Cox proportional hazards models estimated within each racial/ethnic subgroup allowed for possible differences in the association between these reproductive history indicators, hysterectomy, and dementia risk.
Results
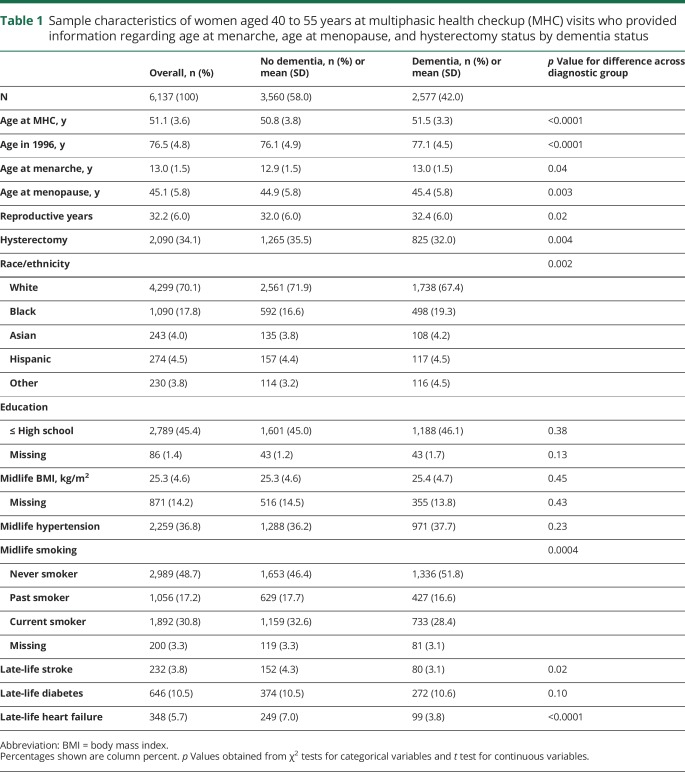
A total of 6,137 women provided information on age at menarche, age at menopause, and hysterectomy status. Among these women, the mean age at the MHC visit was 51.1 years and 76.5 years at the beginning of dementia follow-up in 1996. The mean age at menarche and menopause were 13.0 and 45.1 years, respectively, and the mean duration of reproductive span was 32.2 years (table 1). A total of 2,090 women (34.1%) reported a hysterectomy. Among women who did not report a hysterectomy, the mean age at menarche was 13.0 years, the mean age at menopause was 47.4 years, and the mean duration of reproductive span was 34.4 years. Compared to women without a dementia diagnosis by the end of follow-up, women with a dementia diagnosis had an older average age at menarche, an older average age at menopause, longer reproductive period, and were less likely to have had a hysterectomy. Women with a dementia diagnosis and women without a dementia diagnosis did not significantly differ by education, BMI, midlife hypertension, or late-life diabetes.
Table 1.
Sample characteristics of women aged 40 to 55 years at multiphasic health checkup (MHC) visits who provided information regarding age at menarche, age at menopause, and hysterectomy status by dementia status
Women were followed for an average of 9.1 years (SD 6.3 years; range 0.003–21.7 years). By the end of follow-up, 2,577 (42.0%) women developed dementia, 2,191 (35.7%) women died, 946 (15.4%) women were censored at the beginning of membership gap greater than 90 days, and 423 were followed until the end of the study period.
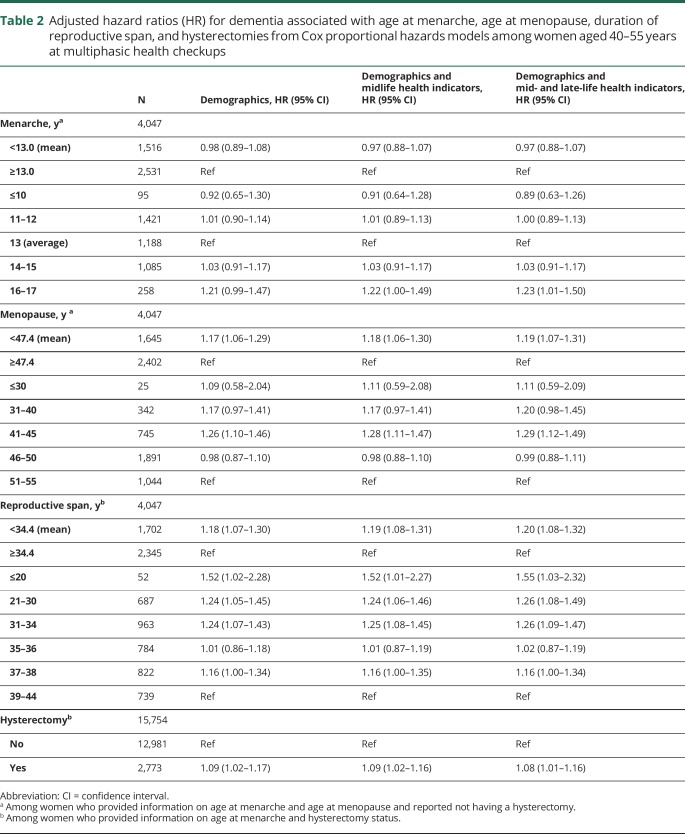
Menarche before age 13 years was not associated with elevated risk of dementia in models adjusting for demographics or in models further adjusting for life course health indicators. In models adjusted for demographics and mid- and late-life health indicators, women who experienced menarche at age 16 or 17 had 23% greater risk of dementia compared to menarche at age 13 (hazard ratio [HR] 1.23; 95% confidence interval [CI] 1.01–1.50).
In models adjusting for demographics and midlife and late-life health indicators, women with natural menopause (i.e., without hysterectomies) before age 47.4 years (mean age at menopause) had 19% elevated dementia risk (HR 1.19; 95% CI 1.07–1.31) (table 2) compared with women who experienced menopause at age 47.4 years or older. Compared to menopause between 51 and 55 years of age, women with menopause between 41 and 45 years of age had 29% elevated dementia risk (HR 1.29; 95% CI 1.12–1.49) and menopause between ages 31 and 40 had a marginal association with elevated dementia risk (HR 1.20; 95% CI 0.98–1.45). There was no evidence of a difference in dementia risk associated with menopause before age 30 years or between 46 and 50 years of age compared to 51–55 years.
Table 2.
Adjusted hazard ratios (HR) for dementia associated with age at menarche, age at menopause, duration of reproductive span, and hysterectomies from Cox proportional hazards models among women aged 40–55 years at multiphasic health checkups
Reproductive span was associated with increased dementia risk, with those having the shortest span having the highest risk. Reproductive spans of less than 34.4 years (the mean duration of reproductive years) were associated with 20% increased dementia risk compared to reproductive spans lasting at least 34.4 years, after adjusting for demographics and life course health indicators (HR 1.20; 95% CI 1.08–1.32). Compared to the longest reproductive span (39–44 years), reproductive spans of 14–20 years were associated with 55% elevated dementia risk (HR 1.55; 95% CI 1.03–2.32), reproductive spans of 21–30 years were associated with 26% elevated risk (HR 1.26; 95% CI 1.08–1.49), and reproductive spans of 31–34 years were associated with a 26% elevated risk (HR 1.26; 95% CI 1.09–1.47).
Among the 15,754 women ages 40 to 55 at MHC who provided information on hysterectomy status and age at menarche, those with a hysterectomy had an 8% increase in dementia risk compared to those without a hysterectomy adjusting for demographics and life course health indicators (HR 1.08; 95% CI 1.01–1.16).
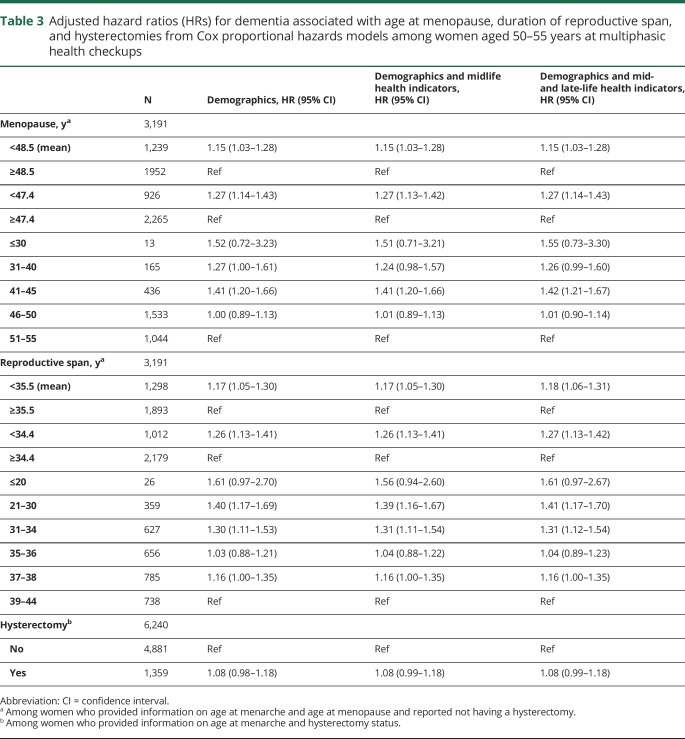
In analyses restricted to women aged 50–55 at MHC, the mean age at natural menopause was 48.5 and the mean duration of reproductive span was 35.5 years (table 3). Experiencing menopause before age 48.5 was associated with 15% elevated risk compared to experiencing menopause at age 48.5 or older (HR 1.15; 95% CI 1.03–1.28) adjusting for demographics and life course health indicators. Compared to menopause between the ages of 51 and 55, menopause between 41 and 45 was associated with 42% elevated risk of dementia (HR 1.42; 95% CI 1.21–1.67) and menopause between 31 and 40 was marginally associated with elevated dementia risk (HR 1.26; 95% CI 0.99–1.60) adjusting for demographics and life course health indicators. Reproductive span of less than 35.5 years was associated with 18% elevated risk of dementia compared to reproductive spans of at least 35.5 years (HR 1.18; 95% CI 1.06–1.31). Compared to reproductive span of 39–44 years, reproductive span of 21–30 years was associated with 41% greater dementia risk (HR 1.41; 95% CI 1.17–1.70) and reproductive span of 31–34 years was associated with 31% greater dementia risk (HR 1.31; 95% CI 1.12–1.54) adjusting for demographics and life course health indicators. Hysterectomies had a marginal association with elevated dementia risk (HR 1.08; 95% CI 0.99–1.18).
Table 3.
Adjusted hazard ratios (HRs) for dementia associated with age at menopause, duration of reproductive span, and hysterectomies from Cox proportional hazards models among women aged 50–55 years at multiphasic health checkups
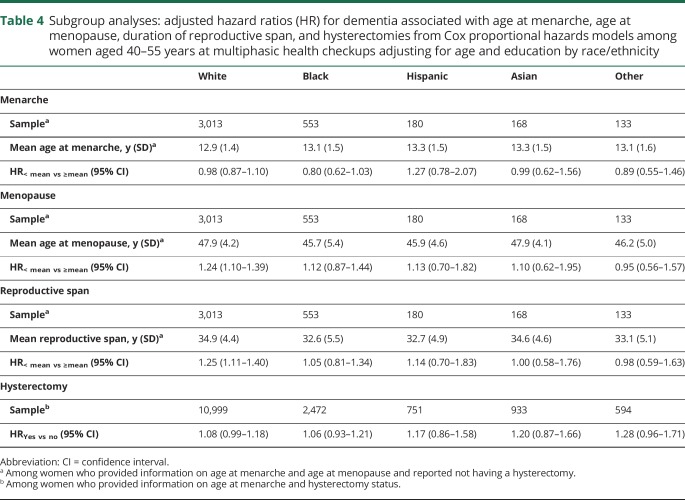
Findings were similar across racial/ethnic groups (table 4) and interaction terms showed no evidence of effect modification by race/ethnicity for age at menarche of 13.0 years (joint test Wald χ2 p value = 0.58), age at menopause below 47.4 years (joint test Wald χ2 p value = 0.41), reproductive span below 34.4 years (joint test Wald χ2 p value = 0.44), or hysterectomies (joint test Wald χ2 p value = 0.70).
Table 4.
Subgroup analyses: adjusted hazard ratios (HR) for dementia associated with age at menarche, age at menopause, duration of reproductive span, and hysterectomies from Cox proportional hazards models among women aged 40–55 years at multiphasic health checkups adjusting for age and education by race/ethnicity
Discussion
In this large diverse cohort of women followed prospectively since the 1960s, we found evidence that indicators of reproductive history, which signal length of exposure to endogenous estrogen, are associated with dementia risk. Overall, our results support the hypothesis that longer exposure to estrogen lowers dementia risk. We found that both components that could shorten a woman's reproductive span (i.e., later age at menarche or earlier age at menopause) as well as the duration of a woman's reproductive span were associated with increased dementia risk. Hysterectomies also were associated with a moderately increased dementia risk. There was no evidence that the associations between indicators of reproductive history and dementia risk varied by race/ethnicity.
Prior literature
Our findings are consistent with a prior case–control study of 115 women with AD at least 55 years old and 1,041 noncognitively impaired controls that showed an increased odds of AD for each additional year of age at menarche.5 Our findings are inconsistent with 2 prior large epidemiologic studies of age at menarche and dementia.6,7 The first included 3,601 female participants of the Rotterdam Study followed for a median of 6.3 years.6 Information regarding age at menarche, age at menopause, and type of menopause was collected during baseline home interviews when participants were at least 55 years old. The study found no difference in dementia risk associated with menarche at age 12 or younger compared to menarche at ages above 14. A separate longitudinal retrospective study by Prince et al.7 examined 8,466 women ages 65+ from Latin America and China followed for a mean of 3.9 years. This study found no association between age at menarche, measured continuously, and dementia risk. Key differences between our study and these 2 longitudinal studies include younger baseline age and narrower range of baseline ages. In both prior studies, women were at least 55 years old when reporting age at menarche, with no upper limit on age at enrollment. Our study also has a longer follow-up period than either study (mean 9.1 vs 3.9 years7; median 8.0 vs 6.2 years6).
Consistent with our findings, a large meta-analysis of case-control, cross-sectional, and longitudinal studies requiring at least a 5-year difference between the oldest vs youngest menopause age groups showed women in the oldest age at menopause group had lower risk of dementia than women in the youngest menopause age group.4 On the other hand, among women who participated in the Rotterdam study, older age at natural menopause was associated with elevated dementia risk.6 Compared to menopause before age 48, menopause between the ages of 50–52 or ages older than 52 were associated with 95% and 78% increased risk of dementia, respectively. There was no evidence of an association between natural menopause and dementia risk in the Latin American and China study7 or in a US study of 1,884 women (91% white),18 both of which examined age at menopause continuously. Possible reasons our results may differ from those of other studies include a younger and more narrow window of age at menopause assessment (40–55 compared to at least 53 years6,7,18) reducing the likelihood of recall bias, longer follow-up period than 2 of the prior studies,6,7 and different operationalization of age at menopause.
Our study is consistent with a prior cross-sectional study demonstrating a protective association between longer reproductive span and cognitive impairment.19 However, findings in other longitudinal studies7,18 have been inconsistent. Prince et al.7 found no association between duration of reproductive period, measured continuously, and dementia risk among women in Latin American and China. On the other hand, the Rotterdam study found longer reproductive periods were associated with increased dementia risk among women with at least one APOE ε4 allele but there was no association among noncarriers.6 We do not have APOE information on our cohort. However, it is unlikely that APOE status fully explains the directional difference in the association between longer reproductive span and dementia risk across the 2 studies (protective vs harmful) given that no association was found among noncarriers in the Rotterdam study.
Our study found that hysterectomies reported during midlife were associated with 8% increased risk of dementia. This is consistent with prior work that has demonstrated an association between hysterectomies,20 as well as other forms of surgical menopause (e.g., unilateral or bilateral oophorectomy),20,21 and elevated risk of dementia or cognitive impairment. Studies suggest that timing of surgical menopause influences dementia risk, with younger age associated with greater risk of dementia or cognitive impairment20–22 and AD pathology.18
Possible biological pathways
Our epidemiologic findings support prior basic science work suggesting neuroprotective effects of estrogen. Age at menarche, age at menopause, and reproductive years may serve as proxies of cumulative exposure to estrogen across the life course. Estradiol is a form of estrogen produced by the developing ovarian follicle and levels fluctuate throughout the menstrual cycle.23 Estradiol has been shown to promote neuronal resilience and repair.2,3 For example, studies have shown that estradiol reduces inflammation, apoptosis, and tau hyperphosphorylation.2,3 Our findings do not directly examine possible benefits of hormone therapy on dementia risk. However, our findings that longer duration of reproductive span is protective against dementia risk tangentially support evidence that hormones near the time of menopause, as opposed to late in life, may reduce dementia risk.24–26 Another possible pathway is progesterone, which is also produced cyclically in menstruating women and has neuroprotective features.27
Strengths and limitations
This study includes several measures of reproductive history that may serve as proxies to estrogen exposure at different points in the life course as well as an indicator of cumulative exposure. Other strengths of this study include a large diverse sample, equal access to an integrated health care delivery system, and prospectively collected data on a wide range of health indicators from midlife into late life. Another important strength of the study is the long dementia follow-up period ranging up to 21 years. However, we are unable to account for other factors that would affect lifetime estrogen exposure, such as number of pregnancies, hormone replacement use, or use of hormonal forms of birth control. Furthermore, the study does not include direct measures of circulating estrogens and therefore we are unable to directly examine the association between different forms of estrogen and dementia risk. We are unable to assess the accuracy of self-reported age at menopause and age at menarche. However, prior work has shown moderate to high levels of correlations between repeated measures of self-reported age at menarche (correlation coefficient: 0.6628–0.8629) and age at menopause (correlation coefficient: 0.7029–0.8830). Random measurement error in age at menarche or age at menopause would bias our estimates of the association between these exposures and dementia towards the null. Although there is some evidence that the reliability of age at menopause decreases with time,29,31 women were, on average, 51.1 years old when they provided information regarding age at menopause. It is unclear if these results are generalizable to other populations. We also did not have information regarding APOE status, which has previously been shown to modify the relationship between reproductive span and dementia risk.6 We did not have information regarding the timing of hysterectomy; earlier age at hysterectomy has previously been associated with greater cognitive decline.18 We likely underestimate the association between premenopausal hysterectomies and dementia risk. Nor did we have information related to possible oophorectomy or hormone replacement therapy in conjunction with hysterectomies, both of which affect women's estrogen exposure. A prior study reported that 25% of hysterectomies performed in 1965 also included bilateral oophorectomy.32 Some women may not have gone through menopause at the time of their MHC interview when they were between the ages of 40 and 55. However, analyses restricted to women ages 50 to 50 at MHC continued to find elevated risk of dementia among women who experienced menopause between ages 41 and 45 compared to those who experienced menopause between ages 51 and 55.
Our results suggest that endocrine events that signal less exposure to estradiol, such as shorter reproductive span, younger age at menopause, and hysterectomy, increase dementia risk. While these risk factors are not modifiable, timing of these events may signal how and when women could most benefit from preventative efforts or points of intervention. Additional large prospective studies including diverse populations are needed to reflect the increasing diversity of the aging population in the United States. Furthermore, studies with very long follow-up periods are needed to examine the individual and interacting roles of endogenous and exogenous estrogens and other hormones across the life span modulating dementia risk. Future work examining the associations between aspects of reproductive history and dementia risk should also incorporate data regarding early-onset dementia and leverage information from imaging and neuropathology. Understanding biological pathways through which reproductive history may modulate dementia risk could identify potential points of intervention. Continuing to examine sex-specific risk factors for dementia in women is critical given the large, disproportionate burden of dementia experienced by women.
Glossary
- AD
Alzheimer disease
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- ICD-9
International Classification of Diseases–9
- ICD-10
International Classification of Diseases–10
- KPNC
Kaiser Permanente Northern California
- MHC
multiphasic health checkups
Author contributions
R.A. Whitmer and P. Gilsanz contributed to the conception and design of the study. P. Gilsanz was responsible for the statistical analysis. All coauthors interpreted the data and contributed significantly to the manuscript.
Study funding
Funding provided by the National Institute on Aging (RF AG052132 PI: R.A. Whitmer). Dr. Gilsanz was supported by the University of California, San Francisco Training for Research on Aging and Chronic Disease (T32 AG049663).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015;11:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol 2009;30:239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab 2011;22:467–473. [DOI] [PubMed] [Google Scholar]
- 4.Georgakis MK, Kalogirou EI, Diamantaras AA, et al. Age at menopause and duration of reproductive period in association with dementia and cognitive function: a systematic review and meta-analysis. Psychoneuroendocrinology 2016;73:224–243. [DOI] [PubMed] [Google Scholar]
- 5.Hong X, Zhang X, Li H. A case-control study of endogenous estrogen and risk of Alzheimer's disease [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 2001;22:379–382. [PubMed] [Google Scholar]
- 6.Geerlings MI, Ruitenberg A, Witteman JC, et al. Reproductive period and risk of dementia in postmenopausal women. JAMA 2001;285:1475–1481. [DOI] [PubMed] [Google Scholar]
- 7.Prince MJ, Acosta D, Guerra M, et al. Reproductive period, endogenous estrogen exposure and dementia incidence among women in Latin America and China: a 10/66 population-based cohort study. PLoS One 2018;13:e0192889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer's risk in a cohort of British women. Psychoneuroendocrinology 2013;38:2973–2982. [DOI] [PubMed] [Google Scholar]
- 9.Gordon NP. Similarity of the Kaiser Permanente Senior Member Population in Northern California to the Non-Kaiser Permanente Covered and General Population of Seniors in Northern California: Statistics from the 2009 California Health Interview Survey. Oakland: Kaiser Permanente Northern California Division of Research; 2012. [Google Scholar]
- 10.Collen MF. Multiphasic Health Testing Services. New York: John Wiley; 1978. [Google Scholar]
- 11.Friedman GD, Seltzer CC, Siegelaub AB, Feldman R, Collen MF. Smoking among white, black, and yellow men and women: Kaiser-Permanente multiphasic health examination data, 1964–1968. Am J Epidemiol 1972;96:23–35. [DOI] [PubMed] [Google Scholar]
- 12.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 14.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71:1057–1064. [DOI] [PubMed] [Google Scholar]
- 15.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Association between birth in a high stroke mortality state, race, and risk of dementia. JAMA Neurol 2017;74:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katon WJ, Lin EH, Williams LH, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med 2010;25:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bove R, Secor E, Chibnik LB, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014;82:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasgon NL, Magnusson C, Johansson AL, Pedersen NL, Elman S, Gatz M. Endogenous and exogenous hormone exposure and risk of cognitive impairment in Swedish twins: a preliminary study. Psychoneuroendocrinology 2005;30:558–567. [DOI] [PubMed] [Google Scholar]
- 20.Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis 2012;10:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083. [DOI] [PubMed] [Google Scholar]
- 22.Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener Dis 2008;5:257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson VW. Aging, estrogens, and episodic memory in women. Cogn Behav Neurol 2009;22:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WR A, QC P, Jufen Z, Kristine Y. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol 2011;69:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA, Group MS. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry 2005;76:103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao H, Breitner JC, Whitmer RA, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology 2012;79:1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh M. Progesterone-induced neuroprotection. Endocrine 2006;29:271–274. [DOI] [PubMed] [Google Scholar]
- 28.Slanger T, Mutschelknauss E, Kropp S, Braendle W, Flesch-Janys D, Chang-Claude J. Test-retest reliability of self-reported reproductive and lifestyle data in the context of a German case-control study on breast cancer and postmenopausal hormone therapy. Ann Epidemiol 2007;17:993–998. [DOI] [PubMed] [Google Scholar]
- 29.Lord C, Duchesne A, Pruessner JC, Lupien SJ. Measuring indices of lifelong estrogen exposure: self-report reliability. Climacteric 2009;12:387–394. [DOI] [PubMed] [Google Scholar]
- 30.Bosetti C, Tavani A, Negri E, Trichopoulos D, La Vecchia C. Reliability of data on medical conditions, menstrual and reproductive history provided by hospital controls. J Clin Epidemiol 2001;54:902–906. [DOI] [PubMed] [Google Scholar]
- 31.Lucas R, Azevedo A, Barros H. Self-reported data on reproductive variables were reliable among postmenopausal women. J Clin Epidemiol 2008;61:945–950. [DOI] [PubMed] [Google Scholar]
- 32.Pokras R, Hufnagel VG. Hysterectomy in the United States, 1965–84. Am J Public Health 1988;78:852–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data are available to qualified investigators from the KPNC IRB Institutional Data Access/Ethics Committee for the purposes of replicating procedures and results.