Abstract
Objective
To assess functional changes in lymphocyte repertoire and subsequent clinical implications during delayed-release dimethyl fumarate (DMF) treatment in patients with multiple sclerosis.
Methods
Using peripheral blood from several clinical trials of DMF, immune cell subsets were quantified using flow cytometry. For some patients, lymphocyte counts were assessed after DMF discontinuation. Incidence of adverse events, including serious and opportunistic infections, was assessed.
Results
In DMF-treated patients, absolute lymphocyte counts (ALCs) demonstrated a pattern of decline followed by stabilization, which also was reflected in the global reduction in numbers of circulating functional lymphocyte subsets. The relative frequencies of circulating memory T- and B-cell populations declined and naive cells increased. No increased incidence of serious infection or malignancy was observed for patients treated with DMF, even when stratified by ALC or T-cell subset frequencies. For patients who discontinued DMF due to lymphopenia, ALCs increased after DMF discontinuation; recovery time varied by ALC level at discontinuation. T-cell subsets closely correlated with ALCs in both longitudinal and cross-sectional analyses.
Conclusions
DMF shifted the immunophenotype of circulating lymphocyte subsets. ALCs were closely correlated with CD4+ and CD8+ T-cell counts, indicating that lymphocyte subset monitoring is not required for safety vigilance. No increased risk of serious infection was observed in patients with low T-cell subset counts. Monitoring ALC remains the most effective way of identifying patients at risk of subsequently developing prolonged moderate to severe lymphopenia, a risk factor for progressive multifocal leukoencephalopathy in DMF-treated patients.
Trial registration numbers
EUDRA CT 2015-001973-42, NCT00168701, NCT00420212, NCT00451451, and NCT00835770.
Delayed-release dimethyl fumarate (DMF) is an oral treatment approved for patients with relapsing-remitting multiple sclerosis (RRMS). In patients treated with DMF, absolute lymphocyte count (ALC) decline typically occurs within the first year of treatment and stabilizes.1 Early ALC drops have been associated with later development of severe, prolonged lymphopenia (ALC <0.5 × 109/L for >6 months) while on treatment; however, degree of change in ALC is not indicative of response, as patients with and without lymphopenia demonstrated similar reductions in relapse rate vs placebo in pivotal trials.1–3 In addition, no overall increased incidence of serious infection, including opportunistic infections, was observed with DMF vs placebo in the controlled pivotal trials.2–5 Rare cases of progressive multifocal leukoencephalopathy (PML) associated with DMF usage have been reported in the presence of moderate to severe, prolonged lymphopenia.6
Preliminary data indicate that DMF-associated lymphopenia results in a shift in immunophenotype, causing a reduction in circulating central and effector memory T cell number and concomitant increase in naive T cells.7–9 We present data from an integrated analysis of long-term data from DMF clinical studies (BG00012 Monotherapy Safety and Efficacy Extension Study in Multiple Sclerosis [ENDORSE] integrated analysis) and 6-month interim findings from a prospective trial (PROCLAIM) to evaluate the early dynamics of the lymphocyte repertoire and compare patients with long-term DMF exposure and varying degrees of lymphopenia to further understand the effect of these changes on the benefit–risk profile of DMF.
Methods
Study design and participants
PROCLAIM analysis
PROCLAIM (EUDRA CT 2015-001973-42 [clinicaltrialsregister.eu/ctr-search/search?query=2015-001973-42]; 6-month interim analysis) is an ongoing, prospective, open-label, 96-week, phase 3b study evaluating DMF 240 mg 2 times daily (BID) in adult patients aged 18–65 years with RRMS. Lymphocyte subset dynamics were characterized within the first 6 months of DMF treatment for patients from PROCLAIM. Key patient inclusion criteria included age 18–65 years and a RRMS diagnosis (McDonald criteria).10 The main exclusion criteria were previous exposure to DMF; positive serology for HIV or hepatitis B or C; history of drug or alcohol abuse within 1 year before screening; any clinically significant infectious illness within 30 days of screening; a history of clinically significant comorbid disorders or conditions; leukocytes <3.5 × 109/L, ALC values ≤ lower limit of normal (LLN, 0.91 × 109/L), or alanine transaminase/serum glutamic pyruvic transaminase or aspartate transaminase/serum glutamic oxaloacetic transaminase ≥2 × the upper limit of normal at screening; or exposure to cyclosporine, azathioprine, methotrexate, mycophenolate mofetil, IV immunoglobulin (Ig), plasmapheresis, or another investigational drug within 6 months of baseline. Patients with ≥6 months of follow-up or who discontinued study treatment at any time point were included in this analysis.
Blood samples were collected at baseline and every 4 weeks for the first 3 months, followed by every 12 weeks through the first year, every 24 weeks in the second year, and at the last follow-up visit, for a total of 104 weeks. An analysis of the major lymphocyte subsets and functional phenotypes of each subset was performed to characterize the dynamics of changes in the immune cell repertoire during early DMF treatment in patients participating in PROCLAIM who had ≥24 weeks of follow-up or who discontinued study treatment at any time point.
The primary endpoint of PROCLAIM is the change in lymphocyte subsets over the initial 48 weeks of DMF treatment. Secondary and exploratory endpoints included the change in ALC and Ig antibodies over 48 weeks of DMF treatment and safety. Safety was measured by adverse events (AEs) and serious AEs (SAEs) and based on the 24-week interim analysis.
ENDORSE integrated analysis
An integrated analysis of the phase 2b study (NCT00168701 [clinicaltrials.gov/ct2/show/NCT00168701]), pivotal phase 3 Efficacy and Safety of Oral BG00012 in Relapsing-Remitting Multiple Sclerosis (DEFINE) (NCT00420212 [clinicaltrials.gov/ct2/show/NCT00420212]), Efficacy and Safety Study of Oral BG00012 With Active Reference in Relapsing-Remitting Multiple Sclerosis (CONFIRM) (NCT00451451 [clinicaltrials.gov/ct2/show/NCT00451451]) studies, and the ongoing phase 3 ENDORSE (NCT00835770 [clinicaltrials.gov/ct2/show/NCT00835770]) extension study of the DEFINE/CONFIRM parent studies was conducted to assess long-term data, referred to as the ENDORSE integrated analysis throughout this article. Data starting from the first exposure to DMF are included in the analysis. The data cutoff date for ENDORSE was October 1, 2016. Details about study designs and objectives for DEFINE/CONFIRM have been published previously.2–4,11
During the ENDORSE extension study, patients who had received DMF 240 mg BID or 3 times daily in the parent studies continued with this dosage, and those receiving placebo (DEFINE/CONFIRM) or glatiramer acetate (CONFIRM) were re-randomized 1:1 to DMF 240 mg BID or 3 times daily. Following marketing authorization of DMF, all patients switched to open label DMF 240 mg BID.
Changes in ALC were assessed using blood samples collected at baseline and throughout treatment with DMF. ALC data were collected at baseline and every 4 weeks (phase 2b), every 4 weeks for the first 3 months, and every 12 weeks thereafter for 2 years (DEFINE/CONFIRM), and at least every 12 weeks for up to 12 years (ENDORSE).
For patients in ENDORSE who discontinued DMF for any reason, and had an ALC < LLN at their last study visit, blood samples were collected every 12 weeks for 48 weeks after their last dose, or until ALC was ≥ LLN, whichever was earlier. Similar to the on-treatment ALC monitoring schedule, the introduction of a stopping rule, which required patients to discontinue treatment if severe, prolonged lymphopenia had occurred, was introduced after the study had been initiated and was applied retrospectively, thus the on-treatment duration of lymphopenia varies for patients who discontinued therapy with DMF and remained on-treatment for ALC follow-up.
The ENDORSE integrated analysis of lymphocyte subsets is cross-sectional in nature, and the first samples for the exploratory subset analysis were collected after all active patients had been in ENDORSE for at least ∼4 years. For this analysis, patients with an ALC ≥ LLN had a single blood sample taken, patients with an ALC >0.5 to 0.91 × 109/L had blood samples collected every 12 weeks, and patients with an ALC ≤0.5 × 109/L had blood samples collected every 4 weeks. This cross-sectional analysis was performed using blood samples from the first time point that both hematology and flow cytometry were performed. Thus, the actual duration of DMF exposure varies for each patient.
The primary objective of ENDORSE is to evaluate the long-term safety profile of DMF. Exploratory objectives include evaluating the effects of DMF on lymphocyte subsets, as well as describing lymphocyte reconstitution after discontinuation of DMF.
Hematology and flow cytometric analyses
ALC were measured by routine clinical laboratory studies using complete blood count (CBC) differential procedure. Immune cell subsets were quantified in whole blood by multiparameter flow cytometry analysis (table e-1; doi.org/10.5061/dryad.d065rr9). Fluorescence-activated cell scanning assays were designed based on Human Immunophenotyping Consortium–recommended standardized immunophenotyping panels12 and were optimized and validated for whole blood sample analysis with sample stability in sodium heparin vacutainers established for up to 48–72 hours post collection (table e-2; doi.org/10.5061/dryad.d065rr9). Data acquisition was performed by LabCorp Clinical Trials on a BD FACSCanto II (BD Biosciences, San Jose, CA) flow cytometer.
Statistical analysis
PROCLAIM
For the longitudinal PROCLAIM analysis, the change in lymphocyte subsets during DMF treatment over 24 weeks and the median (25%, 75% quartiles) percentage change from baseline at week 24 were calculated. The corresponding p values were calculated using Wilcoxon signed rank test. Mean and standard error were calculated for Ig antibodies.
ENDORSE integrated analysis
A comprehensive analysis of the safety profile of DMF was assessed for patients in the ENDORSE integrated analysis with the use of descriptive statistics for the safety population (all patients who received ≥1 dose of the study drug) to calculate incidence of AEs and SAEs.
Change in ALC over time also was assessed for the ENDORSE integrated analysis, but only included patients with a baseline ALC, and ≥1 ALC recorded during treatment. Moderate, prolonged lymphopenia was defined as ALC 0.5–<0.8 × 109/L for ≥6 months and severe, prolonged lymphopenia as ALC <0.5 × 109/L for ≥6 months. The assessment of lymphocyte subsets was limited to only patients in ENDORSE. Patients were analyzed according to ALC category. The correlation between ALC and CD4+ and CD8+ T-lymphocyte counts was assessed using Pearson and Spearman correlation coefficients.
For the analysis of ALC following DMF discontinuation, patients were eligible for inclusion in the analysis if they had ≥1 ALC recorded following the discontinuation of DMF, regardless of reason for discontinuation.
Standard protocol approvals, registrations, and patient consents
Studies were approved by the relevant institutional review board for each study site, and each study was conducted in accordance with the Declaration of Helsinki, International Conference of Harmonisation–Good Clinical Practice guidelines, and all applicable laws and regulations. All participants provided written informed consent before study procedures.
Data availability
PROCLAIM, the phase 2b study, DEFINE, CONFIRM, and ENDORSE were registered with ClinicalTrials.gov (EUDRA CT 2015-001973-42, NCT00168701, NCT00420212, NCT00451451, and NCT00835770). Requests for data supporting this article should be submitted to the Biogen Clinical Data Request Portal (biogenclinicaldatarequest.com).
Results
Demographic and baseline clinical characteristics
For the PROCLAIM analysis of the first 6 months of DMF treatment, 163 patients were included in the interim analysis (table 1). Demographic and baseline characteristics for the 2,513 patients were broadly consistent among all 4 studies in the ENDORSE integrated analysis, as previously presented.2–4,11 Patients in PROCLAIM were older compared with those in DEFINE/CONFIRM; 62% of patients in PROCLAIM were aged ≥40 years at the time of enrollment, compared with 46% in the ENDORSE integrated analysis. The mean (SD) duration of DMF treatment was 30.2 (10.3) weeks for patients in PROCLAIM and 201 (154) weeks for the ENDORSE integrated analysis.
Table 1.
Patient demographics and disease characteristics
Lymphocytes
Monitoring of ALCs
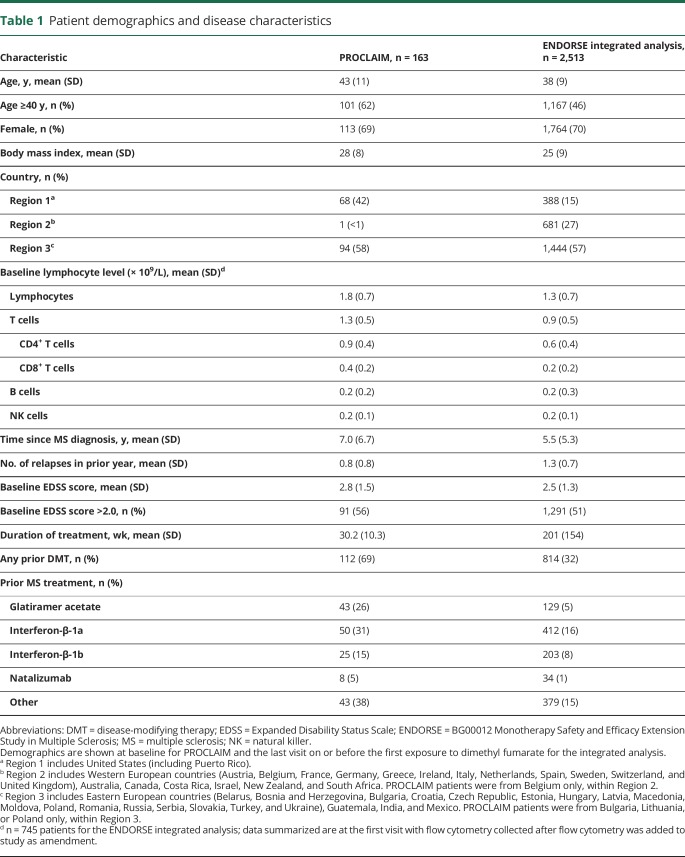
For the ALC analysis, 2,470 patients had ≥1 postbaseline ALC value and therefore were included in the ENDORSE integrated analysis. The pattern of initial decline followed by stabilization of ALC after week 24 was generally maintained during 10 years of follow-up in ENDORSE, and the majority (60.2%) of patients remained > LLN (0.91 × 109/L; figure 1A). Patients with moderate, prolonged lymphopenia remained on treatment for mean (SD) of 4.0 (2.3) years; 2.9 (1.7) years for patients with severe, prolonged lymphopenia.
Figure 1. Absolute lymphocyte counts (ALCs) over 8 years of dimethyl fumarate (DMF) treatment and lymphocyte subsets by ALC categories.
(A) ALCs over 8 years of DMF treatment. Mean (± SE) and 95% confidence intervals (CIs) are presented (n = 2,470). Lower limit of normal (LLN) (0.91 × 109/L) is signified by the dotted line. aData not presented for <10 patients. (B) Lymphocyte subsets by ALC categories CD4+ LLN = 0.2 × 109/L; CD8+ LLN = 0.1 × 109/L. Median (values inside the bars) and 95% CIs are presented. Data were stratified by all ALCs collected during the study (ALC 0.5 to <0.8 × 109/L for ≥6 months [moderate, prolonged lymphopenia]; ALC <0.5 × 109/L for ≥6 months [severe, prolonged lymphopenia]; ALC always ≥0.91 × 109/L [always ≥ LLN]). Patients were categorized as having moderate to severe prolonged lymphopenia if they met these criteria at any time during the study. NK = natural killer.
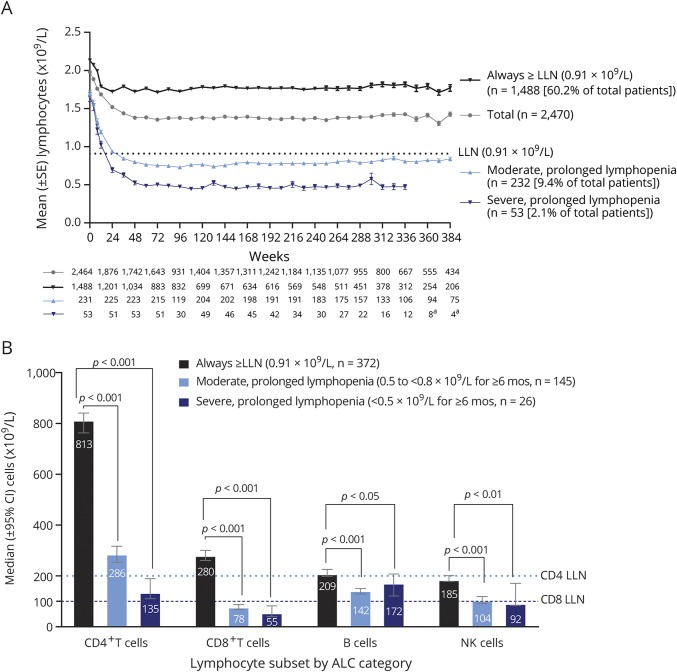
In PROCLAIM, median ALC was 1.82 × 109/L at baseline and 1.24 × 109/L at week 24, representing a median −33.6% change from baseline (figure 2), with reductions observed as early as week 4. Patients will continue to be followed through year 2 to assess the long-term dynamics of ALC.
Figure 2. Immune cell repertoire changes over 24 weeks of dimethyl fumarate (DMF) treatment in PROCLAIM.
For each box plot, the top and bottom box edges correspond to the first and third quartiles, n = 163. The black line inside the box represents the median. The top and bottom whisker lines mark the maximum and minimum values of the dataset, respectively. Dotted lines represent lower limit of normal (LLN) (for those subsets with established cutoff values). Absolute lymphocyte count (ALC) LLN = 0.91 × 109/L; CD4+ LLN = 0.2 × 109/L; CD8+ LLN = 0.1 × 109/L.7,26 the corresponding p values were calculated using Wilcoxon signed-rank test. Significant p values are marked with asterisks (*p < 0.05 vs baseline; **p < 0.01 vs baseline; ***p < 0.001 vs baseline; ****p < 0.0001 vs baseline). NK = natural killer.
Dynamics of lymphocyte subset changes during early DMF treatment
In PROCLAIM, the number of circulating major lymphocyte subsets declined following initiation of DMF treatment, consistent with the decline in ALC (figure 2). B-cell counts initially experienced the greatest rate and proportion of decline, detected as early as 4 weeks after initiation of treatment (−10.4% median change from baseline [CFB], p < 0.0001), before reaching their nadir after 12 weeks (−29.9% median CFB, p < 0.0001), remaining below baseline. By week 8, reduced circulating numbers of CD4+ and CD8+ T and natural killer (NK) cells were observed (median CFB, p value: −12.6%, p < 0.0001; −16.4%, p < 0.0001; and −6.1%, NS). Consistent with the pattern in B cells, the decline in NK cell counts appeared to stabilize after 12 weeks (−11.2% median CFB, p = 0.001), remaining below normal, whereas CD4+ and CD8+ T cell counts continued to decline from baseline through week 24 and CD8+ T cells had the greatest median percentage reduction. By week 24, evidence of a disproportionate effect on T-cell subsets was observed; CD8+ T cells demonstrated a 25% greater relative decline than CD4+ T cells from baseline (CD8+: −44%; CD4+: −33%, p < 0.0001). Monocyte and granulocyte cell counts significantly increased during the first 24 weeks of treatment, though only slightly (<10%, p < 0.001).
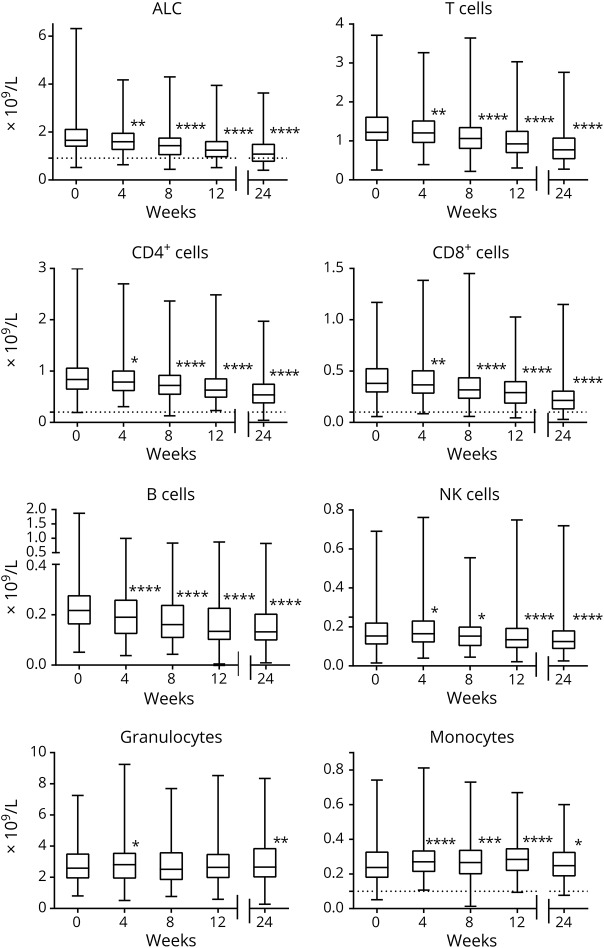
Each major lymphocyte subset (T, B, and NK cells) was further characterized by refined immunophenotyping of functional subsets as depicted in figure 3. As expected, a reduction in the absolute counts of functional subsets was observed at week 24, with the greatest median percentage reduction from baseline in T- and B-cell memory populations and the least effect on naive T- and B-cell subsets (figure 3). While the greatest median percentage reduction by 24 weeks was observed in CD138+ plasma cells (−100%, p < 0.0001), this also is the most difficult population to reliably and accurately measure due to normally very low circulating numbers.13 However, the overall reduction in the terminally differentiated plasma cell population is supported by the concomitant reduction of circulating CD27+ memory B cells, both nonclass-switched (CD27+IgD+) and class-switched (CD27+IgD−) subsets. Notably, among T cells, the greatest median percentage reduction by week 24 was observed in the memory T-cell compartment: central memory (CD45RA−/CCR7+) CD8+ T cells (−73%, p < 0.0001), effector memory (CD45RA−/CCR7−) CD8+ T cells (−65%, p < 0.0001), and effector memory (CD45RA−/CCR7−) CD4+ T cells (−61%, p < 0.0001). Substantial reductions in absolute counts of T helper (Th) 1 (CXCR3+/CCR6−) and Th17 (CXCR3−/CCR6+) CD4+ T cells also were observed compared with the Th2-enriched population (CXCR3−/CCR6−). The numbers of naive (CD45RA+/CCR7+) CD4+ (−7%, NS) and CD8+ (−29%, p < 0.0001) T cells, as well as naive regulatory (CD25+/CD127low/−) CD4+ T cells (0%, p < 0.05), were least affected by DMF treatment by week 24. Interestingly, only 2 populations expanded during DMF treatment by week 24: the numbers of transitional (CD24highCD38high) B cells (+29%, p < 0.0001) and CD56bright NK cells (+25%, p < 0.0001).
Figure 3. Absolute count median percentage change of immune subsets from baseline to week 24 in PROCLAIM.
For each box plot, the top and bottom box edges correspond to Q1 and Q3. The black line inside the box represents the median. The corresponding p values were calculated using Wilcoxon signed-rank test. Significant p values are marked with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). Ig = immunoglobulin; NK = natural killer; Q = quartile; Th = T helper.
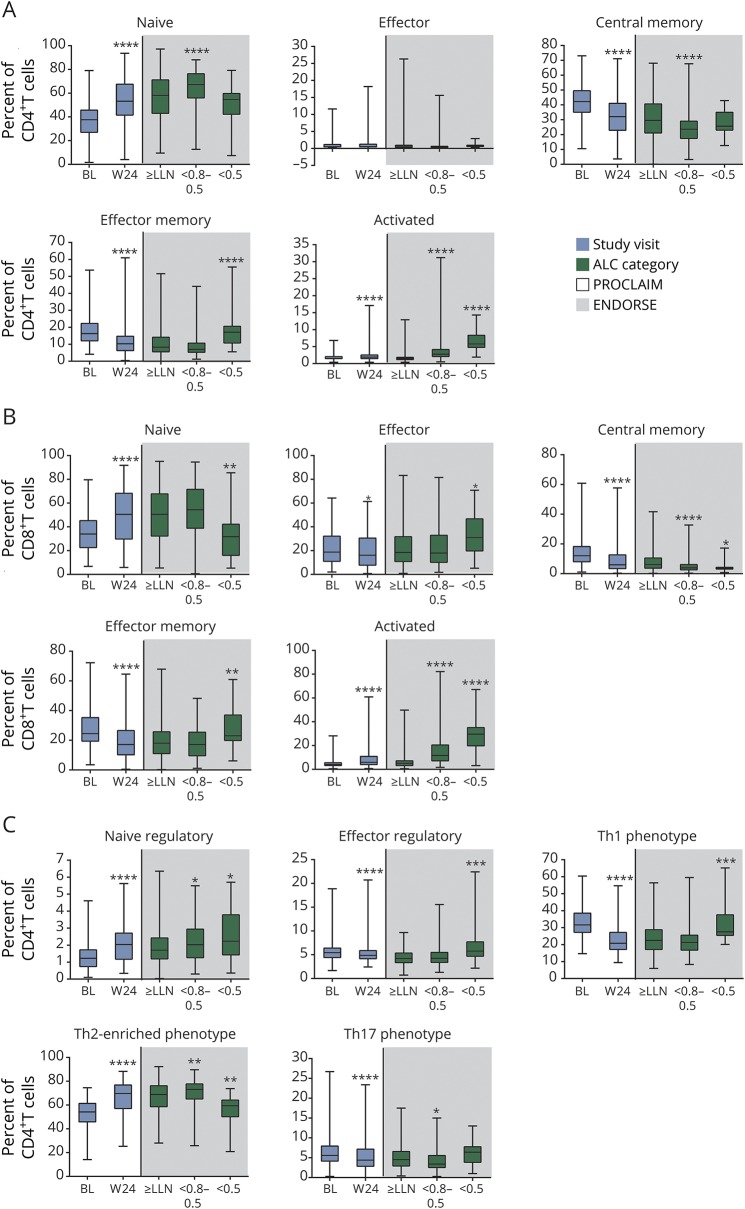
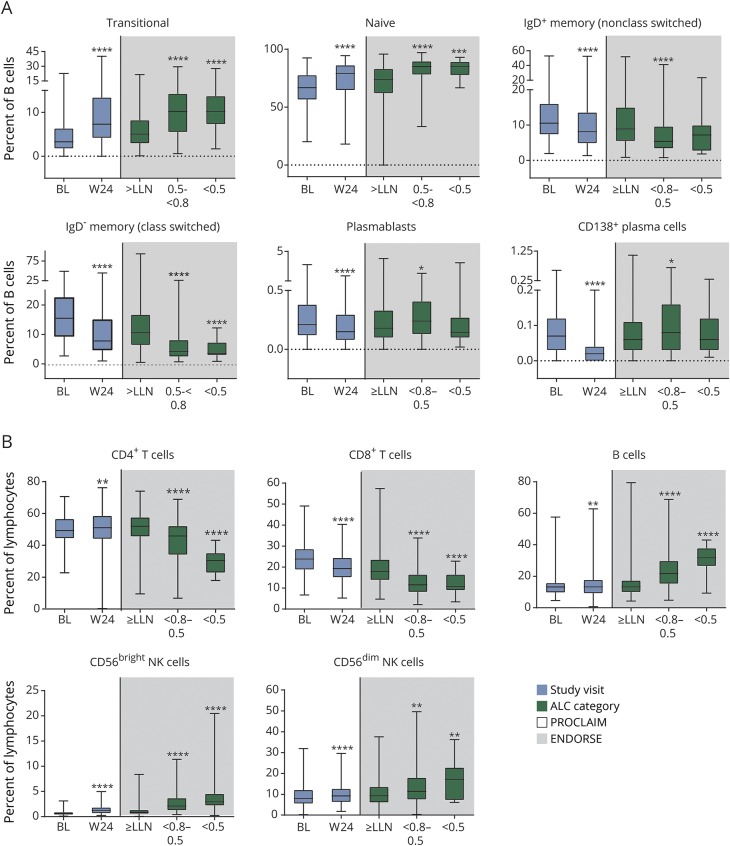
In addition to assessing the changes in lymphocyte subset numbers, the functional composition of the remaining lymphocyte repertoire was evaluated by assessing the relative proportions of each subset population. Within both CD4+ and CD8+ T-cell compartments, at week 24, there was a significant relative decrease in the proportion of central memory and effector memory phenotypes and a corresponding significant increase in the naive phenotype (figure 4, A and B). While the proportion of effector CD8+ T cells was only slightly reduced by week 24, the proportion of CD8+ T cells with an activated phenotype (CD38+HLA-DR+) significantly increased compared with baseline (51%, median CFB, p < 0.0001). Similarly, the proportion of CD4+ T cells with an activated phenotype (CD38+HLA-DR+) also increased (17% median CFB at week 24, p < 0.0001). Importantly, the relative proportion of the total regulatory T cells among the CD4+ T-cell population was stable during DMF treatment (7.7% median CFB at week 24, p < 0.0001) (figure 4C). Correspondingly, there was a relative decline in the proinflammatory Th1 and Th17 subsets (figure 4C) and an increase in the Th2-enriched phenotype. Among the B-cell compartment, the relative proportion of transitional and naive B cells increased while nonclass switched (IgD+) and class switched (IgD−) memory B-cell subsets significantly decreased from baseline to week 24 (figure 5A). Finally, in the immunoregulatory CD56bright NK cell population, there was both an absolute (25% median CFB, p < 0.0001) and relative (87% median CFB at week 24, p < 0.0001) increase (figures 3 and 5B). Overall, DMF treatment appears to selectively modulate proinflammatory and antigen-experienced lymphocytes, with many of these changes observed as early as 4 weeks after DMF initiation. In a parallel assessment, intracellular cytokine staining was also performed. Due to the analytical variability of the data, these findings are not shown and were not statistically significant; however, the patterns observed were consistent with the cell chemokine receptor-defined T-helper subtypes identified by cell surface staining (e.g., CXCR3).
Figure 4. Proportions of T-cell subsets during early (PROCLAIM) and long-term (ENDORSE integrated analysis) dimethyl fumarate (DMF) treatment.
PROCLAIM (n = 163) and the BG00012 Monotherapy Safety and Efficacy Extension Study in Multiple Sclerosis (ENDORSE) integrated analysis (n = 694) are shown. For each box plot, the top and bottom box edges correspond to the first and third quartiles. The black line inside the box represents the median. The top and bottom whisker lines mark the maximum and minimum values of the dataset, respectively. For PROCLAIM (shaded white), the box plots represent the longitudinal trajectory in lymphocyte subsets during early treatment (from baseline [BL] to week 24 [W24]). For the ENDORSE integrated analysis (shaded gray), the box plots represent the cross-sectional observations in lymphocyte subsets in patients who had been exposed to DMF for ∼5 years, stratified by absolute lymphocyte count (ALC) category. For the ENDORSE integrated analysis, data were stratified by ALC categories (ALC always ≥0.91 × 109/L [≥lower limit of normal (LLN)]; ALC <0.8–0.5 × 109/L for ≥6 months [moderate, prolonged lymphopenia, <0.8–0.5]; ALC <0.5 × 109/L for ≥6 months [severe, prolonged lymphopenia, <0.5]). Patients were categorized as having moderate to severe prolonged lymphopenia if they met these criteria at any time during the study. p values were calculated using Wilcoxon signed-rank test, compared the absolute change in relative proportion of lymphocyte subsets at week 24 with baseline for PROCLAIM, were calculated using a pairwise 2-sample Wilcoxon test, and compared the absolute change in relative proportion of lymphocyte subsets for moderate and severe, prolonged lymphopenia compared with ALCs ≥ LLN for the BG00012 Monotherapy Safety and Efficacy Extension Study in Multiple Sclerosis (ENDORSE) integrated analysis. Significant p values are marked with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001) vs baseline for PROCLAIM week 24 or vs ≥ LLN for ENDORSE integrated analysis ALC categories. (A) Naive CD4+ T cells (CD4+/CD45RA+/CCR7+), effector CD4+ T cells (CD4+/CD45RA+/CCR7−), central memory CD4+ T cells (CD4+/CD45RA−/CCR7+), effector memory CD4+ T cells (CD4+/CD45RA−/CCR7−), and activated CD4+ T cells (CD4+/CD38+/HLA-DR+). (B) Naive CD8+ T cells (CD8+/CD45RA+/CCR7+), effector CD8+ T cells (CD8+/CD45RA+/CCR7−), central memory CD8+ T cells (CD8+/CD45RA−/CCR7+), effector memory CD8+ T cells (CD8+/CD45RA−/CCR7−), and activated CD8+ T cells (CD8+/CD38+/HLA-DR+). (C) Naive regulatory T cells (CD4+/CD25+/CD127low/-/CD45RO−), effector regulatory T cells (CD4+/CD25+/CD127low/CD45RO+), Th1 phenotype (CD4+/CXCR3+/CCR6−), Th2-enriched phenotype (CD4+/CXCR3−/CCR6−), and Th17 phenotype (CD4+/CXCR3−/CCR6+). Th = T helper.
Figure 5. Proportions of immune cell subsets during early (PROCLAIM) and long-term (ENDORSE integrated analysis) dimethyl fumarate (DMF) treatment.
PROCLAIM (n = 163) and the BG00012 Monotherapy Safety and Efficacy Extension Study in Multiple Sclerosis (ENDORSE) integrated analysis (n = 694) are shown. For each box plot, the top and bottom box edges correspond to the first and third quartiles. The black line inside the box represents the median. The top and bottom whisker lines mark the maximum and minimum values of the dataset, respectively. For PROCLAIM (shaded white), the box plots represent the longitudinal trajectory in lymphocyte subsets during early treatment (from baseline [BL] to week 24 [W24]). For the ENDORSE integrated analysis (shaded gray), the box plots represent the cross-sectional observations in lymphocyte subsets in patients who had been exposed to DMF for ∼5 years, stratified by absolute lymphocyte count (ALC) category. For the ENDORSE integrated analysis, data were stratified by ALC categories (ALC always ≥0.91 × 109/L [≥lower limit of normal (LLN)]; ALC <0.8–0.5 × 109/L for ≥6 months [moderate, prolonged lymphopenia, <0.8–0.5]; ALC <0.5 × 109/L for ≥6 months [severe, prolonged lymphopenia, <0.5]). Patients were categorized as having moderate to severe, prolonged lymphopenia if they met these criteria at any time during the study. p values were calculated using Wilcoxon signed-rank test, compared the absolute change in relative proportion of lymphocyte subsets at week 24 with baseline for PROCLAIM, were calculated using a pairwise 2-sample Wilcoxon test, and compared the absolute change in relative proportion of lymphocyte subsets for moderate and severe, prolonged lymphopenia compared with ALCs ≥ LLN for the ENDORSE integrated analysis. Significant p values are marked with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001) vs baseline for PROCLAIM week 24 or vs ≥LLN for ENDORSE integrated analysis ALC categories. (A) Transitional B cells (CD19+/CD24hi/CD38hi), naive B cells (CD19+/CD27−/IgD+), IgD+ memory B cells (non-class switched) (CD19+/CD27+/IgD+), IgD− memory B cells (class switched) (CD19+/CD27+/IgD−), plasmablasts (CD19+/CD27++/CD38++), and CD138+ plasma cells (CD19+/CD27++/CD38++/CD138+). (B) CD4+ T cells (CD3+/CD4+), CD8+ T cells (CD3+/CD8+), B cells (CD19+), CD56bright natural killer (NK) cells (CD19−/CD3−/CD16+/CD56hi), and CD56dim NK cells (CD19−/CD3−/CD16+/CD56low). Ig = immunoglobulin.
Lymphocyte subsets by ALC count during long-term DMF treatment
To assess the long-term effects of DMF on lymphocyte function, as well as to identify any differences in patients with prolonged lymphopenia, a cross-sectional analysis was conducted in ENDORSE patients with at least 4 years of DMF exposure. At the time of the first flow cytometry sample, the mean (SD) exposure to DMF was 6.6 (1.3) years for patients with moderate, prolonged and 6.0 (1.3) years for severe, prolonged lymphopenia. Patients with prolonged moderate (n = 145) or severe lymphopenia (n = 26), had lower absolute cell counts of CD4+ and CD8+ T-, B-, and NK cell subsets compared with patients with ALC always above normal (n = 372, figure 1B). Importantly, there was a high correlation between CD4+ counts and ALC and CD8+ counts and ALC (Spearman correlation coefficient, 0.90 and 0.81, respectively). Of note, the majority (>50%) of patients with severe, prolonged lymphopenia had CD4+ and CD8+ T-cell counts below 0.2 × 109/L and 0.1 × 109/L, respectively (median, CD4+ T-cell count: 0.135 × 109/L and CD8+ T-cell count: 0.055 × 109/L).
The functional phenotypes for each major lymphocyte subset also were assessed across ALC categories. Overall, the changes to the functional composition of the T-, B-, and NK cell compartments observed early in DMF treatment in PROCLAIM were consistent in patients with long-term DMF exposure in ENDORSE. In an ALC-dependent manner, the relative proportion of the CD4+ and CD8+ central memory T cells declined (table e-3; doi.org/10.5061/dryad.d065rr9) while the proportion of activated CD4+ and CD8+ T cells increased. Within the B-cell compartment, the proportions of transitional and naive B cells increased, while memory B-cell subsets declined further in an ALC-dependent manner. In addition, the proportion of CD56bright and CD56dim NK cells increased in an ALC-dependent manner. Importantly, across all ALC categories during long-term treatment with DMF, there is a sustained maintenance or increase in the relative proportion of immunoregulatory cells: this includes CD4+ regulatory T, CD56bright NK, and potentially transitional B cells, which have a phenotype that overlaps with IL-10+ B cells, which have been speculated to be implicated in regulatory function.14
Interestingly, there were some substantial divergences in the functional immune repertoire specifically among patients with severe, prolonged lymphopenia. Notably, the proportion of naive CD4+ and CD8+ T cells declined, while the proportion of CD4+ and CD8+ effector memory, CD8+ effector, effector regulatory T, and Th1 T cells increased only among patients with severe, prolonged lymphopenia.
Median percentage changes from baseline in absolute counts and levels of Ig antibodies
Despite changes in the circulating B-cell repertoire with DMF treatment, there was little to no effect on IgM, IgA, IgG, and IgG subclasses 1–4 levels by 24 weeks, which remained stable throughout the observation period (figure e-1 and table e-4; doi.org/10.5061/dryad.d065rr9).
Safety
On-treatment clinical implications of lymphopenia
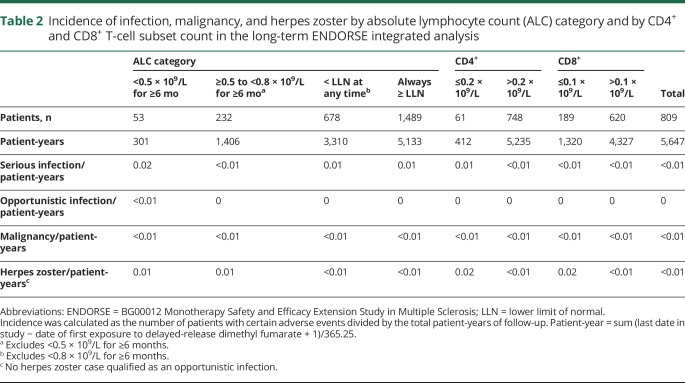
For patients who were exposed to DMF for up to 10 years (10,196 total patient-years), the rate of serious infection, herpes zoster infection, and malignancy in the ENDORSE integrated analysis was low overall, and similar across groups when patients were stratified by on-treatment ALC levels (table 2). Likewise, no increased incidence of serious infections or herpes zoster was observed between groups when stratified by on-treatment CD4+ and CD8+ T-cell counts (table 2). One opportunistic infection (a fatal case of PML) occurred in the setting of severe lymphopenia (ALC range, 0.3–0.6 × 109/L) of 3.5 years' duration and has been previously reported5; there were no other opportunistic infections reported to date.
Table 2.
Incidence of infection, malignancy, and herpes zoster by absolute lymphocyte count (ALC) category and by CD4+ and CD8+ T-cell subset count in the long-term ENDORSE integrated analysis
The safety profile AEs (in 79% of patients) and SAEs (in 4% of patients) in PROCLAIM were consistent with the known safety profile of DMF.2,3,6 As of the 6-month follow-up, no opportunistic infections or serious infections have been reported during the study, though duration of exposure is limited.
Lymphocyte discontinuation and reconstitution in the integrated analysis of ENDORSE
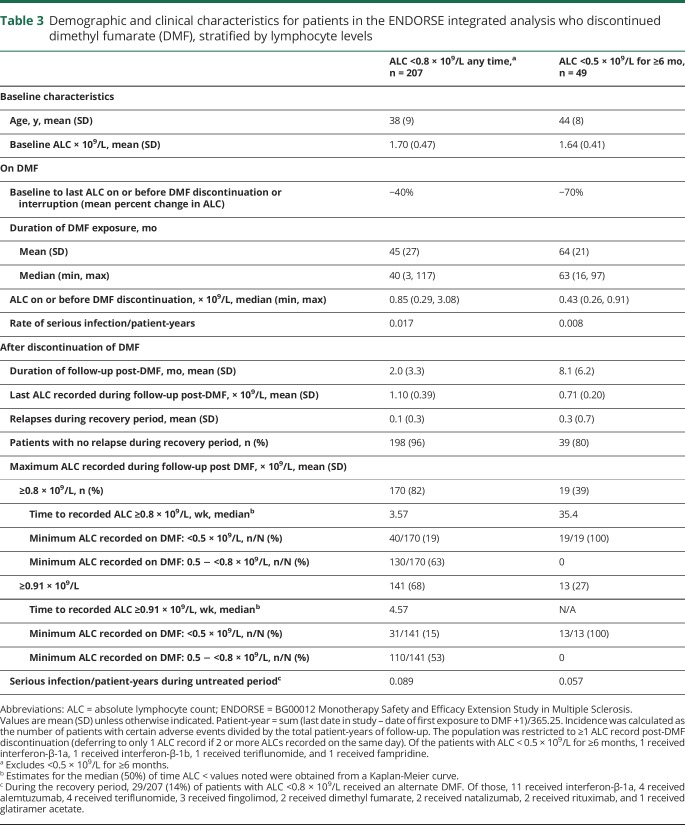
Limited data are available to assess the reconstitution of lymphocytes after discontinuation of DMF. In an analysis of patients with ≥1 ALC <0.8 × 109/L while on treatment with DMF (excluding patients with <0.5 × 109/L for ≥6 months), and ≥1 ALC after DMF discontinuation (n = 207), the majority of patients achieved an ALC of ≥0.8 × 109/L (82%) or 0.91 × 109/L (LLN; 68%) after DMF discontinuation; 63% and 53% of these patients reached those levels within 4–5 weeks (table 3). However, it was not possible to assess lymphocyte subset reconstitution for these patients, as data were limited.
Table 3.
Demographic and clinical characteristics for patients in the ENDORSE integrated analysis who discontinued dimethyl fumarate (DMF), stratified by lymphocyte levels
Patients with severe, prolonged lymphopenia were analyzed separately due to the prolonged duration of lymphopenia while on treatment with DMF. Once these 49 patients had an ALC <0.5 × 109/L for 6 months, they continued to receive treatment with DMF for a mean of 2.9 years before discontinuing therapy. This duration of lymphopenia was prolonged relative to current clinical practice due to amendments to prescribing guidelines. However, of these patients, 15 had ≥3 separate measurements of T-cell subsets recorded after discontinuation of DMF. Of the 15 patients with data available, select individual patient plots to describe the reconstitution of T-cell subsets in the context of ALC reconstitution are presented (figure e-2; doi.org/10.5061/dryad.d065rr9). The high correlation observed between ALC and T-cell subsets while on treatment may suggest a persistent effect following discontinuation; however, due to the spontaneity of collection and small patient numbers, it was not feasible to do a correlative analysis and this could not be confirmed.
Discussion
These findings from a total of 2,633 DMF-treated patients with RRMS in 2 clinical trials, some of whom had long-term treatment with DMF, continue to reflect the well-described pattern of lymphocyte count decline followed by stabilization and corroborate previous smaller studies reporting reductions in circulating lymphocyte subsets corresponding with ALC decline early in the course of treatment,1,7–9,15,16 further demonstrating a maintenance of these changes during long-term treatment. These data indicate that subset changes are closely aligned with the dynamics of overall ALC change, and specifically demonstrate a strong correlation between ALC levels and T-cell subset counts, which underscores the clinical utility and sufficiency of monitoring ALC levels by a routine CBC during DMF treatment.
CD8+ T cells demonstrated a greater relative decline compared to CD4+ T cells, in line with previous observations.17 It has been well-described that CD8+ T lymphocytes are critical for cell-mediated antiviral immunity.7,18 However, with the exception of rare PML in the setting of moderate to severe, prolonged lymphopenia observed in clinical trial and real-world settings, the data from this large population of clinical trial patients treated for many years with DMF did not demonstrate any increased incidence of serious infection or opportunistic infection, regardless of ALC or T-cell subset levels.
These datasets also confirm that DMF shifts the immune repertoire of circulating T and B cells, resulting in a relative expansion of naive and immunoregulatory cells.1,7–9,15,19 The relative proportion of the total regulatory T cells among the CD4+ T-cell population was stable during DMF treatment, suggesting that an appropriate T-reg:T-effector ratio would be maintained to support immune tolerance mechanisms. Interestingly, there also was a relative increase in the activation status of CD8+ and CD4+ T cells. The increased activation status of CD4+ and CD8+ T cells may reflect increased homeostatic expansion.20–23 This increase in an “activated” phenotype is likely indicative of active immune reconstitution in response to the decline in T cells in patients treated with DMF. Notably, the most significant decline during exposure to DMF appears to be on CD8+ central memory, CD8+ and CD4+ effector memory T cells, and class-switched memory B cells, which may explain why no increased incidence of infection was observed in DMF-treated patients. This DMF-mediated shift to an anti-inflammatory state may lead to improvements in disease activity, similar to what has been noted for the B-cell compartment.19 These relative changes are observed regardless of absolute cell counts or ALC category. Because clinical data suggest the presence of lymphopenia is not required for MS relapse-reduction efficacy, the level of ALC reduction or prolonged lymphopenia is not likely to be the primary mechanistic driver of DMF; the contributory effects of cumulative subset-specific changes must still be further explored. While some research has been done to propose specific mechanisms responsible for immune cell loss,7,8,15,24,25 there are likely multiple mechanisms of action for DMF, as well as patient-intrinsic factors, that are currently not well-understood and lead to the heterogeneous effect of DMF on the immune system.
Despite challenges to collecting data in patients after discontinuation from study drug as the majority of patients discontinuing study drug simultaneously discontinue the clinical trial, this dataset provides the first opportunity to describe post-DMF ALC dynamics in a population of >200 patients. Following DMF treatment discontinuation in patients with moderate lymphopenia while on treatment, lymphocyte counts generally increased over time. Most patients achieved an ALC >0.8 × 109/L approximately 1 month after discontinuation. By contrast, the time to reconstitution was longer for patients with severe, prolonged lymphopenia, likely due to the longer time that the patients had severe, prolonged lymphopenia while on treatment. The increase in the proportion of CD4+ and CD8+ effector memory, CD8+ effector, effector regulatory T, and Th1 T cells only among patients with severe, prolonged lymphopenia, together with the increase in activated T, transitional B, and CD56bright NK cells, likely reflect the active dynamics of attempted immune system reconstitution during a chronically lymphopenic state.
The current analysis was subject to inherent limitations associated with integrating data from trials that may have important differences in design, patient population, data extraction procedures, and statistical methodology. In addition, patients enrolled into clinical trials may not fully reflect patients treated in clinical practice, thus continued use of DMF in a real-world population will further describe the benefit–risk profile of DMF. Despite these limitations, amalgamating patient data for this integrated analysis provided an increased sample size and provided consistent observed outcomes.
Taken together, these data provide critical context for the clinical implications of T-cell subset changes in DMF-treated patients and taken together with the strong correlation between ALC and T-cell subsets, continue to support that additional monitoring of T-cell subsets is not required or necessary for safety surveillance in routine clinical practice for patients treated with DMF. The results of these analyses reinforce initial observations documenting the immunomodulatory effects of DMF on lymphocyte subsets. Most importantly, the safety profile of DMF in these studies is consistent with its known effects in adult patients with relapsing forms of MS, and continues to support the positive benefit–risk profile observed in both clinical trial and real-world patients treated with DMF.
Acknowledgment
The authors thank the study participants. Biogen provided funding for medical writing support in the development of this article; Kristen DeYoung from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the article to the authors. The authors had full editorial control of the article, and provided their final approval of all content.
Glossary
- AE
adverse event
- ALC
absolute lymphocyte count
- CBC
complete blood cell count
- CFB
change from baseline
- CONFIRM
Efficacy and Safety Study of Oral BG00012 With Active Reference in Relapsing-Remitting Multiple Sclerosis
- DEFINE
Efficacy and Safety of Oral BG00012 in Relapsing-Remitting Multiple Sclerosis
- DMF
delayed-release dimethyl fumarate
- ENDORSE
BG00012 Monotherapy Safety and Efficacy Extension Study in Multiple Sclerosis
- Ig
immunoglobulin
- LLN
lower limit of normal
- NK
natural killer
- PML
progressive multifocal leukoencephalopathy
- RRMS
relapsing-remitting multiple sclerosis
- SAE
serious adverse event
- Th
T helper
Appendix. Authors
Footnotes
Editorial, page 696
Study funding
This study was funded by Biogen.
Disclosure
D. Mehta is an employee of and holds stock/stock options in Biogen. C. Miller is an employee of and holds stock/stock options in Biogen. D. Arnold has received consulting fees and/or grants from Acorda, Adelphi, Alkermes, Biogen, Celgene, Genentech, Genzyme, Hoffman-La Roche, Immune Tolerance Network, Immunotec, International Progressive MS Alliance, MedDay, MS Society of Canada, Novartis, Pfizer, Receptos, Roche, and Sanofi-Aventis, and has equity interest in NeuroRx Research. E. Bame is an employee of and holds stock/stock options in Biogen. A. Bar-Or has participated as a speaker in meetings sponsored by and received consulting fees and/or grant support from Atara, Biogen, Celgene/Receptos, Genentech/Roche, GlaxoSmithKline, MedImmune, Merck/EMD Serono, Novartis, and Sanofi-Genzyme. R. Gold has received honoraria from Bayer HealthCare, Biogen, Merck Serono, Novartis, and Teva Neuroscience; research support from Bayer HealthCare, Biogen, Merck Serono, Novartis, and Teva Neuroscience; and compensation from Sage for serving as editor of Therapeutic Advances in Neurologic Disorders. J. Hanna is an employee of and holds stock/stock options in Biogen. L. Kappos' institution (University Hospital Basel) received in the last 3 years and used exclusively for research support at the department: steering committee, advisory board, and consulting fees from Actelion, Alkermes, Almirall, Bayer HealthCare, Biogen, Celgene/Receptos, df-mp, Excemed, GeNeuro SA, Genzyme, Japan Tobacco, Merck, Minoryx, Mitsubishi, Novartis, Roche, Sanofi-Aventis, Santhera, Teva, and Vianex, and royalties for Neurostatus-UHB products. The research of the MS Center in Basel has been supported by grants from Bayer HealthCare, Biogen, the European Union, Novartis, Roche Research Foundations, the Swiss MS Society, and the Swiss National Research Foundation. S. Liu is an employee of and holds stock/stock options in Biogen. A. Matta is an employee of and holds stock/stock options in Biogen. J. Phillips has received consulting fees from Acorda, Biogen, Genzyme, EMD Serono, and Sanofi-Aventis. D. Robertson has been a consultant for Biogen, EMD Serono, Genentech, Genzyme, Novartis, and Teva; has received honoraria or speaker fees from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Mallinckrodt, Novartis, and Teva; and has received research grant support from Actelion, Biogen, EMD Serono, Genentech, Genzyme, Mallinckrodt, MedImmune, Novartis, Parexel, Sun, and TG Therapeutics. C. von Hehn is an employee of and holds stock/stock options in Biogen. J. Campbell is an employee of and holds stock/stock options in Envision Pharma Group. K. Spach is an employee of and holds stock/stock options in Envision Pharma Group. L. Yang is an employee of and holds stock/stock options in Biogen. R. Fox has received consulting fees from Biogen, MedDay, Novartis, Questcor, Teva, and Xenoport; has been a part of advisory boards for Biogen and Novartis; and has received research grant funding from Novartis. Go to Neurology.org/N for full disclosures.
References
- 1.Fox RJ, Chan A, Gold R, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate-treated patients with MS: patient management considerations. Neurol Clin Pract 2016;6:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–1097. [DOI] [PubMed] [Google Scholar]
- 3.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. [DOI] [PubMed] [Google Scholar]
- 4.Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008;372:1463–1472. [DOI] [PubMed] [Google Scholar]
- 5.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med 2015;372:1476–1478. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Tecfidera [prescribing information]. Cambridge, MA: Biogen; 2017. [Google Scholar]
- 7.Longbrake EE, Ramsbottom MJ, Cantoni C, Ghezzi L, Cross AH, Piccio L. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler 2016;22:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischer V, Friedrich M, Rezk A, et al. Treatment response to dimethyl fumarate is characterized by disproportionate CD8+ T cell reduction in MS. Mult Scler 2018;24:632–641. [DOI] [PubMed] [Google Scholar]
- 9.Ghadiri M, Rezk A, Li R, et al. Dimethyl fumarate–induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol Neuroimmunol Neuroinflamm 2017;4:e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: interim analysis of ENDORSE, a randomized extension study. Mult Scler 2017;23:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol 2012;12:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caraux A, Klein B, Paiva B, et al. Circulating human B and plasma cells: age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologica 2010;95:1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair PA, Noreña LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010;32:129–140. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Wang Q, Mao G, Dowling CA, Lundy SK, Mao-Draayer Y. Dimethyl fumarate selectively reduces memory T cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol 2017;198:3069–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zecca C, Antozzi CG, Torri Clerici V, et al. Severe multiple sclerosis reactivation during prolonged lymphopenia after dimethyl fumarate discontinuation. Acta Neurol Scand 2018;137:623–625. [DOI] [PubMed] [Google Scholar]
- 17.Khatri BO, Garland J, Berger J, et al. The effect of dimethyl fumarate (Tecfidera) on lymphocyte counts: a potential contributor to progressive multifocal leukoencephalopathy risk. Mult Scler Relat Disord 2015;4:377–379. [DOI] [PubMed] [Google Scholar]
- 18.Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol 2011;85:7256–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Rezk A, Ghadiri M, et al. Dimethyl fumarate treatment mediates an anti-inflammatory shift in B cell subsets of patients with multiple sclerosis. J Immunol 2017;198:691–698. [DOI] [PubMed] [Google Scholar]
- 20.Karnell FG, Lin D, Motley S, et al. Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin Exp Immunol 2017;189:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espinosa E, Romero-Rodriguez DP, Cantoral-Diaz MT, Reyes-Teran G. Transient expansion of activated CD8(+) T cells characterizes tuberculosis-associated immune reconstitution inflammatory syndrome in patients with HIV: a case control study. J Inflamm 2013;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanjako D, Ssewanyana I, Mayanja-Kizza H, et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis 2011;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonelli LR, Mahnke Y, Hodge JN, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood 2010;116:3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatri BO, Tarima SS, Essig B, Sesing J, Olapo T. Delayed lymphocyte re-population following discontinuation of dimethyl fumarate and after switching to other disease modifying drug therapies. Mult Scler Relat Disord 2017;18:60–64. [DOI] [PubMed] [Google Scholar]
- 25.Hoglund RA, Polak J, Vartdal F, Holmoy T, Lossius A. B-cell composition in the blood and cerebrospinal fluid of multiple sclerosis patients treated with dimethyl fumarate. Mult Scler Relat Disord 2018;26:90–95. [DOI] [PubMed] [Google Scholar]
- 26.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015;2:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
PROCLAIM, the phase 2b study, DEFINE, CONFIRM, and ENDORSE were registered with ClinicalTrials.gov (EUDRA CT 2015-001973-42, NCT00168701, NCT00420212, NCT00451451, and NCT00835770). Requests for data supporting this article should be submitted to the Biogen Clinical Data Request Portal (biogenclinicaldatarequest.com).