Abstract
Objective
To cross-sectionally relate multiple small vessel disease (SVD) neuroimaging markers to cognition among older adults.
Methods
Vanderbilt Memory & Aging Project participants free of clinical dementia and stroke (n = 327, age 73 ± 7 years, 59% male, 40% with mild cognitive impairment) completed neuropsychological assessment and 3T MRI to measure white matter hyperintensities (WMH), perivascular spaces (PVS), cerebral microbleeds (CMBs), and lacunes. Linear regressions related each SVD marker to neuropsychological performances and adjusted for age, sex, race/ethnicity, education, cognitive diagnosis, APOE ε4 presence, Framingham Stroke Risk Profile, and intracranial volume.
Results
WMH related to the most neuropsychological measures, including the Boston Naming Test, Animal Naming, Coding, Number Sequencing, Executive Function Composite, and Hooper Visual Organization Test performances (p ≤ 0.01). PVS related to multiple information processing and executive function performances (p ≤ 0.02). Lacunes and CMBs related to fewer measures than expected. Combined models simultaneously testing multiple statistically significant SVD predictors suggested that WMH, PVS, and CMBs each independently related to information processing and executive function performances; however, compared to other SVD markers, PVS remained statistically significant in models related to information processing and executive functioning performances.
Conclusions
As expected, increased WMH corresponded to poorer performances across multiple cognitive domains. PVS, previously considered a benign neuroimaging feature in older adults, may have important clinical implications because PVS was related to information processing and executive function performances even in combined models. On the basis of models with multiple SVD predictors, WMH, PVS, and CMBs may each reflect a separate pathway of small vessel injury.
Cerebral small vessel disease (SVD) encompasses diverse pathologic processes affecting the microvasculature. Common neuroimaging markers of SVD include white matter hyperintensities (WMH) reflecting hypoperfusion, demyelination, and axonal degeneration1; cerebral microbleeds (CMBs) reflecting hypertensive damage and indicated by deposits of hemosiderin-laden macrophages2,3; and lacunes reflecting infarction due to arterial occlusion from vessel wall disorganization and hyaline accumulation.2,4 Among aging adults, these SVD markers relate to incident stroke,5,6 dementia,5,7,8 and increased mortality.9,10 In the absence of clinical dementia, markers such as WMH,11 CMBs,12 and lacunes13 relate to poorer neuropsychological performance. Perivascular spaces (PVS) are radiologically visible fluid-filled spaces adjacent to cerebral vessels associated with hypertension,14 suggesting a link to arteriolosclerosis. Historically, PVS were considered benign, with limited research investigating their clinical significance15; however, PVS are an emerging marker of SVD given that they correlate with hypertension14 and coexist on MRI-histological correlation with WMH.16
It remains unknown whether PVS relate to cognition and whether the association between different SVD markers (e.g., WMH, PVS, CMBs, lacunes) and cognition reflects a common vs unique pathway of small vessel injury. Here, we relate SVD markers to cognition in older adults without clinical dementia or stroke to determine whether SVD markers (WMH, PVS, CMBs, lacunes) contribute uniquely to diverse cognitive functions. On the basis of the literature,17 we hypothesize that among all SVD markers, WMH will have the most common and strongest associations with cognition. When simultaneously considered in the same statistical model, we hypothesize that SVD markers will represent a common pathologic pathway of small vessel injury given that different SVD markers have previously been shown to correlate with one another.14,18
Methods
Study cohort
The Vanderbilt Memory & Aging Project is a longitudinal observational study based in Nashville, TN, investigating vascular health and brain aging. Participants were recruited from the community through postal mailings, clinical referrals, radio advertisements, newsletters, research distribution e-mails, community events, websites, and word of mouth. Inclusion in the study required participants be ≥60 years of age, to speak English, to have intact auditory and visual acuity, and to have a study partner. At eligibility, participants underwent a medical history and record review, clinical interview (including Clinical Dementia Rating19 and functional questionnaire), and neuropsychological assessment for cognitive diagnosis. Participants were included only if they met criteria for a cognitive diagnosis of normal cognition, early mild cognitive impairment,20 or mild cognitive impairment.21 Enrollment exclusions consisted of MRI contraindication, history of neurologic disease (e.g., stroke), clinical heart failure, major psychiatric illness, head injury with loss of consciousness >5 minutes, and a systemic or terminal illness that could affect future follow-up participation. At enrollment, participants completed a comprehensive evaluation that included fasting blood work, physical examination, clinical interview, medication review, echocardiogram, cardiac magnetic resonance imaging, neuropsychological assessment, and brain MRI. Participants were excluded from the current study in a pairwise fashion for missing brain MRI, covariate, or neuropsychological data (figure 1).
Figure 1. Participant selection details.
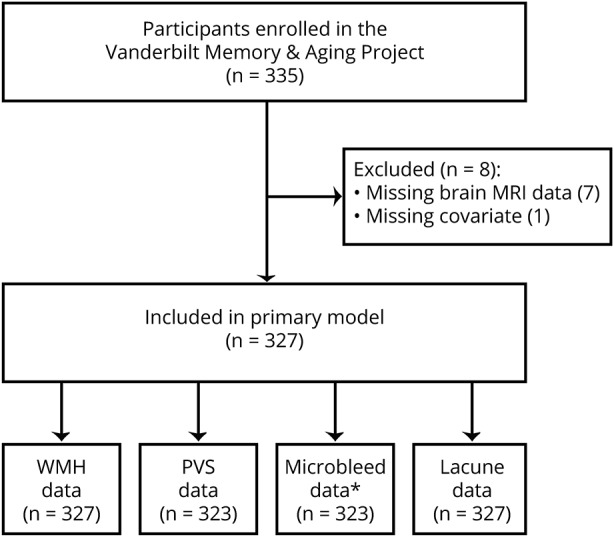
Missing brain MRI data include 7 participants for whom brain MRI was not performed. Missing covariate data include 1 participant missing left ventricular hypertrophy data required for Framingham Stroke Risk Profile calculation. Brain MRI data failing quality control (QC) includes 4 participants for whom susceptibility-weighted imaging (used to identify microbleeds) failed QC. Because of missing neuropsychological outcomes, each model ranged from n = 323 to n = 327. Secondary models required participants to have no missing data for all statistically significant predictors and thus included n = 327 (Wechsler Adult Intelligence Scale IV Coding, Delis-Kaplan Executive Function System [DKEFS] Number Sequencing, Hooper Visual Organization Test) or n = 322 (DKEFS Letter-Number Switching, DKEFS Color-Word Inhibition). PVS = perivascular space; WMH = white matter hyperintensity.
Standard protocol approvals, registrations, and patient consents
The protocol was approved by the Vanderbilt University Medical Center Institutional Review Board. Written informed consent was obtained from all participants before data collection.
Neuropsychological assessment
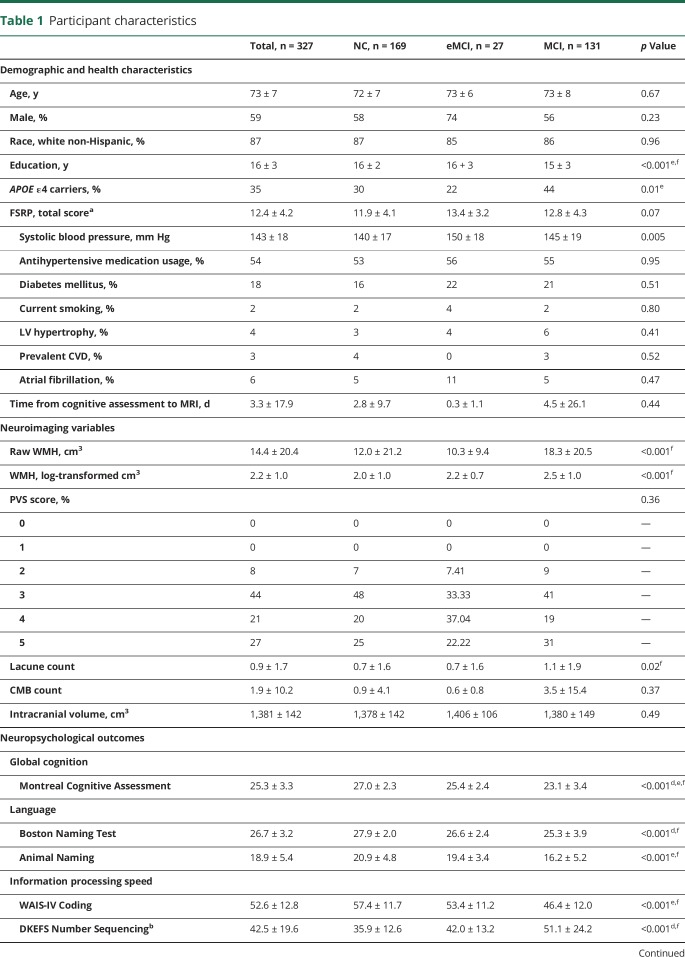
Participants underwent a detailed neuropsychological protocol performed by experienced technicians assessing global cognition, language, visuospatial skills, information processing speed, executive function, and episodic memory (table 1). Measures were carefully selected to avoid floor or ceiling effects and were not used for screening, diagnosis, or selection of participants for the study. Two cognitive composites were calculated from latent variable models to minimize multiple comparisons. As previously described,22 a memory composite was calculated with item-level data from the California Verbal Learning Test–Second Edition and the Biber Figure Learning Test, and an Executive Function Composite was calculated with item-level data from the Delis-Kaplan Executive Function System (DKEFS) Color-Word Inhibition Test, DKEFS Tower Test, Letter Fluency (phonemic verbal fluency) Test, and DKEFS Letter-Number Switching Test. The final composites represent z scores.
Table 1.
Participant characteristics
Brain MRI
Participants were scanned at the Vanderbilt University Institute of Imaging Science on a 3T Philips Achieva system (Best, the Netherlands) with 8-channel SENSE reception. Parameters and postprocessing steps have been detailed elsewhere23 and briefly include T1-weighted magnetization-prepared rapid gradient-echo images (isotropic spatial resolution 1 mm), T2-weighted fluid-attenuated inversion recovery (FLAIR) images (0.45 × 0.45 × 4 mm3), and susceptibility-weighted images (0.75 × 0.75 × 1.5 mm3).
FLAIR data were postprocessed with the use of an automated pipeline to quantify WMH using the Lesion Segmentation Tool toolbox for Statistical Parametric Mapping 8 (SPM8) excluding the cerebellum and brainstem. Manual corrections were then confirmed by a board-certified neuroradiologist (L.T.D.) blinded to clinical information using the Medical Image Processing, Analysis, and Visualization application (mipav.cit.nih.gov).
The remaining SVD markers (PVS, CMBs, lacunes) were manually detected through raw and postprocessed image review by a board-certified neuroradiologist (L.T.D.) blinded to clinical information. PVS were counted in the basal ganglia using T1 and FLAIR sequences according to a semiquantitative method selected because it takes into account regional variations in PVS and total PVS count.24 If a PVS persisted through several slices, it was counted as a single PVS. Manual counts were coded as follows: 0 = present only in the substantia innominata and <5 on either side, 1 = present only in the substantia innominata and >5 on either side, 2 = <5 in the lentiform nucleus on either side, 3 = 5 to 10 in the lentiform nucleus or <5 in the caudate nucleus on either side, 4 = >10 present in the lentiform nucleus but <5 in the caudate nucleus on either side, and 5 = >10 present in the lentiform nucleus and >5 in the caudate nucleus on either side. CMBs were counted with susceptibility-weighted imaging scans. Focal round hypointensities between 1 and 10 mm in diameter that were within the parenchyma and distinct from vascular elements were considered CMBs. Lacunes ranging in size from 2 to 20 mm were manually identified with a combination of T1 and FLAIR images if the lesion had signal on T1 approaching that of CSF and appeared hypointense on FLAIR with a rim of hyperintensity. These size and signal features distinguish lacunes from PVS in accordance with published SVD neuroimaging standards.25 Furthermore, because the PVS of interest for this study were located in the basal ganglia, a PVS would not have a rim of T2-FLAIR hyperintense signal because any adjacent white matter disease would increase the distinction between lacunes and PVS measurements.
Analytical plan
Systolic blood pressure represented the mean of 2 measurements. Diabetes mellitus was defined as hemoglobin A1c ≥6.5%, fasting blood glucose ≥126 mg/dL, or oral hypoglycemic medication or insulin use. Medication review was used to document antihypertensive medication use. Left ventricular hypertrophy was determined from an echocardiogram (left ventricle mass index >95 g/m2 in women, >115 g/m2 in men). Self-reported atrial fibrillation was validated by 1 or more of the following sources: echocardiogram, cardiac magnetic resonance, documented prior atrial fibrillation ablation/procedure, or relevant medication use. Current cigarette smoking (yes/no within 1 year before baseline) was established by self-report. Prevalent cardiovascular disease (CVD) from self-report with supporting medical record evidence included angina, coronary heart disease, or myocardial infarction (heart failure was a parent study exclusion). Framingham Stroke Risk Profile score was determined by calculating points by sex for age, systolic blood pressure accounting for antihypertensive medication use, diabetes mellitus, current cigarette smoking, left ventricular hypertrophy, CVD, and atrial fibrillation.26 APOE genotyping was performed with DNA extracted from whole-blood samples, and APOE ε4 carrier status was defined as positive (ε2/ε4, ε3/ε4, ε4/ε4) or negative (ε2/ε2, ε2/ε3, ε3/ε3). WMH volume and 1 neuropsychological outcome, Letter-Number Switching, were log-transformed before analyses for improved data distribution. PVS were modeled as a categorical predictor with the scoring system described above.
Before analyses, scatterplots with linear fit and locally estimated scatterplot smooth fit were visually inspected for linearity. Semipartial Spearman rank correlations adjusted for age were used to quantify associations not explained by age between each pair of SVD markers, including log-WMH volume, PVS score, CMB count, and lacune count. To assess whether a high burden of each SVD marker corresponded to a high burden of all other markers, each marker was dichotomized as follows: (1) WMH: low raw WMH defined as <25th percentile (equivalent to 3.4-cm3 WMH) and high raw WMH defined as ≥75th percentile (equivalent to 15.4-cm3 WMH), (2) PVS score: low (score ≤2) and high (score ≥3), (3) CMB: presence (≥1) or absence (0), and (4) lacunes: presence (≥1) or absence (0). In unadjusted analyses using Wilcoxon rank-sum test (for continuous outcomes) and Pearson χ2 test (for the dichotomous outcome), we compared each dichotomous SVD marker group to all other SVD features. Continuous outcomes included raw WMH, CMB count, and lacune count. PVS score was treated as a dichotomous outcome (low = score ≤2, high = score ≥3). For hypothesis testing, linear regression models with ordinary least-square estimates related each SVD marker (log-WMH volume, PVS score, CMB count, and lacune count) to each neuropsychological outcome, adjusting for age, education, sex, race/ethnicity, cognitive diagnosis, APOE ε4 presence, intracranial volume, and Framingham Stroke Risk Profile (minus points for age). Ordinary least-square estimates was selected as a modeling strategy because it does not require assumptions of normality of data. For each neuropsychological outcome that related to >1 SVD predictor, a combined linear regression model was performed including all statistically significant SVD predictors for the selected outcome (with identical covariates) to determine whether SVD markers represent a common or unique pathologic pathway of small vessel injury. Finally, an increase in R2 between nested models was used to determine the combined variance explained by multiple SVD markers and unique variance explained by each individual SVD marker. Significance was set a priori at p < 0.05. Analyses were conducted with R version 3.2.3 (r-project.org).
Data availability
Because of participant consent restrictions in data sharing, a subset of data are available to others for purposes of reproducing the results or replicating procedures. These data, analytic methods, and study materials can be obtained by contacting the corresponding author.
Results
Participant characteristics
Participants included 327 adults 60 to 92 years (73 ± 7 years) of age; 59% were male; and 87% identified as non-Hispanic white. Mean time elapsed between neuropsychological testing and MRI acquisition was 3.3 ± 17.9 days. WMH volume ranged from 0.04 to 194.2 cm3. PVS scores ranged from 2 to 5, with 27% of participants receiving a score of 5. CMBs ranged from 0 to 100, with ≤12 CMBs present in 98% of participants. Lacunes ranged from 0 to 13. Table 1 provides details.
SVD marker correlations and co-occurrence
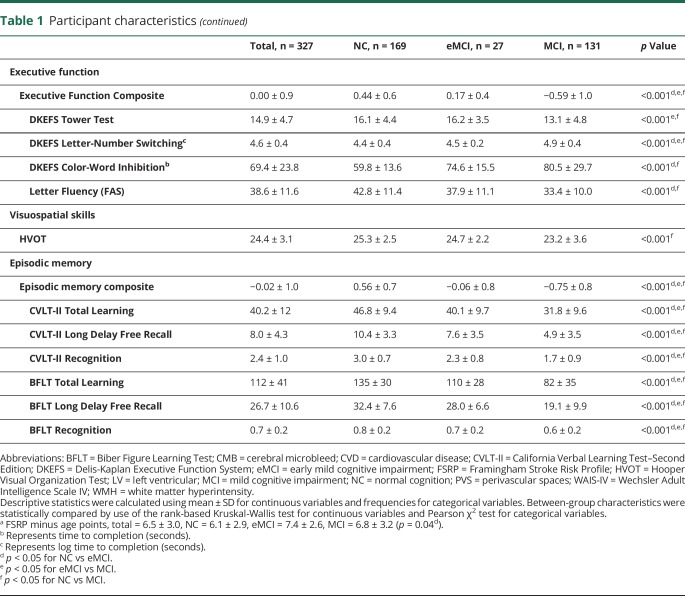
Log-WMH were associated with PVS (r = 0.30, p < 0.001), CMBs (r = 0.25, p < 0.001), and lacunes (r = 0.38, p < 0.001). PVS were associated with CMBs (r = 0.13, p = 0.02) and lacunes (r = 0.32, p < 0.001). CMBs were associated with lacunes (r = 0.24, p < 0.001). Co-occurrence of SVD marker burden suggested that high WMH burden corresponded to higher PVS score, presence of CMBs, and presence of lacunes (p < 0.01 for all). Greater PVS burden corresponded to greater WMH and presence of lacunes (p < 0.001 for both) but not presence of CMBs (p = 0.75). Presence of CMBs corresponded to higher WMH burden and presence of lacunes (p < 0.001 for both) but not PVS score (p = 0.63). Presence of lacunes corresponded to greater WMH, higher PVS score, and presence of CMBs (p < 0.001 for all). Table 2 provides details.
Table 2.
SVD marker frequency
WMH and neuropsychological outcomes
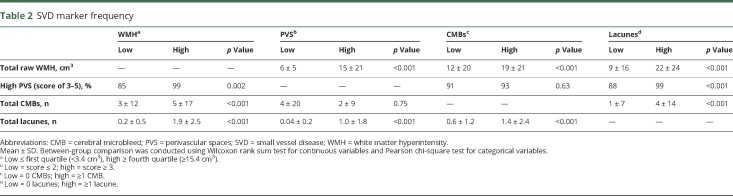
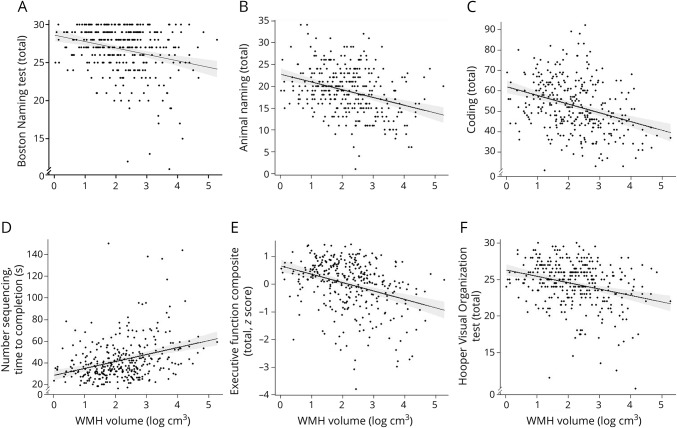
Increased log-WMH were associated with worse Boston Naming Test (β = −0.47, p = 0.009), Animal Naming (β = −0.90, p = 0.002), Coding (β = −2.13, p = 0.002), Number Sequencing (β = 3.09, p = 0.006), and Hooper Visual Organization Test (HVOT; β = −0.50, p = 0.006) performances. Increased log-WMH related to worse Executive Function Composite performance (β = −0.16, p < 0.001).
Examining the individual tests making up the Executive Function Composite showed that increased log-WMH related to worse performance on Letter-Number Switching (β = 0.07, p = 0.002) and Color-Word Inhibition (β = 3.81, p = 0.006). An association emerged between log-WMH and California Verbal Learning Test, 2nd edition Long Delay Free Recall but did not reach the a priori threshold for significance (β = −0.37, p = 0.08). Table 3 provides details, and figure 2 gives illustrations. Models were repeated excluding outliers with similar results.
Table 3.
SVD neuroimaging markers and neuropsychological outcomes
Figure 2. WMH and neuropsychological outcomes.
(A–F) Solid black line reflects unadjusted values of neuropsychological outcomes (y-axis) corresponding to log–white matter hyperintensity (WMH) volume (x-axis). Shading reflects 95% confidence interval.
PVS and neuropsychological outcomes
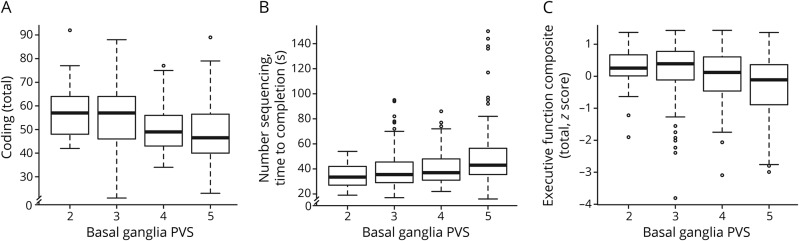
PVS related to worse Coding (β = −6.98, p = 0.02) and Number Sequencing (β = 10.65, p = 0.005) performances. PVS also related to worse Executive Function Composite performance (β = −0.41, p = 0.004).
In an examination of the individual tests making up the Executive Function Composite, PVS related to worse Color-Word Inhibition performance (β = 11.91, p = 0.008) (table 3 and figure 3). To interpret the magnitude of these associations, Color-Word Inhibition is used as an example in which total score was lower, on average, by 11.9 points for an increase in PVS score of 1 point, a magnitude similar to the mean increase in Color-Word Inhibition seen with 15 years of advanced age. Associations also emerged for Letter-Number Switching (β = 0.16, p = 0.06), HVOT (β = −1.45, p = 0.07), and Biber Figure Learning Test Recognition (β = −0.11, p = 0.06) but did not reach the a priori significance threshold. Models were repeated excluding outliers with similar results.
Figure 3. PVS and neuropsychological outcomes.
(A–C) Box reflects unadjusted values of neuropsychological outcomes (y-axis) corresponding to perivascular spaces (PVS) score (x-axis). Bar reflects 95% confidence interval.
CMBs and neuropsychological outcomes
CMBs related to worse Executive Function Composite performance (β = −0.01, p = 0.007). In models examining the individual tests making up the composite, CMBs were associated only with worse Letter-Number Switching performance (β = 0.005, p = 0.002, table 3). Models were repeated excluding outliers with similar results.
Lacunes and neuropsychological outcomes
Lacunes were associated with worse performance on Number Sequencing (β = 1.13, p = 0.04) and HVOT (β = −0.18, p = 0.047). Lacunes also related to worse Executive Function Composite performance (β = −0.06, p = 0.01).
Examination of the individual tests making up the Executive Function composite showed that lacunes were associated only with worse Letter-Number Switching performance (β = 0.02, p = 0.02; table 3). Models were repeated excluding outliers with similar results.
SVD markers as competing predictors of neuropsychological outcomes
One composite (i.e., Executive Function Composite) and 5 individual neuropsychological measures (i.e., Coding, Number Sequencing, Letter-Number Switching, Color-Word Inhibition, and HVOT) had >1 SVD marker as a predictor of cognitive performance. To determine whether the SVD markers represent a common pathologic pathway of small vessel injury, a linear regression model was fitted for each of the neuropsychological outcomes with all statistically significant SVD predictors included for that particular outcome.
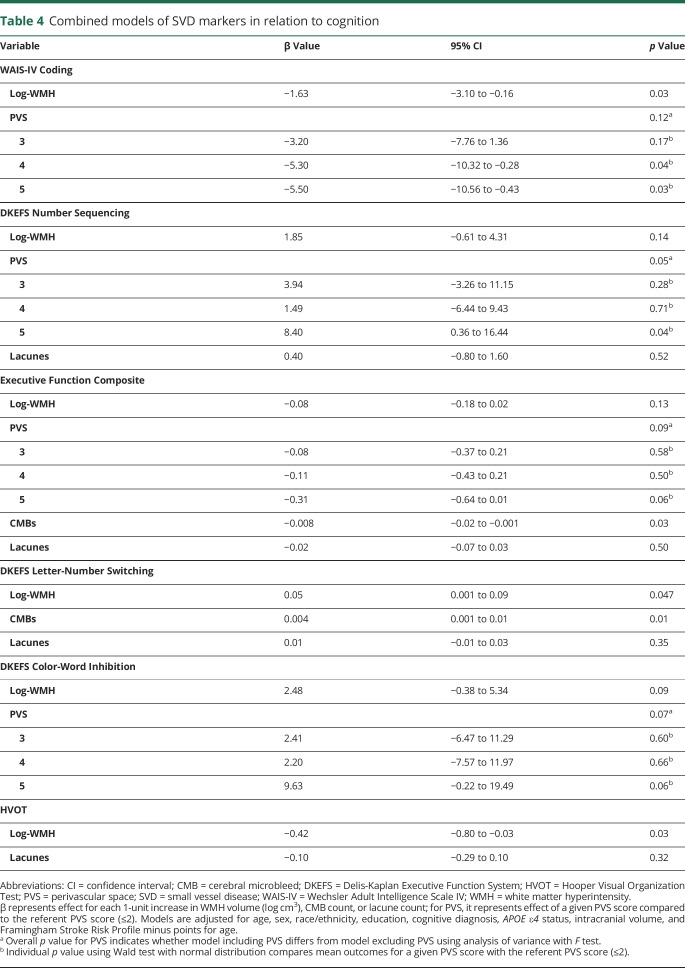
For Coding, the 2 highest PVS stages (p < 0.04 for both) and log-WMH (p = 0.03) persisted in their association with performance. To compare the effect size of associations across SVD markers, a change in R2 analysis was generated for the competing models. For Coding, the addition of log-WMH contributed 1.9% of variance beyond covariates, and the addition of PVS contributed 2.2%. Together, WMH and PVS contributed 3.1% of variance beyond covariates.
For Number Sequencing, the highest PVS stage (p = 0.04) persisted in its association, but results modeling log-WMH and lacunes were attenuated (p > 0.14 for all). The addition of log-WMH contributed 1.7% of variance beyond covariates, while PVS contributed 2.9% and lacunes contributed 0.9%. Combined, these 3 markers contributed 3.7% of variance beyond covariates.
For the Executive Function Composite, CMBs (p = 0.03) persisted in their association with performance. Associations emerged for the highest PVS stage (p = 0.06) and PVS overall (p = 0.09) but did not reach the a priori threshold for significance. Log-WMH and lacunes results were attenuated (p ≥ 0.13 for both). When PVS were placed in a model with CMBs or lacunes separately (2 predictors in each model), the association between PVS and performance persisted (p < 0.03). When examining the change in R2, log-WMH contributed 2.0%, PVS contributed 2.1%, microbleeds contributed 1.3%, and lacunes contributed 1.0% of variance beyond covariates. Combined, all markers contributed 4.0% of variance.
For Letter-Number Switching, log-WMH (p = 0.047) and CMBs (p = 0.01) persisted in their association with performance, but results for lacunes were attenuated (p = 0.35). In an examination of the change in R2, the addition of log-WMH contributed 1.7%, microbleeds contributed 1.7%, and lacunes contributed 0.9% of variance beyond covariates. Together, they contributed 3.0% of variance. For Color-Word Inhibition, PVS (p = 0.07) and log-WMH results were attenuated (p = 0.09) with no predictor related to performance. The addition of log-WMH contributed 1.8% and PVS contributed 2.8% of variance beyond covariates. Together, they contributed 3.5% of variance.
For HVOT, log-WMH (p = 0.03) persisted in their association with performance, but results for lacunes were attenuated (p = 0.32). The addition of log-WMH contributed 1.7% and lacunes contributed 0.9% of variance beyond covariates. Together, they contributed 2.0% of variance. Table 4 gives competing marker details.
Table 4.
Combined models of SVD markers in relation to cognition
Discussion
Among community-dwelling older adults free of clinical dementia and stroke, SVD markers, especially WMH, PVS, and lacunes, relate to worse cognitive performance. PVS appear especially clinically meaningful in that PVS demonstrated the largest contribution to variance in cognitive performance in several combined models testing multiple SVD predictors. Furthermore, combined models offer novel evidence suggesting that WMH, PVS, and CMBs affect cognition in this cohort through unique small vessel injury pathways compared to lacunes. Collectively, these results are statistically independent of shared vascular risk factors, CVD, and atrial fibrillation, adding to growing evidence that SVD is an important public health issue that adversely affects cognitive aging.
A primary observation from this study is that PVS related to numerous information processing speed and executive function outcomes. The maximum magnitude of these associations was equivalent to 15 years of advancing age, a clinically meaningful effect. The robust associations and large effect sizes for a given increase in PVS score reported here may be due to the anatomic specificity of critical watershed areas implicated in PVS. That is, higher scores are assigned for PVS in the caudate falling along the border between the anterior cerebral and lenticulostriate blood supply.27 Thus, higher PVS burden may represent SVD and related tissue damage in this vulnerable region with important implications for information processing speed and executive function skills.28 Furthermore, of the 4 competing models evaluating multiple SVD predictors in which PVS were included, PVS remained a predictor in 2 of 4 models. In a third model, an association was noted for PVS, but it did not meet our a priori significance threshold. One way in which PVS may be unique from other SVD markers is that PVS are thought to reflect fluid extravasation from increased intraluminal pressure and pulsatility29 or ex vacuo dilation.30 While WMH and PVS are both thought to relate to arteriolosclerosis, they do not consistently co-occur on MRI-histologic correlation.1 Therefore, PVS may consequently represent sequelae of increased arterial pulsatility, whereas WMH may reflect hypoperfusion from arterial thickening and decreased permeability.1 Despite being previously considered a benign imaging finding,31 PVS have been linked to both chronic hypertension when in the basal ganglia and cerebral amyloid angiopathy when present in the white matter.32 Results presented here suggest that the presence of basal ganglia PVS on neuroimaging may have critical relevance to cognitive health (especially information processing speed and executive function) before the onset of clinical dementia or stroke, through pathologic mechanisms distinct from other SVD markers. Additional research is needed to replicate and advance these findings.
As expected and consistent with prior work,33 WMH related to more cognitive measures, including language, information processing speed, executive function, and visuospatial skills, compared to all other SVD markers. Such a pervasive association between WMH and multiple cognitive domains may be accounted for by compromised white matter integrity in cortical-subcortical circuits critical to cognition and behavior.28,34 In competing models in which WMH were tested head to head with other SVD markers predictive of performance on the same cognitive measure, WMH contributed uniquely to information processing speed. This observation may be due to the known relation of WMH in frontal-subcortical circuits to worse information processing speed.35 However, in combined models of Executive Function Composite and visuospatial measures, the associations between WMH and cognition were attenuated. These attenuated results may be due to WMH representing heterogeneous pathology ranging from mild gliosis to frank tissue necrosis,30 some of which may overlap with other SVD markers such as lacunes. Collectively, these findings suggest that while WMH may have some overlap with other SVD markers, they are not simply a surrogate of the same pathophysiology of other SVD markers but also appear to have a distinct mechanism of injury.
The remaining SVD markers (CMBs and lacunes) had fewer associations with cognition than expected given the existing literature.36,37 First, lacunes related to information processing, executive function, and visuospatial performances. However, when included in competing models with other statistically significant SVD predictors (including WMH, PVS, and CMBs), lacunes no longer statistically contributed to any cognitive performances. The prevalence of lacunes in the current study is similar to that in population-based cognitively normal aging adult cohorts,36 but relative to other SVD markers examined here, the lacune burden is less common. While prior literature suggests that lacunes relate to executive function and memory,38 we found minimal associations between lacunes and cognition, presumably due to our cognitively normal or only mildly impaired cohort. We did observe that participants with mild cognitive impairment had significantly more lacunes than participants with normal cognition, which aligns with studies in more cognitively compromised cohorts and suggests that lacunes relate to cognition in the setting of greater symptoms.39 Lacunes tend to co-occur with WMH,4 and given the attenuation of lacunes in combined models with WMH, it is plausible that in cohorts with little to no cognitive impairment, WMH may be more predictive of cognition than lacunes. These 2 SVD markers may reflect a shared pathway of injury, representing different sequelae (hypoperfusion in WMH vs vessel occlusion in lacunes) of the same underlying process of arteriolosclerosis.1,2 While this common arteriosclerosis pathway may also relate to the presence of PVS (as mentioned above) and SVD markers have been demonstrated to form a unitary imaging construct,40 the current study suggests that PVS contribute uniquely to cognition through either distinct vessel pathology or downstream effects of earlier insults. Regardless of the pathway of injury, the frequent associations of PVS with cognition in the current study highlight that in individuals with little cognitive impairment, PVS (in addition to WMH) may hold higher clinical relevance than lacunes. Future research using longitudinal data would be beneficial to investigate the possibility of a causal chain between SVD markers.
Second, CMBs were related to only the Executive Function Composite with follow-up models implicating Letter-Number Switching performance specifically. In a combined model examining Letter-Number Switching, CMBs remained the only predictor. Although the effect size was modest, these results suggest that CMBs might contribute a unique pathway of vascular injury to cognition separate from WMH, PVS, and lacunes. Cortical and subcortical CMBs have been associated with worse executive function, presumably through injury to frontal-subcortical circuits, not unlike the pathway by which WMH affect cognition.12 However, it is possible that CMBs also relate uniquely to cognition through a strategic location effect,12 given that set shifting and sequencing behaviors are associated with specific brain regions.41 The clinical implications of the anatomic distributions of SVD should be investigated further. It is noteworthy that more frequent cognitive associations have been reported with CMBs in other population-based studies.37,42 The discrepancy between prior findings and the current results might be expected if the presence of additional SVD burden or other brain pathology in the general population modifies relations between CMBs and cognition. Replication of and further inquiry into the significance of CMBs in different populations are warranted.43
Finally, it is interesting that none of the SVD markers related to episodic learning or memory performance. The prevalence of SVD in our cohort is similar to that of other community-based studies,44 suggesting that the lack of association between SVD and memory is not due to a low SVD burden in the present sample. Among older adults without dementia, SVD markers tend to more strongly relate to information processing speed and executive function skills than episodic memory,45 and our findings are aligned with this prior work. It is plausible that among individuals free of clinical dementia or prior cerebrovascular events such as the current cohort, SVD markers may not have a consistent, clinically relevant association with episodic memory functions. Further inquiry is warranted.
Our study has several strengths, including a reliable neuroimaging technique for quantifying WMH; use of a board-certified neuroradiologist for manual coding of PVS, CMBs, and lacunes; stringent quality control procedures for neuroimaging and neuropsychological methods; and use of a core imaging laboratory with clinically blinded readers. However, several limitations should be considered. Cross-sectional results cannot address causality. Multiple comparisons raise the possibility of a false-positive finding. Participants were well educated, were racially/ethnically homogeneous, and had less CVD burden than the general population, limiting generalizability. The relative healthier nature of the cohort may have affected the extent of SVD pathology present in the cohort, thus limiting possible associations with cognition. We hypothesize that among a cohort with more medical or cerebrovascular comorbid conditions, the associations reported here might be amplified. In addition, given the low burden of vascular risk in this cohort, future research is needed to formally examine the effect of vascular risk or prevalent CVD on the relationship between SVD and cognition. Manual scoring of PVS, CMBs, and lacunes was also performed by a single neuroradiologist. Finally, there is no gold standard PVS measurement. The method used here considers both PVS frequency and location using proposed SVD pathophysiology,24,46 with PVS further along the penetrating arteries assigned a higher (worse) score. This method also aligns with SVD imaging standards put forth by an international working group.25 While the current study did not assess PVS in other regions such as white matter, in similar cohorts and those with symptomatic stroke, basal ganglia PVS have related to cognition while white matter PVS have not,15 and white matter PVS have been associated with cerebral amyloid angiopathy.47 However, future research should ascertain best practices for quantifying PVS and the significance of regional differences in PVS.
The current results offer a more integrated understanding of underlying SVD changes in the context of cognitive aging. PVS appear to be a clinically meaningful marker of SVD affecting cognition in older adults without dementia. While it is well known that WMH relate to cognition, WMH, PVS, and CMBs may contribute unique pathways of cerebrovascular injury in this population. Future research should examine anatomic patterns of SVD markers and longitudinally characterize SVD marker progression in relation to cognitive decline to determine whether these cross-sectional associations have implications longitudinally.
Acknowledgment
The authors thank the dedicated Vanderbilt Memory & Aging Project participants, their loved ones, and the devoted staff and trainees who contributed to recruitment, screening, and enrollment of the cohort.
Glossary
- CMB
cerebral microbleed
- CVD
cardiovascular disease
- DKEFS
Delis-Kaplan Executive Function System
- FLAIR
fluid-attenuated inversion recovery
- HVOT
Hooper Visual Organization Test
- PVS
perivascular spaces
- SVD
small vessel disease
- WMH
white matter hyperintensities
Footnotes
See page 551
Author contributions
B.S. Passiak: study concept and design, interpretation of data, manuscript composition. D. Liu: statistical analysis, provided intellectual input on final draft. H.A. Kresge and F.E. Cambronero: interpretation of data, drafted manuscript, provided intellectual input on final draft. K.R. Pechman: acquisition of data, interpretation of data, provided intellectual input on final draft. K.E. Osborn: interpretation of data, provided intellectual input on final draft. K.A. Gifford and T.J. Hohman: acquisition of data, interpretation of data, study supervision, provided intellectual input on final draft. M.S. Schrag: interpretation of data, provided intellectual input on final draft. L.T. Davis: analysis of data, interpretation of data, provided intellectual input on final draft. A.L. Jefferson: obtained study funding, study concept and design, acquisition of data, analysis and interpretation of data, study supervision, manuscript composition, provided intellectual input on final draft.
Study funding
Supported by Alzheimer's Association IIRG-08-88733 (A.L.J.), R01-AG034962 (A.L.J.), R01-HL111516 (A.L.J.), R01-AG056534 (A.L.J.), R01-NS100980 (A.L.J.), K24-AG046373 (A.L.J.), K23-AG030962 (A.L.J.), F32-AG058395 (K.E.O.), K01-AG049164 (T.J.H.), K12-HD043483 (K.A.G., T.J.H.), K23-AG045966 (K.A.G.), T32-MH064913 (F.E.C.), R25-GM062459 (F.E.C.), UL1-TR000445, S10-OD023680, and the Vanderbilt Memory & Alzheimer's Center.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Fazekas F, Kleinert R, Offenbacher H, et al. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol 1991;12:915–921. [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw JM. What is a lacune? Stroke 2008;39:2921–2922. [DOI] [PubMed] [Google Scholar]
- 3.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 2011;32:528–534. [DOI] [PubMed] [Google Scholar]
- 4.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol 1968;12:1–15. [DOI] [PubMed] [Google Scholar]
- 5.Kaffashian S, Soumaré A, Zhu YC, Mazoyer B, Debette S, Tzourio C. Long-term clinical impact of vascular brain lesions on magnetic resonance imaging in older adults in the population. Stroke 2016;47:2865–2869. [DOI] [PubMed] [Google Scholar]
- 6.Thijs V, Lemmens R, Schoofs C, et al. Microbleeds and the risk of recurrent stroke. Stroke 2010;41:2005–2009. [DOI] [PubMed] [Google Scholar]
- 7.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 8.Jokinen H, Gouw AA, Madureira S, et al. Incident lacunes influence cognitive decline: the LADIS study. Neurology 2011;76:1872–1878. [DOI] [PubMed] [Google Scholar]
- 9.van der Holst HM, van Uden IW, Tuladhar AM, et al. Factors associated with 8-year mortality in older patients with cerebral small vessel disease: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) study. JAMA Neurol 2016;73:402–409. [DOI] [PubMed] [Google Scholar]
- 10.Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging 2009;30:450–456. [DOI] [PubMed] [Google Scholar]
- 11.Tosto G, Zimmerman ME, Carmichael OT, Brickman AM. Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA Neurol 2014;71:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werring DJ, Frazer DW, Coward LJ, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain 2004;127:2265–2275. [DOI] [PubMed] [Google Scholar]
- 13.Edwards JD, Jacova C, Sepehry AA, Pratt B, Benavente OR. A quantitative systematic review of domain-specific cognitive impairment in lacunar stroke. Neurology 2013;80:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yakushiji Y, Charidimou A, Hara M, et al. Topography and associations of perivascular spaces in healthy adults: the Kashima Scan Study. Neurology 2014;83:2116–2123. [DOI] [PubMed] [Google Scholar]
- 15.Riba-Llena I, Nafria C, Mundet X, et al. Assessment of enlarged perivascular spaces and their relation to target organ damage and mild cognitive impairment in patients with hypertension. Eur J Neurol 2016;23:1044–1050. [DOI] [PubMed] [Google Scholar]
- 16.Matsusue E, Sugihara S, Fujii S, Ohama E, Kinoshita T, Ogawa T. White matter changes in elderly people: MR-pathologic correlations. Magn Reson Med Sci 2006;5:99–104. [DOI] [PubMed] [Google Scholar]
- 17.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 2000;14:224–232. [DOI] [PubMed] [Google Scholar]
- 18.Udaka F, Sawada H, Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann NY Acad Sci 2002;977:411–415. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 20.Aisen PS, Petersen RC, Donohue MC, et al. Clinical core of the Alzheimer's Disease Neuroimaging Initiative: progress and plans. Alzheimers Demen 2010;6:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kresge HA, Khan OA, Wegener MA, et al. Subclinical compromise in cardiac strain relates to lower cognitive performances in older adults. J Am Heart Assoc 2018;7:e007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferson AL, Gifford KA, Acosta LM, et al. The Vanderbilt Memory & Aging Project: study design and baseline cohort overview. J Alzheimers Dis 2016;52:539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 25.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication: the Framingham study. Stroke 1994;25:40–43. [DOI] [PubMed] [Google Scholar]
- 27.Wong EH, Pullicino PM, Benedict R. Deep cerebral infarcts extending to the subinsular region. Stroke 2001;32:2272–2277. [DOI] [PubMed] [Google Scholar]
- 28.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez J, Rundek T, Ekind MS, Sacco RL, Wright CB. Perivascular spaces are associated with atherosclerosis: an insight from the Northern Manhattan Study. AJNR Am J Neuroradiol 2013;34:1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly: a morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 1991;114:761–774. [DOI] [PubMed] [Google Scholar]
- 31.Jungreis CA, Kanal E, Hirsch WL, Martinez AJ, Moossy J. Normal perivascular spaces mimicking lacunar infarction: MR imaging. Radiology 1988;169:101–104. [DOI] [PubMed] [Google Scholar]
- 32.Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017;88:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041. [DOI] [PubMed] [Google Scholar]
- 34.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology 2006;66:217–222. [DOI] [PubMed] [Google Scholar]
- 35.Duering M, Gesierich B, Seiler S, et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology 2014;82:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey CL, Kramer JH, Josephson SA, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke 2008;39:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 2012;78:326–333. [DOI] [PubMed] [Google Scholar]
- 38.Benisty S, Gouw AA, Porcher R, et al. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry 2009;80:478–483. [DOI] [PubMed] [Google Scholar]
- 39.Mungas D, Jagust WJ, Reed BR, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology 2001;57:2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 2015;36:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry ME, McDonald CR, Hagler DJ, Jr., et al. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia 2009;47:2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding J, Sigurðsson S, Jónsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology 2017;88:2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charidimou A, Blacker D, Viswanathan A. Context is everything: from cardiovascular disease to cerebral microbleeds. Int J Stroke 2018;13:6–10. [DOI] [PubMed] [Google Scholar]
- 44.Pinter D, Enzinger C, Fazekas F. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J Neurol 2015;262:2411–2419. [DOI] [PubMed] [Google Scholar]
- 45.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol 2006;63:246–250. [DOI] [PubMed] [Google Scholar]
- 46.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 2007;50:1–13. [DOI] [PubMed] [Google Scholar]
- 47.Charidimou A, Meegahage R, Fox Z, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry 2013;84:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because of participant consent restrictions in data sharing, a subset of data are available to others for purposes of reproducing the results or replicating procedures. These data, analytic methods, and study materials can be obtained by contacting the corresponding author.