Abstract
Objective
To determine the association of insulin sensitivity and metabolic status with declining cognition in HIV-infected individuals.
Methods
We conducted targeted clinical and metabolic measures in longitudinal plasma samples obtained from HIV-infected patients enrolled in the Central Nervous System HIV Anti-Retroviral Therapy Effects Research Study (CHARTER). Findings were validated with plasma samples from the Multicenter AIDS Cohort Study (MACS). Patients were grouped according to longitudinally and serially assessed cognitive performance as having stably normal or declining cognition.
Results
Patients with declining cognition exhibited baseline hyperinsulinemia and elevated plasma c-peptide levels with normal c-peptide/insulin ratios, suggesting that insulin production was increased, but insulin clearance was normal. The association of hyperinsulinemia with worsening cognition was further supported by low high-density lipoprotein (HDL), high low-density lipoprotein/HDL ratio, and elevated cholesterol/HDL ratio compared to patients with stably normal cognition.
Conclusions
These findings suggest that hyperinsulinemia and impaired insulin sensitivity are associated with cognitive decline in antiretroviral therapy–treated HIV-infected patients.
HIV-associated neurocognitive disorder (HAND) encompasses 3 stages of neurocognitive impairments: asymptomatic neurocognitive impairment, mild neurocognitive disorder, and HIV-associated dementia, which reflect increasingly severe cognitive, motor, functional, and behavioral abnormalities.1,2 HAND frequently manifests as impairments in the domains of memory and executive function and is associated with progressive neurologic damage, including persistent glial and innate immune activation, brain volume loss, inflammation, synaptodendritic damage, and disruptions in white matter integrity.3–6 Although the underlying pathophysiologic mechanisms of antiretroviral therapy (ART)–insensitive HAND are not well understood, the neuropathologic changes coincide with shifts in brain energy use that appear early in the course of HAND and become progressively dysregulated with aging.7–9
Brain insulin signaling regulates energy metabolism, neuronal survival, cognition, memory, and learning.10,11 Impairments of insulin signaling are associated with cognitive decline and a more rapid onset and progression of neurodegenerative diseases.12,13 Both HIV infection and ART have been independently associated with an increased risk for developing metabolic abnormalities, including insulin resistance,14–16 which has been associated with increased risk for cognitive impairment.17,18 Insulin resistance is an impaired cellular response to insulin that results in increased insulin secretion from the pancreas and hyperinsulinemia. Insulin resistance is accompanied by reductions in insulin sensitivity, which is manifested by lipolysis of adipose tissue, release of fatty acids into the circulation, and subsequent storage in ectopic tissues. In this study, we sought to determine whether hyperinsulinemia and markers of insulin sensitivity were associated with changes of cognition in HIV-infected patients.
Methods
Patient selection and cognitive assessment
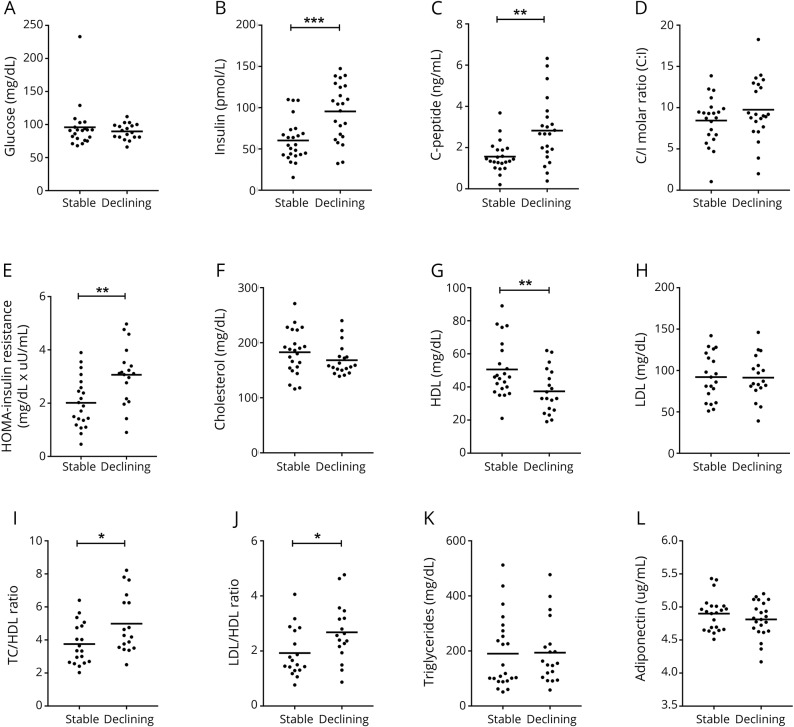
Plasma samples for the discovery phase of this study were obtained from the Central Nervous System HIV Anti-Retroviral Therapy Effects Research (CHARTER) case-control study after a systematic case review of 430 study participants. Patients with psychiatric comorbid conditions, current recreational drug use, and diabetic and cholesterol-lowering medications were excluded from this study. Changes in ART were permitted if only a single drug was changed within the same drug class.19 A neuropsychological testing battery covering 7 cognitive domains—executive function, learning, delayed recall, working memory, verbal fluency, speed of information processing, and motor skills3—was conducted at each of study visit interspersed by ≈6 months. The best available normative standards were used to convert the scores to demographically corrected standard scores (T scores), which correct for effects of age, education, sex, and ethnicity. The presence and severity of cognitive impairment were determined with the Global Deficit Score approach. A Global Deficit Score ≥0.5 was considered impaired.20 All follow-up visits were corrected for practice effects.21 Neurocognitive change was determined with a multivariable change score approach, as previously described.19,21 The z scores were generated for each of 15 neuropsychological test variables and then summed to provide a summary regression score. A summary regression change score from visit 1 to visit 2 was generated for each patient. Patients with a summary regression change distribution in the central 80% were defined as stable; the top 10% were defined as improving; and the bottom 10% were defined as declining.19 Patients were then selected on the basis of matching clinical and demographic characteristics, including age, education, sex, ethnicity, AIDS status, hepatitis C (HCV) serostatus, ART use, and duration of current ART regimen, as previously described.19,22 The discovery cohort included patients with stably normal (n = 24) and declining (n = 23) cognitive function with a first study visit between June 2006 and September 2007. Plasma samples from the validation phase of this study were obtained from 72 HIV-infected men (stably normal n = 39, declining cognition n = 33) enrolled in the Multicenter AIDS Cohort Study (MACS), who were selected from a systematic review of 600 individuals who participated in a case-control longitudinal neuropsychological test battery to assess the effects of HIV on the brain and nervous system between November 2002 and October 2007.23 Inclusion criteria were similar to those for CHARTER. MACS patients were matched to CHARTER patients on age, duration of HIV infection, HCV infection status, nadir CD4+ T-cell count, and plasma HIV-1 viral load (table 1). Fasting blood samples were collected from all participants in this study. Baseline plasma samples for the discovery and validation phases of this study were analyzed as prognostic indicators for longitudinal changes in cognition.
Table 1.
Baseline demographics of discovery (CHARTER) and validation (MACS) cohorts
Standard protocol approvals, registration, and patient consents
An institutional review board/ethical standards committee approved the use of human participants for this study at each performance site. Written informed consent was obtained from all study participants.19
Laboratory assessments
Clinical laboratory assessments of blood counts, metabolic and chemistry panels, HCV antibody, and flow cytometry for CD4+ T-cell count were performed at CHARTER and MACS sites.
Targeted plasma metabolite measurement
Measurements of plasma insulin, c-peptide, and adiponectin were conducted with commercial ELISA kits according to the manufacturer's instructions (EMD Millipore Corp, Billerica, MA).
Statistical analysis
Student t tests for continuous variables and χ2 tests for categorical variables were used to examine group differences. Logistic regression analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC).
Data availability
All data for the analyses in this report are available through the CHARTER and MACS websites at charternntc.org/and statepi.jhsph.edu/macs/macs.html.
Results
Patient characteristics in the discovery and validation cohorts
Patients with stably normal and declining cognition within the discovery cohort (CHARTER) were similar in age, education, ethnicity, HCV infection status, duration of ART regimen, and body mass index (BMI). Current and nadir CD4+ T-cell counts were lower in patients with declining cognition compared to patients with stably normal cognition (table 1). Patients with stably normal and declining cognitive in the validation (MACS) cohort were demographically and clinically similar (table 1) and were similar to the discovery cohort with the exceptions of higher education and higher current and nadir CD4+ T-cell counts compared to patients in the discovery cohort (table 1).
Evidence for impaired insulin sensitivity in HIV-infected patients with declining cognition
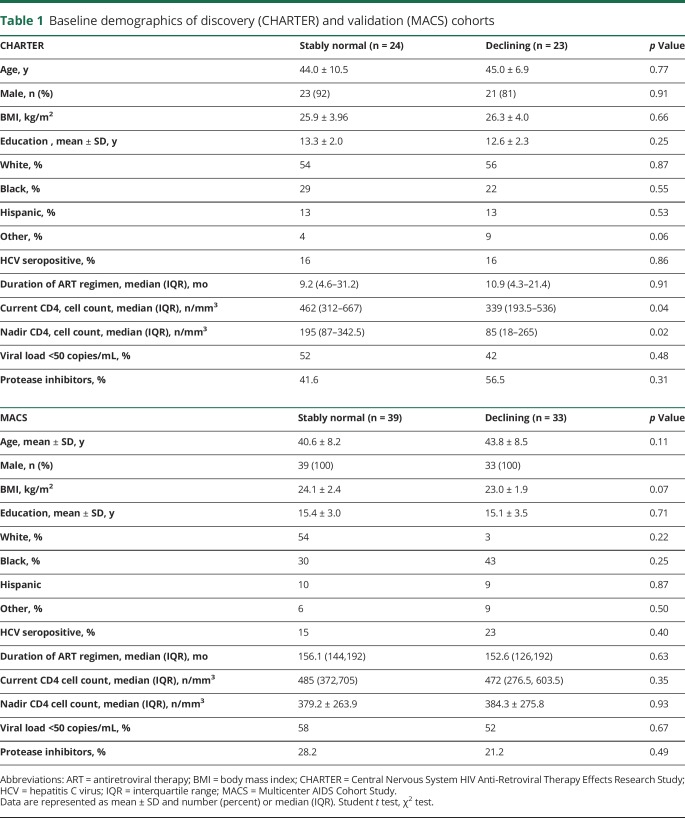
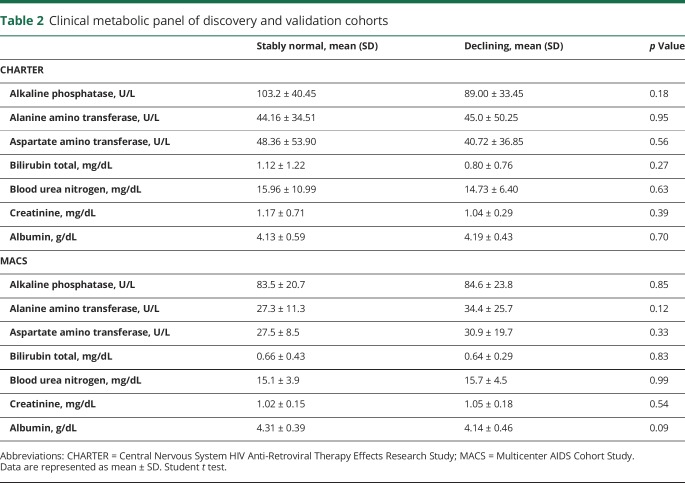
At baseline, there were no group differences in glucose levels. Insulin levels were elevated in patients with declining cognition compared to patients with stably normal cognition (95.5 ± 34.6 vs 60.3 ± 25.2 pmol/L) (figure 1, A and B). C-peptide levels were higher in patients with declining cognition compared to patients with stably normal cognition (2.8 ± 1.6 vs 1.5 ± 0.74 ng/mL) (figure 1C). C-peptide/insulin ratios were similar in patients with declining cognition and stably normal cognition (figure 1D), suggesting normal liver clearance of insulin in both groups. Indeed, metabolic measures of kidney and liver function (creatinine, albumin, bilirubin, alkaline phosphatase, and electrolytes) were within normal ranges in both groups (table 2). The homeostatic model assessment of insulin resistance (HOMA-IR) revealed higher HOMA-IR levels in patients with declining cognition compared to patients with stably normal cognition (3.0 ± 1.1 vs 2.0 ± 0.9) (figure 1E). A clinical lipoprotein panel showed no significant differences in total cholesterol (TC) and low-density lipoprotein (LDL) cholesterol between patients with declining cognition and stably normal cognition (figure 1, F and H). High-density lipoprotein (HDL) cholesterol levels were lower in patients with declining cognition compared to patients with stably normal cognition (figure 1G). Cholesterol ratios of LDL/HDL and TC/HDL were elevated at baseline in patients with declining compared to stably normal cognition (figure 1, J and I). Adiponectin levels were similar (figure 1L). In aggregate, these results provide evidence that impaired insulin sensitivity precedes cognitive decline in HIV-infected patients. A multivariable logistic regression model showed that BMI and protease inhibitor (PI) use were not predictors of cognition. Insulin was the only predictor of cognitive status (p = 0.003). A 10-point increase in insulin levels was associated with 1.44 greater odds of having declining vs normal cognition (95% confidence interval [CI] 1.14–1.83). Insulin explained 31% of the variance. A stepwise logistic regression model showed that current CD4+ cells but not nadir CD4+ T cells were a significant predictor of cognitive status (0.80, 95% CI 0.65–0.99, p = 0.04 per 50-unit increase) and explained 14% of the variance. We performed a multivariable logistic regression analysis including both current CD4+ T cells and insulin as predictor variables and cognitive status as the outcome measure. In this model, insulin was the only significant predictor of cognitive status (1.43, 95% CI 1.13–1.8, p = 0.003 per 10-unit increase) compared to current CD4+ T cells (p = 0.14). This model explained 41% of the variance. Specifically, insulin explained 27% of the variance. When nadir and current CD4+ T cells were forced into the model, neither was significant, and insulin remained a significant predictor of cognitive status (p = 0.003).
Figure 1. Evidence for impaired insulin sensitivity in a discovery cohort of HIV-infected patients with declining cognition (CHARTER cohort).
Plasma levels of (A) glucose, (B) insulin, (C) c-peptide, (D) c-peptide/insulin (C/I) molar ratio, (E) homeostatic model assessment (HOMA) of insulin resistance, (F) total cholesterol (TC), (G) high-density lipoprotein (HDL), (H) low-density lipoprotein (LDL), (I) TC/HDL ratio, (J) LDL/HDL ratio, (K) triglycerides, and (L) adiponectin. Individual data points from each patient and group means are shown. CHARTER = Central Nervous System HIV Anti-Retroviral Therapy Effects Research Study. Student t test, *p < 0.05, **p < 0.01, ***p < 0.001.
Table 2.
Clinical metabolic panel of discovery and validation cohorts
Validation of impaired insulin sensitivity in a second cohort of HIV-infected patients with declining cognition
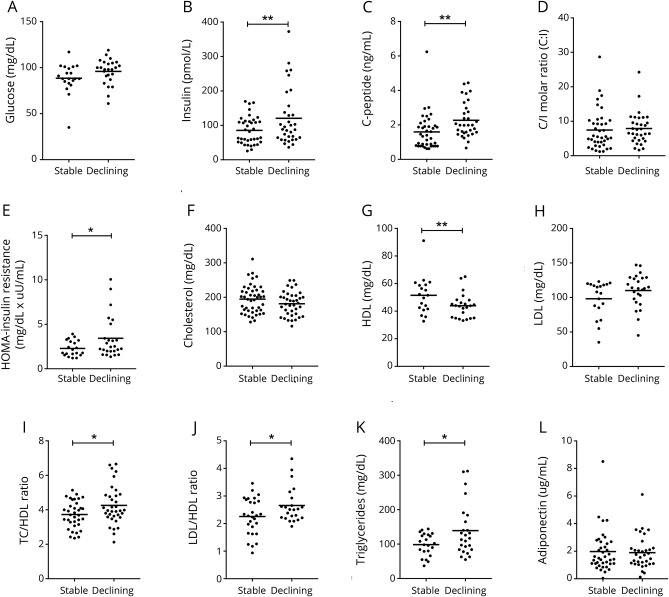
We next sought to confirm that hyperinsulinemia and impaired insulin sensitivity were associated with worsening cognition in an independent set of plasma samples obtained from the MACS cohort. All metabolic results observed in the discovery cohort were validated in the MACS cohort with the single exception that triglyceride levels that were higher in patients with declining cognition compared to stably normal patients in MACS (figure 2K; no difference was apparent in CHARTER). There were no differences in glucose levels between stably normal and declining patients. Insulin levels were elevated in patients with declining cognition compared to those with stably normal cognition (120.5 ± 82.3 vs 85.5 ± 40.0 pmol/L). C-peptide levels were higher in patients with declining cognition compared to patients with stably normal cognition (2.3 ± 1.0 vs 1.6 ± 1.0 ng/mL), and c-peptide/insulin ratios were similar in patients with declining cognition and stably normal cognition (figure 2, A–D). Kidney function and liver function were within normal ranges in patients with declining cognition and stably normal cognition (table 2). HOMA-IR levels in patients with declining cognition were higher compared to patients with stably normal cognition (3.4 ± 2.4 vs 2.2 ± 0.86) (figure 2E). HDL levels were lower in patients with declining cognition compared to those with stably normal cognition with no differences in TC and LDL (figure 2, G and H). LDL/HDL and TC/HDL ratios were higher in patients with declining cognition compared to patients with stably normal cognition (figure 2, J and I). Adiponectin levels were similar among patients with declining cognition and stably normal cognition (figure 2L). A multivariable logistic regression controlling for BMI and PI did not change the pattern of associations between insulin levels and cognitive status (p = 0.04). Although insulin was a significant predictor of cognitive status in CHARTER, it was not significant in MACS (p = 0.057). This could be due to differences in sample size and clinical and demographic characteristics of the 2 cohorts. A 10-point increase in insulin levels was associated with 1.08 greater odds of having declining cognition vs being stably normal (95% CI 0.99–1.17). Insulin explained ≈8% of the variance.
Figure 2. Evidence for impaired insulin sensitivity in a validation cohort of HIV-infected patients with declining cognition (MACS cohort).
Plasma levels of (A) glucose (B) insulin, (C) c-peptide, (D) c-peptide/insulin (C/I) molar ratio, (E) homeostatic model assessment (HOMA) of insulin resistance, (F) total cholesterol (TC), (G) high-density lipoprotein (HDL), (H) low-density lipoprotein (LDL), (I) TC/HDL ratio, (J) LDL/HDL ratio, (K) triglycerides, and (L) adiponectin. Individual data points from each patient and group means are shown. MACS = Multicenter AIDS Cohort Study. Student t test, *p < 0.05, **p < 0.01.
Discussion
Our data show that impaired insulin sensitivity in ART-using HIV-infected individuals precedes declining cognitive function. As a group, HIV-infected individuals with declining cognition exhibited an elevated HOMA-IR index, hyperinsulinemia, and elevated levels of c-peptide and triglycerides with decreased HDL at baseline. C-peptide/insulin ratios were normal, suggesting that insulin production was increased, but insulin clearance was not impaired. Although differences in insulin levels were not significant in the validation cohort, other markers of insulin resistance were present in both cohorts These validated observations suggest that interventions targeting insulin signaling could be useful therapeutic strategies, especially in individuals who exhibit progressive decline in cognitive function.
Brain insulin regulates feeding behavior, body weight homeostasis, mood, neuronal development, neuronal growth and survival, synaptic stability, dendritic arborization, neural circuitry, learning, and memory.24–27 These diverse effects are regulated through the insulin receptor, which is widely expressed in brain with high expression in the hypothalamus, hippocampus, and cerebral cortex.26,28 The insulin receptor possesses intrinsic tyrosine kinase activity that is activated in a dose-dependent manner by insulin binding. This tyrosine kinase activity leads to the phosphorylation of the insulin receptor substrate protein family, which in turn activates the phosphoinositide 3-kinase/Akt and extracellular signal-regulated kinase/mitogen-activated protein kinase signaling cascades, which target multiple downstream effectors, including the mammalian target of rapamycin complex 1, glycogen synthase kinase-3β, and the FOXO family of transcription factors.
The brain has a very high energy demand that requires rapid regional shifts in glucose and lactate uptake to adapt metabolism to neural activity (see review29). This energy source is provided primarily by glucose or lactate that is shuttled from astrocytes to neurons to support glycolytic and oxidative metabolism.30 Astrocyte uptake of peripheral glucose occurs primarily through glucose transporter (GLUT) 1 and GLUT3 glucose transporter proteins in endothelial cells. Insulin is transported from the periphery into the brain, although the mechanisms for insulin transport into the brain are less clear but appear to involve a saturable transport system31 that requires insulin binding to insulin receptors in brain endothelium.32,33 Abnormalities in insulin sensitivity downregulate both endothelial GLUT transporters and insulin receptors34 with consequent reductions in brain uptake of glucose and insulin.35,36 Reduced insulin sensitivity, hyperinsulinemia, and downregulated endothelial expression of GLUT and insulin receptors have been implicated as contributing to the progression of neurodegenerative diseases such as Alzheimer disease12,37 and Parkinson disease.38 Our data suggest that hyperinsulinemia in HIV-infected patients is associated with progressive cognitive impairment. Indeed, several decades of research has shown that insulin has multiple actions in the brain that may regulate many of the same neural pathways perturbed by HIV infection. Neuronal injury, glial activation, persistent low levels of inflammation, and perturbations in energy metabolism are pathologic hallmarks of cognitive impairment that can persist in combination ART–adherent, virologically controlled patients (see review39). In the present study, we found that hyperinsulinemia and reduced insulin sensitivity were prognostic indicators for cognitive decline in 2 independent cohorts of HIV-infected patients. HIV infection and ART are well known to cause alterations in lipid distribution, glucose homeostasis, and energy metabolism, which are associated with metabolic syndrome and insulin resistance.40–42 HIV-infected individuals are at greater risk for hyperinsulinemia, impaired insulin sensitivity, and dyslipidemia compared to uninfected individuals,43,44 and fluorodeoxyglucose-PET studies have demonstrated lower cerebral glucose consumption in HIV-infected patients in the absence of structural abnormalities on MRI.2 In the present study, we found that plasma levels of insulin, c-peptide, HOMA-IR index, and HDL/LDL and TC/HDL ratios were higher at baseline in HIV-infected patients with declining cognition compared to patients with stably normal cognition. The HOMA-IR is a strong index for the assessment of insulin resistance that takes the relationship between fasting glucose and insulin into account, making it a more informative measure of insulin resistance than plasma insulin and glucose levels alone.45 Although the BMIs of HIV-infected patients were on the high end of the normal range, diabetic patients were excluded from the study, and there were no group differences in ART regimens. Thus, obesity, frank diabetes, and differences in ART regimens were not likely to be responsible for the observed interactions of impaired insulin sensitivity with worsening cognitive function. From a clinical standpoint, this is arguably the most important group to identify because individuals showing evidence of neurologic worsening may be targeted for specific adjunctive therapies or more regular neurologic follow-up.
When insulin resistance is defined in the general population, the risk factors of increased BMI, visceral fat, and age are considered. Several additional factors may contribute to the risk of developing insulin resistance in HIV-infected patients such as duration of HIV infection, combination ART regimen, abnormalities in lipid metabolism, and HIV/HCV coinfection.43,46 Before the advent of ART, low blood levels of TC, LDL, and HDL with elevated levels of triglycerides were reported in HIV-infected patients, suggesting that viral infection itself can produce abnormalities in lipid metabolism and insulin sensitivity.43 ART regimens, notably PIs and nucleoside reverse transcriptase inhibitors, are known to further increase the risk for insulin resistance in HIV-infected individuals as a result of the mitochondrial toxicity of nucleoside reverse transcriptase inhibitors– and PI-associated blockade of glucose transport.47,48
Several potential shortcomings of the study should be noted. Patients with declining cognition had lower CD4+ T-cell counts compared to patients with stably normal cognition. However, a multivariable logistic regression model demonstrated that BMI, PI use, and CD4+ T-cell counts were not predictors of cognitive decline. Elevated plasma insulin was a significant predictor of cognitive decline. Although a thorough and careful multidisciplinary selection process was applied to our study to increase confidence in the group assignments, we believe that the generalizability of this data may be restricted by the clinical and demographic characteristics used to match patients in the current study. Individuals assessed in the period from 2002 to 2007 may not generalize to contemporary populations with HIV in the United States. Although we validated primary results in a second cohort of patients, the sample size was limited. The discovery cohort (CHARTER) included very few women, and MACS is a cohort of all gay/bisexual men. Clinical data and plasma samples were obtained between 2002 and 2007. As ART regimens continue to evolve, newer therapies may have less of an impact on insulin resistance. Socioeconomic status and World Health Organization/Centers for Disease Control and Prevention stage data were not controlled for in the present study.
Our data suggest that hyperinsulinemia and abnormalities in lipid and cholesterol metabolism are important factors associated with worsening cognition in HIV-infected patients. Interventions that restore brain insulin sensitivity could be useful therapies for HIV-associated impairments in cognition.
Acknowledgment
The CHARTER group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; and Washington University, St. Louis; is headquartered at the University of California, San Diego; and includes the following: director: Igor Grant, MD; codirectors: Scott L. Letendre, MD, Ronald J. Ellis, MD, PhD, Thomas D. Marcotte, PhD; center manager: Donald Franklin, Jr; neuromedical component: Ronald J. Ellis, MD, PhD (principal investigator [PI]), J. Allen McCutchan, MD; laboratory and virology component: Scott Letendre, MD (co-PI), Davey M. Smith, MD (co-PI); neurobehavioral component: Robert K. Heaton, PhD (PI), J. Hampton Atkinson, MD, Matthew Dawson; imaging component: Christine Fennema-Notestine, PhD (PI), Michael J. Taylor, PhD, Rebecca Theilmann, PhD; data management component: Anthony C. Gamst, PhD (PI), Clint Cushman; statistics component: Ian Abramson, PhD (PI), Florin Vaida, PhD; Johns Hopkins University site: Ned Sacktor (PI), Vincent Rogalski; Icahn School of Medicine at Mount Sinai site: Susan Morgello, MD (Co-PI), and David Simpson, MD (Co-PI), Letty Mintz, NP; University of California, San Diego site: J. Allen McCutchan, MD (PI); University of Washington, Seattle site: Ann Collier, MD (Co-PI) and Christina Marra, MD (co-PI), Sher Storey, PA-C; University of Texas, Galveston site: Benjamin Gelman, MD, PhD (PI), Eleanor Head, RN, BSN; and Washington University, St. Louis site: David Clifford, MD (PI), Muhammad Al-Lozi, MD, Mengesha Teshome, MD. MACS with centers at Baltimore (U01-AI35042): Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Adrian Dobs, Michelle Estrella, W. David Hardy, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr, Sudhir Penugonda, John P. Phair, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (PI), Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Lawrence A. Kingsley, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D'Souza (PI), Alison Abraham, Keri Althoff, Michael Collaco, Priya Duggal, Sabina Haberlen, Eithne Keelaghan, Heather McKay, Alvaro Muñoz, Derek Ng, Anne Rostich, Eric C. Seaberg, Sol Su, Pamela Surkan, Nicholas Wada. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute of Mental Health. Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute and the National Institute on Deafness and Communication Disorders. MACS data collection is also supported by UL1-TR001079 (Johns Hopkins Institute for Clinical and Translational Research) from the National Center for Advancing Translational Sciences, a component of the NIH, and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH, Johns Hopkins Institute for Clinical and Translational Research, or National Center for Advancing Translational Sciences. The MACS website is located at aidscohortstudy.org/.
Glossary
- ART
antiretroviral therapy
- BMI
body mass index
- CHARTER
Central Nervous System HIV Anti-Retroviral Therapy Effects Research
- CI
confidence interval
- GLUT
glucose transporter
- HAND
HIV-Associated Neurocognitive Disorders
- HCV
hepatitis C virus
- HDL
high-density lipoprotein
- HOMA-IR
homeostatic model assessment of insulin resistance
- LDL
low-density lipoprotein
- MACS
Multicenter AIDS Cohort Study
- PI
protease inhibitor
- TC
total cholesterol
Author contributions
Saja Khuder, PhD: conducted analysis and interpretation of data, contributed to writing the manuscript, and contributed to preparation of figures. S. Chen, PhD: conducted sample extraction and mass spectrometry. J.C. McArthur, MBBS, and Ned Sacktor, MD: contributed to interpretation of findings and clinical aspects of the study, contributed to seeking grant funding for this research, contributed to writing of the manuscript. Leah Rubin, PhD: contributed to the statistical analysis of the data. S. Letendre, MD: contributed to writing of the manuscript and patient selection. T. Marcotte, PhD: contributed to writing of the manuscript, preparation of figures, and patient selection. I. Grant, MD: provided samples from the CHARTER cohort, contributed to interpretation of data and editing of the manuscript. Joseph Margolick, MD, PhD: provided samples and contributed to writing of the manuscript. L.P. Jacobson, PhD, D. Franklin, G. D'Souza, PhD, V Stosor, MD, Jordan Lake, MD, and G. Rapocciolo, PhD: provided samples and contributed to writing of the manuscript. A.M. Dickens, PhD: developed mass spectrometry methods, conducted analysis of samples, contributed to writing of the manuscript. N.J. Haughey, PhD: oversaw experimental design, analyses, data interpretation and contributed to writing of the manuscript.
Study funding
This work was supported by NIH awards AA0017408, MH077542, MH075673, AG034849 (N.J.H. and J.C.M.), MH071150 (N.S.), and K23AI110532 (J.E.L.). CHARTER was supported by awards N01 MH22005, HHSN271201000036C, and HHSN271201000030C (I.G., T.M., S.L.).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors: Multicenter AIDS Cohort Study. Neurology 1993;43:2245–2252. [DOI] [PubMed] [Google Scholar]
- 2.Pascal S, Resnick L, Barker WW, et al. Metabolic asymmetries in asymptomatic HIV-1 seropositive subjects: relationship to disease onset and MRI findings. J Nucl Med 1991;32:1725–1729. [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas V, Meyerhoff D, Studholme C, et al. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol 2009;15:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurtray A, Nakamoto B, Shikuma C, Valcour V. Cortical atrophy and white matter hyperintensities in HIV: the Hawaii Aging with HIV Cohort Study. J Stroke Cerebrovasc Dis 2008;17:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire JL, Gill AJ, Douglas SD, Kolson DL; CNS HIV Antiretroviral Therapy Effects Research CHARTER Group. The complement system, neuronal injury, and cognitive function in horizontally-acquired HIV-infected youth. J Neurovirol 2016;22:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen AB, Law I, Krabbe KS, et al. Cerebral FDG-PET scanning abnormalities in optimally treated HIV patients. J Neuroinflammation 2010;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borjabad A, Morgello S, Chao W, et al. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog 2011;7:e1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickens AM, Anthony DC, Deutsch R, et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS 2015;29:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem 2011;96:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyer S, Henneberg N, Knapp S, Lannert H, Martin E. Brain glucose metabolism is controlled by amplification and desensitization of the neuronal insulin receptor. Ann NY Acad Sci 1996;777:374–379. [DOI] [PubMed] [Google Scholar]
- 12.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease: is this type 3 diabetes? J Alzheimers Dis 2005;7:63–80. [DOI] [PubMed] [Google Scholar]
- 13.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012;78:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattler FR, He J, Letendre S, et al. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr 2015;68:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valcour V, Maki P, Bacchetti P, et al. Insulin resistance and cognition among HIV-infected and HIV-uninfected adult women: the Women's Interagency HIV Study. AIDS Res Hum Retroviruses 2012;28:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geroldi C, Frisoni GB, Paolisso G, et al. Insulin resistance in cognitive impairment: the InCHIANTI study. Arch Neurol 2005;62:1067–1072. [DOI] [PubMed] [Google Scholar]
- 18.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam study. Neurology 2010;75:1982–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcotte TD, Deutsch R, Michael BD, et al. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J Neuroimmune Pharmacol 2013;8:1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004;26:307–319. [DOI] [PubMed] [Google Scholar]
- 21.Cysique LA, Franklin D Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol 2011;33:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickens AM, Anthony DC, Deutsch R, et al. CSF metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in the cognitive states of HIV-infected subjects. AIDS 2015;29:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker JT, Kingsley LA, Molsberry S, et al. Cohort profile: recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol 2015;44:1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wozniak M, Rydzewski B, Baker SP, Raizada MK. The cellular and physiological actions of insulin in the central nervous system. Neurochem Int 1993;22:1–10. [DOI] [PubMed] [Google Scholar]
- 25.Cheng CM, Mervis RF, Niu SL, et al. Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res 2003;73:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Chen H, Xu H, et al. Brain insulin receptors and spatial memory: correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem 1999;274:34893–34902. [DOI] [PubMed] [Google Scholar]
- 27.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997;275:661–665. [DOI] [PubMed] [Google Scholar]
- 28.van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin-binding sites in the rat brain: in vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology 1979;105:666–673. [DOI] [PubMed] [Google Scholar]
- 29.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron 2012;75:762–777. [DOI] [PubMed] [Google Scholar]
- 30.Hall CN, Klein-Flugge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 2012;32:8940–8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz MW, Sipols A, Kahn SE, et al. Kinetics and specificity of insulin uptake from plasma into cerebrospinal fluid. Am J Physiol 1990;259:E378–E383. [DOI] [PubMed] [Google Scholar]
- 32.Baura GD, Foster DM, Porte D Jr, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. J Clin Invest 1993;92:1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides 1997;18:1257–1262. [DOI] [PubMed] [Google Scholar]
- 34.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 2003;20:255–268. [DOI] [PubMed] [Google Scholar]
- 35.Grillo CA, Tamashiro KL, Piroli GG, et al. Lentivirus-mediated downregulation of hypothalamic insulin receptor expression. Physiol Behav 2007;92:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mielke JG, Taghibiglou C, Liu L, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem 2005;93:1568–1578. [DOI] [PubMed] [Google Scholar]
- 37.Zhao WQ, De Felice FG, Fernandez S, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 2008;22:246–260. [DOI] [PubMed] [Google Scholar]
- 38.Moroo I, Yamada T, Makino H, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson's disease. Acta Neuropathol 1994;87:343–348. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol 2005;5:69–81. [DOI] [PubMed] [Google Scholar]
- 40.Hughes CA, Cashin RP, Eurich DT, Houston S. Risk factors for new-onset diabetes mellitus in patients receiving protease inhibitor therapy. Can J Infect Dis Med Microbiol 2005;16:230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobson DL, Tang AM, Spiegelman D, et al. Incidence of metabolic syndrome in a cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey). J Acquir Immune Defic Syndr 2006;43:458–466. [DOI] [PubMed] [Google Scholar]
- 42.Feleke Y, Fekade D, Mezegebu Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J 2012;50:221–230. [PubMed] [Google Scholar]
- 43.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992;74:1045–1052. [DOI] [PubMed] [Google Scholar]
- 44.Constans J, Pellegrin JL, Peuchant E, et al. Plasma lipids in HIV-infected patients: a prospective study in 95 patients. Eur J Clin Invest 1994;24:416–420. [DOI] [PubMed] [Google Scholar]
- 45.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 46.Wanke CA, Falutz JM, Shevitz A, Phair JP, Kotler DP. Clinical evaluation and management of metabolic and morphologic abnormalities associated with human immunodeficiency virus. Clin Infect Dis 2002;34:248–259. [DOI] [PubMed] [Google Scholar]
- 47.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet 1998;351:871–875. [DOI] [PubMed] [Google Scholar]
- 48.McComsey GA, Lo Re V III, O'Riordan M, et al. Effect of reducing the dose of stavudine on body composition, bone density, and markers of mitochondrial toxicity in HIV-infected subjects: a randomized, controlled study. Clin Infect Dis 2008;46:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data for the analyses in this report are available through the CHARTER and MACS websites at charternntc.org/and statepi.jhsph.edu/macs/macs.html.