Abstract
Objective
To estimate effects of vitamin D levels on incident delirium hospital admissions using inherited genetic variants in mendelian randomization models, which minimize confounding and exclude reverse causation.
Methods
Longitudinal analysis using the UK Biobank, community-based, volunteer cohort (2006–2010) with incident hospital-diagnosed delirium (ICD-10 F05) ascertained during ≤9.9 years of follow-up of hospitalization records (to early 2016). We included volunteers of European descent aged 60-plus years by end of follow-up. We used single-nucleotide polymorphisms previously shown to increase circulating vitamin D levels, and APOE variants. Cox competing models accounting for mortality were used.
Results
Of 313,121 participants included, 544 were hospitalized with delirium during follow-up. Vitamin D variants were protective for incident delirium: hazard ratio = 0.74 per 10 nmol/L (95% confidence interval 0.62–0.87, p = 0.0004) increase in genetically instrumented vitamin D, with no evidence for pleiotropy (mendelian randomization–Egger p > 0.05). Participants with ≥1 APOE ε4 allele were more likely to develop delirium (e.g., ε4ε4 hazard ratio = 3.73, 95% confidence interval 2.68–5.21, p = 8.0 × 10−15 compared to ε3ε3), but there was no interaction with vitamin D variants.
Conclusions and relevance
In a large community-based cohort, there is genetic evidence supporting a causal role for vitamin D levels in incident delirium. Trials of correction of low vitamin D levels in the prevention of delirium are needed.
Delirium is an acute decline in cognitive function, with a rapid onset of symptoms.1 It is relatively common in later life and is associated with substantially increased morbidity and mortality.1,2 Interest has grown in the potential role of vitamin D in protecting against cognitive decline. A recent meta-analysis reported that low vitamin D levels are associated with poorer cognition and accelerated cognitive decline,3 perhaps through vitamin D's anti-inflammatory properties.4,5 In observational studies, low levels of vitamin D have been associated with an increased likelihood of delirium in patients with hip fracture6 and for hospital-acquired, new-onset delirium.7 Genetically increased levels of vitamin D have been linked to reduced risk of Alzheimer disease in one study.8 However, evidence for the potential role of genetically increased vitamin D levels in reducing risk of incident delirium in large population-based studies is lacking.
Studies of outcomes in later life are susceptible to confounding and reverse causation, when associations with a risk factor are due to the effects of underlying pathology rather than being causal. Inherited genetic variants can provide stronger evidence for potential causal effect on an outcome—a technique known as mendelian randomization (MR).9
To apply genetic analyses, variants altering risk factor status are needed, which have their mode of action through the risk factor only.9 Here, we applied MR approaches to test vitamin D associations with incident delirium in an exceptionally large sample of older UK Biobank participants of European descent, in order to clarify the likely causal significance. We also accounted for APOE status.
Methods
Between 2006 and 2010, UK Biobank recruited 503,325 community-based volunteers (aged 40–70 years) from across the United Kingdom.10 Genetic data were available on 488,377 UK Biobank participants, of whom 451,427 participants were identified as of European ancestry using self-report and genetics data.11 Given that 7.5% of the participants were from non-European ancestries and from diverse lineages, we focused on participants from European ancestry.
Standard protocol approvals, registrations, and patient consents
Participants provided informed consent for data linkage to hospital inpatient admissions, cancer registrations, and death registrations. Ethical approval for the UK Biobank study was obtained from the North West Multi-Centre Research Ethics Committee. The current analysis was part of UK Biobank–approved project 14631 on aging well.
Delirium diagnosis was ascertained using ICD-10 code F05 in hospital discharge data. Participants who had a delirium episode before baseline interview were excluded from analyses (n = 51). Participants were eligible for inclusion if they reached the age of 60 within the follow-up period (up to February 16, 2016) (excluded n = 138,251). Participants with incident delirium had to be aged 60 years or older at diagnosis because incident cases before age 60 years are rare (excluded n = 3). One participant was excluded because they were missing an assessment date. This left 313,121 participants for the analysis.
Genotyping quality control and imputation were performed centrally by UK Biobank. Genetic variants rs429358 and rs7412 were used to define APOE haplotypes. Genetic variants for circulating 25(OH)D concentration (vitamin D) were extracted from 2 studies: 4 from Vimaleswaran et al.12 and 6 from a more recent report by Jiang et al.,13 which included a larger sample size but log-transformed the vitamin D levels (the 4 loci from Vimaleswaran are included in the 6 from Jiang) (only one variant from each locus was included, and only if the final meta-analysis p value was <5 × 10−8; all included variants had sufficient imputation quality [>0.4] and no deviation from Hardy-Weinberg equilibrium [p > 1 × 10−6] in the UK Biobank participants). We checked for consistency across both results and used the weights from Vimaleswaren to obtain the hazard ratio (HR) per nanomoles/liter of vitamin D. To confirm that the genetic risk score (GRS) was predictive for vitamin D, we used data from the InCHIANTI Study, a cohort study of aging in the Tuscany region of Italy in which both vitamin D and genetic variants were available.
The primary MR analysis methods were performed using R (v3.4.1) package “MendelianRandomization” (v0.2.2). Genetic variants were individually assessed for their association with incident delirium, and the natural log-transformed subhazard ratios (sHRs) were aligned with the vitamin D–raising allele and effect from the previously published studies and submitted to the “mr_input()” function. We used the penalized robust inverse-variance weighted (IVW) regression result as the primary analysis, and checked the penalized weighted median and penalized robust MR-Egger analyses for consistency in the effect estimate, and finally whether the MR-Egger intercept significantly differed from zero to test for potential pleiotropy (i.e., whether the effect of the single-nucleotide polymorphisms [SNPs] on delirium is via a pathway other than the risk factor under assessment14). The exponential of the IVW regression coefficient was derived (due to the earlier logging of the estimates for the MR analysis) to give the risk change (HR) per unit of vitamin D (nmol/L). The UK National Institute for Clinical Excellence concluded that “there is consensus that levels below 25 nmol/L (10 ng/ml) qualify as ‘deficient,’ but beyond this there is currently no standard definition of ‘optimal’ 25(OH)D levels. Some sources suggest that levels above 50 nmol/L (30 ng/ml) are ‘sufficient,’ while 70–80 nmol/L (28–32 ng/ml) is ‘optimal.’”15 We therefore chose to present the effect of genetically instrumented vitamin D levels per 10 nmol/L increase, as this is a clinically relevant level. To derive the risk of incident delirium per 10 nmol/L of vitamin D, we raised the HR to the power 10.
In secondary analyses of vitamin D, we created a GRS by summing the number of vitamin D-increasing alleles carried by each UK Biobank participant, weighted by the effect on circulating vitamin D levels using PLINK v1.916; we used the 6 loci from Jiang et al.13 We confirmed that our vitamin D GRS was associated with vitamin D levels in blood assays from 1,161 participants from the InCHIANTI aging study, using a linear regression model adjusted for age, sex, and study site, with natural log-transformed vitamin D levels as the outcome (coefficient = 1.29, 95% confidence interval [CI] 0.80–1.78, p = 3 × 10−7).
Estimates of associations between the vitamin D variants and incident delirium were from Fine and Gray competing risks regression17 adjusted for age, sex, genotyping array, and principal components 1–5 of the genotyping data to account for population structure. Estimates of associations between APOE haplotype and incident delirium were adjusted for age, sex, genotyping array, and principal components 1–5. In sensitivity analyses, we investigated the effects of adjusting for self-reported time spent in the sun in the summer, excluding one of each pair of participants related to the third degree or closer (identified by KING kinship analysis18), adjusting for calcium GRS (n = 5 of 7 variants published in reference 19 as rs1550532 were not available in the UK Biobank version 2 genetic data release, and we excluded rs1570669 because it is located at the same locus as rs6013897 in the vitamin D score on chromosome 20), excluding participants with diagnosed dementia from analyses (ICD-10 codes F00, F01, F02, F03, and G30), investigating the effect of a rare variant in CYP2R1 (rs117913124)20 with incident delirium, and rerunning the analysis using a noncompeting risk Cox model. To investigate whether the vitamin D-increasing variants may be acting via other pathways, we first took advantage of recent large pQTL (protein quantitative trait loci) studies to determine whether these loci are associated with proteins other than vitamin D21,22 and, second, the catalog of published genome-wide association studies.23 Stata v14.1 (StataCorp, College Station, TX) was used for competing risks regression.
Data availability
Data are available from the UK Biobank after submitting an application (ukbiobank.ac.uk/register-apply/). The syntax for conducting the analysis is available on request.
Results
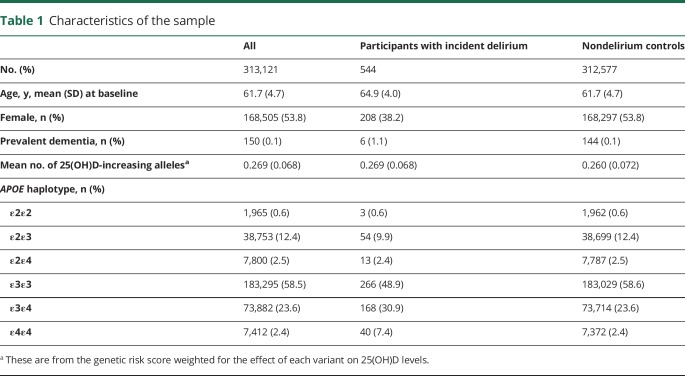
Of 313,121 participants included in analyses, 544 had at least one incident episode of delirium recorded in hospital discharge data. The mean time to delirium diagnosis was 4.6 years (SD 1.6 years). Mean age at baseline for all the participants was 61.7 years (SD 4.7 years) and 53.8% of the sample were women (table 1). The mean age at delirium diagnosis was 71.6 years (SD 4.12 years), the minimum and maximum age was 60.0 and 77.8 years, respectively. The overall follow-up was up to 9.9 years. There were 11,669 deaths during the follow-up period (198 deaths after delirium). Within 1 year after the delirium diagnosis, 151 (27.8%) had died, and within 5 years, 196 (36.3%) had died.
Table 1.
Characteristics of the sample
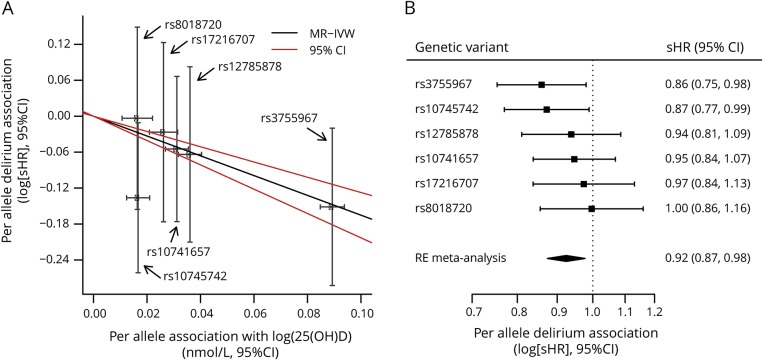
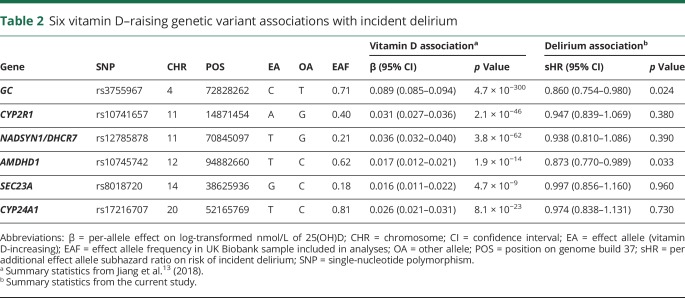
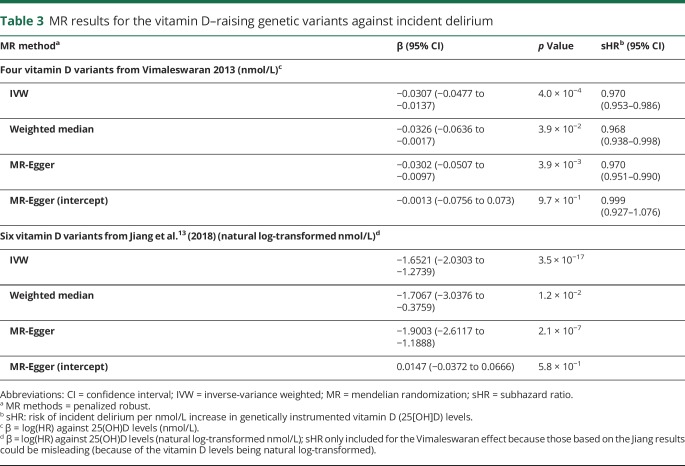
Vitamin D-increasing alleles were associated with decreased risk of delirium (figure, A and B and table 2): the IVW regression coefficient was −1.65 (95% CI −2.03 to −1.27, p = 3.5 × 10−17) (table 3). We observed consistent effect directions in the other MR methods (penalized weighted median coefficient = −1.71, 95% CI −3.04 to −0.38) (penalized robust MR-Egger coefficient = −1.90, 95% CI −2.61 to −1.19), and no significant pleiotropy, as tested by the MR-Egger method (p > 0.05).
Figure. Vitamin D-increasing genetic variants are associated with reduced risk of incident delirium.
Jiang et al.13 (2018) found 6 genetic variants to affect circulating 25(OH)D (vitamin D) levels. (A) The effect of previously published per-allele effect of each variant on vitamin D levels is plotted on the x-axis, and the association of the same variant with incident delirium (logged hazard ratio) is plotted on the y-axis, with the mendelian randomization inverse-weighted (MR-IVW) regression line and 95% confidence intervals (CIs) also show. (B) The per-allele association with incident delirium is shown for each vitamin D–raising genetic variant, with a random-effects (RE) meta-analysis of the 6 variants also shown. sHR = subhazard ratio.
Table 2.
Six vitamin D–raising genetic variant associations with incident delirium
Table 3.
MR results for the vitamin D–raising genetic variants against incident delirium
Because the units from Jiang et al. are natural log-transformed vitamin D levels and therefore are not easily interpretable, we also applied MR methods to the 4 largest-effect variants published by Vimaleswaran et al.12 who provide the per-allele effect on each nanomole/liter of vitamin D levels. This relationship was consistent when we used 4 vitamin D-increasing alleles from Vimaleswaran et al.12 (table 3). The sHR for incident delirium per unit increase in genetically instrumented 25(OH)D levels (nmol/L) was 0.970 (the exponential of the penalized robust IVW regression coefficient −0.0307; table 3) (95% CI 0.953–0.986): incident delirium risk is therefore reduced by 3% per nmol/L increase in genetically instrumented vitamin D. The risk reduction per 10 nmol/L increase was 0.736 (95% CI 0.620–0.873), calculated by raising 0.970 to the power 10. We observed consistent effect directions in the other MR methods (penalized weighted median sHR = 0.968, 95% CI 0.938–0.998) (penalized robust MR-Egger sHR = 0.970, 95% CI 0.951–0.990), and no significant pleiotropy, as tested by the MR-Egger method (p > 0.05) (table 3). Using a GRS for vitamin D, we found a consistent association (sHR = 0.88 per SD of vitamin D GRS, 95% CI 0.81–0.96, p = 0.0044).
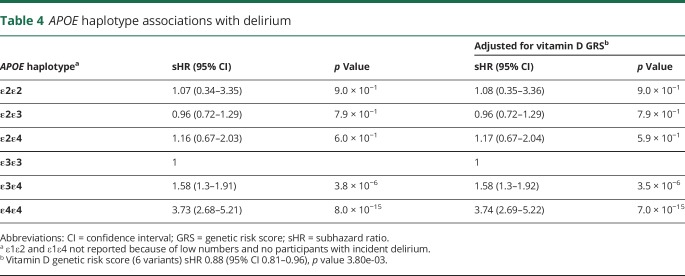
Participants with one or more APOE ε4 allele were at a substantial increased risk of incident delirium (table 4). Those homozygote for APOE ε4 (i.e., 2 ε4 copies present) had markedly increased risks (sHR = 3.73, 95% CI 2.68–5.21, p = 8.0 × 10−15); ε4 heterozygotes (i.e., ε3ε4) were at intermediate risk (sHR = 1.58, 95% CI 1.30–1.91, p = 3.8 × 10−6). The APOE associations were independent of the vitamin D genetic risks and were not substantively changed (table 4). There was no interaction with vitamin D variants.
Table 4.
APOE haplotype associations with delirium
Sensitivity analyses
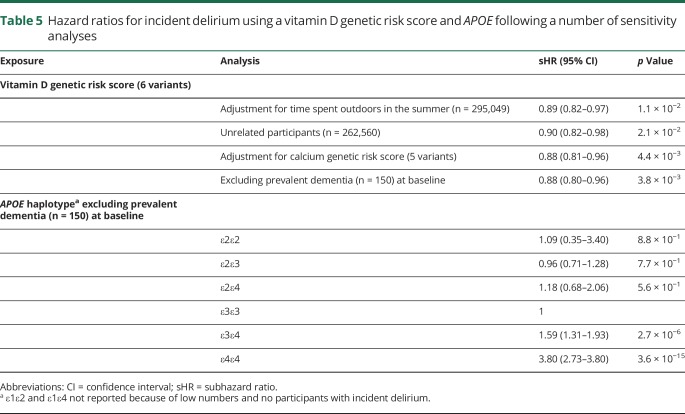
To test the robustness of the vitamin D association with delirium, we adjusted for self-reported time spent outdoors in the summer; excluded one of each pair of participants related to the third degree or closer; and adjusted for a GRS of 5 known calcium level–altering variants that have not been reported to be associated with vitamin D levels. Results were not substantively changed (table 5) and the calcium-altering variants were not associated with delirium. Excluding 150 cases of dementia diagnosed before the assessment visit had no effect on the association between the vitamin D GRS, APOE genotype, and incident delirium (table 5). We tested the association between a low-frequency variant in CYP2R1 (rs117913124, vitamin D–decreasing minor allele frequency 2.77% in the studied sample) identified in a separate study19 and incident delirium, and found there was no significant association (sHR 1.05, 95% CI 0.74–1.49, p = 0.78). However, because we had only 80% power to detect an effect of 1.6 (at α = 0.05, with 544 patients with delirium and 312,577 controls, using the genetic association study power calculator24), this variant may have a smaller effect on delirium that we were not powered to detect. In noncompeting risk models, the point estimates for the 6 vitamin D genetic variants with incident delirium were slightly different, although the overall conclusion remained the same (rs3755967 HR 0.86, 95% CI 0.76–0.98; rs10745742 HR 0.87, 95% CI 0.77–0.99; rs12785878 HR 0.94, 95% CI 0.81–1.08; rs10741657 HR 1.06, 95% CI 0.93–1.19; rs17216707 HR 1.03, 95% CI 0.88–1.19; rs8018720 HR 1.00, 95% CI 0.86–1.17). The MR analyses using results from noncompeting risk models were also consistent when using the 4 variants from Vimaleswaran: the HR for incident delirium per 10 nmol/L increase in genetically instrumented 25(OH)D levels was 0.737 (95% CI 0.620–0.872). Finally, we found that vitamin D-increasing loci (or proxies) are not associated with other protein levels in 2 recent large pQTL studies,21,22 although one of the 6 SNPs (rs17216707) is also associated with estimated glomerular filtration rate.25 Excluding this SNP from the MR analysis makes no meaningful difference (new penalized robust MR-IVW regression estimate using only 5 variants = −1.6399, 95% CI −1.887 to −1.392, p = 1.4 × 10−38).
Table 5.
Hazard ratios for incident delirium using a vitamin D genetic risk score and APOE following a number of sensitivity analyses
Discussion
In this large-scale prospective study, participants with genetically determined higher vitamin D levels had substantially lower risks of incident hospitalized episodes of delirium. Because MR analysis greatly reduces confounding and excludes reverse causation,9,13 our results suggest that interventional trials to increase vitamin D levels may be justified to reduce risk of delirium. In addition, participants carrying an APOE ε4 allele were at increased risk of incident delirium, likely contributing to the increased risk of dementia observed after an episode of delirium. However, the effect of vitamin D–related variants was not altered by APOE status.
The association between vitamin D and delirium is plausible as vitamin D receptors are distributed within the hippocampus, hypothalamus, cortex, and subcortex, and evidence suggests a variety of neurophysiologic and neuroprotective mechanisms.26 In addition, behavioral and attention disorders, and accelerated aging have been documented in vitamin D receptor knockout transgenic mice models.27–29 Furthermore, evidence indicates that vitamin D may modulate serotonin synthesis.30 Serotonin is involved in regulating a range of behaviors and brain function and has been indicted to have a role in delirium.31 Therefore, the effect of vitamin D on delirium may be in part attributable to its effect on serotonin synthesis.30,31 Low levels of vitamin D have been observationally associated with an increased likelihood of delirium in patients with hip fracture and for hospital-acquired, new-onset delirium.6,7
A number of studies have used MR approaches to estimate the effects of low vitamin D levels on cognitive outcomes. Of note, a recent analysis showed no association between vitamin D and global or memory cognition using an MR approach, although the vitamin D score (synthesis) was composed of 2 SNPs.29 Genetically increased levels of vitamin D (4 SNPs score), however, were associated with a reduced risk of Alzheimer disease at nominal statistical significance.8 Here, we use MR approaches to estimate the effects of vitamin D levels on risk of delirium, thereby minimizing confounding and excluding reverse causation. Although we cannot rule out pleiotropy (the vitamin D-increasing loci affect delirium risk via other mechanistic routes), we found no evidence in 2 large recent pQTL studies21,22 that these loci affect other protein levels, and excluding the one variant associated with estimated glomerular filtration rate in another genome-wide association study25 did not significantly change the result.
A recent meta-analysis reported that there was no association between APOE and delirium, although the number of delirium cases included was smaller than in the present study.28 Here, we showed carriers of an APOE ε4 allele were at an increased risk of delirium independent of a dementia diagnosis in our analyses, suggesting that APOE may constitute a shared mechanism between the 2 conditions. APOE ε4 status may therefore be a useful addition to estimating risk of developing delirium, especially in designing intervention trials.
There are a number of limitations with our study. First, serum vitamin D levels were not available for the UK Biobank participants and therefore the genetic variants could not be assessed concurrently with vitamin D levels and delirium. However, we confirmed that the genetic variants we studied were strongly associated with blood levels of vitamin D in the InCHIANTI Study (see methods). Second, MR analysis has important assumptions and limitations, in particular that the genetic instruments are only associated with the outcome via the exposure under examination, and the association between the exposure and outcome is linear; however, MR-Egger analysis provided no evidence of pleiotropy, and variants were not associated with covariates in our analyses. Finally, UK Biobank participants tended to be healthier than the UK general population at baseline, and therefore our number of delirium cases is lower than expected and estimates may be less applicable to frail older groups.32
In the data available, the vast majority of the participants had one delirium episode; 32 participants had ≥2 delirium episodes. Our analysis is based on the first delirium episode. Future studies could examine risk factors for recurrent events. The methods in this analysis were used to estimate the linear effect of vitamin D levels around the population average; future studies could examine the nonlinear effects of vitamin D and delirium.
In this study, genetic evidence suggests that higher vitamin D levels may be a substantial causal protective factor for incident delirium. Clinical studies are needed to confirm this. Delirium and dementia share a common risk factor in APOE genotype, which may contribute to the high rates of incident dementia observed after an episode of delirium.
Acknowledgment
This research has been conducted using the UK Biobank Resource, under application 14631. The authors thank the UK Biobank participants and coordinators for this unique dataset and Dr. Andrew R. Wood for his work identifying the UK Biobank participants of European descent.
Glossary
- CI
confidence interval
- GRS
genetic risk score
- HR
hazard ratio
- ICD-10
International Classification of Diseases, Tenth Revision
- IVW
inverse-variance weighted
- MR
mendelian randomization
- pQTL
protein quantitative trait loci
- sHR
subhazard ratio
- SNP
single-nucleotide polymorphism
Footnotes
Editorial, page 553
CME Course: NPub.org/cmelist
Author contributions
K. Bowman and L.C. Pilling conducted the statistical analysis. K. Bowman: design or conceptualization of the study, analysis or interpretation of the data, drafting or revising the manuscript for intellectual content. L. Jones: design or conceptualization of the study, analysis or interpretation of the data, drafting or revising the manuscript for intellectual content. L.C. Pilling: design or conceptualization of the study, analysis or interpretation of the data, drafting or revising the manuscript for intellectual content. J. Delgado: drafting or revising the manuscript for intellectual content. G.A. Kuchel: drafting or revising the manuscript for intellectual content. L. Ferrucci: drafting or revising the manuscript for intellectual content. R.H. Fortinsky: drafting or revising the manuscript for intellectual content. D. Melzer: design or conceptualization of the study, analysis or interpretation of the data, drafting or revising the manuscript for intellectual content.
Study funding
Supported by UK Medical Research Council award MR/M023095/1 to D.M., and L.F. is supported by the Intramural Research Program of the NIH National Institute on Aging.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157–1165. [DOI] [PubMed] [Google Scholar]
- 2.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med 2002;162:457. [DOI] [PubMed] [Google Scholar]
- 3.Goodwill AM, Szoeke C. A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc 2017;65:2161–2168. [DOI] [PubMed] [Google Scholar]
- 4.Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflamm Res 2014;63:803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res 2014;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torbergsen AC, Watne LO, Frihagen F, Wyller TB, Brugaard A, Mowe M. Vitamin deficiency as a risk factor for delirium. Eur Geriatr Med 2015;6:314–318. [Google Scholar]
- 7.Quraishi SA, Litonjua AA, Elias KM, et al. Association between pre-hospital vitamin D status and hospital-acquired new-onset delirium. Br J Nutr 2015;113:1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christopher SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS. Modifiable pathways in Alzheimer's disease: mendelian randomisation analysis. BMJ 2017;359:j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paternoster L, Tilling K, Davey Smith G. Genetic epidemiology and mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLoS Genet 2017;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilling LC, Kuo CL, Sicinski K, et al. Human longevity: 25 genetic loci associated in 389,166 UK Biobank participants. Aging 2017;9:2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional mendelian randomization analysis of multiple cohorts. PLoS Med 2013;10:e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, O’Reilly PF, Aschard H, et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun 2018;9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowden J, Smith GD, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Research UK. Expert Paper 3: vitamin D. Support Doc NICE PH32 [Internet]. 2010;25. Available at: nice.org.uk/guidance/ph32/documents/expert-paper-3-vitamin-d2. Accessed August 2018. [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 18.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics 2010;26:2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Seaghdha CM, Wu H, Yang Q, et al. Meta-analysis of genome-wide association studies identifies six new loci for serum calcium concentrations. PLoS Genet 2013;9:e1003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manousaki D, Dudding T, Haworth S, et al. Low-frequency synonymous coding variation in CYP2R1 has large effects on vitamin D levels and risk of multiple sclerosis. Am J Hum Genet 2017;101:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun BB, Maranville JC, Peters JE, et al. Genomic atlas of the human plasma proteome. Nature 2018;558:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao C, Chen G, Song C, et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun 2018;9:3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacArthur J, Bowler E, Cerezo M, et al. The new NHGRI-EBI catalog of published genome-wide association studies (GWAS catalog). Nucleic Acids Res 2017;45:D896–D901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson JL. GAS Power Calculator. 2017. Available at: csg.sph.umich.edu/abecasis/gas_power_calculator. Accessed August 2018.
- 25.Pattaro C, Teumer A, Gorski M, et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun 2016;7:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aspell N, Lawlor B, O'Sullivan M. Is there a role for vitamin D in supporting cognitive function as we age? Proc Nutr Soc 2018;77:124–134. [DOI] [PubMed] [Google Scholar]
- 27.Keisala T, Minasyan A, Lou YR, et al. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol 2009;115:91–97. [DOI] [PubMed] [Google Scholar]
- 28.Tuohimaa D, Meagher D, Williams J, Mulligan O, McCarthy G. A systematic review and meta-analysis of the association between the apolipoprotein E genotype and delirium. Psychiatr Genet 2016;26:53–59. [DOI] [PubMed] [Google Scholar]
- 29.Maddock J, Zhou A, Cavadino A, et al. Vitamin D and cognitive function: a mendelian randomisation study. Sci Rep 2017;7:13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J 2015;29:2207–2222. [DOI] [PubMed] [Google Scholar]
- 31.van Mast RC, Fekkes D. Serotonin and amino acids: partners in delirium pathophysiology? Semin Clin Neuropsychiatry 2000;5:125–131. [DOI] [PubMed] [Google Scholar]
- 32.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Allen N. OP41 The representativeness of the UK Biobank cohort on a range of sociodemographic, physical, lifestyle and health-related characteristics. BMJ 2016;70(suppl 1):2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the UK Biobank after submitting an application (ukbiobank.ac.uk/register-apply/). The syntax for conducting the analysis is available on request.