Abstract
The growing number of publications concerning postoperative cognitive decline (POCD) after cardiac surgery is indicative of the health-related and economic-related importance of this intriguing issue. Significantly, the reported POCD incidence over the years has remained steady due to various unresolved challenges regarding the examination of this multidisciplinary topic. In particular, a universally accepted POCD definition has not been established, and the pathogenesis is still vaguely understood. However, numerous recent studies have focused on the role of the inflammatory response to a surgical procedure in POCD occurrence. Therefore, this traditional narrative review summarizes and evaluates the latest findings, with special attention paid to the difficulties of defining POCD as well as the involvement of inflammation in POCD development. We searched the MEDLINE, Scopus, PsycINFO and CENTRAL databases for the best evidence, which was classified according to the Oxford Centre for Evidence-based Medicine. To our knowledge, this is the first narrative review that identified class-1 evidence (systematic review of randomized trials), although most evidence is still at class-2 or below. Furthermore, we revealed that defining POCD is a very controversial matter and that the inflammatory response plays an important role in the mutually overlapping processes included in POCD development. Thus, developing the definition of POCD represents an absolute priority in POCD investigations, and the inflammatory response to cardiac surgery merits further research.
MeSH Keywords: Cardiac Surgical Procedures, Cardiopulmonary Bypass, Cognition Disorders, Neuropsychological Tests, S100 Calcium Binding Protein beta Subunit, Systemic Inflammatory Response Syndrome
Background
Although the reports of postoperative cognitive decline (POCD) after cardiac surgery date back to the first years of the introduction of cardiopulmonary bypass (CPB), many questions remain open, making this topic very important [1]. Furthermore, because of improvements in medical techniques, the rates of major complications (e.g., mortality) following cardiac surgery are currently low; however, the incidence of POCD is still unchanged, and has become the most common postoperative complication [2]. Nevertheless, some investigators underestimate the importance of POCD, believing it to be more a myth than a true complication, but present evidence has proven the significant health-related and economic-related importance of this phenomenon [3,4]. Apart from cognitive deterioration in the form of memory difficulties and a general slowing of information processing, the occurrence of POCD is associated with markedly adverse outcomes, such as prolonged hospitalization and rehabilitation, including increased mortality, diminished quality of life, and more common working disability and early retirement, thereby producing a significant burden on the healthcare system [5,6].
POCD incidence drops over time, with the highest rate being 30 to 70% at hospital discharge, followed by 20 to 30% 6 months after surgery and 15 to 25% after 12 months of follow-up [7–9]. Late POCD is considered an extension of early POCD, but with more significant outcomes [10–13]. Distinguishing postoperative delirium from early POCD as a closely related diagnosis is particularly important. The former, which is considered a strong predictor of POCD development, usually occurs within the first 3 postoperative days [14,15]. However, POCD occurs at the end of the first week and has no effect on consciousness, and its duration may be significantly prolonged [16,17].
Lesions underlying POCD are probably in the hippocampus, which is pronouncedly sensitive to hypoxic injury [16,18,19]. Because of the subtle nature of POCD disorders, the diagnosis requires the administration of a specific and sensitive neuropsychological test battery that examines different cognitive domains [5]. The absence of a gold-standard definition for diagnosing POCD represents a major obstacle in POCD investigations. Thus, the reported POCD incidence varies widely, potentially resulting in misleading conclusions and making this topic very confusing [2,20].
Early research noted that POCD is more frequent after cardiac surgery than after other types of surgery [10,21]. POCD was long thought to be a repercussion of the physiological disturbances prompted by CPB management, hence the term “pump-head” [10,22,23]. However, recent well-randomized studies revealed that POCD incidence is similar, regardless of whether CPB was performed during the surgical procedure [16,19,23–25]. Therefore, the current theories of POCD development following cardiac surgery have been updated and now include surgery perfusion-, patient- and anesthesia-related risk factors [3].
The purpose of this review is to examine and discuss the recent literature concerning POCD after cardiac surgery, with an emphasis on controversies that follow the definition of POCD as well as on the evidence of a plausible role of the inflammatory response in POCD pathogenesis.
Methods
Two reviewers (SG and GK) independently searched across the MEDLINE, Scopus, PsycINFO and CENTRAL databases for all literature written in English and published up to October 2018 using the following medical subject heading (MeSH) terms: cardiac surgical procedures; coronary artery bypass; cardiopulmonary bypass; coronary artery bypass, off-pump; cognition disorders; cognitive dysfunction; neuropsychological tests; postoperative complications; inflammation; systemic inflammatory response syndrome; and biomarkers. Additionally, due to the variable terminology in this area an in-depth list of plain text terms was used; however, a systematic search methodology of the available literature was not carried out. Relevant references from the selected articles were also retrieved for additional analysis. We first browsed for human studies, but in the absence of evidence, we looked for animal data. To limit the reference number, inclusion of articles in our research was based on a combination of the level of evidence and the time of publication. Disagreements concerning study selection were resolved by consulting with NK, who approved the final list of included studies.
In this traditional narrative review, the evidence level was stratified according to the recommendation of the Oxford Centre for Evidence-based Medicine [26]. Because of growing interest among researchers regarding this topic, class-1 evidence (systematic review of randomized trials) has finally been obtained. Nevertheless, the majority of human studies still provide class-2 evidence or weaker, while a significant amount of evidence is derived from animal data.
Neuropsychological Assessment
Given that POCD diagnosis includes time-consuming and exhausting neuropsychological tests and trained experts for conducting these tests [27], there is substantial interest among investigators to provide reliable biochemical markers of cognitive functions, such as S100β protein and neuron-specific enolase. However, despite numerous clinical trials, their sensitivity is still insufficient [10,28–31].
A significant obstacle of neuropsychological assessment represents the absence of a worldwide-accepted POCD definition [20,27], as the Statement of Consensus on Assessment of Neurobehavioral Outcomes After Cardiac Surgery that was formed at a conference in Fort Lauderdale, Florida, USA in 1994 [32] has not been consistently adopted [5,33]. Subsequently, because statistical criteria and methods used to define POCD, neuropsychological test selection and assessment time have been primarily left to authors’ discretion, POCD incidence varies extensively across different studies [13,34]. Moreover, similarly designed studies report contradictory results; thus, a comparison of studies is difficult [20,35].
In particular, most studies have used cut-off values for cognitive deterioration and normal variation in postoperative cognitive functions between one to 2 standard deviations, and the commonly used minimal number of tests showing a decline out of the total number of administered tests has been set between one to 3 tests [1,3,21,23]. Furthermore, preferable statistical methods include an absolute decline from baseline scores expressed as a standard deviation or a percentage change, reliable change index and standardized regression-based method [21]. The first represents the method of choice to detect POCD incidence in interventional studies; however, this method results in a low study strength, is unable to control for measurement reliability, learning effect or regression toward the mean, and has a low sensitivity for assessing an individual cognitive domain [3,13]. In contrast, the reliable change index and standardized regression-based methods are more sensitive to detecting cognitive deterioration in a specific domain [34]. Additionally, some researchers have introduced a nonsurgical or noncardiac surgical control group to minimize the learning effect of the tests, but the inclusion of control groups also has some issues, particularly regarding the complicated physiological processes attributed to cardiac surgery and CPB [16,21]. Specifically, we identified more than 100 different neuropsychological tests among published studies that have been used to diagnose POCD. Domains of memory, attention and psychomotor speed are commonly impaired following cardiac surgery; therefore, selected tests must have adequate sensitivity and specificity to cover different cognitive domains without overlapping but must also not exhaust the patient in the early postoperative period [5,20]. Furthermore, if a parallel form of the test is available, its application is highly recommended to reduce the learning effect across sessions [4]. Additionally, tests must be free of interfering confounders, such as ceiling, floor and basement effects [1,3]. Early postoperative cognitive evaluation should be performed at least 7 days after the procedure to avoid the effects of sedatives, postsurgical pain, sleep deprivation, patient fatigue and the hospital environment on the test results [12,23,32]. Although early POCD is commonly thought to be a harbinger of later cognitive deterioration [11,13], neuropsychological test results have been shown to be similar between patients who underwent cardiac surgery 5 years ago and a well-matched patient control group [36], most likely because of the effects of natural aging, the progression or new silent incidence of cardiovascular disease or cerebrovascular disease, or the development of dementia or Alzheimer’s disease [10,33,36]. Therefore, in our opinion, considering available data, cognitive functions should be assessed preoperatively, in the early postoperative period (seven to 14 days after procedure), and no later than 6 to 9 months following surgery.
Interestingly, previous studies have reported that preoperative depression with an incidence between 25 and 30% is a more significant factor for POCD development after cardiac surgery than perioperative factors [37,38]. Therefore, to exclude the effects of depression and anxiety on the neuropsychological test results, appropriate tests must be applied [35]. Next, preoperative neurological examination is highly recommended [32]. Finally, the literature also emphasizes the importance of conducting tests in a standardized fashion on the same time of day and in the same, quiet room [2,5].
Surgery Perfusion-Related Risk Factors
Currently, a very decisive hypothesis of POCD development includes systemic inflammatory response syndrome (SIRS) induced by cardiac surgery and CPB itself [1,2,19]. The latter causes specific activation of the inflammatory response by the immune system following contact between blood and the artificial materials of the bypass circuit, by ischemia-reperfusion injury, by complement activation, and by heparin neutralization with protamine [19]. In contrast, cardiac surgery nonspecifically initiates an inflammatory response [39]. Significantly, Parolari et al. reported similarity of the inflammatory marker levels after cardiac surgery with or without CPB [40], whereas several recent studies demonstrated that avoiding CPB does not improve cognitive functions (up to class-1 evidence) [16,19,23–25]. Furthermore, despite the general opinion that open-heart surgery compared to coronary artery bypass graft surgery is more prone to POCD occurrence, recent findings suggest otherwise (class-3 evidence) [11].
SIRS contributes to blood-brain barrier leakage as well as to the development of cerebral edema and cerebral inflammation [19,41], possibly with a crucial role in POCD pathogenesis [42]. To support the involvement of SIRS in POCD, several recent studies administered different types and doses of corticosteroids during the perioperative period to attenuate the inflammatory response and thereby decrease POCD incidence, but the results are contradictory [29,43–46]. The latest findings are encouraging, suggesting that optimal steroid type (i.e., dexamethasone), dose (i.e., 0.1 mg·kg−1) and time of administration (i.e., 10 h before surgery) have been identified (class-2 evidence) [29]; however, further larger multicentral studies to confirm these results and to investigate the long-term impact of corticosteroids on cognitive functions are mandatory.
The stress response to cardiac surgery has also been investigated as a possible factor involved in POCD pathogenesis [47], due to the U-curve relationship between glucocorticoid levels and memory [48], i.e., high glucocorticoid levels may be toxic for neural structures, especially the hippocampus, which is closely related to memory [8]. However, a recent study with a longitudinal assessment of perioperative cortisol levels did not support this theory, revealing that a prolonged and pronounced cortisol response merely reflects the stress response to the surgical procedure, while the real cause of POCD after cardiac surgery lies in another mechanism (class-3 evidence) [49].
Another proposed explanation of POCD development includes the incidents of cerebral microemboli originating during CPB as well as careless perfusionist procedures; however, beating-heart surgery was unable to reduce POCD incidence (up to class-1 evidence) [11,23,24,35,50].
Additionally, several intraoperative issues related to POCD occurrence have been described in the literature. Mean arterial pressure has been shown to need to be maintained between 80 and 90 mmHg during CPB, without intraoperative pressure dropping more than 32 mmHg [51], because brain hypoperfusion may lead to POCD [52]. Similarly, surgical manipulation during beating-heart surgery may reduce cardiac output; therefore, decreasing systemic blood pressure might reduce the brain perfusion index [12,53]. Although hypothermia is associated with decreased oxygen consumption and carbon dioxide production, studies have shown opposite effects of spontaneous hypothermia (32–35°C) during surgical procedures on cognitive functions [54,55], probably because of the rewarming effect after weaning off CPB [35]. Furthermore, performing a deep hypothermic circulatory arrest on animal models stimulates neuronal death [56], for which human studies have demonstrated an association with pronounced POCD [57]. Hemodilution also has an impact on cognitive functions [19,58], i.e., intraoperative hematocrit levels between 15 to 17% and a greater than 12% decline in hematocrit level from the baseline level represent a threshold for POCD occurrence [59]. Additionally, intraoperative and early postoperative hypercoagulability may cause temporary occlusion of tiny brain blood vessels, which is associated with POCD development [60].
Next, because impaired cerebral autoregulation prompted by surgical procedure may contribute to intraoperative cerebral ischemia, this mechanism has also been proposed as a significant part of POCD development [1,3]. Slater et al. showed that intraoperative cerebral desaturation is associated with POCD occurrence, i.e., the patients who did not desaturate below the 50% threshold had no meaningful incidence of POCD [61]. Furthermore, a multicenter randomized NORMOSAT study introduced the near infrared-reflected spectroscopy guided intervention algorithm that monitors and, as needed, enhances cerebral oxygen saturation, and accordingly, prevents prolonged and pronounced intraoperative as well as early postoperative cerebral desaturation [62]. Moreover, recent studies have proved that these manipulations during cardiac surgical procedures are useful in reducing POCD incidence (class-2 evidence) [12,63].
Finally, to improve cognitive outcomes following cardiac surgery, several novel techniques have been introduced, such as the “no-touch” approach [64], the single-clamp technique [65], ultrafiltration- and leukocyte-depletion methods [19], pulsatile flow [31], minimized extracorporeal circulation [2] and cell saver devices [24], while the alpha-stat technique compared to the pH-stat technique [27], as well as a shorter clamp and CPB duration [66], have been considered preferable.
Patient-Related Risk Factors
A frequently coexisting factor of cardiovascular disease is atherosclerosis, which, due to possible plaque rupture and artery stenosis and, consequently, intraoperative brain hypoperfusion as well as microemboli of carotid and cerebral arteries, may play a significant role in POCD pathogenesis [12,13,67]. Furthermore, magnetic resonance brain imaging has revealed that a significant portion of the elderly population has experienced asymptomatic stroke that altered cerebral autoregulation, predisposing them to POCD occurrence [11]. Additionally, coronary artery disease represents an independent risk factor for POCD [66,68], as cognitive deterioration was shown to be similar if the patients were surgically or conservatively treated [69]. However, in patients with a preoperative low cardiac output, the surgical procedure improves cognitive functions [70]. Advanced peripheral artery disease is also considered to be a POCD risk factor [66], but evidence of an association between intermittent claudication and cognitive disturbances is contradictory [71].
Hypertension as well as diabetes through disruption of cerebral autoregulation and developed vascular encephalopathy may underlie POCD occurrence [1,2]. Moreover, diabetes is associated with increased inflammatory markers, suggesting a possible alternative pathway of cognitive impairment [72,73]. Interestingly, type I diabetes does not contribute more significantly to cerebrovascular incidents than does type II diabetes [74]. Nevertheless, intraoperative hyperglycemia over 10 mmol·L−1, even in patients without diabetes, is associated with POCD occurrence, likely through SIRS activation [75]. Next, chronic obstructive pulmonary disease due to altered cerebral blood flow [76,77], as well as chronic kidney disease and liver disease accompanied by vascular encephalopathy, disruption of the blood-brain barrier, high ammonia levels and anemia may be included in the pathogenesis of POCD [76,78].
Finally, old age represents a well-recognized POCD risk factor because of structural brain changes, dementia development, and lower cognitive reserves, as well as the limited recovery potential of the depleted brain [4,16]. Female sex is considered a predictor of POCD in the elderly population because of the role of estrogen in preserving brain homeostasis [27,79]. Genetic studies have revealed that certain combinations of C-reactive protein, interleukin-6, tumor necrosis factor-alpha and P-selectin polymorphisms, through modulation of the inflammatory response, contribute to the pathogenesis of POCD [1,27]. A relationship of lower education [6,80], smoking history [66], loneliness [4] and hospital environment [20] with POCD occurrence is also described in the literature.
Anesthesia-Related Risk Factors
The recent findings that suggest that anesthesia impacts POCD occurrence are particularly questionable. The selection of general or regional anesthesia does not seem to modify POCD incidence [16]. Furthermore, the effects of repeated exposure to anesthesia and depth of anesthesia during the procedure on POCD occurrence are also unclear [81]. However, the duration of mechanical ventilation in the intensive care unit is clearly associated with POCD development [29]. Interestingly, animal studies have described a neurotoxic effect of fentanyl [82]; however, the same effect has not been found in human subjects [3]. Ketamine administration during anesthesia induction is related to a lower rate of postoperative delirium and POCD incidence, probably because of the anti-inflammatory effect of the drug (class-2 evidence) [83]. However, a higher POCD incidence was observed after cardiac surgery managed with propofol compared to that with sevoflurane, regardless of the anti-inflammatory effect of propofol [12,84]. Kanbak et al. demonstrated greater postoperative cognitive functions following anesthesia maintained with isoflurane than that maintained with sevoflurane and desflurane [85].
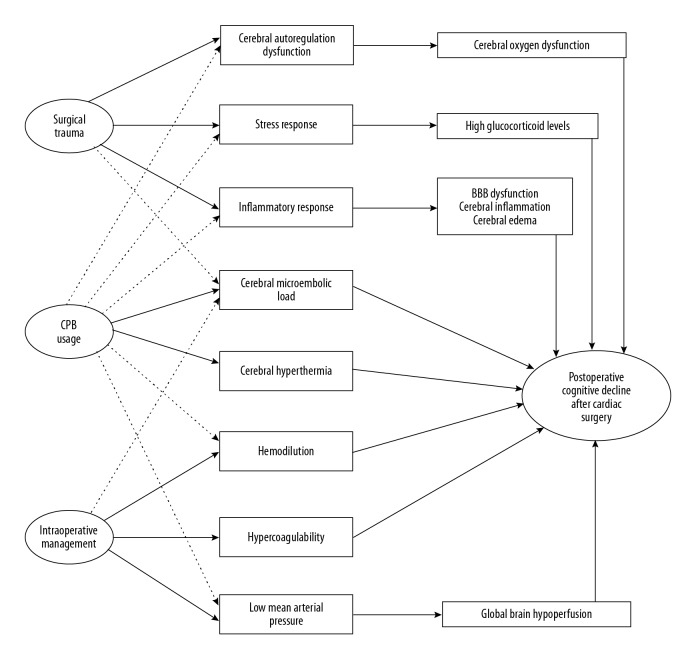
Overall, the present evidence suggests that surgery perfusion- and patient-related risk factors have a greater impact on cognitive functions than anesthesia-related risk factors (Table 1). Possible mechanisms that may underlie POCD development after cardiac surgery are shown in Figure 1.
Table 1.
Modifiable, partially modifiable and nonmodifiable risk factors that may be involved in the occurrence of postoperative cognitive decline following cardiac surgery.
| Preoperative | Intraoperative | Postoperative | |
|---|---|---|---|
| Modifiable | Arterial pressure control [2] | Surgical manipulation [64] | Duration of mechanical ventilation [29] |
| Glycemic control [73] | Cardiopulmonary bypass [23] | Sedation, analgesia and delirium management [15] | |
| Alcohol, nicotine or substance abuse [27,66] | Temperature management [54] | ||
| Hematocrit management [59] | |||
| Arterial pressure management [51] | |||
| Glycemic control [75] | |||
| Partially modifiable | Neurocognitive reserve [12] | Anesthetic management and bispectral index control [81] | Postoperative complications [3] |
| Depression and anxiety [38] | Cerebral oxygen control [61] | Patient frailty [1] | |
| Education level [80] | Duration of surgery [16] | Sleep disturbance [16] | |
| Social adjustment [4] | |||
| Nonmodifiable | Old age [16] | Type of surgery [11] | Hospital environment [20] |
| Female sex [79] | |||
| Genetics [27] | |||
| Dementia [4] | |||
| Neurodegenerative disease [3] | |||
| Underlying vascular disease [69] | |||
| Kidney and liver disease [76,78] | |||
| Pulmonary disease [77] |
References attached to risk factors are listed in the reference list at the end of article. Please see the body of the article text for additional discussion.
Figure 1.
A schematic diagram of the possible mechanisms included in the development of postoperative cognitive decline following cardiac surgery. The full-line arrow compared to dashed line arrow indicates a more significant relationship between the 2 terms. The extent of the possible impact of each mechanism as well as their interaction effect in the pathogenesis of postoperative cognitive decline after cardiac surgery is beyond the scope of this article. For additional discussion, please see the body of the article text. CPB – cardiopulmonary bypass; BBB – blood-brain barrier.
Next Steps
Our understanding of POCD has expanded significantly since the first reports back in the 1970s [21]; therefore, we must reevaluate our knowledge to overcome the present gaps and set directions for future research. Recent findings suggest that the inflammatory response is a key figure in the complex pathogenesis of POCD [1,2,29]; however, further studies are warranted. To better illustrate the importance of this topic for researchers, the effects of numerous interesting interventions with different classes of evidence have been described in the literature on cognitive functions, such as the effects of valerian root [86], minocycline [87], statins [88], lidocaine [89], noble gas xenon [90], and others, predominantly through their anti-inflammatory activity. Because preoperative cognitive impairment is present in 20 to 46% of elderly patients, Gerriets et al. proposed that an obligatory neuropsychological evaluation prior to cardiac surgery is a good investment of time and money to detect at-risk patients [91]. Indeed, a short preoperative cognitive training regime may reduce POCD rates [4,12].
Mahanna et al. demonstrated a significant implication of selected POCD definition on the study outcome, i.e., selection of 5 different criteria to diagnosis cognitive deterioration after cardiac surgery on the same patient group resulted in POCD incidence rates between 1 to 35% [92]. Therefore, the most challenging issue will be to set a unique and validated POCD definition that includes an adequate statistical approach, a standardized neuropsychological test battery and an appropriate assessment time. To achieve this task, another conference of leading authorities in this field with the goal of agreeing upon a new updated consensus will likely be necessary and represents an imperative for conducting further investigations of this multidisciplinary topic. This standardized approach ensures the credibility of study findings, simplifies comparisons among studies and guarantees research excellence.
Conclusions
Because POCD investigators are faced with a methodological mixture of cognitive impairment definitions that may have crucial impacts on the study findings, this issue requires attention. Present evidence suggests the significant involvement of the inflammatory responses to cardiac surgical procedures in the complicated nature of the pathogenesis of POCD. However, future research must provide more insights about the role and association of inflammation with other mutually interdependent mechanisms that underlie POCD development.
Footnotes
Source of support: Departmental sources
Conflicts of interest.
None.
References
- 1.Berger M, Terrando N, Smith SK, et al. Neurocognitive function after cardiac surgery: from phenotypes to mechanisms. Anesthesiology. 2018;129:829–51. doi: 10.1097/ALN.0000000000002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhamidipati D, Goldhammer JE, Sperling MR, et al. Cognitive outcomes after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2017;31:707–18. doi: 10.1053/j.jvca.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 3.van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67:280–93. doi: 10.1111/j.1365-2044.2011.07008.x. [DOI] [PubMed] [Google Scholar]
- 4.Keage HA, Smith A, Loetscher T, Psaltis P. Cognitive outcomes of cardiovascular surgical procedures in the old: An important but neglected area. Heart Lung Circ. 2016;25:1148–53. doi: 10.1016/j.hlc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Polunina AG, Golukhova EZ, Guekht AB, et al. Cognitive dysfunction after on-pump operations: Neuropsychological characteristics and optimal core battery of tests. Stroke Res Treat. 2014;2014 doi: 10.1155/2014/302824. 302824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan X, Zhu T, Chen C, et al. Serum glial cell line-derived neurotrophic factor levels and postoperative cognitive dysfunction after surgery for rheumatic heart disease. J Thorac Cardiovasc Surg. 2018;155:958–65.e1. doi: 10.1016/j.jtcvs.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 7.Ghaffary S, Ghaeli P, Talasaz AH, et al. Effect of memantine on post-operative cognitive dysfunction after cardiac surgeries: A randomized clinical trial. Daru. 2017;25:24. doi: 10.1186/s40199-017-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinger RY, James OG, Borges-Neto S, et al. 18F-florbetapir positron emission tomography-determined cerebral beta-amyloid deposition and neurocognitive performance after cardiac surgery. Anesthesiology. 2018;128:728–44. doi: 10.1097/ALN.0000000000002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth E, Vig K, Racz K, et al. Influence of the postoperative inflammatory response on cognitive decline in elderly patients undergoing on-pump cardiac surgery: A controlled, prospective observational study. BMC Anesthesiol. 2017;17:113. doi: 10.1186/s12871-017-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kok WF, Koerts J, Tucha O, et al. Neuronal damage biomarkers in the identification of patients at risk of long-term postoperative cognitive dysfunction after cardiac surgery. Anaesthesia. 2017;72:359–69. doi: 10.1111/anae.13712. [DOI] [PubMed] [Google Scholar]
- 11.Knipp SC, Weimar C, Schlamann M, et al. Early and long-term cognitive outcome after conventional cardiac valve surgery. Interact Cardiovasc Thorac Surg. 2017;24:534–40. doi: 10.1093/icvts/ivw421. [DOI] [PubMed] [Google Scholar]
- 12.Sheth KN, Nourollahzadeh E. Neurologic complications of cardiac and vascular surgery. Handb Clin Neurol. 2017;141:573–92. doi: 10.1016/B978-0-444-63599-0.00031-4. [DOI] [PubMed] [Google Scholar]
- 13.Selnes OA, Goldsborough MA, Borowicz LM, McKhann GM. Neurobehavioural sequelae of cardiopulmonary bypass. Lancet. 1999;353:1601–6. doi: 10.1016/S0140-6736(98)07576-X. [DOI] [PubMed] [Google Scholar]
- 14.Brown CHt, Probert J, Healy R, et al. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology. 2018;129:406–16. doi: 10.1097/ALN.0000000000002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer AC, Veldhuijzen DS, Ottens TH, et al. Association between delirium and cognitive change after cardiac surgery. Br J Anaesth. 2017;119:308–15. doi: 10.1093/bja/aex053. [DOI] [PubMed] [Google Scholar]
- 16.Steinmetz J, Rasmussen LS. Peri-operative cognitive dysfunction and protection. Anaesthesia. 2016;71:58–63. doi: 10.1111/anae.13308. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Ding S, Tao X, et al. The quality of life of patients developed delirium after coronary artery bypass grafting is determined by cognitive function after discharge: A cross-sectional study. Int J Nurs Pract. 2017;23(5) doi: 10.1111/ijn.12563. [DOI] [PubMed] [Google Scholar]
- 18.Wan Y, Xu J, Ma D, et al. Postoperative impairment of cognitive function in rats: A possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–43. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Salameh A, Dhein S, Dahnert I, Klein N. Neuroprotective strategies during cardiac surgery with cardiopulmonary bypass. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111945. pii: E1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glumac S, Kardum G, Karanovic N. Reply to: Dexamethasone and postoperative cognitive decline. Eur J Anaesthesiol. 2018;35:635–36. doi: 10.1097/EJA.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 21.Evered L, Silbert B, Scott DA. Pre-existing cognitive impairment and post-operative cognitive dysfunction: Should we be talking the same language? Int Psychogeriatr. 2016;28:1053–55. doi: 10.1017/S1041610216000661. [DOI] [PubMed] [Google Scholar]
- 22.Shaw PJ, Bates D, Cartlidge NE, et al. Neurologic and neuropsychological morbidity following major surgery: Comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18:700–7. doi: 10.1161/01.str.18.4.700. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy ED, Choy KC, Alston RP, et al. Cognitive outcome after on- and off-pump coronary artery bypass grafting surgery: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2013;27:253–65. doi: 10.1053/j.jvca.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Patel N, Minhas JS, Chung EM. Intraoperative embolization and cognitive decline after cardiac surgery: A systematic review. Semin Cardiothorac Vasc Anesth. 2016;20:225–31. doi: 10.1177/1089253215626728. [DOI] [PubMed] [Google Scholar]
- 25.Lamy A, Devereaux PJ, Prabhakaran D, et al. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. 2013;368:1179–88. doi: 10.1056/NEJMoa1301228. [DOI] [PubMed] [Google Scholar]
- 26.OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine; http://www.cebm.net/index.aspx?o=5653. [Google Scholar]
- 27.Arrowsmith JE, Grocott HP, Reves JG, Newman MF. Central nervous system complications of cardiac surgery. Br J Anaesth. 2000;84:378–93. doi: 10.1093/oxfordjournals.bja.a013444. [DOI] [PubMed] [Google Scholar]
- 28.Silva FP, Schmidt AP, Valentin LS, et al. S100B protein and neuron-specific enolase as predictors of cognitive dysfunction after coronary artery bypass graft surgery: A prospective observational study. Eur J Anaesthesiol. 2016;33:681–89. doi: 10.1097/EJA.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 29.Glumac S, Kardum G, Sodic L, et al. Effects of dexamethasone on early cognitive decline after cardiac surgery: A randomised controlled trial. Eur J Anaesthesiol. 2017;34:776–84. doi: 10.1097/EJA.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 30.Kumpaitiene B, Svagzdiene M, Drigotiene I, et al. Correlation among decreased regional cerebral oxygen saturation, blood levels of brain injury biomarkers, and cognitive disorder. J Int Med Res. 2018;46:3621–29. doi: 10.1177/0300060518776545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozturk S, Sacar M, Baltalarli A, Ozturk I. Effect of the type of cardiopulmonary bypass pump flow on postoperative cognitive function in patients undergoing isolated coronary artery surgery. Anatol J Cardiol. 2016;16:875–80. doi: 10.14744/AnatolJCardiol.2015.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–95. doi: 10.1016/0003-4975(95)00106-u. [DOI] [PubMed] [Google Scholar]
- 33.Patel N, Minhas JS, Chung EM. Risk factors associated with cognitive decline after cardiac surgery: a systematic review. Cardiovasc Psychiatry Neurol. 2015;2015 doi: 10.1155/2015/370612. 370612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kneebone AC, Andrew MJ, Baker RA, Knight JL. Neuropsychologic changes after coronary artery bypass grafting: Use of reliable change indices. Ann Thorac Surg. 1998;65:1320–25. doi: 10.1016/s0003-4975(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 35.Indja B, Fanning JP, Maller JJ, et al. Neural network imaging to characterize brain injury in cardiac procedures: the emerging utility of connectomics. Br J Anaesth. 2017;118:680–88. doi: 10.1093/bja/aex088. [DOI] [PubMed] [Google Scholar]
- 36.van Dijk D, Moons KG, Nathoe HM, et al. Cognitive outcomes five years after not undergoing coronary artery bypass graft surgery. Ann Thorac Surg. 2008;85:60–64. doi: 10.1016/j.athoracsur.2007.08.068. [DOI] [PubMed] [Google Scholar]
- 37.Kadoi Y, Kawauchi C, Ide M, et al. Preoperative depression is a risk factor for postoperative short-term and long-term cognitive dysfunction in patients with diabetes mellitus. J Anesth. 2011;25:10–17. doi: 10.1007/s00540-010-1072-5. [DOI] [PubMed] [Google Scholar]
- 38.Stroobant N, Vingerhoets G. Depression, anxiety, and neuropsychological performance in coronary artery bypass graft patients: A follow-up study. Psychosomatics. 2008;49:326–31. doi: 10.1176/appi.psy.49.4.326. [DOI] [PubMed] [Google Scholar]
- 39.Thomaidou E, Argiriadou H, Vretzakis G, et al. Perioperative use of erythromycin reduces cognitive decline after coronary artery bypass grafting surgery: a pilot study. Clin Neuropharmacol. 2017;40:195–200. doi: 10.1097/WNF.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 40.Parolari A, Camera M, Alamanni F, et al. Systemic inflammation after on-pump and off-pump coronary bypass surgery: A one-month follow-up. Ann Thorac Surg. 2007;84:823–28. doi: 10.1016/j.athoracsur.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 41.Merino JG, Latour LL, Tso A, et al. Blood-brain barrier disruption after cardiac surgery. Am J Neuroradiol. 2013;34:518–23. doi: 10.3174/ajnr.A3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abrahamov D, Levran O, Naparstek S, et al. Blood–brain barrier disruption after cardiopulmonary bypass: Diagnosis and correlation to cognition. Ann Thorac Surg. 2017;104:161–69. doi: 10.1016/j.athoracsur.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 43.Royse CF, Saager L, Whitlock R, et al. Impact of methylprednisolone on postoperative quality of recovery and delirium in the steroids in cardiac surgery trial: A randomized, double-blind, placebo-controlled substudy. Anesthesiology. 2017;126:223–33. doi: 10.1097/ALN.0000000000001433. [DOI] [PubMed] [Google Scholar]
- 44.Ottens TH, Dieleman JM, Sauer AM, et al. Effects of dexamethasone on cognitive decline after cardiac surgery: A randomized clinical trial. Anesthesiology. 2014;121:492–500. doi: 10.1097/ALN.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 45.Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:1243–53. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 46.Dieleman JM, Nierich AP, Rosseel PM, et al. Intraoperative high-dose dexamethasone for cardiac surgery: A randomized controlled trial. JAMA. 2012;308:1761–67. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 47.Mu DL, Li LH, Wang DX, et al. High postoperative serum cortisol level is associated with increased risk of cognitive dysfunction early after coronary artery bypass graft surgery: A prospective cohort study. PLoS One. 2013;8:e77637. doi: 10.1371/journal.pone.0077637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lupien SJ, Maheu F, Tu M, et al. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Glumac S, Kardum G, Karanovic N. A prospective cohort evaluation of the cortisol response to cardiac surgery with occurrence of early postoperative cognitive decline. Med Sci Monit. 2018;24:977–86. doi: 10.12659/MSM.908251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu YH, Wang DX, Li LH, et al. The effects of cardiopulmonary bypass on the number of cerebral microemboli and the incidence of cognitive dysfunction after coronary artery bypass graft surgery. Anesth Analg. 2009;109:1013–22. doi: 10.1213/ane.0b013e3181aed2bb. [DOI] [PubMed] [Google Scholar]
- 51.Siepe M, Pfeiffer T, Gieringer A, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg. 2011;40:200–7. doi: 10.1016/j.ejcts.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 52.Kumpaitiene B, Svagzdiene M, Sirvinskas E, et al. Cerebrovascular autoregulation impairments during cardiac surgery with cardiopulmonary bypass are related to postoperative cognitive deterioration: Prospective observational study. Minerva Anestesiol. :2018. doi: 10.23736/S0375-9393.18.12358-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Song Z, Fu P, Chen M, Bi Q. Association of CT perfusion and postoperative cognitive dysfunction after off-pump coronary artery bypass grafting. Neurol Res. 2016;38:533–37. doi: 10.1080/01616412.2016.1187830. [DOI] [PubMed] [Google Scholar]
- 54.Nathan HJ, Rodriguez R, Wozny D, et al. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: Five-year follow-up of a randomized trial. J Thorac Cardiovasc Surg. 2007;133:1206–11. doi: 10.1016/j.jtcvs.2006.09.112. [DOI] [PubMed] [Google Scholar]
- 55.Arrowsmith JE, Dunning JL. Normothermic cardiopulmonary bypass is beneficial for cognitive brain function after coronary artery bypass grafting-a prospective randomized trial. Eur J Cardiothorac Surg. 2001;19:732–34. doi: 10.1016/s1010-7940(01)00641-8. [DOI] [PubMed] [Google Scholar]
- 56.Kin H, Ishibashi K, Nitatori T, Kawazoe K. Hippocampal neuronal death following deep hypothermic circulatory arrest in dogs: Involvement of apoptosis. Cardiovasc Surg. 1999;7:558–64. doi: 10.1016/s0967-2109(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 57.Rungatscher A, Luciani GB, Linardi D, et al. Temperature variation after rewarming from deep hypothermic circulatory arrest is associated with survival and neurologic outcome. Ther Hypothermia Temp Manag. 2017;7:101–6. doi: 10.1089/ther.2016.0037. [DOI] [PubMed] [Google Scholar]
- 58.Del Felice A, Tessari M, Formaggio E, et al. Hemoglobin concentration affects electroencephalogram during cardiopulmonary bypass: An indication for neuro-protective values. Artif Organs. 2016;40:169–75. doi: 10.1111/aor.12533. [DOI] [PubMed] [Google Scholar]
- 59.Mathew JP, Mackensen GB, Phillips-Bute B, et al. Effects of extreme hemodilution during cardiac surgery on cognitive function in the elderly. Anesthesiology. 2007;107:577–84. doi: 10.1097/01.anes.0000281896.07256.71. [DOI] [PubMed] [Google Scholar]
- 60.Lo B, Fijnheer R, Nierich AP, et al. Activation of hemostasis is associated with early cognitive decline after off-pump coronary artery bypass surgery. J Thromb Haemost. 2005;3:2114–17. doi: 10.1111/j.1538-7836.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- 61.Slater JP, Guarino T, Stack J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44. doi: 10.1016/j.athoracsur.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 62.Deschamps A, Hall R, Grocott H, et al. Cerebral oximetry monitoring to maintain normal cerebral oxygen saturation during high-risk cardiac surgery: A randomized controlled feasibility trial. Anesthesiology. 2016;124:826–36. doi: 10.1097/ALN.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 63.Chan MJ, Chung T, Glassford NJ, Bellomo R. Near-infrared spectroscopy in adult cardiac surgery patients: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2017;31:1155–65. doi: 10.1053/j.jvca.2017.02.187. [DOI] [PubMed] [Google Scholar]
- 64.Szwed K, Pawliszak W, Anisimowicz L, et al. Short-term outcome of attention and executive functions from aorta no-touch and traditional off-pump coronary artery bypass surgery. World J Biol Psychiatry. 2014;15:397–403. doi: 10.3109/15622975.2013.824611. [DOI] [PubMed] [Google Scholar]
- 65.Hammon JW, Stump DA, Butterworth JF, et al. Single crossclamp improves 6-month cognitive outcome in high-risk coronary bypass patients: The effect of reduced aortic manipulation. J Thorac Cardiovasc Surg. 2006;131:114–21. doi: 10.1016/j.jtcvs.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 66.Perez-Belmonte LM, Florido-Santiago M, Millan-Gomez M, et al. Research long-term cognitive impairment after off-pump versus on-pump cardiac surgery: Involved risk factors. J Am Med Dir Assoc. 2018;19:639–40.e1. doi: 10.1016/j.jamda.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Trubnikova O, Tarasova I, Barbarash O. The influence of low and moderate carotid stenosis on neurophysiologic status of patients undergoing on-pump coronary artery bypass grafting. Front Neurol. 2012;3:1. doi: 10.3389/fneur.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ottens TH, Hendrikse J, Nathoe HM, et al. Brain volume and cognitive function in patients with revascularized coronary artery disease. Int J Cardiol. 2017;230:80–84. doi: 10.1016/j.ijcard.2016.12.079. [DOI] [PubMed] [Google Scholar]
- 69.Selnes OA, Grega MA, Bailey MM, et al. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88:445–54. doi: 10.1016/j.athoracsur.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bornstein RA, Starling RC, Myerowitz PD, Haas GJ. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurol Scand. 1995;91:260–65. doi: 10.1111/j.1600-0404.1995.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 71.Rafnsson SB, Deary IJ, Fowkes FG. Peripheral arterial disease and cognitive function. Vasc Med. 2009;14:51–61. doi: 10.1177/1358863X08095027. [DOI] [PubMed] [Google Scholar]
- 72.Chung CC, Pimentel D, Jor’dan AJ, et al. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015;85:450–58. doi: 10.1212/WNL.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang N, Jiang R, Wang X, et al. Insulin resistance plays a potential role in postoperative cognitive dysfunction in patients following cardiac valve surgery. Brain Res. 2017;1657:377–82. doi: 10.1016/j.brainres.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 74.Thourani VH, Weintraub WS, Stein B, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045–52. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 75.Schricker T, Sato H, Beaudry T, et al. Intraoperative maintenance of normoglycemia with insulin and glucose preserves verbal learning after cardiac surgery. PLoS One. 2014;9:e99661. doi: 10.1371/journal.pone.0099661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghaffary S, Hajhossein Talasaz A, Ghaeli P, et al. Association between perioperative parameters and cognitive impairment in post-cardiac surgery patients. J Tehran Heart Cent. 2015;10:85–92. [PMC free article] [PubMed] [Google Scholar]
- 77.Ortapamuk H, Naldoken S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: Comparison with cognitive impairment. Ann Nucl Med. 2006;20:99–106. doi: 10.1007/BF02985621. [DOI] [PubMed] [Google Scholar]
- 78.Mullen KD. Review of the final report of the 1998 Working Party on definition, nomenclature and diagnosis of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25:11–16. doi: 10.1111/j.1746-6342.2006.03216.x. [DOI] [PubMed] [Google Scholar]
- 79.Olver TD, Hiemstra JA, Edwards JC, et al. Loss of female sex hormones exacerbates cerebrovascular and cognitive dysfunction in aortic banded miniswine through a neuropeptide Y-Ca2+-activated potassium channel-nitric oxide mediated mechanism. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007409. pii: e007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliveira FR, Oliveira VH, Oliveira IM, et al. Hypertension, mitral valve disease, atrial fibrillation and low education level predict delirium and worst outcome after cardiac surgery in older adults. BMC Anesthesiol. 2018;18:15. doi: 10.1186/s12871-018-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110:i98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 82.Kofke WA, Garman RH, Garman R, Rose M. Opioid neurotoxicity: Role of neurotransmitter systems. Neurol Res. 2000;22:733–37. doi: 10.1080/01616412.2000.11740748. [DOI] [PubMed] [Google Scholar]
- 83.Hudetz JA, Iqbal Z, Gandhi SD, et al. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand. 2009;53:864–72. doi: 10.1111/j.1399-6576.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 84.Chen F, Duan G, Wu Z, et al. Comparison of the cerebroprotective effect of inhalation anaesthesia and total intravenous anaesthesia in patients undergoing cardiac surgery with cardiopulmonary bypass: A systematic review and meta-analysis. BMJ Open. 2017;7:e014629. doi: 10.1136/bmjopen-2016-014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanbak M, Saricaoglu F, Akinci SB, et al. The effects of isoflurane, sevoflurane, and desflurane anesthesia on neurocognitive outcome after cardiac surgery: A pilot study. Heart Surg Forum. 2007;10:E36–41. doi: 10.1532/HSF98.20061076. [DOI] [PubMed] [Google Scholar]
- 86.Hassani S, Alipour A, Darvishi Khezri H, et al. Can Valeriana officinalis root extract prevent early postoperative cognitive dysfunction after CABG surgery? A randomized, double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2015;232:843–50. doi: 10.1007/s00213-014-3716-x. [DOI] [PubMed] [Google Scholar]
- 87.Fan L, Wang TL, Xu YC, et al. Minocycline may be useful to prevent/treat postoperative cognitive decline in elderly patients. Med Hypotheses. 2011;76:733–36. doi: 10.1016/j.mehy.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 88.Abbasi SH, Mohammadinejad P, Shahmansouri N, et al. Simvastatin versus atorvastatin for improving mild to moderate depression in post-coronary artery bypass graft patients: A double-blind, placebo-controlled, randomized trial. J Affect Disord. 2015;183:149–55. doi: 10.1016/j.jad.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 89.Leng T, Gao X, Dilger JP, Lin J. Neuroprotective effect of lidocaine: Is there clinical potential? Int J Physiol Pathophysiol Pharmacol. 2016;8:9–13. [PMC free article] [PubMed] [Google Scholar]
- 90.Al Tmimi L, Van de Velde M, Herijgers P, et al. Xenon for the prevention of postoperative delirium in cardiac surgery: Study protocol for a randomized controlled clinical trial. Trials. 2015;16:449. doi: 10.1186/s13063-015-0987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerriets T, Schwarz N, Bachmann G, et al. Evaluation of methods to predict early long-term neurobehavioral outcome after coronary artery bypass grafting. Am J Cardiol. 2010;105:1095–101. doi: 10.1016/j.amjcard.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 92.Mahanna EP, Blumenthal JA, White WD, et al. Defining neuropsychological dysfunction after coronary artery bypass grafting. Ann Thorac Surg. 1996;61:1342–47. doi: 10.1016/0003-4975(95)01095-5. [DOI] [PubMed] [Google Scholar]