Abstract
Background: Inositol polyphosphate 4-phosphatase type II (INPP4B) has been identified as a negative regulator of phosphatidyl inositol 3-kinase (PI3K)/Akt signaling in human several cancers. However, the expression, clinical significance and biological function of INPP4B in human hepatocellular carcinoma (HCC) clinical tissues and cell lines are little known.
Materials and methods: We evaluated the expression of INPP4B in 86 cases of paired human HCC samples by immunohistochemistry, and the clinical significance of INPP4B expression was analyzed. The expression of INPP4B in five HCC cell lines was detected through using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western blot analyses. The role of INPP4B gene on HCC cell proliferation, apoptosis, migration, invasion as well as epithelial-to-mesenchymal transition (EMT) and chemoresistance was examined via INPP4B mammalian expression vector and small interfering RNA (siRNA) transfection in vitro. Western blot analysis was used to explore the downstream molecules modulated by INPP4B.
Results: Immunohistochemistry analysis revealed that INPP4B was significantly downregulated in HCC tissues compared with the corresponding normal tissues. The rate of INPP4B-positive staining was markedly lower in metastatic samples than in those of non-metastatic samples. Univariate analysis showed that INPP4B expression was indicated to have a marked association with histological grades, tumor size and tumor metastasis. Moreover, INPP4B overexpression suppressed cell proliferation, migration, invasion and EMT, but induced cell apoptosis and chemosensitivity in human HCC cell lines. In contrast, INPP4B knockdown had the opposite effects on the biological behaviors of HCC cells. Furthermore, INPP4B was found to inhibit the activation of PI3K/Akt signaling in HCC cells.
Conclusion: Our findings suggest that INPP4B is a tumor suppressing gene in human HCC, and might act as a novel therapeutic target for HCC patients.
Keywords: INPP4B, hepatocellular carcinoma, proliferation, invasion, chemoresistance
Introduction
Liver cancer is the second most common cause of cancer-related death worldwide and has an incidence of approximately 8,50,000 new cases per year.1 Hepatocellular carcinoma (HCC) represents approximately 90% of all cases of primary malignancies of the liver.1 Accumulating evidence suggests that HCC is a complex disease caused by viral hepatitis (chronic hepatitis B and hepatitis C), alcoholism, non-alcoholic steatohepatitis and aflatoxin.2–4 The disease is characterized by its diagnosis at a late stage and aggressiveness, as well as poor overall outcome with dismal survival rate.5 In addition, patients with advanced HCC have a notoriously poor response rate to traditional drugs; and there is no established, effective treatment prescription at present.6,7 Hence, a better understanding of the etiology and pathogenesis of HCC may result in novel early diagnosis and therapeutic strategies.
Molecular researches have shown that a number of genetic and epigenetic abnormalities and several key signaling pathways are involved in the progression and metastasis of HCC.8–10 Various growth factors and their receptors as well as hepatitis viruses are found to mediate these pathways, such as Ras/Raf/mitogen-activated protein kinase (MAPK), Wnt/β-catenin and phosphatidyl inositol 3-kinase (PI3K)/Akt signaling cascades.11–14 Inositol polyphosphate 4-phosphatase type II (INPP4B), a phosphoinositide phosphatase, is a negative regulatory factor of the PI3K/Akt signaling.15 The identification of INPP4B as a potential tumor suppressor gene was reported in the cases of prostate cancer,16 breast and ovarian cancers,17,18 thyroid neoplasm15,19 and cervical cancer.20 Conversely, several reports indicated that INPP4B may act as an oncogene in acute myeloid leukemia (AML),21 melanoma22 and colon cancer.23 In addition, INPP4B is driving chemoresistance in AML by a novel gain-of-function mechanism independent of its phosphatase functions.21,24 Although the function and expression of INPP4B in human different cancer remain controversial, INPP4B and INPP4B-mediated PI3K/Akt pathway really play an important role in tumorigenesis and metastasis.
Recently, it has been demonstrated that miR-765 promotes HCC cell proliferation through targeting INPP4B mRNA transcripts.25 In the present study, we identify that INPP4B expression is markedly decreased in human HCC tissues compared with the adjacent non-tumorous tissues, and INPP4B downexpression is significantly associated with histological grades, tumor size and metastasis. In addition, INPP4B overexpression inhibits cell proliferation, migration, invasion, epithelial-to-mesenchymal transition (EMT), but induces cell apoptosis and chemosensitivity through the regulation of PI3K/Akt signaling in human HCC. Our findings suggest that INPP4B plays a critical role in suppressing HCC growth, metastasis and chemotherapy resistance, and may provide new insights into a novel therapeutic target.
Materials and methods
Tissue samples
Totally, 86 paired tumorous and adjacent non-tumorous liver tissues from HCC patients were obtained from the Shanghai Outdo Biotech Co. Ltd (Shanghai, China). Patients had not received radiotherapy or chemotherapy prior to surgery. All of the samples were obtained with written informed consent and analyzed anonymously. This study was approved by the Medical Ethical Committee of the Affiliated Jiangyin People’s Hospital of Southeast University Medical College. The clinical characteristics of all patients are shown in Table S1.
Table S1.
Clinical characteristics of 86 HCC patients
| Parameter | HCC patients (%) |
|---|---|
| Gender | |
| Male | 62 (72.1) |
| Female | 24 (27.9) |
| Age | |
| ≤45 | 20 (23.3) |
| >45 | 66 (76.7) |
| Histological grade | |
| Well | 21 (24.4) |
| Moderate | 41 (47.7) |
| Poor | 24 (27.9) |
| Tumor size (cm) | |
| ≤5 | 52 (60.5) |
| >5 | 34 (39.5) |
| Metastasis | |
| Absent | 70 (81.4) |
| Present | 16 (18.6) |
| HBsAg | |
| Negative | 13 (15.1) |
| Positive | 73 (84.9) |
| Cirrhosis | |
| Absent | 54 (62.8) |
| Present | 32 (37.2) |
Abbreviation: HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma.
Cell lines and culture conditions
The liver cancer cell lines Hep3B, Huh7, HepG2, SK-HEP1 and SMMC-7721 were obtained from the Cell bank of Chinese Academy of Sciences (Shanghai, China). All cells are maintained in DMEM or MEM medium (GIBCO Laboratories, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, USA) and 1% penicillin–streptomycin at 37ºC in a humidified incubator with 5% CO2.
DNA constructs and cell transfection
The full-length human INPP4B gene was amplified from the template INPP4B cDNA clone, and then was embeded in pcDNA3.1+N-HA expression vector (GenSscript Biotechnology, China). Huh7 and SK-HEP1 cells were seeded at 70% confluence and were transiently transfected with the indicated vectors with Lipofectamine® 3000 (Invitrogen, USA) following the manufacturer’s instructions. The empty pcDNA vector was used as the negative control. In addition, INPP4B-specific small interfering RNA (siRNA) oligonucleotides (Cat: SR305819, OriGene, USA) and negative control siRNA (OriGene, USA) in OptiMEM (Invitrogen, USA) were transfected into liver cancer cell lines using Lipofectamine RNAiMAX (Invitrogen, USA) according to the manufacturer’s protocol. The effective INPP4B siRNA sequence is 5ʹ-AGUACAUACAGCGAUGAAAUUGGAA-3ʹ, and the control-siRNA sequence is 5ʹ-CGUUAAUCGCGUAUAAUACGCGUAT-3ʹ.
RNA extraction and qRT-PCR
For the RNA extraction and quantitative reverse transcription polymerase chain reaction (qRT-PCR), analyses were performed as previously described.26 In brief, total RNA extraction was using TRIzol reagent (Invitrogen, USA). The obtained RNA was used to synthesize cDNA via Superscript first strand synthesis kit according to the instructions of manufacturer (TakaRa, Japan). qRT-PCR analysis was carried out using the SYBR® Premix Ex Taq™ II (TaKaRa, Japan) according to the manufacturer’s protocol. The mix was preheated at 95°C (45s), and then amplified at 95°C (10s) and 60°C (40s) for 40 cycles. The relative expression was evaluated by the comparative (2−ΔΔCT) method. The sequences of primers in this section are listed in Table S2.
Table S2.
The primer sequences
| INPP4B | Forward | 5’-AGCAACCCATGCAGACCTAC-3’ |
| Reverse | 5’-ATGCAGTTCAGGCTCTTCCC-3’ | |
| E-cadherin | Forward | 5’-GAACGCATTGCCACATACAC-3’ |
| Reverse | 5’-GAATTCGGGCTTGTTGTCAT-3’ | |
| N-cadherin | Forward | 5’-TGCCAGTGTGACTCCAACG-3’ |
| Reverse | 5’-GCAGGATGATGATGCAGAGC-3’ | |
| Twist | Forward | 5’-GGCCAGGTACATCGACTTCC-3’ |
| Reverse | 5’-CATCCTCCAGACCGAGAAGG-3’ | |
| Slug | Forward | 5’-GACTACCGCTGCTCCATTCC-3’ |
| Reverse | 5’-TGGTCCTTGGAGGAGGTGTC-3’ | |
| GAPDH | Forward | 5’-GAAGGTGAAGGTCGGAGTC-3’ |
| Reverse | 5’-GAAGATGGTGATGGGATTTC-3’ |
Western blot analysis
Western blots were performed using anti-INPP4B (Abcam, UK), anti-E-cadherin (Abcam, UK), anti-N-cadherin (Abcam, UK), anti-twist (OriGene, USA), anti-slug (OriGene, USA), anti-Cleaved caspase-3 (Cell Signaling Technology, USA), anti-Cleaved PARP (Cell Signaling Technology, USA), anti-Phospho-Akt (Invitrogen, USA), anti-Akt (Invitrogen, USA), anti-Phospho-PI3K (Sigma-Aldrich, USA), anti-PI3K (OriGene, USA), anti-p53 (Invitrogen, USA) and anti-GAPDH (Immunology Consultants Laboratory, USA) as previously described.27
Immunohistochemical assay
Paired paraformaldehyde-fixed paraffin tissue chips were subjected to immunohistochemistry (IHC) assay, and staining evaluation was performed as previously reported.27,28 Briefly, staining signal intensities according to the chromatosis intensity, no staining, light yellow, buffy and brown are scored 0, 1, 2 and 3, respectively. And the percentage of positive cells was categorized as the following grades: 0 (less than 5%), 1 (5–25%), 2 (26–50%) and 3 (>51%) accordingly. The final weighed expression score was ranged from 0 to 9 through multiplying the staining intensity score and the percentage score. The INPP4B expression levels are: 0–2, negative expression and 3–9, positive expression.
Cell proliferation and clonogenic assays
Huh7 and SK-HEP1 transfected cells were plated in 96-well plates at a density of 1×104/well with triplicate, and the cell proliferation was measured with a Cell Counting Kit-8 (CCK-8) assay kit (Dojindo, Kumamoto, Japan) for 4 days as previously described.29 About Lapatinib-induced apoptosis, Huh7 and SK-HEP1 transfected cells (1×104/well) were treated with 0 µM, 5 µM, 10 µM, 20 µM and 40 µM lobaplatin for 48 hrs. 10 μl CCK-8 solution was added to each well and incubated for additional 2.5 hrs at 37°C. The optical density was measured with an ELISA reader (BioTek, Winooski, VT, USA). The clonogenic ability was assessed by clone formation assay as described previously.30 Triplicate independent experiments were performed.
Flow cytometry analysis
Cell apoptosis assay was performed using the Annexin V-propidium iodide (PI) apoptosis detection kit (Biouniquer, China) following the manufacturer’s instructions. Briefly, cells were harvested and washed with cold phosphate-buffered saline (PBS) twice, incubated with Annexin V-Fluorescein isothiocyanate (FITC) and PI for 10 mins at room temperature without light, and determined by the flow cytometry analysis. The data were analyzed by ModFit 3.3 software.
Scratch wound-healing and invasion assays
Scratch wound-healing was used to detect cell migration and performed as previously described.26 Briefly, Huh7 and SK-HEP1 transfected cells were seeded in 6-well plates at 5×105/well. Then, micropipette tip was made across the center of cell monolayer, and washed with PBS to remove debris. After 12 hrs, images were taken under a microscope and migrating cells were counted. Invasion assays were performed as previously reported.28
Immunofluorescence
INPP4B-overexpression Huh7/SK-HEP1 and corresponding control cells were digested and attached to coverslips, and then fixed with 4% paraformaldehyde solution for 30 mins. All samples were incubated 30 mins with TritonX-100. Then, the samples were blocked with 5% bovine serum albumin (BSA) and incubated with the following primary antibodies overnight at 4ºC: rabbit anti-E-cadherin (1:200, Abcam, UK) and mouse anti-N-cadherin (1:200, Abcam, UK). After washed with PBS twice, the samples were incubated with Alexa Fluor-594 goat anti-rabbit (1:500, Invitrogen, USA) and Alexa Fluor-488 goat anti-mouse (1:500, Invitrogen, USA) secondary antibodies for 30 mins at 37ºC. After washing, nuclei were stained with DAPI. Finally, samples were observed and photographed under an inverted fluorescence microscope.
Statistical analysis
The independent Student’s t-test for two groups or one-way analysis of variance (ANOVA) for three or more groups was used to evaluate the statistical significance. To determine correlations between variables, Pearson’s correlation coefficient was calculated. A P-value <0.05 was considered statistically significant. Statistical analyses were computed using SPSS 19.0 and GraphPad Prism 5.0. All values are represented as the mean ± SEM from three independent experiments.
Results
INPP4B expression is frequently decreased in human liver cancer tissues
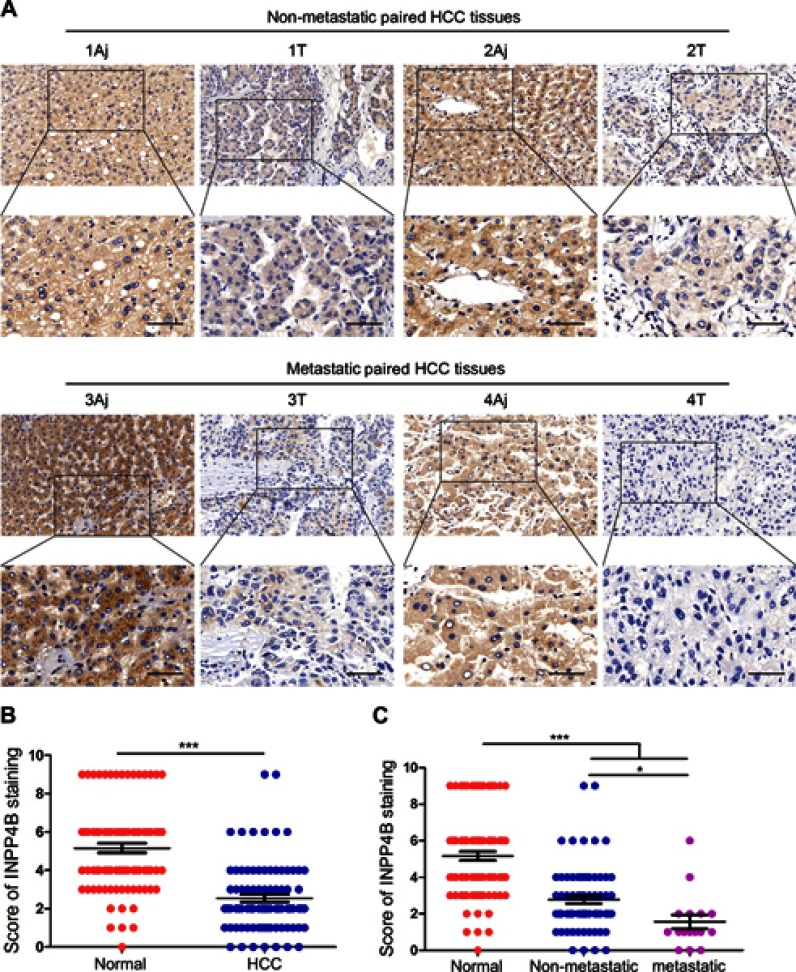
To explore whether there is a difference in INPP4B expression between normal liver tissues and HCC tissues, 86 pairs of HCC and matched tumor-adjacent samples were analyzed by IHC analysis. As a result, INPP4B staining was predominantly located in the cell membrane and cytoplasm (Figure 1A). The level of INPP4B expression was evidently higher in normal liver tissues than in HCC tissues (Figure 1A and B; Table S3). In addition, we found that INPP4B was more highly expressed in non-metastatic HCC samples than in those with metastasis (Figure 1A and C). The variables tested on univariate analysis showed that INPP4B expression was markedly associated with histological grades, tumor size and metastasis (Table 1). These data suggest that impaired expression of INPP4B may play an important role in the progression of human HCC.
Figure 1.
INPP4B is markedly downregulated in HCC tissues. (A) IHC analysis of INPP4B expression in 86 paired human HCC tissues. Representative pictures. Aj, adjacent normal tissue; T, HCC tissue. (B and C) Results of immunohistochemical staining were evaluated by the staining scores in different HCC tissues. Scale bar, 100 μm. *P<0.05; ***P<0.001.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; IHC, immunohistochemistry; HCC, hepatocellular carcinoma.
Table S3.
INPP4B is significantly downexpressed in HCC tissues
| Groups | Cases | INPP4B expression | P-value | ||
|---|---|---|---|---|---|
| Negative | Positive | Positive rate | |||
| Hepatocellular carcinoma | 86 | 52 | 34 | 39.54% | <0.001 |
| Adjacent normal tissues | 86 | 9 | 77 | 89.53% | |
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma.
Table 1.
Correlations between INPP4B expression and clinicopathological factors in 86 HCC patients
| Characteristics | Total (N) | INPP4B expression | P-value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Gender | 0.8102 | |||
| Male | 62 | 37 | 25 | |
| Female | 24 | 15 | 9 | |
| Age | 0.3195 | |||
| ≤45 | 20 | 14 | 6 | |
| >45 | 66 | 38 | 28 | |
| Histological grade | 0.0014* | |||
| Well-moderate | 62 | 31 | 31 | |
| Poor | 24 | 21 | 3 | |
| Tumor size (cm) | 0.0031* | |||
| ≤5 | 52 | 38 | 14 | |
| >5 | 34 | 14 | 20 | |
| Metastasis | 0.0142* | |||
| Absent | 70 | 38 | 32 | |
| Present | 16 | 14 | 2 | |
| HBsAg | 0.9316 | |||
| Negative | 13 | 8 | 5 | |
| Positive | 73 | 44 | 29 | |
| Cirrhosis | 0.7664 | |||
| Absent | 54 | 32 | 22 | |
| Present | 32 | 20 | 12 | |
Note: *P<0.05.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, human hepatocellular carcinoma; HBsAg, hepatitis B surface antigen.
INPP4B expression in human HCC cell lines
Next, the level of INPP4B in five human HCC cell lines, HepG2, Hep3B, Huh7, SK-HEP1 and SMMC-7721, was examined by qRT-PCR and western blot analyses. As a result, the lowest level of INPP4B expression was found in HepG2 cells, while the highest level was found in Hep3B cells (Figure 2). Considering that Huh7 and SK-HEP1 cells have a moderate level of INPP4B expression, we chose to upregulate and silence INPP4B expression in these cell lines. Then, we explore the biological function of INPP4B on HCC cells.
Figure 2.
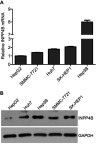
The level of INPP4B in HCC cell lines. (A) The expression of INPP4B mRNA in fiver human HCC cell lines was assessed by qRT-PCR. (B) Western blot analysis of INPP4B protein expression in different HCC cell lines.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
INPP4B inhibits cell proliferation and colony formation
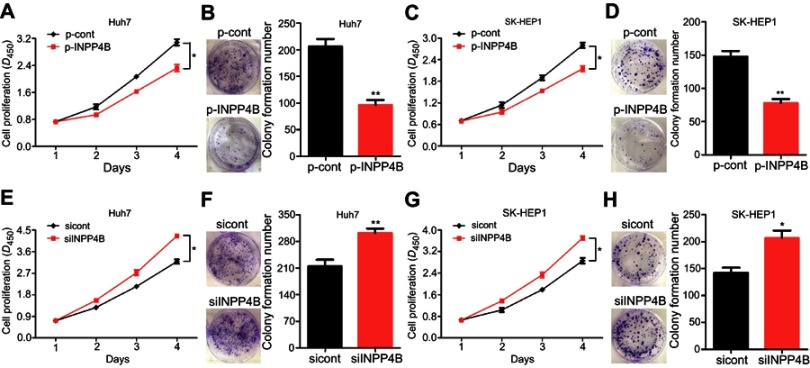
Subsequently, we constructed an INPP4B-overexpression vector (named p-INPP4B) and a negative control vector (named p-Cont), and then transiently transfected into human HCC cell lines Huh7 and SK-HEP1, respectively. To determine the effects of INPP4B on HCC cells growth in vitro, we performed CCK-8 and colonigenic assays. As a result, INPP4B overexpression obviously inhibited Huh7 cells proliferation and colony formation (Figure 3A and B). Similar results had also been shown in SK-HEP1 cells (Figure 3C and D). By contrast, INPP4B knockdown by siRNA markedly enhanced cell proliferation and colony formation (Figure 3E–H). Collectively, these findings indicate that INPP4B suppresses HCC cell viability and colony formation.
Figure 3.
INPP4B inhibits HCC cell proliferation and colonigenic ability in vitro. (A) The proliferation ability of Huh7 cells after transfected with p-Cont and p-INPP4B vectors was detected by CCK-8 assay. (B) The colony formation of Huh7 cells was significantly decreased in p-INPP4B group compared with p-Cont group. (C and D) The proliferation and colonigenic ability of SK-HEP1 cells after transfected with p-Cont and p-INPP4B vectors was assessed (two clones, A and B). (E) Silenced INPP4B expression obviously enhanced the proliferation ability of Huh7 cells. (F) The colony formation analysis of INPP4B knockdown and negative control Huh7 cells. (G and H) The proliferation and colonigenic ability of SK-HEP1 cells was assessed after transfected with specific siRNA for INPP4B and negative control siRNA (two clones, E and F). The data are representative of at least three different experiments ± SEM. *P<0.05; **P<0.01.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma; CCK-8, Cell Counting Kit-8; siRNA, small interfering RNA.
INPP4B induces apoptosis and enhances the chemosensitivity of HCC cell
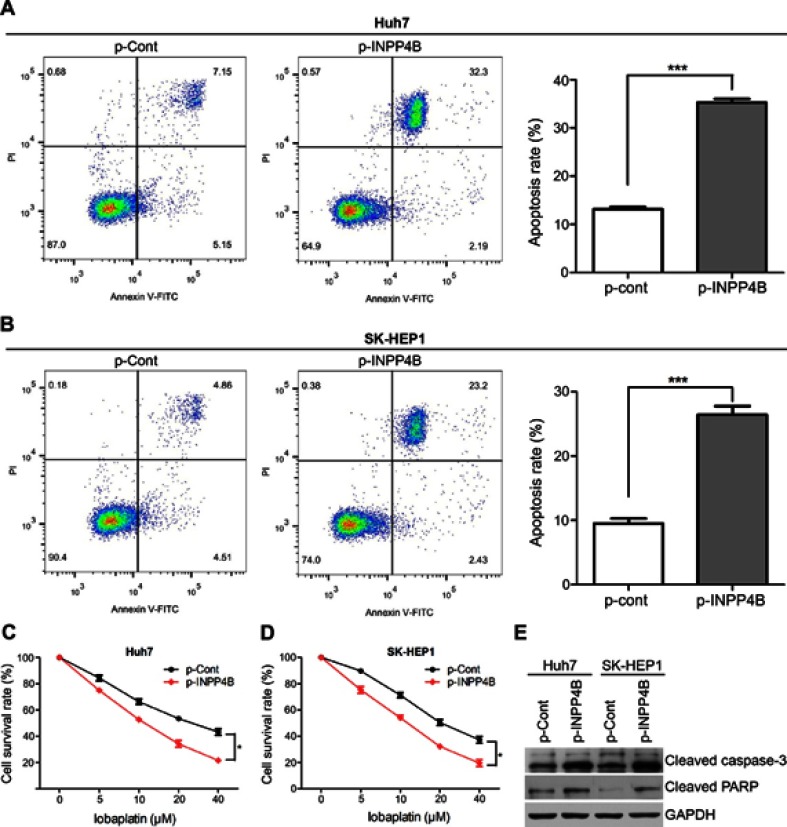
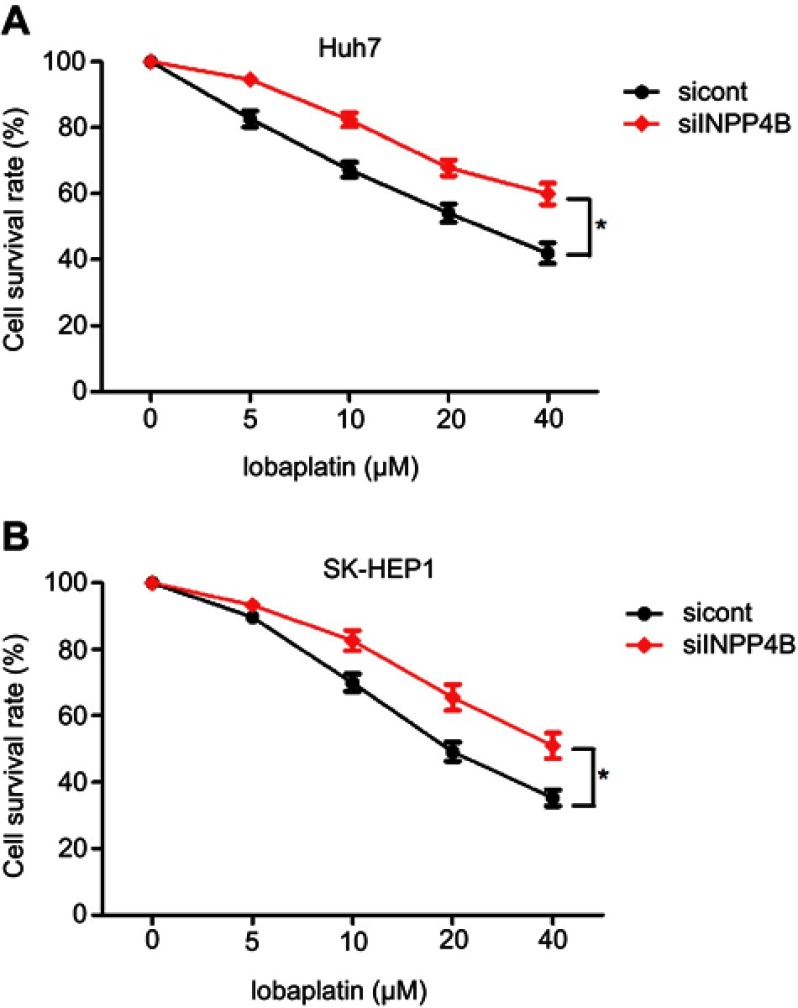
We next detected the effect of INPP4B on apoptosis and chemosensitivity of HCC cells. For the apoptosis assays, Huh7 and SK-HEP1 cells were harvested after 48 hrs of incubation, stained with 50 μg/mL PI and FITC and subsequently analyzed via flow cytometry. INPP4B overexpression resulted in a significant increase in the percentages of cells undergoing apoptosis (Figure 4A and B). Lapatinib, a tyrosine kinase inhibitor, has been used in HCC clinical treatment and cell culture studies.31,32 To explore the effects of INPP4B on the chemosensitivity of HCC cells, various lobaplatin concentrations were adopted. Huh7 and SK-HEP1 cells were transfected with INPP4B-overexpression vectors 48 hrs before the treatment of lobaplatin, and the anti-proliferative effects were measured using the CCK-8 assay the treatment with lobaplatin for 48 hrs. The results revealed that the INPP4B overexpression noticeably induced the chemosensitivity of HCC cells to lobaplatin-based therapy (Figure 4C and D), whereas INPP4B knockdown had the opposite effect (Figure S1). Considering poly ADP-ribose polymerase (PARP) and caspase-3 are two important molecules in death receptor-mediated apoptosis pathways,33 we further detected the expression of these factors in HCC cells treated with lobaplatin. As a result, an increased activating cleavage of PARP and caspase-3 was induced by lobaplatin in INPP4B-overexpression HCC cells (Figure 4E). These results reveal that INPP4B expression induces apoptosis and plays a favorite role in the chemosensitivity of HCC cells.
Figure 4.
INPP4B promotes apoptosis and chemosensitivity of HCC cell. (A and B) Induction of apoptosis in INPP4B overexpression Huh7 and SK-HEP1 cells compared with the control groups. (C and D) INPP4B overexpression induced lobaplatin-induced anti-proliferative effects in Huh7 and SK-HEP1 cells via CCK-8 assays. (E) Western blot analysis for cleavage of caspase-3 and PARP in INPP4B overexpression Huh7 and SK-HEP1 cells and corresponding control groups after treated with lobaplatin (20 µM) for 48 hrs. The data are representative of at least three different experiments ± SEM. *P<0.05; ***P<0.001.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma; CCK-8, Cell Counting Kit-8; PARP, poly ADP-ribose polymerase.
Figure S1.
INPP4B knockdown induces the chemoresistance of HCC cell. (A) Downregulation of INPP4B inhibits lobaplatin-induced anti-proliferative effects in Huh7 cell. (B) INPP4B knockdown promotes resistance of lobaplatin-based treatment in SK-HEP1 cells. The data are representative of at least three different experiments ± SEM. *P<0.05.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma.
INPP4B suppresses cell migration and invasion
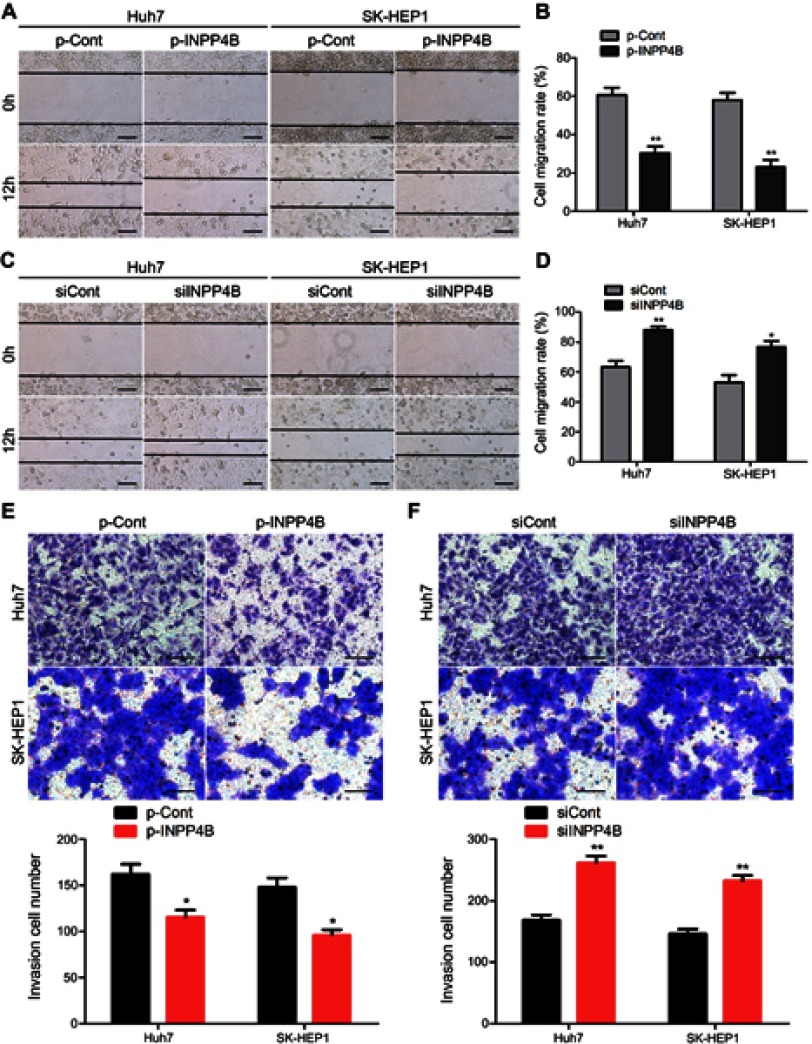
To explore whether INPP4B affects the metastatic phenotype of HCC cells, Huh7 and SK-HEP1 cells were transfected with INPP4B-overexpression vectors and specific siRNA. Scratch wound-healing assays confirmed that overexpression of INPP4B obviously inhibited cell migration (Figure 5A and B). Conversely, downreglation of INPP4B significantly promoted the migration of Huh7 and SK-HEP1 cells (Figure 5C and D). Transwell assays demonstrated that INPP4B overexpression markedly suppressed cell invasion, whereas INPP4B knockdown had the opposite effects on HCC cell invasion (Figure 5E and F). These results suggest that INPP4B inhibits HCC cells metastasis in vitro.
Figure 5.
INPP4B reduces HCC cell migration and invasion in vitro. (A) The migration of Huh7 and SK-HEP1 cells after transfected with p-Cont and p-INPP4B vectors was detected by scratch wound-healing assays. (B) The cell migration rate in INPP4B overexpression and corresponding control groups was statistically analyzed. (C and D) INPP4B knockdown could significantly promote the migration of Huh7 and SK-HEP1 cells (two clones, A and B). (E) Invasion assays of Huh7 and SK-HEP1 cells after transfected with p-Cont and p-INPP4B vectors. (F) INPP4B knockdown could enhance the invasion of Huh7 and SK-HEP1 cells. The data are representative of at least three different experiments ± SEM. Scale bar, 100 μm. *P<0.05; **P<0.01.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma.
INPP4B overexpression inhibits cell EMT in vitro
The EMT programme has a crucial role in the progression and metastasis of human HCC.34 EMT is a complex process that could acquire mesenchymal phenotypes with increased migratory and invasive capabilities.35 During the process, epithelial markers E-cadherin and β-catenin is decreased, whereas the expression of mesenchymal markers (such as N-cadherin and fibronectin) is increased.36,37 In addition, several multipotent transcription factors (TFs) such as snail, twist and zeb families are involved in the regulation of EMT programme and tumor metastasis.38
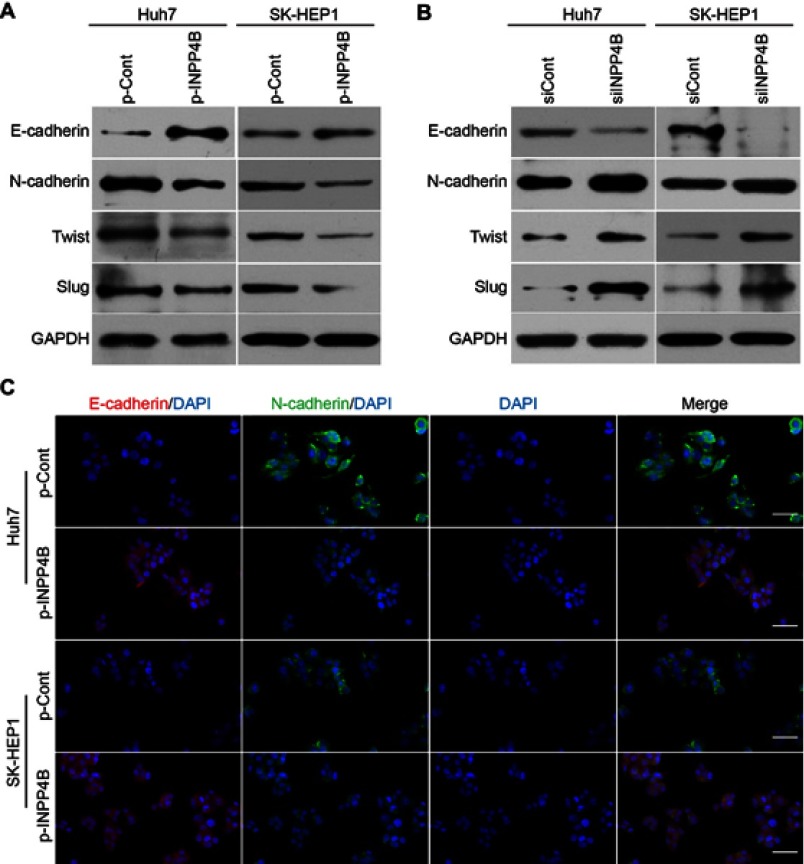
To explore the potential role of INPP4B in EMT process of HCC cells, we assessed the expression of EMT markers in INPP4B-overexpression and knockdown HCC cells. Western blot and qRT-PCR analysis showed a marked decrease in the expression of N-cadherin and a significant increase in the expression of E-cadherin in INPP4B-overpression Huh7 and SK-HEP1 cells, compared with the corresponding control cells (Figure 6A; Figure S2 A and B). Conversely, INPP4B knockdown resulted in a decrease in the expression of E-cadherin and an increase in the expression of N-cadherin in Huh7 and SK-HEP1 cells (Figure 6B; Figure S2C and D). These changes in the expression of E-cadherin and N-cadherin were confirmed by an immunofluorescent assay (Figure 6C). Additionally, INPP4B overexpression significantly inhibited the expression of multipotent TFs such as twist and slug, whereas INPP4B knockdown had the opposite effect (Figure 6A and B; Figure S2). These data indicate that INPP4B plays a suppressive role in the EMT-derived invasive phenotype in HCC cells.
Figure 6.
INPP4B inhibits EMT in HCC cells. (A) Western blot showing a higher E-cadherin expression and a lower N-cadherin, twist and slug expression in INPP4B overexpression Huh7 and SK-HEP1 cells than that of the corresponding control groups. (B) Western blot analyses of E-cadherin, N-cadherin, twist and slug expression in INPP4B knockdown Huh7/SK-HEP1 cells and the corresponding control cells. (C) Immunofluorescence assays for E-cadherin and N-cadherin expression in INPP4B overexpression Huh7/SK-HEP1 cells and the corresponding control cells. Scale bar, 150 μm.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma; EMT, epithelial-to-mesenchymal transition.
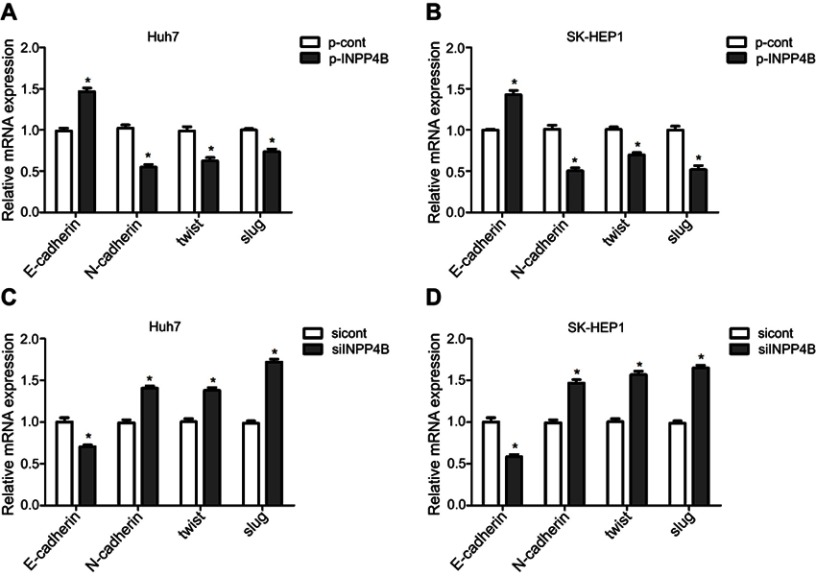
Figure S2.
INPP4B expression reduces EMT of HCC cells. (A and B) qRT-PCR analysis showing relative increased level of E-cadherin mRNA and decreased level of N-cadherin, twist and slug mRNA in INPP4B overexpression Huh7 and SK-HEP1 cells compared with the corresponding control groups. (C and D) The mRNA expression of E-cadherin, N-cadherin, twist and slug in INPP4B knockdown Huh7/SK-HEP1 cells and the corresponding control cells was detected by qRT-PCR. The data are representative of at least three different experiments ± SEM. *P<0.05.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma; EMT, epithelial-to-mesenchymal transition; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
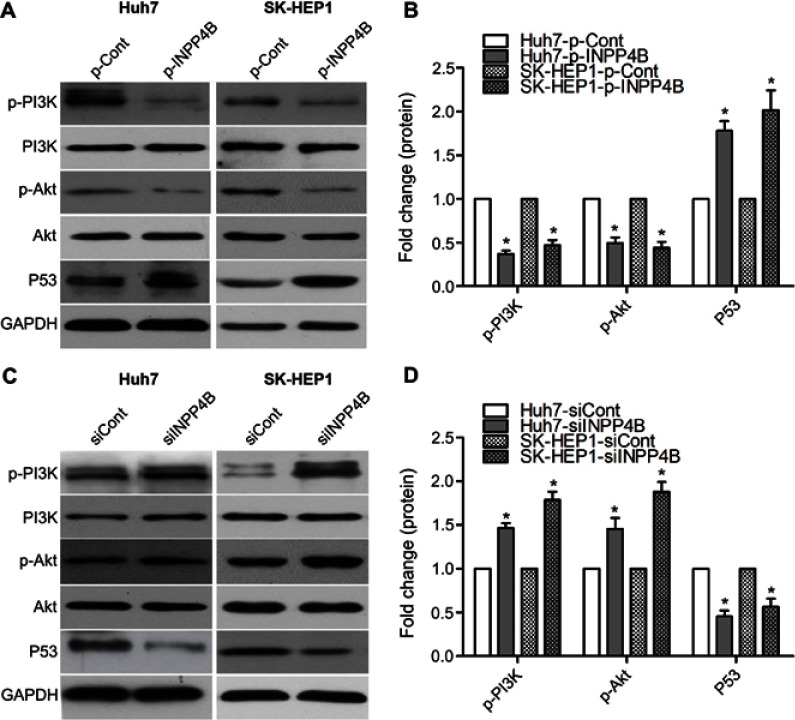
INPP4B negatively regulates PI3K/Akt signaling in HCC cell
It has been shown that INPP4B is a negative regulatory factor of PI3K/Akt pathway.15 To further explore the potential mechanism of INPP4B inhibiting HCC cell growth and metastasis, we detected its downstream effectors, PI3K and Akt. INPP4B overexpression in Huh7 and SK-HEP1 cell lines markedly decreased phosphorylation of PI3K and Akt (Figure 7A and B), whereas INPP4B knockdown had the opposite effect (Figure 7C and D). p53 is well established as a tumor suppressor and regulated by the PI3K/Akt signaling.39 Moreover, an increased p53 expression was found in INPP4B-overexpression cells compared with the control cells, but a decreased p53 expression in INPP4B silenced cells compared with the control cells (Figure 7). As shown in Figure 6A and B, INPP4B was found to affect the expression of twist and slug in HCC cells. These data suggest that INPP4B reduces HCC cells growth and metastasis via regulating the PI3K/Akt pathway and its downstream-related TFs.
Figure 7.
INPP4B negatively regulates PI3K/Akt signaling in HCC cell. (A) INPP4B overexpression reduced the activation of PI3K/Akt pathway and the expression of p53 in human HCC cell lines. (B) The levels of phosphor‑PI3K, phosphor‑Akt, p53 were calculated in INPP4B-overexpression and control cells. (C) INPP4B knockdown induced the activation of PI3K/Akt pathway and the expression of p53 in human HCC cell lines. (D) The levels of phosphor‑PI3K, phosphor‑Akt, p53 were calculated in INPP4B knockdown and control cells. The data are representative of at least three different experiments ± SEM. *P<0.05.
Abbreviations: INPP4B, inositol polyphosphate 4-phosphatase type II; HCC, hepatocellular carcinoma; PI3K, phosphatidyl inositol 3-kinase.
Discussion
For a long time, studies about INPP4B have mainly focused on its suppressive role in the regulation of PI3K/Akt signaling and cell growth. Recently, several researches suggest that INPP4B exerted dual effects on tumorigenesis and metastasis in different cancers.15–23 A previous report found that miR-765 promoted HCC cell proliferation by downregulating INPP4B expression.25 However, the expression of INPP4B in human HCC samples and its biological function on HCC cell apoptosis, chemosensitivity, invasion and EMT is still little known. In the present study, we confirmed that INPP4B was significantly downregulated in HCC tissues. In addition, INPP4B was found to inhibit cell proliferation, migration, invasion and EMT, while induce cell apoptosis and chemosensitivity in human HCC by deactivation of the PI3K/Akt pathway.
INPP4B has been frequently identified to be decreased in the cases of human prostate, breast and ovarian cancers.16,17,40 Conversely, there are several reports indicating that INPP4B was overexpressed in AML,21 melanoma22 and colon cancer.23 In this study, we demonstrated the loss expression of INPP4B in most HCC tissues compared with the corresponding normal liver tissues. In addition, the level of INPP4B expression was higher in non-metastatic HCC samples than in those with metastasis. Univariate analysis showed that downregulation of INPP4B expression was obviously associated with clinical parameters, including histological grades, tumor size and metastasis (all P<0.05). Our results provide new evidence that INPP4B may act as a tumor suppressive role in the progression and metastasis of human HCC.
Of note, a recent study has shown that miR-765 enhanced cell proliferation through targeting INPP4B expression in human HCC.25 Additionally, INPP4B expression was indicated to have marked association with chemoresistance in AML.21,24 Then, we confirmed that INPP4B overexpression significantly suppressed cell proliferation, but played a favorite role in cell apoptosis and chemosensitivity in HCC. By contrast, INPP4B knockdown promoted cell proliferation, but reduced apoptosis and chemosensitivity of HCC cells. Further studies had shown that the upregulation of INPP4B obviously inhibited cell migration and invasion, whereas the downregulation of INPP4B had the opposite effects.
Of further interest, we detected the expression of EMT-related marks and multipotent TFs in INPP4B-overexpression/knockdown Huh7 and SK-HEP1 cells, and the corresponding control cells to explore the role of INPP4B in the EMT of HCC cells. Obviously, INPP4B expression positively regulated the expression of E-cadherin, but negatively regulated the expression of N-cadherin and EMT-related multipotent TFs, such as twist and slug. These findings shed new light on the role of INPP4B in suppressing cancer cells' invasion via the regulation of EMT.
It has been identified that INPP4B affects cancer cell growth and metastasis by regulating the activation of PI3K/Akt signaling.19,20,23 To further investigate how INPP4B was involved in cell proliferation and migration of human HCC, we detected the downstream targets modulated by INPP4B. As a result, INPP4B overexpression induced the deactivation of PI3K/Akt pathway, whereas INPP4B knockdown had the opposite effects in human HCC cells. In addition, p53 inactivation is a great risk factor for human HCC,41 which is one of the important downstream TFs of PI3K/Akt pathway.39 Fortunately, increased expression of p53 was found in INPP4B-overexpression HCC cell lines. These data suggested that INPP4B suppressed cell growth and metastasis through modulating the downstream TFs including twist, slug and p53 regulated by PI3K/Akt signaling in human HCC.
Conclusion
Our study indicates that INPP4B expression is downregulated in human HCC tissues, and significantly related with the progression of HCC. In addition, INPP4B expression inhibits cell growth, metastasis and chemoresistance in human HCC through the deactivation of PI3K/Akt signaling. The present study demonstrated that INPP4B might be an attractive target for therapeutic intervention against the growth, metastases and chemoresistance of human HCC.
Acknowledgments
This study was supported by grants from the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX17_0179), the Scientific Research Foundation of Graduate School of Southeast University (YBJJ1798) and the Scientific Research Projects from Wuxi City Commission of Health and Family Planning (MS201642).
Author contributions
Chenbo Ding conceived and designed the experiments. Wendong Tang, Liwen Yang, Taoyu Yang, Min Liu, Yanjie Zhou, Jiang Lin and Ke Wang performed the experiments and analyzed the data. Chenbo Ding and Liwen Yang wrote the paper. Ke Wang and Chenbo Ding supervised the entire experimental work. All authors have read and approved the final manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 2.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353(9160):1253–1257. doi: 10.1016/S0140-6736(98)09148-X [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5 Suppl 2):S72–S78. doi: 10.1097/00004836-200211002-00002 [DOI] [PubMed] [Google Scholar]
- 4.Della Corte C, Aghemo A, Colombo M. Individualized hepatocellular carcinoma risk: the challenges for designing successful chemoprevention strategies. World J Gastroenterol. 2013;19(9):1359–1371. doi: 10.3748/wjg.v19.i9.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016;22(1):7–17. doi: 10.3350/cmh.2016.22.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 7.Khemlina G, Ikeda S, Kurzrock R. The biology of hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16(1):149. doi: 10.1186/s12943-017-0712-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Jiang L, Guan XY. The genetic and epigenetic alterations in human hepatocellular carcinoma: a recent update. Protein Cell. 2014;5(9):673–691. doi: 10.1007/s13238-014-0065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moeini A, Cornellà H, Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer. 2012;1(2):83–93. doi: 10.1159/000342405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda M, Sugimoto H, Kodera Y. Genetic and epigenetic aspects of initiation and progression of hepatocellular carcinoma. World J Gastroenterol. 2015;21(37):10584–10597. doi: 10.3748/wjg.v21.i37.10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benn J, Schneider RJ. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci U S A. 1994;91(22):10350–10354. doi: 10.1073/pnas.91.22.10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology. 2004;39(6):1683–1693. doi: 10.1002/hep.20245 [DOI] [PubMed] [Google Scholar]
- 13.Lee YI, Kang-Park S, Do SI, Lee YI. The hepatitis B virus-X protein activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2001;276(20):16969–16977. doi: 10.1074/jbc.M011263200 [DOI] [PubMed] [Google Scholar]
- 14.Chuma M, Terashita K, Sakamoto N. New molecularly targeted therapies against advanced hepatocellular carcinoma: from molecular pathogenesis to clinical trials and future directions. Hepatol Res. 2015;45(10):E1–E11. doi: 10.1111/hepr.12459 [DOI] [PubMed] [Google Scholar]
- 15.Kofuji S, Kimura H, Nakanishi H, et al. INPP4B is a PtdIns(3,4,5)P3 phosphatase that can act as a tumor suppressor. Cancer Discov. 2015;5(7):730–739. doi: 10.1158/2159-8290.CD-14-1329 [DOI] [PubMed] [Google Scholar]
- 16.Hodgson MC, Shao LJ, Frolov A, et al. Decreased expression and androgen regulation of the tumor suppressor gene INPP4B in prostate cancer. Cancer Res. 2011;71(2):572–582. doi: 10.1158/0008-5472.CAN-10-2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedele CG, Ooms LM, Ho M, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci U S A. 2010;107(51):22231–22236. doi:10.1073/pnas.1015245107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16(2):115–125. doi: 10.1016/j.ccr.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Chew C, Lunardi A, Gulluni F, et al. In vivo role of iNPP4B in tumor and metastasis suppression through regulation of PI3K-AKT signaling at endosomes. Cancer Discov. 2015;5(7):740–751. doi: 10.1158/2159-8290.CD-14-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Sun Z, Qi M, et al. INPP4B restrains cell proliferation and metastasis via regulation of the PI3K/AKT/SGK pathway. J Cell Mol Med. 2018;22(5):2935–2943. doi: 10.1111/jcmm.13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzneladze I, He R, Woolley JF, et al. INPP4B overexpression is associated with poor clinical outcome and therapy resistance in acute myeloid leukemia. Leukemia. 2015;29(7):1485–1495. doi:10.1038/leu.2015.51 [DOI] [PubMed] [Google Scholar]
- 22.Chi MN, Guo ST, Wilmott JS, et al. INPP4B is upregulated and functions as an oncogenic driver through SGK3 in a subset of melanomas. Oncotarget. 2015;6(37):39891–39907. doi: 10.18632/oncotarget.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo ST, Chi MN, Yang RH, et al. INPP4B is an oncogenic regulator in human colon cancer. Oncogene. 2016;35(23):3049–3061. doi: 10.1038/onc.2015.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rijal S, Fleming S, Cummings N, et al. Inositol polyphosphate 4-phosphatase II (INPP4B) is associated with chemoresistance and poor outcome in AML. Blood. 2015;125(18):2815–2824. doi: 10.1182/blood-2014-09-603555 [DOI] [PubMed] [Google Scholar]
- 25.Xie BH, He X, Hua RX, et al. Mir-765 promotes cell proliferation by downregulating INPP4B expression in human hepatocellular carcinoma. Cancer Biomark. 2016;16(3):405–413. doi: 10.3233/CBM-160579 [DOI] [PubMed] [Google Scholar]
- 26.Ding C, Tang W, Fan X, et al. Overexpression of PEAK1 contributes to epithelial-mesenchymal transition and tumor metastasis in lung cancer through modulating ERK1/2 and JAK2 signaling. Cell Death Dis. 2018;9(8):802. doi: 10.1038/s41419-018-0817-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding C, Luo J, Yu W, et al. Gab2 is a novel prognostic factor for colorectal cancer patients. Int J Clin Exp Pathol. 2015;8(3):2779–2786. [PMC free article] [PubMed] [Google Scholar]
- 28.Ding C, Luo J, Li L, et al. Gab2 facilitates epithelial-to-mesenchymal transition via the MEK/ERK/MMP signaling in colorectal cancer. J Exp Clin Cancer Res. 2016;35:5. doi: 10.1186/s13046-015-0280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding C, Li L, Yang T, Fan X, Wu G. Combined application of anti-VEGF and anti-EGFR attenuates the growth and angiogenesis of colorectal cancer mainly through suppressing AKT and ERK signaling in mice model. BMC Cancer. 2016;16(1):791. doi: 10.1186/s12885-016-2834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding C, Luo J, Fan X, et al. Elevated Gab2 induces tumor growth and angiogenesis in colorectal cancer through upregulating VEGF levels. J Exp Clin Cancer Res. 2017;36(1):56. doi: 10.1186/s13046-017-0524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekaii-Saab T, Markowitz J, Prescott N, et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res. 2009;15(18):5895–5901. doi: 10.1158/1078-0432.CCR-09-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding D, Huang H, Jiang W, et al. Reticulocalbin-2 enhances hepatocellular carcinoma proliferation via modulating the EGFR-ERK pathway. Oncogene. 2017;36(48):6691–6700. doi: 10.1038/onc.2017.230 [DOI] [PubMed] [Google Scholar]
- 33.Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171(8):2000–2016. doi: 10.1111/bph.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan L, Xu F, Dai CL. Relationship between epithelial-to-mesenchymal transition and the inflammatory microenvironment of hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37(1):203. doi: 10.1186/s13046-018-0887-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33(14):1755–1763. doi: 10.1038/onc.2013.128 [DOI] [PubMed] [Google Scholar]
- 36.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ansieau S, Collin G, Hill L. EMT or EMT-promoting transcription factors, where to focus the light? Front Oncol. 2014;4:353. doi: 10.3389/fonc.2014.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simabuco FM, Morale MG, Pavan ICB, et al. p53 and metabolism: from mechanism to therapeutics. Oncotarget. 2018;9(34):23780–23823. doi: 10.18632/oncotarget.25267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salmena L, Shaw P, Fans I, et al. Prognostic value of INPP4B protein immunohistochemistry in ovarian cancer. Eur J Gynaecol Oncol. 2015;36(3):260–267. [PubMed] [Google Scholar]
- 41.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 Suppl):S84–S101. doi: 10.1016/j.jhep.2016.02.021 [DOI] [PubMed] [Google Scholar]