Abstract
Background
Little is known about the historic and current risk of Zika virus (ZIKV) infection in Southeast Asia, where the mosquito vector is widespread and other arboviruses circulate endemically. The aim of the study is to explore the long-term circulation of ZIKV in Thailand.
Methods
Centralized ZIKV surveillance began in Thailand in January 2016. Between January 2016 and December 2017, suspected Zika cases had biological samples (serum, plasma, urine) tested for confirmation through PCR. We analyzed the spatial and age distribution of cases. We constructed time-resolved phylogenetic trees using genomes from Thailand and elsewhere to estimate when ZIKV was first introduced.
Findings
There were 368 confirmed cases out of 1,717 symptomatic individuals tested. Cases came from throughout the year and from 29 of the 76 Thai provinces. Individuals had 2.8 (95%CI: 2.3-3.6) times the odds of testing positive if they came from the same district and were sick within the same year of a confirmed case relative to the odds of testing positive anywhere, consistent with focal transmission. The probability of cases being <10y was 0.99 (95%CI: 0.72-1.30) times the probability of being that age in the underlying population. This rose to 1.62 (95%CI 1.33-1.92) among 21-30y and fell to 0.53 (95%CI: 0.40-0.66) for those >50y. This age distribution is consistent with that observed in the ZIKV epidemic in Colombia. Phylogenetic reconstructions suggest persistent circulation within Thailand since at least 2002.
Interpretation
We find evidence that ZIKV has circulated at a low but sustained level for at least 16 years, suggesting that ZIKV can adapt to persistent endemic transmission. Health systems need to adapt to cope with regular occurrences of the severe complications associated with infection.
Introduction
Zika virus (ZIKV) is a flavivirus spread by Aedes mosquitoes that was first discovered in Uganda in 1947 with the first reported presence in Asia in 1966 in Malaysia1,2. Infection in humans usually causes mild disease or no symptoms at all3. However, ZIKV infection has been shown to be associated with the development of Guillain Barré Syndrome and infection during the early stages of pregnancy has been linked to Congential Microcephaly Syndrome4,5. Much of the current interest in ZIKV has been focused on South America, where the virus spread widely following its introduction in 20136. The situation in Asia is much less clear.
It has been proposed that ZIKV has circulated silently in the region for years, as has previously been shown with another arbovirus, chikungunya virus7,8. The poor current understanding of the long-term transmission patterns of ZIKV is not surprising. Even when symptoms do occur, laboratory testing for ZIKV is rarely performed and clinical misdiagnosis is common, especially given the potential for serological cross-reactivity with dengue virus (DENV) which has circulated endemically in much of Southeast Asia for decades9. In support of sustained transmission are a handful of viral isolates obtained in the 1960s in Malaysia, from 2006 in Thailand and from 2012 in the Philippines, all prior to the emergence of the Asian lineage into South America6,10-12. Seroprevalence studies in the 1950s also showed some population immunity to ZIKV, however, interpreting the results of serology is complicated due to flavivirus cross-reactivity3,13. By contrast, the viruses responsible for a recent outbreak in Singapore showed little viral diversity, consistent with recent emergence, however, they were genetically closest to viruses isolated from other Asian countries than to South American strains consistent with an introduction from the surrounding region14.
It is still unknown if there has been widespread long-term circulation of ZIKV in the region, resulting in high levels of population immunity. The epidemic versus endemic nature of ZIKV has important consequences for tackling the pathogen and in particular the risk of severe complications. It has been suggested that ZIKV epidemics are likely to burn themselves out after just a few years, with resulting population immunity leading to absence of the virus for decades15. In such a scenario, efforts to contain the virus and enhanced surveillance for Guillain Barré Syndrome and microcephaly may not be an effective use of resources, given the lead time and logistical constraints in establishing such measures. By contrast, if ZIKV can transition to sustained endemic circulation, this necessitates the development of long-term intervention strategies and the establishment of systematic surveillance for the severe complications associated with infection.
Analyzing the ZIKV situation in Thailand provides an opportunity to understand the long-term epidemic potential of ZIKV. Aedes mosquitoes are found throughout Thailand and all four serotypes of DENV, which is transmitted by the same mosquitoes, circulate endemically throughout the year9,16. Thailand has been identified by phylogenetic studies as a potential source of DENV in the region17. Following the renewed interest in ZIKV globally, in 2016 the National Institute of Health in Thailand, starting the centralized testing of serum, plasma and urine samples of individuals with symptoms consistent with Zika from throughout the country.
Here, we present the results of this surveillance. In addition, we use the age distribution of cases to infer the immunity to ZIKV in the population and use phylogenetic analyses to infer the current diversity in circulating viruses.
Methods
Data collection
From January 2016, the National Institute of Health in Thailand established the centralized testing of suspected Zika cases that presented at hospitals from throughout the country. The symptoms of suspected cases were presence of maculopapular rash and a fever, or just a rash in provinces where Zika cases had already been identified. Acute serum, plasma or urine samples of suspected cases were sent to the National Institute of Health laboratories in Bangkok where they were tested for evidence of infection using PCR. The date of symptom onset, the date of testing, the district of the hospital, the age of the individual and the results of the testing were recorded.
Phylogenetic analyses
In order to assess whether the recent cases of Zika in Thailand represent a re-emergence of ZIKV associated with the large outbreak in South America or sustained transmission within Thailand, we downloaded all available full genome sequences from Thailand (N=8) from GenBank. We also selected a random sample of other sequences from Asia (Cambodia (N=3), Singapore (N=3), Malaysia (N=2)) as well as sequences from French Polynesia (N=5), Brazil (N=9) and Colombia (N=5). We used these sequences to build time-resolved phylogenetic trees using BEAST software with a GTR+G nucleotide substitution model and a random local clock, as has previously been identified as optimal for ZIKV phylogenetic analyses14,23.
To estimate the date that ZIKV first emerged in Thailand, we randomly selected 100 time-resolved trees from the posterior distribution. For each tree, we extracted the date for the most ancient (i.e., furthest back in time) Most Recent Common Ancestor (MRCA) separating any two Thai viruses. We then calculated the mean date and 95% confidence intervals across the 100 trees.
To assess the diversity in viruses within any location over time, we randomly selected 100 trees from the posterior distribution. For each tree we extracted the evolutionary distance separating each pair of tips. For all pairs of viruses coming from the same country and isolated within a year of each other, we calculated the proportion that had an MRCA within different time limits going back in time, ranging from 0.1 years to 10 years in the past. We then calculated the mean proportion over the 100 trees.
The GenBank accession numbers for the sequences used in these analyses can be found in Table S1.
Statistical analysis
Age distribution analysis
We can use the age distribution of cases to gain insight into the level of population immunity. If ZIKV had circulated endemically for decades, cases would be concentrated in children as most adults would be immune from previous infection. By contrast, a novel emergence would result in the case distribution being similar to the age distribution of the population. This assumes that there are no large-scale differences by age in the probability of becoming infected, developing symptoms or seeking care. We used data on the age distribution in Thailand and compared that to the age distribution in Zika cases18. As a comparison we also compared the age distribution of dengue cases that were also sent to the laboratory between 2011-2016. For each virus, we calculate the proportion of cases that were within each ten-year age bin and divided it by the proportion of the underlying population within that age bin. Values of this relative proportion of 1 indicate that individuals in that age category had a similar risk of becoming a case as anyone from the population, consistent with no immunity in the population. Relative proportions greater than 1 in younger individuals and less than 1 in older individuals are consistent with immunity in the population shifting cases to younger individuals19. To assess uncertainty, we used a bootstrap approach. We repeatedly randomly resampled all the cases with replacement and recalculated the relative proportion each time. Ninety-five percent confidence intervals were obtained from the 2.5 and 97.5 percentile of the resultant distribution. To allow us to compare the age distribution to ZIKV in a known emergent setting, we calculate the same value for Zika cases from Colombia between 2015 and 201620.
Spatial analyses
The probability that an individual with Zika symptoms presents to healthcare facilities and the subsequent probability that healthcare facilities send the samples to be tested by the National Institute of Health is likely to differ spatially and temporally. In order to assess whether Zika cases persisted in the same district for many months allowing for spatial and temporal biases in observation, we used the spatial distribution of both the cases and those that tested negative. This test-negative approach assumes that the individuals that tested negative are similar in their healthcare seeking as the cases21. To increase the probability that test-negative individuals were true negatives, we only included individuals who provided biological samples within seven days of symptom onset.
To characterize the spatial dependence among these cases, we calculated the odds of an individual with symptoms within 30 days of an index Zika case that lived within the same district testing positive for ZIKV relative to the odds of anyone testing positive for ZIKV within that time frame. We repeated the analysis considering cases that lived in nearby districts (defined as other districts within 50km) and those that lived in distal districts (districts >50km). We then considered longer time periods (1- 6 months, 6-12 months and >12 months). This approach has previously been used to characterize the spatial and temporal dependence among dengue cases22. To assess uncertainty, we used a bootstrap approach where all the cases were resampled with replacement and the odds ratio recalculated. Ninety-five percent confidence intervals were obtained from the 2.5 and 97.5 percentiles of the resultant distribution.
To assess whether particular regions had a greater risk of ZIKV than others, we divided the country into 6 regions (North, Northeast, Central, South, West and East) and identified the region for each symptomatic individual. We then calculated the odds of testing positive among individuals within each region, relative to the odds of anyone testing positive. Values greater than 1 indicate a greater risk of observing a case in that region compared to the country as a whole whereas values less than 1 indicates a reduced risk.
Ethical approval
This study is based on secondary analysis of data collected as part of Thai governmental surveillance activities during the global Zika emergency. No ethical approval was required because the data were anonymized.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
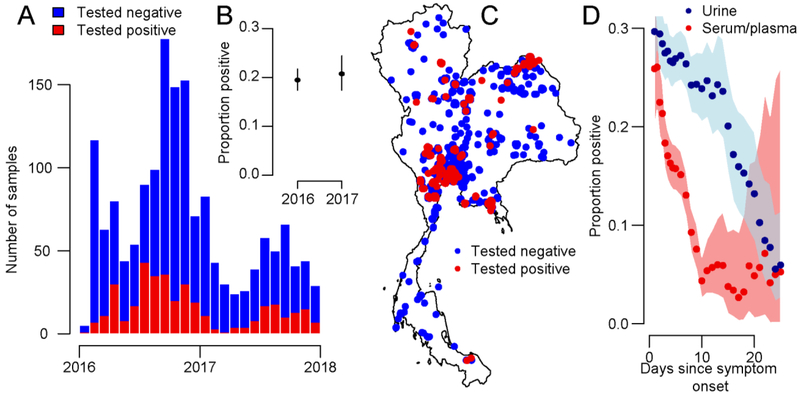
Between January 2016 and December 2017, there were 3,089 samples tested from 1,717 symptomatic individuals for evidence of ZIKV infection. Overall, 21.4% (368/1,717) of individuals tested positive (Figure 1A). There were 1,247 serum/plasma samples (181 positive, 14.5%), 1,831 urine samples (413 positive, 22.5%) and 10 samples from other sources (saliva, CSF with none positive) received, with some patients providing multiple samples. The probability of testing positive fell from 17.1% (150/879) for serum/plasma samples collected <7 days of symptom onset to 7.4% (21/283) in samples collected with longer delays (Figure 1). With urine samples, 27.7% (281/1,015) were positive when collected <7 days compared to 17.1% (111/650) with longer delays. Cases were found throughout the year, although with greater concentration in the second half of the year with 72% (265/368) of cases occurring between July and December. The probability of symptomatic individuals testing positive was consistent in the two years (20.4% [257/1258] in 2016 vs 24.2% [111/459] in 2017, p-value 0.1). While 61% (224/368) of confirmed Zika cases were female, once we accounted for the probability of being tested (62% [1038/1666] of symptomatic individuals sent for testing were female), we observed no difference in the odds ratio of testing positive by sex (OR: 1.0, 95%CI: 0.8-1.3).
Figure 1.
(A) Temporal distribution of the results of testing of symptomatic individuals from throughout Thailand 2016-2017. (B) Proportion of symptomatic individuals testing positive in each year. (C) Spatial distribution of samples. (D) Proportion of samples testing positive as a function of time and type of sample. Each point represent the proportion [positive from a 10-day time window. The shaded area represents 95% exact binomial confidence intervals.
Samples from individuals with symptoms consistent with ZIKV infection were sent from 60 of the 76 provinces in Thailand. Twenty-nine of these provinces (48%), and 77 different districts, covering all regions in the country, had at least one case, consistent with a widespread distribution of the virus (Figure 1B). We observed a small increased risk of a symptomatic individual testing positive for ZIKV in the Northeast (Relative Risk: 1.5, 95%CI: 1.2-1.8) and East (Relative Risk: 1.6, 95%CI: 1.2-2.2) relative to the country as a whole and a reduced risk in the South (Relative Risk: 0.3, 95%CI: 0.03-0.9) (Figure S1). There were 53 confirmed Zika cases from Bangkok, located within the Central region, out of 223 symptomatic individuals tested, with the probability of individuals from the capital testing positive being the same as the country as a whole (Relative Risk: 1.1, 95%CI: 0.8-1.4).
Within a district we observed strong spatial dependence between cases. Symptomatic individuals had 2.8 (95%CI: 2.3-3.6) times the odds of testing positive for ZIKV if they came from the same district and were sick within the same year of a confirmed Zika case relative to the odds of any symptomatic individual testing positive in that time interval (Figure 2). This value fell to 0.9 (95%CI: 0.2-1.8) at time intervals greater than a year. This spatial dependence extended to neighbouring districts too at a reduced scale (Figure 2).
Figure 2.
Odds of a symptomatic individual testing positive for ZIKV when they live at different spatial distances (within district [dark blue], neighbouring district [light blue] and distal district [grey]) of a confirmed Zika case sick within different temporal windows relative to the odds of a symptomatic individual testing positive for ZIKV anywhere within that same temporal window. The box plots represent means with interquartile ranges and 2.5% and 97.5% bootstrapped confidence intervals.
The mean age of cases was 32y, slightly lower than the mean age of the population (37y). Among Zika cases, the probability of being <10 years old, was 0.99 (95%CI: 0.72-1.30) times the probability of being within that age range in the underlying population (Figure 3A). This rose to 1.62 (95%CI 1.33-1.92) among 21-30 year olds and fell to 0.53 (95%CI: 0.40-0.66) for those >50 years. As females of reproductive age may be more likely to seek care due to microcephaly risks, we repeated the analysis using males only, finding consistent results. They were also largely consistent with the age distribution of cases in Colombia, where the relative risk of being a Zika case was lowest in those <10 years (RR: 0.63) and those >50y (RR:0.70) and highest in those 21-30 (RR: 1.58) with broadly similar patterns between females and males (Figure 3B). The mean age of cases in Colombia was 31y, similar to the mean age in the population (30y). As a comparison, dengue cases in Thailand were strongly concentrated in children with a mean age of 12y (Figure 3C). The probability of a dengue case being <10 years old was 4.01 (95%CI: 3.85-4.24) times greater than the probability of being in that age range in the underlying population. Dengue cases were 0.04 (0.01-0.05) times as likely to be >50 years than the underlying population.
Figure 3.
Proportion of Zika cases in (A) Thailand and (B) Colombia that are within each age group relative to the proportion of the underlying population that are within that age group. (C) Proportion of cases of dengue cases in Thailand that are within each age group relative to the proportion of the underlying population that are within that age group. The box plots represent means with interquartile ranges and 2.5% and 97.5% bootstrapped confidence intervals.
Using time-resolved phylogenetic trees we found that the Thai sequences were ancestral to the viral strains from French Polynesia, Brazil and Colombia (Figure 4A). Among these sequences, we estimate the emergence of ZIKV occurred in October 2002 (95%CI: January 2001-April 2004). We also find that the viruses were a lot more diverse in Thailand than in these other locations. In Brazil, if we only considered pairs of viruses isolated within a year of each other, we found that 88% had an ancestor in common within the within the prior two years (Figure 4B). We found similar values for Colombia and French Polynesia. By contrast, no viral pairs from Thailand that came from individuals sick within a year of each other had an ancestor in common within the prior 2 years. It is only when we considered ancestors in the prior 8 years that this value rose to 73%.
Figure 4.
(A) Time-resolved phylogenetic tree of ZIKV sequences available from GenBank. (B) Proportion of pairs of sequences isolated within a year of each other that have their Most Recent Common Ancestor within different time limits by country of origin for the pair of viruses.
Discussion
Here we have presented the results of ZIKV surveillance in Thailand. We demonstrate that within the first few months of surveillance being initiated, ZIKV was found throughout the country. In individual locations, cases exhibited strong spatial dependence, lasting for at least one year. These findings are consistent with sustained focal transmission with occasional longer distance transmission events, as has previously shown with DENV24. These findings are also consistent with sporadic case reports between 2012-2014 of ZIKV infection occurring in locations throughout the country10. Further, while we could not definitely identify the time point in which it first entered into the country, it appears to have circulated since at least 2002, many years prior to the commencement of surveillance. However, this long-term circulation has not yet been enough to result in sufficient immunity to shift transmission to the youngest members of the population, as has occurred with DENV.
Where viruses shift from epidemic to endemic transmission, there is a resultant increase in the genetic diversity among circulating lineages as individual lineages establish sustained transmission chains. For example, with DENV, which has circulated endemically in Thailand for decades, it has been previously shown that fewer than 1% of pairs of viruses infected in Bangkok at around the same time by the same serotype have a common ancestor within the prior 6 months24. Our findings suggest that ZIKV, in Thailand at least, has shifted towards endemic circulation. It remains unclear whether ZIKV can also make the transition in South America, as DENV did in the 1990s25.
The hypothesis that ZIKV transmission would burn itself out is based on assumptions that large-scale population immunity will drive the disease to extinction15. Serosurveys conducted after the 2015-2016 outbreak in South America have found high seroprevalence and are therefore consistent with this hypothesis26,27. While serosurveys will be ultimately necessary to properly quantify the level of population immunity in Thailand, the observed similarity between the age-specific incidence patterns in Thailand as compared to those in Colombia and Puerto Rico, where ZIKV has only recently emerged, suggest limited population immunity20,28. Thus, our findings from Thailand suggest that ZIKV may have found a middle ground - sufficient transmission to maintain itself but not at high enough levels to result in widespread immunity. Understanding why ZIKV exhibits such different transmission dynamics in Thailand as compared to the Americas will require additional studies.
Our findings are also consistent with age-specific differences in symptomatic infection risk or health seeking behavior. We find a reduced risk of being a Zika case among those over 50 years in age and an increased risk in those 21 to 30 years. Similar patterns have been described in the American outbreak20,28.
Our findings highlight the key insight that phylogenetic approaches provide, even with just a handful of sequences. While we cannot definitely rule out that the individual sequences represent the recent offspring from independent introductions into the country, this would necessitate a diverse viral reservoir in the wider region, which has not been observed. This is also inconsistent with the spatially widespread nature of sporadic case reports within Thailand, prior to surveillance initiation10. Our study also demonstrates the key sensitivity of phylogenetic analyses to the underlying sampling of isolates. Using all available sequences from Thailand provides an estimate of the most common recent ancestor in the country around 2002. However, this is largely reliant on a single isolate from 200612. Had this isolate not been present, this estimate would have shifted to 2006 (this would still provide an estimate of 13 years of circulation prior to today). It seems likely that additional sampling would shift the estimated date of emergence further back, although this may not be possible if the offspring of older lineages are now extinct.
Our findings have implications for the ongoing surveillance of ZIKV-related severe health complications. Between January 2016 and August 2018, there were four cases of Guillain Barré Syndrome in individuals with PCR confirmed ZIKV infection in Thailand29. Over the same period, 130 pregnant women were identified with ZIKV infection, of which two had subsequent abortions due to fetal ZIKV infection. A further 119 of these women have since given birth, four of which to babies with microcephaly, although none had signs of fetal ZIKV infection. Over the same period there have been 285 microcephaly cases where ZIKV infection had not previously been identified in the mother; congenital Zika syndrome was identified in three of these cases. Our findings suggest that should ZIKV continue to circulate endemically, there will be regular occurrences of these severe complications and that, in particular, surveillance needs to be able to reliably identify and follow up pregnant women.
We do not know why individuals sought care or had their samples sent to the central laboratory for processing. In particular, there may be differences by age. If older aged cases were less likely to be identified, this would imply even less immunity in the population, whereas reduced probability of detection in the youngest individuals would indicate greater immunity. However, there were no age-specific guidance for testing so any differences are likely to be minor. Further, none of these findings change our inference of long-term circulation of the virus. Population-representative seroprevalence studies, with serological assays that can distinguish between antibody responses to different flaviviruses (particularly ZIKV, DENV and Japanese encephalitis virus) are needed to help quantify the level of circulation and immunity in the population.
Our findings provide strong evidence of the long-term and widespread spread of ZIKV in Thailand, suggesting that ZIKV is able to transition to endemic transmission. These results support the development of long-term sustained interventions and surveillance efforts to tackle both the spread of the virus and the regular occurrences of severe ZIKV-related health outcomes.
Supplementary Material
Research in context.
Evidence before this study
To assess the evidence-base of Zika in Thailand, we searched Google Scholar for all publications prior to September 2018 that had the words “Zika” and “Thailand” in the title. We found 25 documents, mostly consisting of individual case reports. Two articles showed that cases of Zika had been found in different places in Thailand, however, no previous studies have considered the endemicity of the virus in the country and the likely level of underlying immunity. An equivalent search in PubMed yielded 12 results with no additional documents.
Added value of this study
Using data from the national surveillance system, we present a comprehensive assessment of the Zika situation in Thailand. We use information from symptomatic individuals that tested negative for Zika to control for underlying spatial differences in healthcare seeking. We demonstrate that the age distribution of Zika cases are largely consistent with that from a known emergent setting (Colombia) and different from dengue, where population immunity is high. We use time-resolved phylogenetic approaches to demonstrate that ZIKV has circulated in the country for 17 years if not longer.
Implications of all the available evidence
Our findings are consistent with sustained transmission of ZIKV within a single country for many years. This suggests that other countries that have also experienced ZIKV outbreaks may also be affected by the virus in future years. Our findings support the need for the deployment of long-term sustainable surveillance and intervention strategies.
Acknowledgments
Funding
NSF, NIH
Funding: HS, IR-B and DATC were funded by National Science Foundation (DEB-1642174). DATC was also funded by the NIH (R01AI114703).
Footnotes
Conflicts of interest: We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg 1969; 18: 411–5. [DOI] [PubMed] [Google Scholar]
- 2.Dick GWA, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46: 509–20. [DOI] [PubMed] [Google Scholar]
- 3.Lessler J, Chaisson LH, Kucirka LM, et al. Assessing the global threat from Zika virus. Science 2016; 353: aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 2016; 387: 2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao-Lormeau V-M, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 387: 1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faria NR, Azevedo R do S da S, Kraemer MUG, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016; 352: 345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salje H, Cauchemez S, Alera MT, et al. Reconstruction of 60 Years of Chikungunya Epidemiology in the Philippines Demonstrates Episodic and Focal Transmission. J Infect Dis 2016; 213: 604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong V, Dussart P, Buchy P. Zika virus in Asia. Int J Infect Dis 2017; 54: 121–8. [DOI] [PubMed] [Google Scholar]
- 9.Messina JP, Brady OJ, Scott TW, et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 2014; 22: 138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buathong R, Hermann L, Thaisomboonsuk B, et al. Detection of Zika Virus Infection in Thailand, 2012–2014. Am J Trop Med Hyg 2015; 93: 380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015; 21: 722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitatpattana N, Chaiyo K, Rajakam S, et al. Complete Genome Sequence of a Zika Virus Strain Isolated from the Serum of an Infected Patient in Thailand in 2006. Genome Announc 2018; 6 DOI: 10.1128/genomeA.00121-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pond WL. ARTHROPOD-BORNE VIRUS ANTIBODIES IN SERA FROM RESIDENTS OF SOUTH-EAST ASIA. Trans R Soc Trop Med Hyg 1963; 57: 364–71. [DOI] [PubMed] [Google Scholar]
- 14.Ho ZJM, Hapuarachchi HC, Barkham T, et al. Outbreak of Zika virus infection in Singapore: an epidemiological, entomological, virological, and clinical analysis. Lancet Infect Dis 2017; 17: 813–21. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson NM, Cucunubá ZM, Dorigatti I, et al. EPIDEMIOLOGY. Countering the Zika epidemic in Latin America. Science 2016; 353: 353–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraemer MUG, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015; 4: e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabaa MA, Ty Hang VT, Wills B, Farrar J, Simmons CP, Holmes EC. Phylogeography of recently emerged DENV-2 in southern Viet Nam. PLoS Negl Trop Dis 2010; 4: e766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Census International Data Base, https://www.census.gov/data-tools/demo/idb/informationGateway.php (accessed Sept 4, 2018).
- 19.Cummings DAT, lamsirithaworn S, Lessler JT, et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med 2009; 6: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacheco O, Beltrán M, Nelson CA, et al. Zika Virus Disease in Colombia - Preliminary Report. N Engl J Med 2016; published online June 15. DOI: 10.1056/NEJMoa1604037. [DOI] [PubMed] [Google Scholar]
- 21.Jewell NP, Dufault S, Cutcher Z, Simmons CP, Anders KL. Analysis of cluster-randomized test-negative designs: cluster-level methods. Biostatistics 2018; published online Feb 12. DOI: 10.1093/biostatistics/kxy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salje H, Lessler J, Endy TP, et al. Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci U S A 2012; 109: 9535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 2012; 29: 1969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salje H, Lessler J, Maljkovic Berry I, et al. Dengue diversity across spatial and temporal scales: Local structure and the effect of host population size. Science 2017; 355: 1302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Barraquer I, Cordeiro MT, Braga C, de Souza WV, Marques ET, Cummings DAT. From re-emergence to hyperendemicity: the natural history of the dengue epidemic in Brazil. PLoS Negl Trop Dis 2011; 5: e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netto EM, Moreira-Soto A, Pedroso C, et al. High Zika Virus Seroprevalence in Salvador, Northeastern Brazil Limits the Potential for Further Outbreaks. MBio 2017; 8 DOI: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrana JV, Carrillo FB, Burger-Calderon R, et al. Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proceedings of the National Academy of Sciences 2018; 115: 9294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozier M, Adams L, Febo MF, et al. Incidence of Zika Virus Disease by Age and Sex - Puerto Rico, November 1, 2015-October 20, 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1219–23. [DOI] [PubMed] [Google Scholar]
- 29.Thai Ministry of Public Health. Thai. Thai Bureau of Vector Borne Diseases. www.thaivbd.org/n/contents/view/325061 (accessed Aug 28, 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.