Abstract
Background:
Patient registries use standardized methods to systematically gather uniform data for specific groups of patients managed in clinical practice to evaluate specified outcomes.
Aim:
The objective of this study was to identify and describe structures of the identified thalassemia registries in worldwide and summarize their key characteristics.
Methods:
We reviewed the literature on thalassemia registries. A search of PubMed, Scopus, ProQuest, and Science Direct databases was conducted in September 2018. We also reviewed the existing thalassemia registry websites in different countries. The keywords used to our search were as follows: Thalassemia, Hemoglobinopathy, Registry, Database, and Registration System. Some features such as the name of registry, funding source, objectives of the registry, minimum data set, and methods of data collection were determined.
Results:
We identified 16 thalassemia registries operating on a multinational, national, or regional level between1984 and 2016. Most of these aimed to improve the diagnosis and management of control programs. Government funding was the most common funding source for registries. Furthermore, the most common method of data submission was Web-based data entry. The data were entered by a member of the clinical team or a nominated data manager.
Conclusion:
Registries provide a positive return on investment; their establishment and maintenance require ongoing support by government, policy makers, research funding bodies, clinicians, thalassemia patients and their caregivers. However, the results of research suggest the establishment of an international network for coordination and collaboration between thalassemia registries.
Keywords: Thalassemia, Hemoglobinopathy, Surveillance, Registry, Database
1. INTRODUCTION
Thalassemia is a blood related genetic condition which characterized by decreased synthesis of one of the two types of polypeptide chains (α or β) (1). This disorder encompasses the lack of or errors in genes accountable for the construction of hemoglobin, a protein present in the red blood cells (2). The World Health Organization (WHO) announced that the frequency of thalassemia and abnormal hemoglobin carriers is 5.1% with nearly 226 million carriers worldwide (2, 3). Nearly 80% of thalassemia cases worldwide are detected in the area extending from sub-Saharan Africa to the Mediterranean Basin, the Middle East, and South and Southeast Asia (4).
Currently, as a result of important clinical and scientific improvements, thalassemia and other hemoglobin disorders are considered as remediable and preventable in cases where effective national programmers are in place and where there is free access to quality healthcare for people living with these conditions (5). The management of thalassemia patients is compound and requires a multidisciplinary strategy that integrates clinically and the laboratory features (6). As with other chronic disease management, the healthcare procedure is long-lasting and continuous of consisting multiple different parts of the process (7). Therefore, having IT tools such as healthcare systems to assist the progression can improve the healthcare services quality (8). Besides providing more accurate and timely information regarding patient care, it has been found to improve the efficiency of healthcare organization services especially in terms of patient data management (9).
Several approaches are being applied across different healthcare systems around the world, including the use of patient registries, which have been identified as a method of improving quality and cost efficiency in health and healthcare (10, 11). High quality patient registries provide valuable contributions to designate demographics, clinical features and to determine baseline prevalence and variation in practices ’in the real world’ (12-14). They let surveillance of important health conditions, a better understanding of patient health status and requirements, evaluate changing practice and trends over time, and allow predictions for resource requirements (15, 16).
A thalassemia registry encompasses comprehensive information related to thalassemia patients, over many years (17, 18). Moreover, the thalassemia registry provides data on access to and quality of care, and patient outcomes such as survival and Quality of life (19, 20). It has successfully followed changing dynamics and healthcare requirements and allowed detection of health system faults to aids, not only disease research but also the development and evaluation of a prevention program and the creation of clinical strategies (18, 21). In recent years, the thalassemia registry has been considered as an important tool for monitoring and improving the quality of patient care (1, 22). European Medicines Agency (EMA) recognizes the need for common approaches to foster the optimal use of national and multinational registries (23).
2. AIM
The objective of this study was to identify existing thalassemia registries in worldwide and describe their main characteristics including objectives, data sources, responsible institutions, core data set and the process of registration in different countries.
3. METHODS
We reviewed the literature reporting on thalassemia registries. The databases PubMed, Scopus, ProQuest, and Science Direct were searched up until September 2018. In addition, Google and Google Scholar were searched for thalassemia registry websites, unpublished studies and grey literature. There was no restriction on date of publication. The keywords used for the search were the following; Thalassemia, Hemoglobinopathy, Surveillance, Registry and Database. All documents and reports on thalassemia registries were included if they provided details about the program characteristics. We did not apply language constraints and provided a translation service if needed for non-English documents. The references of found articles were used to identify other related articles. In addition, we contacted the authors of the included studies to ask if they were aware of any further registries.
In this study we did not set any language restrictions, we used only English search words. After a complete search, all search results were reviewed separately based on studies title or running title and relevant documents were selected. The duplicated documents were excluded. Afterward agreement on the final included studies was reached, one author independently extracted data using a standard data extraction form, which was then cross-checked by the second reviewer. If the contact details were provided in retrieved sources, an email was sent to a registry manager asking for additional peer-reviewed publications and other resources, such as conference presentations and annual reports. In case of no response, two follow-up reminders were sent. The unknown field for incomplete/not response was entered into the data extraction table.
Based on retrieved peer-reviewed publications and gray literature, the selected thalassemia registries were further analyzed to obtain the following information: (a) region, (b) country, (c) registry name, (d) responsible institutes, (e) Internet home page, (f) type of registry, (g) institution year, (h) funding sources, (i) objectives, (j) diseases coverage, (k) language of collected data, (l) participation type, (m) core data set, (n) data sources, and (o) methods of data collection.
4. RESULTS
Using the search strategies, 149 references were identified and 12 papers (13, 14, 16-20, 22, 24-27) met the inclusion criteria (Figure 1). We identified 16 thalassemia registries (set up from 1984 and 2016). Out of total amount, 13 registries were national, 1 multinational and 2 regional. The identified registries were established in 14 countries distributed in Asia (n=4), Europe (n=9), North America (n=2), and Oceania (n=1). No thalassemia registry was identified in Africa and South America (Table 1). Six registries were specifically designed to collect data on thalassemia patients and 10 were hemoglobinopathies registries including also thalassemia patients.
Figure 1. Preferred reporting items for reviews flow diagram.
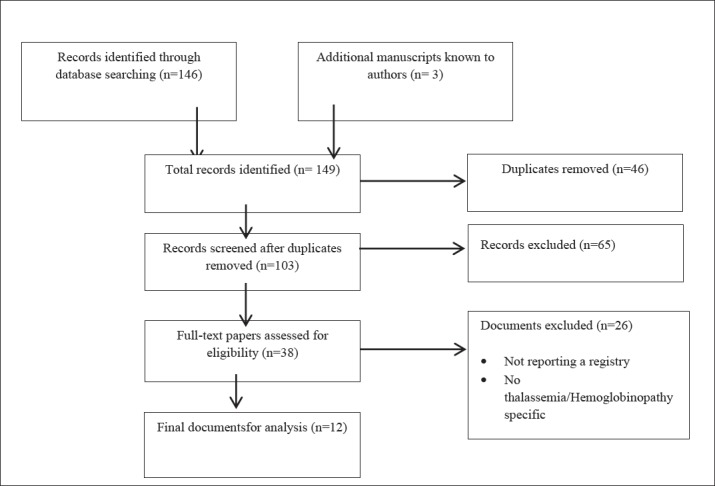
Table 1. General overview of the identified registries.
| Region | Country | Name of registry | Responsible institute | Internet home page |
|---|---|---|---|---|
| Asia | Iran | Electronic Thalassemia Registry (ETR Mazandaran) | Thalassemia Research Center and Mazandaran University of Medical Sciences | http://thr.mazums.ac.ir/ |
| Singapore | National Thalassemia Registry (NTR) | KK Women's and Children's Hospital | https://www.kkh.com.sg | |
| Oman | National Register of Symptomatic Hemoglobinopathies | Genetic Blood Disorders Unit and Ministry of Health | Unknown | |
| Saudi Arabia | Pediatric Non-Malignant Blood DisordersRegistry | King Faisal Specialist Hospital | Unknown | |
| Europe | Bulgaria | National Registry of Patients with Thalassemia in Bulgaria (NRPTB) | Bulgarian Association for Promotion of Education and Science and Information Centre for Rare Diseases | https://www.raredis.org |
| United Kingdom | National Haemoglobinopathy Registry (NHR) | National Health Service | http://nhr.nhs.uk | |
| European Haemoglobinopathy Registry(EHR) | National Health Service | https://www.sicklecellsociety.org/resource/european-haemoglobinopathy-registry/ | ||
| France | Register of Thalassemic Patients in France | National Institute of Health and Medical Research and National Institute of Health Surveillance | https://www.ap-hm.fr | |
| Italy | Italian Multiregional Thalassemia Registry (HTA-Thal) | Consorzio per Valutazioni Biologichee Farmacologiche and Fondazione per la Ricerca Farmacologica Gianni Benzi Onlus | http://www.cvbf.net/tag/hta-thal-registry | |
| Sicilian Registry Thalassemia and Hemoglobinopathies (ReSTE) | Epidemiological Observatory of the Regional Councillorship | http://pti.regione.sicilia.it | ||
| Greece | National Registry for Haemoglobinopathies in Greece (NRHG) | Greek Society of Hematology | http://www.enerca.org/members-centers/center/22/national-center-for-thalassaemia-and-haemoglobinopathies-of-laikon-general-hospital-of-athens-greece | |
| Spain | National Registry of Hemoglobinopathies in Spain (REPHem) | Spanish Society of Pediatric Hematology and Oncology | https://www.e-clinical.org/rephem/index.aspx | |
| Turkey | Turkish Hemoglobinopathy Registry | Turkish Society of Pediatric Hematology | http://www.tphd.org.tr/ | |
| North America | United States | Registry and Surveillance System for Hemoglobinopathies (RuSH) | Centers for Disease Control and National Heart, Lung, and Blood Institute | https://www.cdc.gov |
| Canada | Data Information System for Hemoglobinopathies(DISH) | Children’s Hospital of Eastern Ontario | https://www.project-redcap.org/ | |
| Oceania | Australia | Haemoglobinopathy Registry (HbR) | Monash University | http://www.torc.org.au/hbr |
Moreover, Government funding was the most common funding source for registries. Only the National Hemoglobinopathies Registry in Greece was private funded. The time length of funding was not reported. The objectives of most thalassemia registries were improving diagnosis and management of control programs. Other purposes of thalassemia registries include healthcare planning, epidemiological and clinical research, education, policy making, prevention, and follow up (Table 2). The most common method of data submission was web-based data entry. The data were entered by a member of the clinical team or a nominated data manager (Table 3).
Table 2. Structures of the identified thalassemia registries.
| Registry | Type | Year | Funding | Objectives of registry | Disease Coverage | Language | Participation |
|---|---|---|---|---|---|---|---|
| ETR Mazandaran | Regional | 2016 | Thalassemia Research Center, Uni | Improving diagnosis, Research, Decision making | Thalassemia major | Persian | Voluntary |
| NTR | National | 1992 | Gov | Management of control programs,follow- up, Prevention, Counseling and Screening | Thalassemia major | Unknown | Voluntary |
| National Register of Symptomatic Hemoglobinopathies | National | 2000 | Gov | Improving diagnosis, Control of blood disorders, Research | Thalassemia major, Sickle cell | English | Voluntary |
| Pediatric Non-Malignant Blood Disorders Registry | National | 2008 | Gov | Management of control programs, Follow-up, Improving diagnosis | Thalassemia major, Sickle cell | Arabic | Voluntary |
| NRPTB | National | 2009 | Gov | Improving diagnosis, Follow-up, Prevention, Policy making, Research, Compare management practices | Thalassemia major,Intermedia | Bulgarian | Voluntary |
| NHR | National | 2009 | Gov | Improving care, Management of control programs, Prevention, Research | Thalassemia major, Sickle cell | English | Voluntary |
| EHR | Multi national | 2004 | Public | Improving diagnosis, Follow-up, Prevention, Research, Planning | Thalassemia major, Otherhemoglobinopathy | English | Voluntary |
| Register of Thalassemic Patients in France | National | 2005 | Gov | Improving care, Compare conventional treatment, Research | Thalassemia major, Intermedia | French | Voluntary |
| HTA-Thal | National | 2008 | Gov,Fondazione Giambrone | Improving diagnosis, Management of control programs, Healthcare planning,Research | Thalassemia major | Italian | Voluntary |
| ReSTE | Regional | 1984 | Gov | Management of control programs, Care Planning, Research | Thalassemia major, Intermedia, Sickle cell, Other hemoglobinopathy | Italian | Voluntary |
| NRHG | National | 2009 | Private | Improving care, Monitor treatment, Prevention, Research | Thalassemia, Sickle cell, Hemoglobin lepore | Greek | Voluntary |
| REPHem | National | 2014 | Industrial Association | Improving diagnosis, Improve Treatment, Prevention, Research,Comparison with other registries | Thalassemia major, Intermedia, Sickle cell | Spanish | Voluntary |
| Turkish Hemoglobinopathy Registry | National | 2012 | Gov. Uni | Improving diagnosis, Management of control programs, Research | Thalassemia major, Intermedia, Sickle cell | Turkish | Voluntary |
| RuSH | National | 2010 | Gov | Improving care, Monitoring health care utilization and clinical outcomes, Planning, Research, education | Thalassemia, Sickle cell | English | Voluntary |
| DISH | National | 2014 | Gov | Management of control programs, Research, Improving care | Thalassemia ,Sickle cell, Other Hemoglobinopathies | English | Voluntary |
| HbR | National | 2014 | Industry partners | Improving diagnosis, Follow-up, Research, Monitoring outcomes | Thalassemia major, Sickle cell, Other haemoglobinopathies | English | Voluntary |
Table 3. Data collection onthe identified thalassemia registries.
| Registry | Core minimum data set | Data sources | Data submission |
|---|---|---|---|
| ETR Mazandaran | Demographics, Clinical, Complications, Medication | Haemoglobinopathy centers | Web-based |
| NTR | Demographics, Clinical | Hospitals | On-line data transfer |
| National Register of Symptomatic Hemoglobinopathies | Administrative, Clinical | Hospitals, Tertiary care centers | Paper, On-line data transfer |
| Pediatric Non-Malignant Blood DisordersRegistry | Demographic, Consanguinity, Diagnostic, Laboratory | Hospitals, Haemoglobinopathy centers | Web-based |
| NRPTB | Demographic, Diagnostic, Mortality | Hospitals, Thalassemia centers | Web-based |
| NHR | Patient, Adverse Events, Annual Review | Treatment centers, other bodies such as blood and transplant centers | Web-based |
| EHR | Demographics, Clinical, Treatment, Laboratory | Hospitals, Haemoglobinopathy Centers | On-line data transfer |
| Register of Thalassemic Patients in France | Epidemiological, Clinical, Biological | Hospitals, Pediatric centers, stem cell transplants database | Web-based |
| HTA-Thal | Demographic,Clinical, Complications, Quality of life,Cost | Hospitals, Haemoglobinopathy centers | Web-based |
| ReSTE | Demographics,Clinical | Hospital, Haemoglobinopathy centers | Paper, fax |
| NRHG | Demographic, Disease | Hospitals, Haemoglobinopathy centers | Web-based |
| REPHem | Demographic, Clinical | Hospitals, Haemoglobinopathy centers | Web-based |
| Turkish Hemoglobinopathy Registry | Demographic, Disease | Hemoglobinopathy centers | Web-based |
| RuSH | Administrative, Clinical, Health care utilization | Haemoglobinopathy centers ,Public health records, Clinical records, registries | Paper, On-line data transfer |
| DISH | Demographic, Diagnostic, Hospitalizations, transfusions, Tests, Medication, Bone marrow transplant | Hospitals | Web-based |
| HbR | Demographics, Diagnosis, Laboratory , Complications, Clinical outcomes | Hospitals, Haemoglobinopathy centers, registries, Medical databases | On-line data transfer |
5. DISCUSSION
Thalassemia registries are essential tools and an important resource for planning and evaluating of disease prevention program based on facts (26, 28). In particular they will enhance surveillance of important health conditions, awareness of the prevalence of the disease, better understanding of patient health status, treatment options and detection of shortcomings in the healthcare system (20, 25, 29). This was the first review of its kind to compare existing thalassemia registries in worldwide. We identified 16 thalassemia registries, which consisted of 13 national, one multinational and two regional registries.
An important element in determining the feasibility of developing a new registry relates to funding (21). Registries with good coverage and accuracy will most likely require significant and sustainable funding sources (30, 31). The registries were received funding from various sources, including government agencies, scientific organizations, research collaborators, pharmaceutical manufacturers, accreditation bodies, philanthropic organizations and non-profit organization (32, 33). Based on our study most of the thalassemia registries were funded by the government agencies. This tendency might be explained by the superiority of government funding in terms of continuity and predictability, which is required to sustain a thalassemia registry. Clear objectives are essential to define the structure and process of data collection and to ensure that the registry effectively addresses the important questions through the appropriate outcomes analyses (34, 35). The purposes of the registry have to be obviously defined and approved upon by the registry sponsors (36). Results of this study showed that objectives of most thalassemia registries were improving diagnosis and management of control programs. However, other purposes of thalassemia registries include healthcare planning, epidemiological and clinical research, education, policy making, prevention, and follow-up. Moreover, objectives often overlap, for example, improving diagnosis and prevention can provide improve quality of care. The time and resources needed to collect and process data from a registry can be substantial (36, 37).
Registries should define a core data set of essential data elements and patient outcomes that will address the critical questions anticipated by the purpose and objectives for which it was created (35). Elements of data to be included must have potential value in the context of the current scientific and clinical climate and must be chosen by a team of experts (38). Registries within this review most commonly collected data on: (a) demographics; (b) clinical; (c) complications of disease and therapy; and (d) outcomes. Although, the core minimum data set for thalassemia registries was almost similar in different registries and covered all aspects of quality of care, the number and details of data elements various in different countries.
Accuracy, integrity and completeness of data are the most important elements in the quality and value of any registry (39). Low data quality can be due to inadequate collection of data at reporting sites, inattentive abstracting of information from clinical data sources, poor definition and specificity of data, inadequate understanding of complex data elements by those providing the data and lack of incentive and collaboration among reporting centers (39-41). Comparability of data is essential for interpretation and this in turn, depends on standardization of the methodology and the diagnostic criteria applied (40). Effective quality control using regular internal and external audits and monitoring site visits can help to achieve these foremost goals (33). Data collection of thalassemia registries from diverse and dispersed sites, including general practice, thalassemia clinics, hematopoietic stem cell transplants, and pediatric hematology centers, is a significant challenge. Procedures and policies to ensure completeness and validity of data should be developed before the data collection commence and reviewed at regular intervals. In addition, good quality information systems are needed for effective data collection to support the registry. Integration of the registry infrastructure with the information and communications technology systems already in place within the national health care systems enables automatic data capture, which significantly reduces the burden of data entry. The web-based data entry was a core feature of the majority of registries within this review. In recent years, the web-based registries have become popular because they are user friendly and can be managed from different locations (42, 43).
Additionally, the web-based systems offer the best access to the registry’s data, increase the accuracy of data and facilitates real time data entry, updates, reporting and mapping functionalities (42, 44, 45). The initial cost to develop a web-based registry can be expensive; however web-based data collection was found to yield a shorter case registration time, lower cost per case to maintain the system and lower rate of error occurrence than the paper-based data collection (46). This review had several limitations. The development of thalassemia registries is a new field of research; therefore, the number of included studies is restricted and there was no standard recommendation for reporting the results. We identified a number of gaps in thalassemia registry coverage. Few registries were identified in areas with emerging economies; the majority being based in high income countries, that creating geographic gaps in coverage. In much of the Asia Pacific region, South America and Africa, registries were either absent entirely or had limited data or poor accessibility for outside research. Unfortunately, 2 registries of Cyprus and Malaysia were excluded from the study due to lack of available information.
The use of English only search words might have resulted in the failure to identify registries in some countries. Therefore, we tried to obtain our necessary information from various peer reviewed articles, reports and web sites. In addition, Contact with authors of the included papers provided valuable detail on registries.
6. CONCLUSIONS
This analysis confirms the utility of thalassemia registries for the collection of large set of data. In particular, the considerations derived from this data set highlight how the use of large, well monitored patients’ registries can guide health authorities and health providers to plan cost efficient services and to meet patients’ needs and expectations. We suggest the establishment of an international network for collaboration between thalassemia registries. Global harmonization of data submission methods and minimum data set would facilitate international comparisons. Registries provide a positive return on investment; their establishment and maintenance require ongoing support by government, policy makers, research funding bodies, clinicians and patient with thalassemia.
Acknowledgments:
This manuscript was based on some results of the Ph.D. dissertation on health information management at Tehran University of Medical Sciences. The authors extend thanks to health information management professionals at Tehran University of Medical Sciences.
Author’s contribution:
Each author gave substantial contribution to the conception or design of the work and in the acquisition, analysis and interpretation of data for the work. Each author had role in drafting the work and revising it critically for important intellectual content. Each author gave final approval of the version to be published and they agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest:
There are no conflicts of interest
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Meloni A, Ramazzotti A, Positano V, Salvatori C, Mangione M, Marcheschi P, et al. Evaluation of a web-based network for reproducible T2* MRI assessment of iron overload in thalassemia. Int J Med Inform. 2009;78(8):503–512. doi: 10.1016/j.ijmedinf.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Al-Riyami AZ, Daar S. Transfusion in Haemoglobinopathies: Review and recommendations for local blood banks and transfusion services in Oman. Sultan Qaboos Univ Med J. 2018;18(1):3–12. doi: 10.18295/squmj.2018.18.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazlican E, Celenk O, Kerkez B, Demirhindi H, Akbaba M, Kiremitci M. Evaluation of married haemoglobinopathic carrier couples for prevention of haemoglobinopathic births. Balkan Med J. 2013;30(4):394–399. doi: 10.5152/balkanmedj.2013.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazarbachi AH, Moukhadder H, Bou-Fakhredin R, Roumi J, Chaya B. How I treat and monitor non-transfusion-dependent thalassaemia. Haematologica. 2017;102(1):20–27. [Google Scholar]
- 5.Aguilar Martinez P, Angastiniotis M, Eleftheriou A, Gulbis B, Manu Pereira M, Petrova-Benedict R, et al. Haemoglobinopathies in Europe: health & migration policy perspectives. Orphanet J Rare Dis. 2014;9(97):11–17. doi: 10.1186/1750-1172-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulbis B, Cotton F, Ferster A, Ketelslegers O, Dresse MF, Rongé-Collard E, et al. Neonatal haemoglobinopathy screening in Belgium. J Clin Pathol. 2009;62(1):49–52. doi: 10.1136/jcp.2008.060517. [DOI] [PubMed] [Google Scholar]
- 7.Tubman VN, Fung EB, Vogiatzi M, Thompson AA, Rogers ZR, Neufeld EJ, et al. Guidelines for the standard monitoring of patients with thalassemia: report of the thalassemia longitudinal cohort. J Pediatr Hematol Oncol. 2015;37(3):162–169. doi: 10.1097/MPH.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahraki AD, Safdari R, Shahmoradi L, Malak JS, Pourghaz B. Acute stroke registry planning experiences. J Registry Manag. 2018;45(1):37–42. [PubMed] [Google Scholar]
- 9.Antoniou Z, Schiza EC, Neokleous K, Angastiniotis M, Pattichis CS, Schizas CN. E-health services for the european reference network on rare anaemias (eENERCA) Stud Health Technol Inform. 2015;213(5):153–156. [PubMed] [Google Scholar]
- 10.Lundstrom M, Barry P, Brocato L, Fitzpatrick C, Henry Y, Rosen P, et al. European registry for quality improvement in cataract surgery. Int J Health Care Qual Assur. 2014;27(2):140–151. doi: 10.1108/IJHCQA-10-2012-0101. [DOI] [PubMed] [Google Scholar]
- 11.Sypek MP, Jose MD, McDonald SP. Clinical quality registries for clinician-level reporting: strengths and limitations. Med J. 2018;208(7):322–327. doi: 10.5694/mja17.00581. [DOI] [PubMed] [Google Scholar]
- 12.Crighton G, Wood E, Scarborough R, Ho PJ, Bowden D. Haemoglobin disorders in Australia: where are we now and where will we be in the future? Intern Med J. 2016;46(7):770–779. doi: 10.1111/imj.13084. [DOI] [PubMed] [Google Scholar]
- 13.Inusa BPD, Colombatti R. European migration crises: the role of national hemoglobinopathy registries in improving patient access to care. Pediatric blood & cancer. 2017;64(7):21–32. doi: 10.1002/pbc.26515. [DOI] [PubMed] [Google Scholar]
- 14.Kosaryan M, Karami H, Alipour A, Masoudinejad M, Darvishi Khezri H, Akbarzadeh R, et al. Designing an electronic registry for patients with beta-thalassemia major for Mazandaran province 2016. International Journal of Caring Sciences. 2017;10(1):575–582. [Google Scholar]
- 15.Davoodi S, Haghighi KS, Kalhori SRN, Hosseini NS, Mohammadzadeh Z, Safdari R. Occupational disease registries characteristics and experiences. Acta Inform Med. 2017;25(2):136–140. doi: 10.5455/aim.2017.25.136-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modell B, Khan M, Darlison M, King A, Layton M. A national register for surveillance of inherited disorders: beta-thalassaemia in the United Kingdom. Bull World Health Organ. 2001;79(11):1006–1013. [PMC free article] [PubMed] [Google Scholar]
- 17.Thuret I, Pondarre C, Loundou A, Steschenko D, Girot R, Bachir D, et al. Complications and treatment of patients with beta-thalassemia in France: results of the national registry. Haematologica. 2010;95(5):724–729. doi: 10.3324/haematol.2009.018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conte R, Ruggieri L, Gambino A, Bartoloni F, Baiardi P, Bonifazi D, et al. The Italian multiregional thalassemia registry: centers characteristics, services, and patients’ population. Hematology. 2016;21(7):415–424. doi: 10.1080/10245332.2015.1101971. [DOI] [PubMed] [Google Scholar]
- 19.Cela E, Bellon JM, Cruz M, Belendez C, Berrueco R, Ruiz A, et al. National registry of hemoglobinopathies in Spain (REPHem) Pediatric blood & cancer. 2017;64(7):32–40. doi: 10.1002/pbc.26322. [DOI] [PubMed] [Google Scholar]
- 20.Hoots WK. The registry and surveillance in hemoglobinopathies: improving the lives of individuals with hemoglobinopathies. Am J Prev Med. 2010;38(4):510–511. doi: 10.1016/j.amepre.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Hassan HJ, Morfini M, Taruscio D, Abbonizio F, Giampaolo A, Kodra Y, et al. Current status of Italian registries on inherited bleeding disorders. Blood transfusion. 2014;12(3):576–581. doi: 10.2450/2014.0017-14s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voskaridou E, Ladis V, Kattamis A, Hassapopoulou E, Economou M, Kourakli A, et al. A national registry of haemoglobinopathies in Greece: deducted demographics, trends in mortality and affected births. Ann Hematol. 2012;91(9):1451–1458. doi: 10.1007/s00277-012-1465-7. [DOI] [PubMed] [Google Scholar]
- 23.Patient registry initiative- strategy and mandate of the cross-committee task force. United Kingdom: European medicines agency; 2017. [Google Scholar]
- 24.Rajab AG, Patton MA, Modell B. Study of hemoglobinopathies in Oman through a national register. Saudi Med J. 2000;21(12):1168–1172. [PubMed] [Google Scholar]
- 25.Aydinok Y, Oymak Y, Atabay B, Aydogan G, Yesilipek A, Unal S, et al. A national registry of thalassemia in Turkey: demographic and disease characteristics of patients, achievements, and challenges in prevention. Turk J Haematol. 2018;35(1):12–18. doi: 10.4274/tjh.2017.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK thalassaemia register. Lancet. 2000;355(9220):2051–2052. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- 27.Hulihan MM, Feuchtbaum L, Jordan L, Kirby RS, Snyder A, Young W, et al. State-based surveillance for selected hemoglobinopathies. Genet Med. 2015;17(2):125–130. doi: 10.1038/gim.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burney M, Schunk K, Oundjian NJ, Younge RG. Incomplete follow-up of hemoglobinopathy carriers identified by newborn screening despite reporting in electronic medical records. J Natl Med Assoc. 2011;103(10):852–856. doi: 10.1016/s0027-9684(15)30440-5. [DOI] [PubMed] [Google Scholar]
- 29.Bellgard MI, Macgregor A, Janon F, Harvey A, O’Leary P, Hunter A, et al. A modular approach to disease registry design: successful adoption of an internet-based rare disease registry. Hum Mutat. 2012;33(10):2356–2366. doi: 10.1002/humu.22154. [DOI] [PubMed] [Google Scholar]
- 30.Krysinska K, Sachdev PS, Breitner J, Kivipelto M. Dementia registries around the globe and their applications: A systematic review. Alzheimers Dement. 2017;13(9):1031–1047. doi: 10.1016/j.jalz.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian S, Tangka F, Edwards P, Hoover S. Developing and testing a cost data collection instrument for noncommunicable disease registry planning. Cancer Epidemiol. 2016;45(1):4–12. doi: 10.1016/j.canep.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronbjerg KA. How nonprofit human service organizations manage their funding sources: key findings and policy implications. Nonprofit Manag Leadersh. 1991;2(2):159–175. doi: 10.1002/nml.4130020206. [DOI] [PubMed] [Google Scholar]
- 33.Aljurf M, Rizzo JD, Mohty M, Hussain F, Madrigal A, Pasquini MC, et al. Challenges and opportunities for HSCT outcome registries: perspective from international HSCT registries experts. Bone Marrow Transplant. 2014;49(8):1016–1021. doi: 10.1038/bmt.2014.78. [DOI] [PubMed] [Google Scholar]
- 34.Bellgard MI, Render L, Radochonski M, Hunter A. Second generation registry framework. Source Code Biol Med. 2014;9(14):1–6. doi: 10.1186/1751-0473-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User’s Guide. 3. Rockville: Agency for Healthcare Research and Quality (US); 2014. [PubMed] [Google Scholar]
- 36.Courtney PM, Markel DC. Arthroplasty registries: improving clinical and economic outcomes. J Knee Surg. 2017;30(1):7–11. doi: 10.1055/s-0036-1593615. [DOI] [PubMed] [Google Scholar]
- 37.Abbasi M, Ahmadian L, Amirian M, Tabesh H, Eslami S. The development of a minimum data set for an infertility registry. Perspect Health Inf Manag. 2018;15(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- 38.Workman TA. AHRQ methods for effective health care. engaging patients in information sharing and data collection: the role of patient-powered registries and research networks. 1. rockville: agency for healthcare research and quality; 2013. [PubMed] [Google Scholar]
- 39.O’Reilly GM, Gabbe B, Moore L, Cameron PA. Classifying, measuring and improving the quality of data in trauma registries: A review of the literature. Injury. 2016;47(3):559–567. doi: 10.1016/j.injury.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Kodra Y, Posada M, Coi A, Santoro M, Bianchi F, Ahmed F, et al. Data quality in rare diseases registries. Adv Exp Med Biol. 2017;10(31):149–164. doi: 10.1007/978-3-319-67144-4_8. [DOI] [PubMed] [Google Scholar]
- 41.Khare R, Ruth BJ, Miller M, Tucker J, Utidjian LH, Razzaghi H, et al. Predicting causes of data quality issues in a clinical data research network. AMIA Jt Summits Transl Sci Proc. 2018;20(17):113–121. [PMC free article] [PubMed] [Google Scholar]
- 42.Subhani S, Al-Rubeaan K. Design and development of a web-based Saudi national diabetes registry. J Diabetes Sci Technol. 2010;4(6):1574–1582. doi: 10.1177/193229681000400635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaman B, Khandekar R, Al Shahwan S, Song J, Al Jadaan I, Al Jiasim L, et al. Development of a web-based glaucoma registry at King Khaled eye specialist hospital, Saudi Arabia: a cost-effective methodology. Middle East Afr J Ophthalmol. 2014;21(2):182–5. doi: 10.4103/0974-9233.129773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poffley A, Thomas E, Grace SL, Neubeck L, Gallagher R, Niebauer J, et al. A systematic review of cardiac rehabilitation registries. Eur J Prev Cardiol. 2017;24(15):1596–1609. doi: 10.1177/2047487317724576. [DOI] [PubMed] [Google Scholar]
- 45.Mohammadzadeh Z, Ghazisaeedi M, Nahvijou A, Rostam Niakan Kalhori S, Davoodi S, Zendehdel K. Systematic review of Hospital Based Cancer Registries (HBCRs): necessary tool to improve quality of care in cancer patients. Asian Pac J Cancer Prev. 2017;18(8):2027–2033. doi: 10.22034/APJCP.2017.18.8.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tohira H, Jacobs I, Mountain D, Gibson N, Yeo A. International comparison of regional trauma registries. Injury. 2012;43(11):1924–1930. doi: 10.1016/j.injury.2012.08.024. [DOI] [PubMed] [Google Scholar]


