To the Editor:
Asthma is a chronic lung inflammatory disease with multiple phenotypic manifestations, underlined by several disease endotypes that reflect distinct pathophysiological mechanisms1. A T-helper cell type 2 high (TH2High) and mixed TH2/TH17 endotypes have been associated with severe asthma2. TH2High is characterized by an eosinophilic whereas a TH2/TH17 by a mixed eosinophilic/neutrophilic airway inflammation. TH2/TH17 endotype manifests as a difficult-to-control, steroid-resistant disease3, 4. Past research has demonstrated high sputum levels of IL-6 in patients with mixed eosinophilic/neutrophilic airway inflammation5. IL-6 blockade has been proposed as a treatment for asthma6. The IL-4 receptor alpha chain variant R576 (IL-4Rα-R576) drives mixed TH2/T 17 airway inflammation7, 8. Treatment of Il4raR576 mice with an anti-IL-6 monoclonal antibody (mAb) protected against severe airway inflammation5. There are no reported cases of IL-6 pathway blockade for pediatric asthma. Here, we analyzed the response of two patients with severe persistent, non-atopic asthma with evidence of TH2/TH17 inflammation treated with tocilizumab, a humanized anti-IL-6 receptor (IL-6R) mAb.
Patient 1:
This is a 6-year-old with severe persistent, non-atopic asthma, homozygous for the IL4RR576 allele (mutant allele). He had severe life-threatening asthma (with documented reversibility of airway obstruction on bronchodilation; [Table 1]) with 18 intensive care unit (ICU) admissions (four requiring intubation and multiple requiring non-invasive positive pressure ventilation [NIPPV]). Past workup demonstrated negative testing to aeroallergens (skin prick testing [SPT] and serum allergen-specific IgE [sIgE]), normal total serum IgE, normal immune evaluation, sweat test not suggestive of cystic fibrosis, and mild peripheral eosinophilia without evidence for Churg-Strauss syndrome. His anti-neutrophil cytoplasmic antibodies testing was negative and vocal cord dysfunction was thought to be unlikely. Modified barium swallow (MBS) showed deep laryngeal penetration of thin liquids and rigid bronchoscopy revealed type 1 laryngeal cleft. Flexible bronchoscopy and bronchoalveolar lavage (BAL) revealed columnar epithelium admixed with numerous eosinophils and scattered neutrophils, macrophages, and lymphocytes (consistent with mixed TH2/TH17 inflammation).
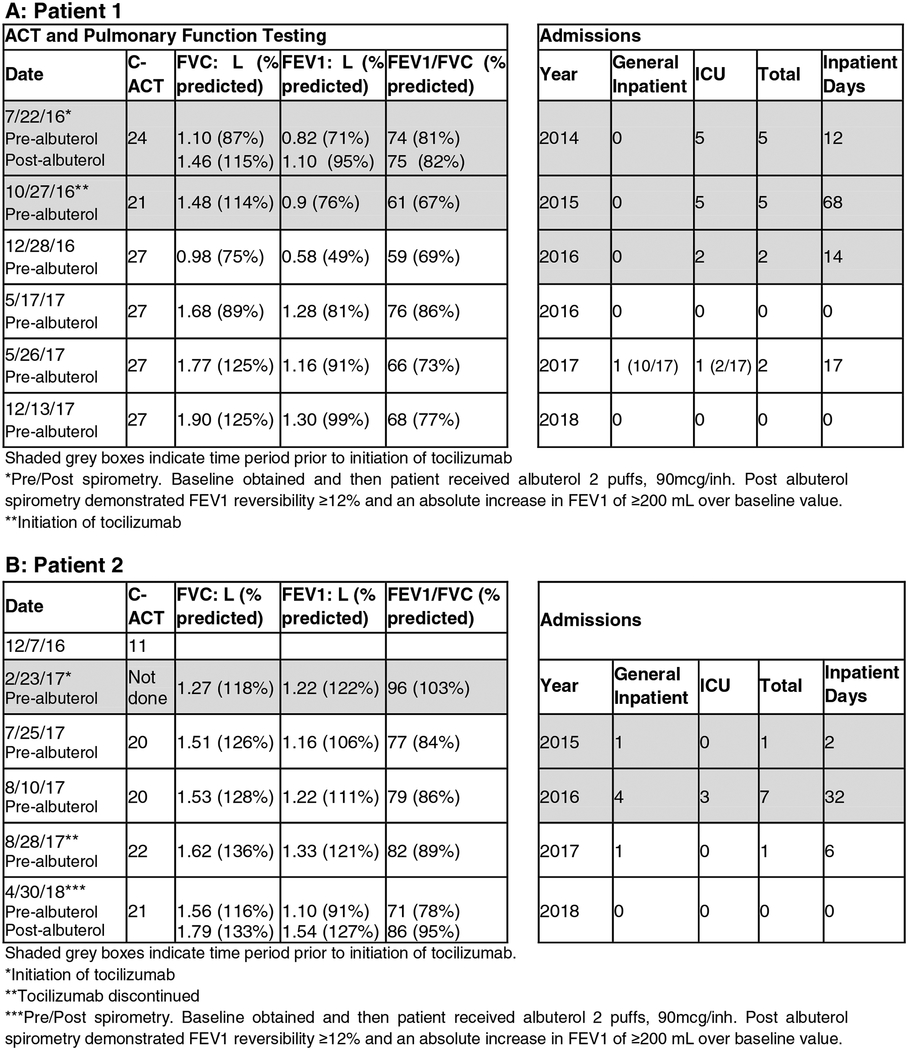
Table 1.
Clinical response: Asthma control test (for children ages 4–11, max score 27), pulmonary function testing, and hospital admissions.
|
Despite fluticasone-salmeterol, montelukast, azithromycin, prednisolone (QOD), omeprazole, nectar-thickened liquids, and careful assessment of adherence to medications in 11/2015 he developed status asthmaticus requiring mechanical ventilation, isoflurane, continuous albuterol, heliox, and terbutaline with a prolonged ICU course. Theophylline and intravenous immunoglobulins [IVIG, at 1g/kg q4wk] were added to his treatment regimen. His insurance denied coverage of omalizumab. Compassionate use for mepolizumab was not-approved. Dupilumab was not yet FDA approved. When stabilized, he underwent successful laryngeal cleft repair. Following repair, his MBS was normal and thickened feeds were discontinued.
While he initially improved following cleft repair and theophylline/IVIG, he had another severe asthma exacerbation in 9/2016 requiring NIPPV. Given ongoing life-threatening exacerbations and known TH2/TH17 airway inflammation, he started tocilizumab on 10/27/16 at 10mg/kg IV q4wk. Tocilizumab was approved through his insurance. Six months later, he was admitted to ICU in status asthmaticus, so tocilizumab was increased to 8mg/kg/dose q2wk and IVIG to 1 gram/kg q2wk. On this regimen he developed neutropenia (absolute neutrophil count: 680cells/μL). Accordingly, the tocilizumab dose was readjusted to 10mg/kg q4wk, on which he is currently maintained, in addition to budesonide/formoterol (160mcg-4.5mcg, 2puff BID), montelukast (10mg QD), azithromycin (200mg three times/wk), theophylline (450mg QD), prednisolone (9mg QOD), and IVIG 1gm/kg q2wk.
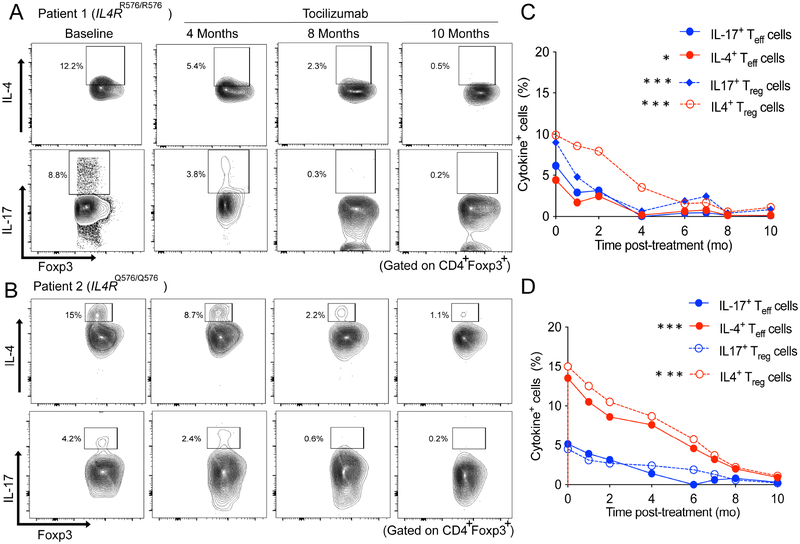
Overall, he exhibited sustained clinical and immunologic responses to tocilizumab over the past 26 months. Clinically, he had decreased hospital admissions, and inpatient hospital days, and improved pulmonary function testing (FEV1 from 76% to 99%) (Table 1 and Fig. E1, A in the Online Repository). He has also had a marked immunological response to tocilizumab with decreased circulating IL-4+Foxp3+ (TH2-like) and IL-17+ Foxp3+ (TH17-like) Treg cells, implicated in disease pathogenesis (Fig 1, A and C)9, as well as decreased TH2 and TH17 effector cells (Fig E2, A, in the Online Repository).
Fig 1.
Analysis of cytokine production by T regulatory (Treg) cells in patient 1 and 2. A and B, Flow cytometric analysis of IL-4 and IL-17 expression in Treg cells just prior to therapy and at 4, 8 and 10 months after tocilizumab treatment in patient 1 (Fig 1, A) and patient 2 (Fig 1, B). C and D, Graphical presentation of IL4 and IL-17 expression in Treg and T effector (Teff) cells in patient 1 (Fig 1, C) and patient 2 (Fig 1, D). *p<0.05, **<0.01, ***<0.001, ****<0.0001 by twoway ANOVA with Tukey post-test analysis.
Patient 2:
This is a 5-year-old with mild atopic dermatitis, eosinophilic esophagitis, and severe persistent, non-atopic asthma who exclusively carried the dominant IL4RQ576 allele. While showing improvement of obstruction on bronchodilation (Table 1), he had persistent severe asthma symptoms despite nectar thick liquids, mometasone/formoterol, fluticasone, montelukast, theophylline, prednisolone (QOD), and omeprazole. Azithromycin was discontinued due to lack of clinical benefit. Theophylline was discontinued due to side effects. Evaluation revealed negative SPT and sIgE to aeroallergens, normal total IgE, reassuring immune evaluation, sweat test not suggestive of cystic fibrosis, normal ciliary biopsy, and negative ABPA work up. He had mild peripheral eosinophilia without evidence for Churg-Strauss syndrome. MBS showed deep laryngeal penetration of thin liquids. His rigid bronchoscopy revealed type 1 laryngeal cleft. He underwent flexible bronchoscopy and BAL that demonstrated airway eosinophilia. BAL culture grew moderate Streptococcus pneumoniae, for which he was treated with Augmentin. Nevertheless, he continued to have persistent exacerbations requiring oral steroids.. Therapies with other biologics were considered but were not available. Omalizumab and anti-IL-5 therapy were not approved for his age (also has non-atopic history). Dupilumab was not at that time FDA approved. His insurance denied IVIG therapy but approved tocilizumab for treatment of asthma.
He started tocilizumab on 2/23/17 at 10mg/kg IV q4wk. Due to ongoing asthma symptoms, tocilizumab was increased to 8mg/kg q2wk on 5/2017. He had one episode of neutropenia (Absolute neutrophil count: 840cells/μL) that spontaneously resolved after tocilizumab was held for 2 wk. He discontinued tocilizumab on 8/28/17 given commitment/challenges of traveling for IV infusions.
While on tocilizumab, the patient demonstrated favorable clinical response with decreased hospital admissions (Table 1 and Fig. E1, B in the Online Repository), and was weaned off oral prednisolone. Flow cytometric analysis at baseline demonstrated TH2high skewing affecting his Treg and Teff cells with a lesser TH17 cell response as compared to patient 1 (Fig 1, B). Tocilizumab therapy suppressed his circulating IL-4+Foxp3+ (TH2-like) and IL-17+Foxp3+ (TH17-like) Treg cells (Fig 1, B and D) and TH2 and TH17 cells (Fig E2, B, in the Online Repository). His immunological improvement remained sustained at 4 months post tocilizumab therapy, the last time his studies were repeated. Since discontinuation of tocilizumab, he underwent laryngeal cleft repair with subsequent normal MBS so nectar thick liquids were discontinued.
In summary, both patients demonstrated clinical and immunological responses to tocilizumab therapy. There were no adverse infections on tocilizumab despite developing mild neutropenia that spontaneously resolved. Neither patients’ peripheral eosinophilia was impacted by tocilizumab therapy (Fig E3 in the Online Repository). Patient 1, homozygous for the IL4RR576 mutant allele, continues on tocilizumab with clinical and immunologic response. Patient 2, homozygous for the dominant IL4RQ576 allele, discontinued tocilizumab but also demonstrated clinical and immunological improvement. Clinical response for both patients must also be evaluated in the setting of other confounding factors including polypharmacy, cleft repair, and the natural history of asthma, which are limitations for this case series.
Tocilizumab therapy may be effective in patients with severe persistent, steroid-resistant asthma by virtue of suppressing both TH2 and TH17 cell responses. Further studies are needed to determine the role of anti-IL-6R therapy in severe asthma and the utility of IL4R genotyping in directing better patient outcomes.
Supplementary Material
Funding Sources:
This work was supported in part by the National Institutes of Health (grant no. R01 AI065617) to Dr. Chatila and (grant no. K24 AI 106822 and UG1HL139124) to Dr. Phipatanakul. Dr. Esty was supported by the Harvard Medical School/CRICO Fellowship in Patient Safety and Quality (Boston, MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: All authors declare no direct financial interest in subject matter or materials discussed in article, or with a company making a competing product.
Clinical Implications: The use of tocilizumab in two children with severe persistent, steroid-resistant asthma resulted in immunological improvement and suggestive of clinical improvement.
References
- 1.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011; 127:355–60. [DOI] [PubMed] [Google Scholar]
- 2.Agache I, Akdis CA. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol Int 2016; 65:243–52. [DOI] [PubMed] [Google Scholar]
- 3.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 2014; 134:1175–86 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakta NR, Erle DJ. IL-17 and “TH2-high” asthma: Adding fuel to the fire? J Allergy Clin Immunol 2014; 134:1187–8. [DOI] [PubMed] [Google Scholar]
- 5.Chu DK, Al-Garawi A, Llop-Guevara A, Pillai RA, Radford K, Shen P, et al. Therapeutic potential of anti-IL-6 therapies for granulocytic airway inflammation in asthma. Allergy Asthma Clin Immunol 2015; 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 2012; 8:1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med 2016; 22:1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Gadir A, Massoud AH, Chatila TA. Antigen-specific Treg cells in immunological tolerance: implications for allergic diseases. F1000Res 2018; 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol 2016; 138:639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.