Abstract
Although donation of bone marrow (BM) or peripheral blood stem cells (PBSC) from children to family members undergoing allogeneic transplantation are well-established procedures, studies detailing levels of pain, symptoms, and long-term recovery are lacking. To address this, we prospectively enrolled 294 donors age <18 at 25 pediatric transplant centers in North America, assessing them pre-donation, peri-donation, 1 month, 6 months, and 1 year after donation. We noted that 71% of children reported pain and 59% other symptoms peri-donation, with resolution to 14% and 12% at 1 month. Both older age (age 13–17 vs. younger) and female sex were associated with higher levels of pain peri-donation, with the highest rates in older females (57% and 17% reporting grades 2–4 and 3–4 pain, respectively). Multivariate analyses showed a 4-fold increase in risk for older females compared to males <13 (p<0.001)). At 1 year, 11% of 13–17 year old females reported grade 2–4 pain compared to 3%, 0%, and 1% of males aged 13–17, females <13, and males <13 (p=0.01). Males and females 13–17 years old failed to return to pre-donation pain levels at 1 year 22% and 23% of the time compared to 3% and 10% in males and females <13 (p=0.002). In conclusion, females age 13–17 are at increased risk for grade 2–4 pain at 1 year and more than 20% of females and males 13–17 do not return to baseline pain levels 1 year after BM donation. Studies aimed at decreasing symptoms and improving recovery in older children are warranted.
Keywords: stem cell transplantation, BM collection toxicities, Donor safety, PBSC collection toxicities
INTRODUCTION
Donation of bone marrow (BM) by children to siblings or other family members has been an accepted procedure for nearly four decades.1 Over the past 2 decades, the practice of G-CSF stimulated collection of peripheral blood stem cells (PBSC) has become the most common method of donation for adult donors. PBSC products from related pediatric donors have been used to some extent in pediatrics (very high risk recipients, reduced intensity regimens, manipulated products, etc.) but the approach is performed in only a minority of donors.2,3 A combination approach, using G-CSF followed by BM donation (“G-primed” BM) has been investigated by some groups as a way of improving engraftment without increasing graft versus host disease (GVHD),4 and makes up a similarly small portion of pediatric donation procedures.
In 2010 the American Academy of Pediatrics issued a policy statement on children as hematopoietic stem cell donors.5 The statement outlined a series of recommendations including conditions under which donation is considered reasonable, a call to use the safest and most appropriate donation approach, and a strong statement encouraging research regarding the physical and psychological outcomes associated with donation. A Worldwide Network for Blood and Marrow Transplantation Association (WBMT) working group similarly outlined the need for more detailed data regarding the donation process in children in order to inform and protect them.6
Unfortunately, well-characterized assessments of the child donor experience are lacking. For BM donation, a single center report published in the 1980s described outcomes of 128 child donors under 10 and 343 between 10 and 19 as part of a description of outcomes of donors of all ages.7 The outcomes described, however, were limited to severe adverse events, “greater than expected” pain, post-spinal headaches, and transfusions (“life threatening complications” were noted in 0.4%). Between that study and 2012, a description of 23 children under age 2,8 and several small,9–11 and 1 larger study2 of side effects of PBSC collection in children were published. Finally, in 2012 a larger prospective study of pediatric donors was performed by the Pediatric Diseases Working Party of the European Blood and Marrow Transplant Group.3 Although these studies documented low rates of serious adverse events (SAEs) and attempted to describe pain, the tools used were basic (pain yes/no with no description of location and minimal description of intensity, etc.) and no longer-term follow up after the procedure was performed.
Data tools that document the donor experience in much greater detail were developed and validated by National Marrow Donor Program (NMDP) over this past decade.12–15 These approaches describe and quantitate pain by specific location as well as describe and grade specific toxicities common to BM and PBSC donors. The tools have been used to assess donors at pre-donation baseline and document recovery, both short and long-term. Using these more precise data approaches in unrelated donors, it has been noted that women experience greater degrees of pain and side effects,13–15 obese donors of PBSC experience more pain,13 and BM donation in adults leads to more SAEs and lingering pain compared to PBSC donation.15
With a lack of detailed data regarding the related HSC donor experience in mind and better donor evaluation tools in hand, a multi-institutional team joined with the NMDP/CIBMTR to implement the Related Donor Safety Study (RDSafe) funded by a grant from the US National Heart, Lung, and Blood Institute (NHLBI). This prospective observational trial enrolled related donors (RD) of all ages at 53 centers in the United States and prospectively collected detailed pre- and post-donation assessments through one year post-procedure. This report details pain, toxicities, and SAEs occurring in 294 pediatric donors < 18 who underwent collection at 25 centers between 2010–2014. Previously published quality of life studies of a large subset of this cohort showed the important findings that 1) a portion of pediatric donors have significant decreases in health-related quality of life (HR-QoL) associated with the procedure16 and 2) there is a disconnect between parents’ and children’s perceptions of the donation process.17 Those papers combined with this report provide the most comprehensive assessment of pediatric HSC donation to date.
METHODS
Parents of pediatric donors were approached for enrollment if their child had been selected to provide either a first or second BM or PBSC donation for a family member. Parental or legal guardian consent was obtained for all donors, and assent obtained for donors above 7 years of age during standard donor counseling sessions at the transplant center. All transplant centers were required to obtain and maintain IRB approval for the study. Donors and/or their parents had to be willing to receive phone calls at 1, 6 and 12 months for follow up interviews by the CIBMTR Survey Research Group. Potential donors unable to speak English, unable to complete a phone interview or who had no access to a telephone were excluded from the study. Donors providing unstimulated peripheral blood stem cells or lymphocytes were not eligible to participate in the study.
All donors underwent a comprehensive medical evaluation including a physical examination, blood tests, and further work up as deemed necessary. Donors were approved to donate per transplant center criteria for donation.
DATA COLLECTION
Eligibility information and donor demographics were reported after donor consent was obtained. A pre-donation form was completed at the time of donor clearance for stem cell donation; for PBSC or G-primed marrow donors this was performed before any mobilizing agent was administered, for BM donors this was performed before the day of collection. This form included questions on donor health history to assess any pre-existing medical conditions (comorbidities) present prior to donation. Details on toxicity and pain were collected at five time points including pre-donation, peri-donation, and 1, 6 and 12 months post-donation. Toxicity was defined by CTC toxicity measures for specific symptoms known to be common with PBSC and BM collection. This approach to assessing toxicities was validated by the NMDP and has been published previously.12–15 The specific scale is called the Modified Toxicity Criteria (MTC) and the symptoms assessed included fever, fatigue, skin rash, local reactions to an injection, nausea, vomiting, anorexia, insomnia, dizziness, and syncope scored based upon the CTC scale allowing categorization as grades 0–4. Pain was reported at 10 body sites (back, bones, head, hip, IV site, joints, limbs, muscles, neck, and throat) and/or other and was scored as 0 (absent), 1 (mild), 2 (moderate), 3 (severe) or 4 (disabling). Pain and MTC toxicities were collected by the transplant center through direct questions to the donor when possible or the parent for children unable to communicate an assessment at pre-donation and peri-donation time points. The CIBMTR Survey Research group was responsible for follow up assessments at 1m, 6m, and 1yr and followed the same procedure of asking direct questions to the donor when possible.
Pain and MTC symptoms for PBSC donors were reported just prior to apheresis on day +5 of G-CSF (known time of peak effects) and if collection occurred on multiple days, pain and symptoms were assessed each day. For marrow donation, pain and MTC symptoms were reported by the donor through a follow up call 24 to 48 hours post-donation. For G- prime marrow donation, pain and MTC symptoms were described just prior to donation and within 48 hours post-donation.
A product specific donation form was completed on the day of donation detailing information on the donation procedure (for example type of anesthesia used, volume of product collected, whether autologous or allogeneic blood transfusions were given for marrow donation, use of central line, days of apheresis, and hypocalcemia symptoms for PBSC collections).
Pre- and post-donation CBCs were reported for all donors. Adverse events were reported as applicable following standard CIBMTR data reporting procedures.
Endpoints
Endpoints for this study included: 1) Incidence of grades 2–4 and 3–4 skeletal pain at peri-collection and post-donation. Skeletal pain represented pain in at least one of the following sites: back, bone, head, hip, joints, limbs, and neck. Severity of skeletal pain was defined as the maximum grade among these pain sites. 2) Incidence of grades 2–4 and 3–4 body symptoms at peri-collection and post-donation. Body symptoms were assessed using the MTC outlined above and the peak toxicity level across all symptoms was analyzed. 3) Recovery to pre-donation levels by 1 year, defined as a pain or symptom score less than or equal to the score at pre-donation. 4) Incidence of blood transfusion after BM donation. 5) Serious adverse events related to donation.
Data Analysis
Analyses were conducted separately by donation type; analyses of PB and G-primed BM were descriptive only due to small numbers. Chi-square tests or Fisher’s Exact tests as appropriate were used to compare the incidences of skeletal pain and MTC symptoms as well as recovery to pre-donation levels for age and sex subgroups in univariate analyses. Multivariate analyses using logistic regression models were conducted to examine prognostic factors for pain and MTC symptoms. The effects were estimated via odds ratios (ORs). Stepwise model selection was used to determine donor characteristics to be included. The following donor and collection characteristics were considered for inclusion in the multivariate model: race, sex, age, BMI, donation year, comorbidity group, pre-donation counts (WBC, platelets, neutrophils, mononuclear cells, hemoglobin), pre-donation symptoms (skeletal pain or max MTC grade), and volume of BM collected per kg donor weight.
RESULTS
Demographics
Table 1 shows demographic details of the 294 participants in the study cohort. As expected, most first donation procedures were unprimed BM donation (92%) with a small percentage of PBSC (5%) and G-primed BM (3%). Second procedures were rare (3.4% of all donations), and were most often PBSC collections. All ages were well represented, with slightly fewer donors in the youngest age group (27%, 38%, and 35% in the 0–6, 7–12, and 13–17 year old groups, respectively). Donor sexes were balanced (48% females). The sample was ethnically diverse: 55% of child donors were Caucasian, 26% were African American, and 13% were Hispanic.
Table 1.
Characteristics of related pediatric (0–17 year olds) donors
| Variable | First BM |
First PBSC |
First G-primed BM |
Second BM |
Second PBSC |
Second G-primed BM |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Number of donors | 261 | 15 | 8 | 6 | 3 | 1 |
| Number of centers | 25 | 14 | 7 | 5 | 3 | 1 |
| Age at donation | ||||||
| 0–6 | 72 (28) | 1 ( 7) | 3 (38) | 1 (17) | 1 (33) | 0 |
| 7–12 | 103 (39) | 5 (33) | 3 (38) | 2 (33) | 0 | 0 |
| 13–17 | 86 (33) | 9 (60) | 2 (25) | 3 (50) | 2 (67) | 1 (100) |
| Median (range) | 10 (0–17) | 14 (6–17) | 10 (4–17) | 13 (2–16) | 16 (7–16) | 15 (.−.) |
| Sex | ||||||
| Female | 128 (49) | 5 (33) | 3 (38) | 3 (50) | 2 (67) | 1 (100) |
| Male | 133 (51) | 10 (67) | 5 (63) | 3 (50) | 1 (33) | 0 |
| BMI (kg/m2)a | ||||||
| Underweight, <5 percentile | 6 ( 2) | 1 ( 8) | 0 | 0 | 0 | 0 |
| Healthy weight, 5th to 85th percentile | 152 (63) | 6 (46) | 6 (75) | 2 (40) | 1 (50) | 1 (100) |
| Overweight, 85th to 95th percentile | 44 (18) | 1 ( 8) | 1 (13) | 1 (20) | 0 | 0 |
| Obese, ≥95th percentile | 41 (17) | 5 (38) | 1 (13) | 2 (40) | 1 (50) | 0 |
| Unknown | 7 (N/A) | 2 (N/A) | 0 (N/A) | 0 (N/A) | 1 (N/A) | 0 (N/A) |
| Not Applicable, child under 2 | 11 (N/A) | 0 (N/A) | 0 (N/A) | 1 (N/A) | 0 (N/A) | 0 (N/A) |
| Race | ||||||
| Caucasian | 137 (52) | 11 (73) | 7 (88) | 4 (67) | 2 (67) | 1 (100) |
| Hispanic | 34 (13) | 2 (13) | 1 (13) | 0 | 0 | 0 |
| African / African American | 72 (28) | 1 ( 7) | 0 | 1 (17) | 1 (33) | 0 |
| Asian / Pacific Islander | 5 ( 2) | 1 ( 7) | 0 | 1 (17) | 0 | 0 |
| Native American | 2 ( 1) | 0 | 0 | 0 | 0 | 0 |
| Multiracial | 8 ( 3) | 0 | 0 | 0 | 0 | 0 |
| Unknown | 3 ( 1) | 0 | 0 | 0 | 0 | 0 |
| Collection related | ||||||
| Year of donation | ||||||
| 2010 | 22 ( 8) | 1 ( 7) | 0 | 0 | 0 | 1 (100) |
| 2011 | 64 (25) | 2 (13) | 4 (50) | 1 (17) | 0 | 0 |
| 2012 | 66 (25) | 4 (27) | 1 (13) | 4 (67) | 3 (100) | 0 |
| 2013 | 69 (26) | 5 (33) | 2 (25) | 0 | 0 | 0 |
| 2014 | 40 (15) | 3 (20) | 1 (13) | 1 (17) | 0 | 0 |
| BM specific | ||||||
| Type of anesthesia | ||||||
| General | 261 (100) | 7 (88) | 6 (100) | 1 (100) | ||
| Spinal | 0 | 1 (13) | 0 | 0 | ||
| Volume of BM product per donor weight (mL/kg) | ||||||
| N Eval | 256 | 7 | 6 | 1 | ||
| Median (Range) | 16 (4–36) | 17 (11–24) | 10 (3–31) | 22 (22–22) | ||
| PBSC specific | ||||||
| Number of days agent administered | ||||||
| 3 | 0 | 1 (13) | 0 | 1 (100) | ||
| 4 | 7 (47) | 0 | 1 (33) | 0 | ||
| 5 | 7 (47) | 7 (88) | 1 (33) | 0 | ||
| 6 | 1 ( 7) | 0 | 0 | 0 | ||
| 7 | 0 | 0 | 1 (33) | 0 | ||
| Mobilizing agents | ||||||
| G-CSF | 15 (100) | 8 (100) | 3 (100) | 1 (100) | ||
| Number of days of collection | ||||||
| 1 | 11 (73) | 2 (67) | ||||
| 2 | 4 (27) | 1 (33) | ||||
| Pre-collection WBC (x109/L) | ||||||
| N Eval | 15 | 3 | ||||
| Median (range) | 29.7 (14.4–75.9) | 46.6 (20.9–51.7) | ||||
| Average daily G-CSF dose per donor weight (μg/kg/day) | ||||||
| N Eval | 13 | 8 | 3 | 1 | ||
| Median (range) | 10.3 (8.0–11.9) | 5.0 (4.8–11.2) | 6.3 (5.1–15.6) | 6.0 (6.0–6.0) | ||
| CD34 prior to collection (x10 /L) | ||||||
| N Eval | 9 | 1 | ||||
| Median (range) | 64.1 (22.1–98.7) | 72.4 (72.4–72.4) | ||||
| Volume of whole blood processed (L) | ||||||
| N Eval | 13 | 3 | ||||
| Median (range) | 18 (5.0–40.0) | 14.8 (10.6–21.0) | ||||
| Central line placement | ||||||
| Yes | 10 (67) | 2 (67) | ||||
| Femoral | 6 | 0 | ||||
| Subclavian | 0 | 1 | ||||
| Internal jugular | 3 | 1 | ||||
| Other sitec | 1 | 0 | ||||
| No | 5 (33) | 1 (33) | ||||
BMI was calculated from height and weight reported at time of enrollment, median time from enrollment to collection was 13 days (range, −12 to 183 days).
Comorbidities categorized based on NMDP guidelines for unrelated donors. Absent: no comorbidities present; Present, acceptable: comorbidity(ies) present but acceptable for donation of product collected; Present, need more information; comorbidity(ies) present but more information would be needed to evaluate if the donor would have been accepted or deferred if evaluated as an unrelated donor for donation of the product collected; Present, defer: comorbidity(ies) present that would have deferred the donor had he/she been evaluated as an unrelated donor for the type of product collected.
Other site: right atrium/superior vena cav (PBSC 1st time).
Date of previous donation was collected as month and year only, the 15th of the month was assumed for purposes of this calculation.
Pain and Common Toxicities Associated with the Donation Procedure
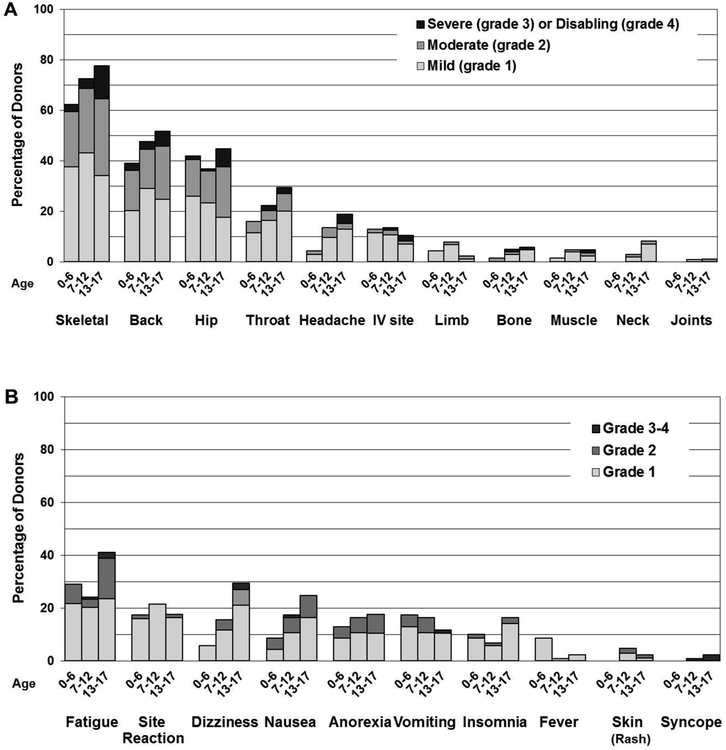
Figure 1A shows pain experienced by unprimed BM donors by age reported 24–48 hours after BM donation. The most common sites of pain included skeletal, back, hip, and throat. More than 60% of even the youngest children reported pain, with nearly a quarter of them experiencing ≥ grade 2 pain. Both the percentage reporting and intensity of pain increased with age, with the 13–17 year old cohort reporting pain nearly 80% of the time with 44% and 13% reporting grade 2–4 and grade 3–4 pain (p= 0.011 and 0.007, respectively, Table 2).
Figure 1. Location and grade of pain and type and grade of collection related toxicities reported by children within 48 hours after bone marrow collection by age group.
A. Sites of pain among BM donors
B. Collection related toxicities among BM donors
Table 2.
Univariate comparisons of skeletal pain and common toxicities experienced by BM pediatric RDSafe donors, by age and by sex at two days, one month, six months, and one year post-donation
| Event and time point | Age 0–12 | Age 13–17 | p-valueb | Male | Female | p-valueb | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (95% CIa) | (95% CIa) | (95% CIa) | (95% CIa) | |||||||
| Pain | ||||||||||
| Baseline, grade 2–4 | (0–3) | (0–6) | 0.551 | (0–3) | (0–6) | 0.240 | ||||
| Baseline, grade 3–4 | (0–3) | (0–4) | 1.000 | (0–3) | (0–4) | 0.490 | ||||
| At collection, grade 2–4 | (21–35) | (33–55) | 0.011 | (20–35) | (30–48) | 0.046 | ||||
| At collection, grade 3–4 | (1–7) | (7–22) | 0.007 | (2–11) | (4–14) | 0.460 | ||||
| At 1 month, grade 2–4 | (0–6) | (2–16) | 0.119 | (1–8) | (2–11) | 0.499 | ||||
| At 1 month, grade 3–4 | (0–4) | (0–5) | 1.000 | (0–3) | (0–5) | 0.498 | ||||
| At 6 months grade 2–4 | (0–5) | (0–9) | 0.602 | (0–6) | (0–7) | 1.000 | ||||
| At 6 months, grade 3–4 | (0–4) | (0–7) | 1.000 | (0–5) | (0–5) | 1.000 | ||||
| At 1 year, grade 2–4 | (0–4) | (2–17) | 0.017 | (0–7) | (1–10) | 0.434 | ||||
| At 1 year, grade 3–4 | (0–3) | (0–5) | (0–4) | (0–4) | ||||||
| Non-recovery at 1 month | (5–16) | (12–32) | 0.032 | (3–14) | (13–28) | 0.010 | ||||
| Non-recovery at 6 months | (6–16) | (9–27) | 0.198 | (4–15) | (10–25) | 0.099 | ||||
| Non-recovery at 1 year | (3–12) | (13–34) | 0.002 | (4–16) | (8–23) | 0.268 | ||||
| Max MTC | ||||||||||
| Baseline, grade 2–4 | (0–2) | (0–8) | 0.109 | (0–4) | (0–4) | 1.000 | ||||
| Baseline, grade 3–4 | (0–2) | (0–6) | 0.332 | (0–3) | (0–4) | 0.494 | ||||
| At collection, grade 2–4 | (11–22) | (17–37) | 0.064 | (14–29) | (11–25) | 0.634 | ||||
| At collection, grade 3–4 | (0–5) | (2–13) | 0.121 | (0–7) | (1–9) | 0.492 | ||||
| At 1 month, grade 2–4 | (2–10) | (1–12) | 1.000 | (2–10) | (2–11) | 1.000 | ||||
| At 1 month, grade 3–4 | (0–4) | (0–5) | 1.000 | (0–5) | 0 | (0–3) | 1.000 | |||
| At 6 months, grade 2–4 | (1–7) | (1–11) | 0.689 | (1–7) | (1–10) | 0.708 | ||||
| At 6 months, grade 3–4 | (0–2) | (0–7) | 0.335 | (0–5) | (0–4) | 1.000 | ||||
| At 1 year, grade 2–4 | (0–5) | (1–12) | 0.338 | (0–7) | (1–9) | 0.677 | ||||
| At 1 year, grade 3–4 | (0–3) | (0–5) | (0–4) | (0–4) | ||||||
| Non-recovery at 1 month | (6–17) | (5–21) | 1.000 | (4–15) | (8–22) | 0.201 | ||||
| Non-recovery at 6 months | (6–17) | (6–22) | 0.823 | (5–16) | (8–22) | 0.396 | ||||
| Non-recovery at 1 year | (5–16) | (4–20) | 8 | (3–15) | (6–20) | 0.349 | ||||
Exact confidence interval
Fisher’s Exact Test p-value
Child donors reported MTC symptoms 24–48 hours after BM donation less often than pain (Figure 1B), with the most common symptoms experienced by donors in the study being fatigue, site reaction, dizziness, and nausea. Notably, the older cohort had a trend towards having more grade 2–4 MTC symptoms compared to younger donors (p = 0.064, Table 2) with 41%, 29%, and 25% of older donors reporting respectively fatigue, dizziness, and nausea, compared to 27%, 12%, and 14% in younger donors.
There were 15 PBSC donors undergoing the procedure for the first time. One donor reported grade 2–4 pain and none reported MTC symptoms with collection. There was a trend for less grade 2–4 pain (p=0.069) and grade 2–4 toxicities (p=0.081) with PBSC collection compared to BM donation, but the numbers are small. G-primed BM numbers were also low (n=8), with 2 donors reporting grade 2–4 pain, while 3 reported grade 2–4 toxicities after donation (data not shown). Of note, PBSC donors on the RDSafe study between ages 18–25 experienced grades 2–4 pain and MTC toxicity levels of 48% and 18%, respectively,18 and earlier studies have shown side effects with PBSC donation to increase with age,2,3 so our small PBSC population is likely not fully reflective of the experience of older children with PBSC.
Univariate Analysis of Pain and Toxicity with BM Donation: Effect of Age and Sex at Donation
The two younger groups reported outcomes that were statistically noted to be similar for both pain and MTC symptoms and were thus combined for univariate analysis (Table 2). Age had a major effect on both pain and toxicity reported within 24–48 hours after donation, with donors in the 13–17 year old group reporting 44 vs. 27% grades 2–4 pain and 13 vs. 4% grades 3–4 pain compared to the younger group (p= 0.011 and 0.007, respectively). In addition, older donors trended toward reporting more grades 2–4 symptoms (26 vs. 16%, p=0.064). A similar effect was noted by sex, with more grade 2–4 pain being reported by female donors (all ages, 39 vs. 27%, p=0.046).
When age and sex were assessed together by univariate analysis, there was a clear effect of being female and older, with 13–17 year old females experiencing grades 2–4 and 3–4 pain 57% and 17% of the time, compared to 13–17 year old males (30% and 9%), <13 year old females (30% and 4%), and <13 year old males (25% and 3%; p=0.004 and 0.023, for grades 2–4 and 3–4 respectively, Table 3). A combined age and sex effect was not observed for non-pain symptoms.
Table 3.
Univariate comparisons of skeletal pain and common toxicities experienced by BM pediatric RDSafe donors, by age and sex at baseline, two days, one month, six months, and one year post-donation
| Event and time point | Male, age 0–12 | Female, age 0–12 | Male, age 13–17 | Female, age 13–17 | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| (95% CIa) | (95% CIa) | (95% CIa) | (95% CIa) | ||||||
| Pain | |||||||||
| Baseline, grade 2–4 | (0–4) | (0–6) | (0–8) | (0–12) | 0.428 | ||||
| Baseline, grade 3–4 | (0–4) | (0–6) | (0–8) | (0–8) | 0.655 | ||||
| At collection, grade 2–4 | (17–36) | (20–41) | (17–46) | (41–72) | 0.004 | ||||
| At collection, grade 3–4 | (1–10) | (1–10) | (3–22) | (7–31) | 0.023 | ||||
| At 1 year, grade 2–4 | (0–8) | (0–6) | (0–16) | (3–27) | 0.011 | ||||
| Non-recovery at 1 month | (2–15) | (6–24) | (2–25) | (17–48) | 0.007 | ||||
| Non-recovery at 1 year | (0–10) | (4–20) | (9–40) | (10–40) | 0.002 | ||||
| Max MTC | |||||||||
| Baseline, grade 2–4 | (0–4) | (0–4) | (0–12) | (0–12) | 0.109 | ||||
| Baseline, grade 3–4 | (0–4) | (0–4) | (0–8) | (0–12) | 0.332 | ||||
| At collection, grade 2–4 | (12–29) | (6–21) | (12–39) | (16–45) | 0.121 | ||||
| At collection, grade 3–4 | (0–8) | (0–7) | (0–12) | (3–23) | 0.113 | ||||
Exact confidence interval
Fisher’s Exact Test p-value
Time to Recovery and Persistence of Pain at 1 year
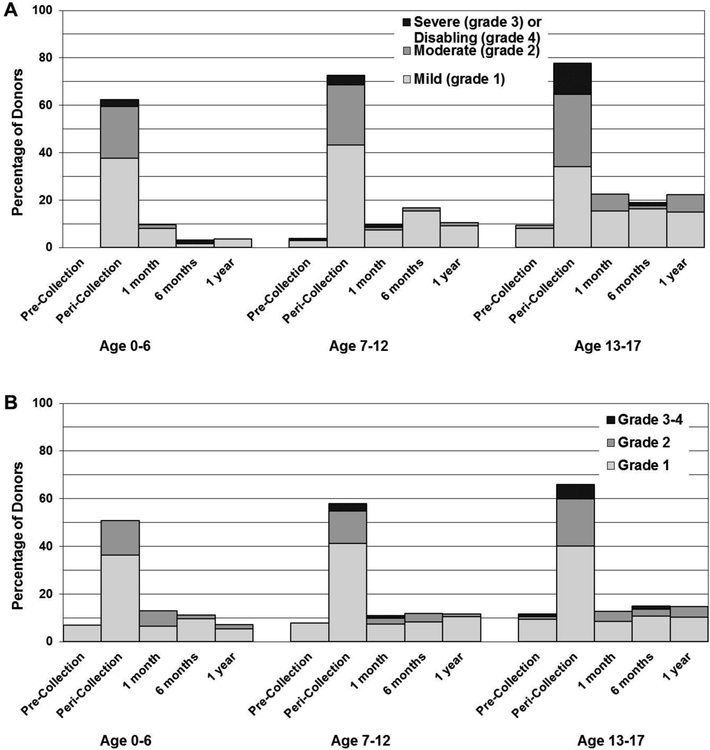
Pre-donation levels of pain and symptoms were low for children at baseline, especially for those in the youngest group (Figure 2). It is notable that a higher percentage of donors in each age group report grade 1–2 pain at one year compared to pre-donation (ages 0–12 2% pre-donation and 8% at 1 year, p=0.02 and ages 13–17 9% pre-donation vs. 22% at 1 year, p=0.05).
Figure 2. Overall highest reported grade of pain and symptoms by age at pre-collection, within 48 hours of collection and at 1month, 6months, and 1 year after first BM collection procedure.
A. Skeletal Pain. Skeletal pain experienced by BM donors, by age, at pre-collection, during the peri-collection period, and post-donation. (Skeletal pain represents pain in at least one of the following sites: back, bone, headache, hip, limb, joint, and neck). The severity of skeletal pain is defined as the maximum grade among these pain sites.
B. Max MTC. Symptoms experienced by BM donors, by age, at pre-collection, during the peri-collection period, and post-donation. (Max MTC represents: fatigue, insomnia, site reaction, dizziness, anorexia, nausea, vomiting, skin (rash), fever, syncope). The severity of max MTC is defined as the maximum grade among these symptoms.
Univariate analyses of pain from pre-donation through 1 year and lack of recovery (return to pre-donation pain/symptom level) showed a similar age and sex effect. Donors age 13–17 reported grade 2–4 pain levels at 1 year of 7% vs. 1% in younger donors (p=0.017). Older donors had a higher risk of failure to return to pre-donation levels at 1 month and 1 year (older 21% vs. younger 10% at 1 month, 22% vs 6% at 1 year; p=0.032 and 0.002, respectively). Twenty percent and 16% of females failed to return to pre-donation pain levels at 1 and 6 months compared to 7% and 8% of males (p=0.010 and 0.099, see Table 2). Combining age and sex variables, 11% of 13–17 year old females reported grade 2–4 pain at 1 year compared to 3%, 0%, and 1% of males aged 13–17, females <13, and males <13 (p=0.011). Males and females 13–17 years old failed to return to pre-donation levels at 1 year 22% and 23% of the time compared to failure of recovery of 3% and 10% in males and females <13 (p=0.002, Table 3).
Comorbidities in Childhood Donors
A variety of comorbidities were reported in children undergoing BM or PBSC donation. Table 4 describes comorbidities reported in first-time BM donors by age, most commonly associated with pulmonary, CNS, psychiatric, or gastrointestinal conditions. We analyzed the effect of these comorbidities in two ways. First, we looked at the effect of any given comorbidity on outcomes. Because each comorbidity was rare, it is not surprising that no significant effects were noted. We then placed the comorbidities into three categories: 1) comorbidities that would clearly lead to deferral based upon NMDP policies, 2) comorbidities that were considered acceptable for donors by NMDP policy, and 3) comorbidities that would have required detailed assessment by the transplant center and further information in order to make a judgment regarding donation. Notably, no pediatric donors had comorbidities that would have been a contraindication to donation according to NMDP standards. Although the presence of comorbidities was associated with a small increase in pre-donation pain (8 vs. 0% grade 2–4, p=0.010), there was no difference in pain, MTC symptoms, and recovery experienced by pediatric donors reporting vs. those not reporting comorbidities.
Table 4.
Classification of comorbidities in related pediatric BM donors by age at donation. (Note that a donor can have more than one comorbidity. All percentages are row percent.)
| Comorbidity | Age 0 to 6 | Age 7 to 12 | Age 13 to 17 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Present, Accept |
Present, indeterminate |
Absent | Present, Accept |
Present, indeterminate |
Absent | Present, Accept |
Present, indeterminate |
||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Overall comorbidities | 61 | (85) | 6 | ( 8) | 5 | ( 7) | 76 | (74) | 11 | (11) | 16 | (16) | 65 | (76) | 9 | (10) | 12 | (14) |
| Pulmonary | 66 | (92) | 1 | ( 1) | 5 | ( 7) | 89 | (86) | 1 | ( 1) | 13 | (13) | 80 | (93) | 0 | 6 | ( 7) | |
| CNS / psychiatric | 72 | (100) | 0 | 0 | 96 | (93) | 5 | ( 5) | 2 | ( 2) | 79 | (92) | 1 | ( 1) | 6 | ( 7) | ||
| Gastrointestinal | 72 | (100) | 0 | 0 | 99 | (96) | 4 | ( 4) | 0 | 82 | (95) | 3 | ( 3) | 1 | ( 1) | |||
| Hematologic | 70 | (97) | 2 | ( 3) | 0 | 101 | (98) | 1 | ( 1) | 1 | ( 1) | 85 | (99) | 1 | ( 1) | 0 | ||
| Cardiovascular | 71 | (99) | 1 | ( 1) | 0 | 101 | (98) | 2 | ( 2) | 0 | 85 | (99) | 1 | ( 1) | 0 | |||
| Endocrine | 72 | (100) | 0 | 0 | 102 | (99) | 1 | ( 1) | 0 | 86 | (100) | 0 | 0 | |||||
| Genitourinary | 71 | (99) | 1 | ( 1) | 0 | 103 | (100) | 0 | 0 | 86 | (100) | 0 | 0 | |||||
| Liver disease | 72 | (100) | 0 | 0 | 103 | (100) | 0 | 0 | 85 | (99) | 1 | ( 1) | 0 | |||||
Blood Transfusions after First Time BM Donation
Allogeneic blood transfusion occurred very rarely in this cohort of children and only in the younger groups (4% and 2% of children ages 0–6 and 7–12, respectively). Only 9% of 13–17 year olds received autologous PRBC transfusions; none of them received allogeneic PRBCs.
Serious Adverse Events
Two serious adverse events were reported in this cohort. A 5-year old donating marrow was admitted for observation for a possible aspiration event after emesis post-extubation and a 13-year old was admitted post-BM donation for observation after rhythm abnormalities and hypertension occurred associated with the procedure. The 13-year old had a pre-existing seizure disorder and experienced a seizure during this night of observation, which further prolonged hospitalization. These two events were considered expected, as anesthesia-related events are known to occur in a small percentage of patients after BM donation.15
Multivariate analysis
Multivariate analysis confirmed that age and sex significantly influenced grade 2–4 pain within 24–48 hours after BM donation, with females age 13–17 having a 4-fold increase in risk compared to younger males (p<0.001, Table 5). Also notable was a decrease in risk during the latter two years of the study (OR 0.52; p = 0.022).
Table 5.
Skeletal pain grade 2–4 at 2 days post collection
| Variable | n (%) | OR (95% CI) | p-value |
|---|---|---|---|
| Age/sex | 0.004 | ||
| 0–12, Male | 87 (0.25) | 1 | |
| 0–12, Female | 84 (0.30) | 1.25 (0.63–2.47) | 0.519 |
| 13–17, Male | 43 (0.30) | 1.35 (0.59–3.07) | 0.472 |
| 13–17, Female | 42 (0.57) | 4.05 (1.84–8.91) | <0.001 |
| Year of collection | |||
| 2010–2012 | 148 (0.39) | 1 | |
| 2013–2014 | 108 (0.25) | 0.52 (0.29–0.91) | 0.022 |
DISCUSSION
Data detailing the experience of normal pediatric BM and PBSC donors is limited, including older studies focused on SAEs,7,8 retrospective experiences of PBSC or G-primed BM collection,9–11,19–22 and a single prospective study from the EBMT.23 The prospective study (Styczynski et al) contained valuable insights regarding complications of anesthesia and apheresis, blood transfusions, pain and hospital stay. However, the study focused only on peri-collection toxicities, pain and toxicity assessments were limited, and no long-term follow up was performed. These reports showed that severe complications in pediatric donation are rare, but they left significant gaps in understanding the pediatric donation experience. Compelling questions include: 1) Where specifically does pain occur and how severe is it? 2) What other symptoms are experienced? 3) How long do the pain and symptoms last? 4) What are the longer-term outcomes (are there children who do not recover fully)? 5) Are there specific factors that increase risk of early pain/symptoms or failure to fully recover? 6) What is the experience like from a psychosocial HR-QoL perspective?
Earlier publications from the pediatric RDSafe HR-QoL cohort documented important psychosocial findings that are currently being assessed in follow up studies.16,17 The RDSafe pediatric medical toxicity study toxicity presented here addresses the other questions enumerated above with compelling findings. We show that the experience of the large majority of children donating HSC includes at most mild to moderate temporary discomfort followed by full recovery. We describe in much more detail a general observation made by other studies23 that as children grow older, higher percentages of them report pain and symptoms, such that by the time they are teenagers they report levels of pain very similar to previously published adult NMDP donors.14 But differing from previous reports, even the youngest donors in our study reported pain more than 60% of the time, and more than 20% of that was grade 2–4 pain. This should serve as a reminder to practitioners involved in pediatric BM donation procedures that the large majority of patients will experience pain, and some will experience significant pain. Therefore, measures should be in place to minimize this (e.g. long-acting local analgesics to collection sites, prophylactic pain medication, etc.). We note that reported pain levels within 24–48 hours of collection decreased over the course of the study, which may have been a result of increased attention focused on RD during the study period.
It is notable that this study is the first to show that sex differences in pain experienced during the BM donation process start early, and that young females age 13–17 are particularly vulnerable to both pain and other toxicities. This finding is consistent with earlier studies of overall levels of pain in adolescents that have reported an approximate doubling of reported pain by females compared to males.24,25 But our finding is even more striking, with multivariate analysis showing the risk of grades 2–4 pain to be 4-fold higher. Adolescent females would be an ideal population for prospectively planned interventions aimed at decreasing up-front discomfort associated with the procedure.
This is the first study in pediatrics to assess donation-associated symptoms at multiple time points after the procedure, and it is important to note that although the large majority of donors experienced a quick and full recovery with no symptoms, some pediatric donors reported minor discomfort and/or persistent symptoms as late as a year after collection. A percentage of both males (22%) and females (23%) aged 13–17 had not fully returned to pre-donation levels at 1 year, and 11% of 13–17 year old girls reported grade 2–4 pain (all grade 2) compared to 2% at pre-donation (p=0.01). Why this low-grade discomfort persists in some donors is unclear; it is mostly back and hip pain, suggesting a relationship with the collection. Whether the pain was continuous over the year is also unclear, as the interviewers did not ask for perceived causes, whether the pain was persistent, or whether the pain was specifically localized the collection site. Further study into this issue is important; if persistent pain is noted to be due to local trauma or nerve damage, interventions could possibly be designed to prevent this prolonged pain. In the meantime, it is important to inform parents and children that in a small percentage of cases, a child donor may have mild persistent skeletal discomfort associated with the donation procedure.
It is notable that although the presence of comorbidities in children was associated with slightly higher pre-donation symptoms, there was no measurable effect of any of the comorbidities reported on the tolerability of the procedure or time to recovery. As expected, the numbers of comorbidities reported by children were relatively small, therefore, further study with larger numbers could possibly identify specific comorbidities that may be important in informing whether pediatric donors with specific comorbidities are at increased risk. Until such data is available, a consensus panel from the WBMT has recommended that potential pediatric HSC donors with chronic medical problems should be seen by a specialist who treats patients with that condition (e.g. a child with diabetes should be seen by a pediatric endocrinologist) and donation should not move forward unless it is determined by the specialist that the child should be able to safely tolerate the anesthesia, temporary discomfort, and anemia associated with HSC donation.6
It is also notable that 27% of children donating marrow received allogeneic blood transfusions in the EBMT experience compared to 0.75% in our experience. The EBMT paper showed a higher risk of transfusions in marrow donors who had more than 20ml/kg collected and recommended that this amount not be exceeded.23 A maximum of 20ml/kg has long been standard practice at most US pediatric marrow donation centers, and our study demonstrates that using this approach, allogeneic blood transfusions after BM harvest in children can be minimized.
In conclusion, this is the most comprehensive study of pediatric HSC donation to date and the first to look at long-term recovery from pain and symptoms. The study showed that although the BM donation procedure is generally safe, almost all children experience pain or other collection related symptoms, and teenage females are especially vulnerable to more severe pain. In addition, a small percentage of child donors have incomplete recovery and persistent mild discomfort as late as 1-year after the procedure. The data presented here should be used as a baseline for informed consent and prospective trials aimed at improving comfort during and recovery after the pediatric HSC donation experience.
highlights.
Children commonly report pain (71%) and symptoms (59%) 24–48 hours after BM donation
Older age and female sex are associated with higher levels of pain peri-donation
Females aged 13–17 are at increased risk for grade 2–4 pain at 1 year.
>20% of donors aged 13–17 don’t return to baseline pain level 1-year post BM donation
ACKNOWLEDGEMENTS
The study was funded by R01 HL085707 through the NHLBI. Additional funding for MAP was provided by 2UG1HL069254 (NHLBI/NCI) and the Johnny Crisstopher Children’s Charitable Foundation St. Baldrick’s Consortium Grant.
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 1U24HL138660 from NHLBI and NCI; a contract HHSH250201700006C with Health Resources and Services Administration (HRSA/DHHS); three Grants N00014-17-1-2388, N00014–17-1-2850 and N00014-18-1-2045 from the Office of Naval Research; and grants from Adaptive Biotechnologies; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; *CytoSen Therapeutics, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite Pharma, Inc.; Medac, GmbH; *Mediware; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Pharmaceuticals Corporation; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES OF CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
REFERENCES
- 1.Pulsipher MA, Nagler A, Iannone R, et al. Weighing the risks of G-CSF administration, leukopheresis, and standard marrow harvest: ethical and safety considerations for normal pediatric hematopoietic cell donors. Pediatr Blood Cancer. 2006;46:422–33. [DOI] [PubMed] [Google Scholar]
- 2.Pulsipher MA, Levine JE, Hayashi RJ, et al. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: the pediatric blood and marrow transplant consortium experience (PBMTC) 1996–2003. Bone Marrow Transplant. 2005;35:361–7. [DOI] [PubMed] [Google Scholar]
- 3.Styczynski J, Balduzzi A, Gil L, et al. Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective European Group for Blood and Marrow Transplantation Pediatric Diseases Working Party study. Blood. 2012;119:2935–42. [DOI] [PubMed] [Google Scholar]
- 4.Frangoul H, Nemecek ER, Billheimer D, et al. A prospective study of G-CSF primed bone marrow as a stem-cell source for allogeneic bone marrow transplantation in children: a Pediatric Blood and Marrow Transplant Consortium (PBMTC) study. Blood. 2007;110:4584–7. [DOI] [PubMed] [Google Scholar]
- 5.Children as hematopoietic stem cell donors. Pediatrics. 2010;125:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitan M, van Walraven SM, Worel N, et al. Determination of Eligibility in Related Pediatric Hematopoietic Cell Donors: Ethical and Clinical Considerations. Recommendations from a Working Group of the Worldwide Network for Blood and Marrow Transplantation Association. Biol Blood Marrow Transplant. 2016;22:96–103. [DOI] [PubMed] [Google Scholar]
- 7.Buckner CD, Clift RA, Sanders JE, et al. Marrow harvesting from normal donors. Blood. 1984;64:630–4. [PubMed] [Google Scholar]
- 8.Sanders J, Buckner CD, Bensinger WI, et al. Experience with marrow harvesting from donors less than two years of age. Bone Marrow Transplant. 1987;2:45–50. [PubMed] [Google Scholar]
- 9.de La Rubia J, Diaz MA, Verdeguer A, et al. Donor age-related differences in PBPC mobilization with rHuG-CSF. Transfusion. 2001;41:201–5. [DOI] [PubMed] [Google Scholar]
- 10.Kawano Y, Takaue Y, Watanabe T, et al. Efficacy of the mobilization of peripheral blood stem cells by granulocyte colony-stimulating factor in pediatric donors. Cancer Res. 1999;59:3321–4. [PubMed] [Google Scholar]
- 11.Watanabe T, Takaue Y, Kawano Y, et al. HLA-identical sibling peripheral blood stem cell transplantation in children and adolescents. Biol Blood Marrow Transplant. 2002;8:26–31. [DOI] [PubMed] [Google Scholar]
- 12.Miller JP, Perry EH, Price TH, et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:29–36. [DOI] [PubMed] [Google Scholar]
- 13.Pulsipher MA, Chitphakdithai P, Miller JP, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009;113:3604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013;121:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Lower risk for serious adverse events and no increased risk for cancer after PBSC vs BM donation. Blood. 2014;123:3655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Switzer GE, Bruce J, Kiefer DM, et al. Health-Related Quality of Life among Pediatric Hematopoietic Stem Cell Donors. J Pediatr. 2016;178:164–170 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Switzer GE, Bruce J, Pastorek G, et al. Parent versus child donor perceptions of the bone marrow donation experience. Bone Marrow Transplant. 2017;52:1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulsipher MA, Logan BR, Chitphakdithai P, et al. The Effect of Aging and Pre-Donation Comorbidities on the Related PBSC Donor Experience: A Report from the Related Donor Safety Study (RDSafe). Biol Blood Marrow Transplant. 2018. November 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Rubia J, de Arriba F, Arbona C, et al. Follow-up of healthy donors receiving granulocyte colony-stimulating factor for peripheral blood progenitor cell mobilization and collection. Results of the Spanish Donor Registry. Haematologica. 2008;93:735–40. [DOI] [PubMed] [Google Scholar]
- 20.Hussein AA, Sharma S, Al-Zaben A, et al. Safety and feasibility of granulocyte colony-stimulating factor (G-CSF) primed bone marrow (BM) using three days of G-CSF priming as stem cell source for pediatric allogeneic BM transplantation. Pediatr Transplant. 2014;18:625–30. [DOI] [PubMed] [Google Scholar]
- 21.Behfar M, Faghihi-Kashani S, Hosseini AS, et al. Long-Term Safety of Short-Term Administration of Filgrastim (rhG-CSF) and Leukophresis Procedure in Healthy Children: Application of Peripheral Blood Stem Cell Collection in Pediatric Donors. Biol Blood Marrow Transplant. 2017. [DOI] [PubMed] [Google Scholar]
- 22.Korbling M, Chan KW, Anderlini P, et al. Allogeneic peripheral blood stem cell transplantation using normal patient-related pediatric donors. Bone Marrow Transplant. 1996;18:885–90. [PubMed] [Google Scholar]
- 23.Styczynski J, Balduzzi A, Gil L, et al. Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective EBMT-PDWP study. Blood. 2011. 2012. March 22;119(12):2935–42. [DOI] [PubMed] [Google Scholar]
- 24.Holden S, Rathleff MS, Roos EM, et al. Pain patterns during adolescence can be grouped into four pain classes with distinct profiles: A study on a population based cohort of 2953 adolescents. Eur J Pain. 2017. [DOI] [PubMed] [Google Scholar]
- 25.Rathleff MS, Roos EM, Olesen JL, et al. High prevalence of daily and multi-site pain--a cross-sectional population-based study among 3000 Danish adolescents. BMC Pediatr. 2013;13:191. [DOI] [PMC free article] [PubMed] [Google Scholar]