Abstract
Background
Nasal polyps influence the burden of aspirin exacerbated respiratory disease (AERD) by contributing to eicosanoid production. AERD is diagnosed through graded aspirin challenges. It is not known how sinus surgery affects aspirin challenge outcomes.
Objective
To investigate the effects of endoscopic sinus surgery (ESS) on aspirin-induced reaction severity and on the levels of eicosanoids associated with these reactions.
Methods
Twenty-eight AERD patients were challenged with aspirin before and 3-4 weeks after ESS. Respiratory parameters and plasma and urine levels of eicosanoids were compared before and after challenges.
Results
Before ESS, AERD diagnosis was confirmed in all study patients by aspirin challenges that resulted in hypersensitivity reactions. After ESS, reactions to aspirin were less severe in all patients and twelve out of twenty-eight patients (43%, p<0.001) had no detectable reaction. A lack of clinical reaction to aspirin was associated with lower peripheral blood eosinophilia [0.1 K/μL (IQR 0.1-0.3) vs. 0.4 K/μL (IQR 0.2-0.8), p=0.006], lower urinary leukotriene E4 (LTE4) levels after aspirin challenge [98 pg/mg creatinine (IQR 61-239) vs. 459 pg/mg creatinine (IQR 141-1344) , p=0.02], and lower plasma prostaglandin D2 to prostaglandin E2 (PGD2/PGE2) ratio [0 (±0) vs. 0.43 (±0.2), p=0.03], compared to those who reacted..
Conclusions
Sinus surgery results in decreased aspirin sensitivity and a decrease in several plasma and urine eicosanoid levels in AERD patients. Diagnostic aspirin challenges should be offered to patients with suspected AERD prior to ESS to increase diagnostic accuracy. Patients with established AERD could undergo aspirin desensitizations after ESS as the severity of their aspirin-induced hypersensitivity reactions lessens.
Keywords: aspirin-exacerbated respiratory disease, eicosanoids, aspirin challenges, eosinophils, endoscopic sinus surgery, nasal polyps
Introduction
Aspirin-exacerbated respiratory disease (AERD) is a subset of asthma characterized by the triad of nasal polyposis, asthma, and respiratory reactions to cyclooxygenase 1 (COX-1)–inhibiting medications.1 AERD is diagnosed by a graded aspirin challenge that results in characteristic upper and lower respiratory symptoms.2,3 Though understanding of the pathogenesis of AERD is limited,4 changes in the respiratory tissue of AERD patients are apparent. Nasal polyp fibroblasts of AERD patients lack prostaglandin E2 (PGE2) synthase and subsequently produce low levels of PGE2; there is also resistance to PGE2 anti-inflammatory effects due to the lack of E-prostanoid 2 (EP2) receptor expression.5–7 The lack of PGE2 and the reduction of its effects on the respiratory tissue is one of the important features of AERD. PGE2 inhibits pro-inflammatory 5-lipoxygenase (5-LO) expression and production of leukotriene B4 (LTB4), while activating the 15-LO pathway.8 This leads to an in increase in 15-hydroxyeicosatetraenoic acid (15-HETE), which is the precursor of another anti-inflammatory eicosanoid – lipoxin A4.8,9 In AERD patients, higher baseline 15-hydroxyeicosatetraenoic acid (15-HETE) levels were associated with symptom improvement during aspirin treatment.10 Levels of lipoxin A4 and 15-epi-lipoxin A4 are significantly lower in AERD patients compared to aspirin-tolerant asthma patients.11,12
In nasal polyp tissue, low PGE2 in conjunction with high PGD2 levels and the resulting high PGD2/PGE2 ratio are characteristic of AERD.6, 13–15 The levels of several eicosanoids change during acute hypersensitivity reaction to aspirin challenge: both LTE4 and PGD2 increase after the reaction locally and systemically.16–18 High urinary PGDM levels are associated with a more severe bronchospasm during aspirin challenges.13,19,20 Urinary LTE4 levels are positively correlated with reaction severity during aspirin challenges.21
These observations suggest that the removal of the nasal polyps might alleviate AERD disease burden, at least temporarily. However, the effects of nasal polyp excision by endoscopic sinus surgery (ESS) on clinical reactions to aspirin and on eicosanoid levels during aspirin challenges have not been well described. Except for the decrease in urinary LTE4 levels,22 it is not known whether ESS leads to changes in eicosanoid levels in plasma or urine, and if so, whether such changes are associated with changes in severity of clinical reactions during aspirin challenges. In this study we sought to examine the influence of ESS on aspirin challenge clinical outcomes. We hypothesized that ESSvwould lead to attenuation of the aspirin-induced hypersensitivity reactions during aspirin challenges in AERD patients and to a decrease in urinary LTE4 and PGD2, and an increase in plasma lipoxin A4 levels.
Methods
Patients
Patients aged 18 years or older with a diagnosis of AERD were recruited into the study. Inclusion criteria comprised documented history of asthma and nasal polyps, chronic rhinosinusitis, and at least one clinical adverse reaction to any COX-inhibitor, stable asthma (baseline FEV1 >70% of predicted and no asthma exacerbations for at least two months before the first challenge), stable asthma treatment for a minimum of four weeks, and oral montelukast 10 mg daily for at least one week prior to aspirin challenges. All patients had nasal polyps and were planning to have aspirin desensitization as part of their routine medical care. All patients signed an informed consent at the time of enrollment, which was approved by the Institutional Review Board at Albert Einstein College of Medicine.
Study protocol
This was a prospective observation trial. All patients underwent a diagnostic oral graded aspirin challenge to confirm aspirin sensitivity. For the graded challenge, 40 mg of aspirin (dissolved Original Alka-Seltzer) was initially administered and then the dose was doubled every 60 minutes (80, 160, and 325 mg).23 The diagnosis of AERD was established by the presence of a positive aspirin challenge, defined as characteristic sinonasal symptoms and/or ≥15% decline in FEV1. Patients were treated on the basis of their specific symptoms after aspirin-induced hypersensitivity reactions. After confirming the diagnosis, patients were scheduled for ESS within 12 months from the time of the initial aspirin challenge. Three to four weeks after ESS, patients had another aspirin challenge and subsequent desensitization to aspirin. At least one week prior to each of the two challenges, all patients took 10 mg of montelukast daily.20 No participants were taking zileuton before challenges. Patients were required to be off oral corticosteroids for at least one week before the challenges (unless on chronic oral prednisone).24 All patients continued using their prescribed inhaled corticosteroids (ICS) with or without long-acting beta-agonists (LABA) at the time of the study and there was no change in their medications between the two challenges. Patients were observed for upper and lower respiratory symptoms, ocular injection, rash, and abdominal pain.
Baseline data included measurements of fractional exhaled nitric oxide (FeNO), spirometry, and nasal inspiratory peak flow (NPF). The FeNO values were recorded by using NIOX VERO (Aerocrine AB, Morrisville, NC) according to the manufacturer guidelines, before performing spirometry and measuring NPF. Spirometry was performed with the Puritan Bennett Renaissance® II spirometer (Pleasanton, CA) to measure forced expiratory volume in 1 second (FEV1). NPF was measured following manufacturer guidelines with an In-Check™ Nasal inspiratory flow device (Clement International, Ltd., Essex, UK). The best of three efforts was recorded for spirometry and NPF. Blood was collected at baseline for measurement of the complete blood count (CBC) and eosinophil count (Sysmex XN-Series Automated Hematology Analyzer, Lincolnshire, IL).
Urinary eicosanoid measurements
Urine was collected at baseline (before aspirin administration), and at ~1 to 2 hours after the onset of the aspirin-induced reaction. Urine samples were stored at −80Ό and analyzed by enzyme-linked immunosorben t assay, (Cayman Chemical Company, Ann Arbor, MI) for tetranor prostaglandin D2 metabolite (PGDM) and LTE4. All measurements were made in triplicate. The results are expressed as picograms of analyte per milligram of creatinine.
Plasma eicosanoid measurements
Blood samples were collected into EDTA-containing collection tubes. Plasma was separated and frozen at −80°C. Samples were ran domized and eicosanoids were extracted by blinded technicians. Eicosanoids were isolated by liquid:liquid extraction as previously described.25, 26 Online liquid chromatography of extracted samples was performed with an Agilent 1200 Series capillary HPLC (Agilent Technologies, Santa Clara, Calif). To measure lipoxins, we employed reverse-phase-based chiral HPLC-MS/MS as previously described.27 Concentrations of eicosanoids were measured and are reported as picograms analyte per milliliter (pg/mL).
Endoscopic sinus surgery (ESS)
Patients were deemed surgical candidates if they had medically refractory disease defined as unsuccessful symptom resolution after previous medical therapy with systemic, broad-spectrum or culture-directed antibiotics (≥2 weeks duration) and either topical nasal corticosteroid sprays (≥3 week duration) or at least a 5-day trial of systemic corticosteroid therapy.28, 29 ESS was performed as part of standard of care based on medical needs. All but one patient had steroid-releasing sinus implant (Propel™, Intersect ENT, Palo Alto, CA) placement during ESS.30
Statistical Analysis
Patient characteristics comparison between the two visits was done by using a paired t-test or Wilcoxon signed-rank test (if data were non-normally distributed). All summary statistics were expressed as means ± standard error of the means (SEM) or as medians and interquartile ranges (IQR). Comparison between those who reacted to the post-ESS aspirin challenge and those who did not react was done by using 2-tailed t-test or Wilcoxon rank-sum test (for non-normally distributed data). The proportion of asymptomatic (“silent”) challenges was compared before and after ESS using the McNemar test for correlated proportions. Categorical data were analyzed by chi-square or Fisher’s exact tests, as appropriate. Pearson correlation analyses were used for measuring linear dependence and expressed as Pearson coefficient. All statistical analyses were performed with STATA 14.2 software (StataCorp, College Station, TX). P-values of <0.05 were considered significant for all analyses.
Results
Twenty-eight AERD patients were enrolled in to the study. More than a half were women and the majority were either African-American or Hispanic/Latino (Table I). All patients had clinical symptoms (upper and/or lower respiratory symptoms) during the hypersensitivity reaction to the challenge before ESS. After ESS, twelve out of 28 patients (43%) had no clinically obvious signs of reaction (Table II, p<0.001).
Table I.
Patients’ characteristics
| AERD patients, N=28 | |
|---|---|
| Age (y), mean ±SE | 46 ±2.7 |
| Female patients, N (%) | 18 (67) |
| Race, N (%) | |
| African American | 14 (50) |
| Latino | 7 (25) |
| White | 7 (25) |
| ICS/LABA* use, N (%) | 23 (82) |
| Oral corticosteroids use, N (%) | 3 (11) |
ICS/LABA – inhaled corticosteroids/long-acting beta-agonists
Table II.
Characteristics of patients during the aspirin challenge before and after surgery.
| Before surgery | After surgery | P-value | |
|---|---|---|---|
| Baseline FEV1, % predicted (±SEM) | 83.7 ± 4.3 | 83.9 ± 4.3 | 0.9 |
| Number of patients with no clinical symptoms during aspirin challenge, N (%) | 0 | 12 (43) | <0.001 |
| Decrease in FEV1-% predicted during hypersensitivity reaction to aspirin, % median (IQR) | −10.0 (−19.0 to −3.8) | −8.6 (−14.0 to −2.4) | 0.02 |
| Minimal FEV1-% value during hypersensitivity reaction to aspirin, % (±SEM) | 68.0 ± 3.9 | 78.0 ± 4.9 | 0.04 |
| Baseline nasal peak flow, L/min (±SEM) | 107.0 ± 8.6 | 141.0 ± 9.0 | 0.003 |
| Decrease in nasal peak flow, % median (IQR) | −26 (−45 to −16) | −13 (−20 to 4) | 0.001 |
| Minimal nasal peak flow during hypersensitivity reaction to aspirin, L/min, median (IQR) | 80 (30–100) | 130 (90–170) | <0.001 |
| FeNO*, ppb median (IQR) | 45 (28–69) | 28 (16–44) | 0.006 |
| FeNO decrease during aspirin desensitization, % median (IQR) | −23 (−41 to −17) | −13 (−32 to −2) | 0.04 |
| Time in minutes to the onset of the hypersensitivity reaction after provoking dose, mean (±SEM) | 43 (± 5.7) | 38 (± 5.0) | 0.5 |
| Number of aspirin doses before hypersensitivity reaction onset, median (IQR) | 2 (1–2) | 2 (1–2) | 0.8 |
| Aspirin provoking dose (mg), median (IQR) | 80 (40–80) | 80 (40–80) | 0.5 |
| Number of aspirin doses before hypersensitivity reaction onset, median (IQR) | 2 (1–2) | 2 (1–2) | 0.8 |
| Baseline peripheral blood eosinophil counts, K/μL, median (IQR) | 0.7 (0.4–0.8) | 0.3 (0.1–0.5) | 0.003 |
FeNO = fractioned exhaled nitric oxide
Comparison of clinical outcomes to aspirin challenge before and after ESS
There was no significant difference in baseline FEV1 %-predicted before and after ESS: 83.7%-predicted (±4.3) vs. 83.9%-predicted (±4.3), respectively (p=0.9, Table II). As expected, there was a significant difference between baseline nasal peak inspiratory flow (NPF) values before and after ESS: 107 (±8.6) L/min before vs. 141 (±9.0) L/min, respectively (p=0.003, Table II). There was also a significant decrease in baseline FeNO from 45 (IQR 28-69) before to 28 (IQR 16-44) after ESS, (p=0.006, Table II), and in baseline peripheral blood eosinophil count from 0.7 (IQR 0.4-0.8) K/μL to 0.3 ( IQR 0.1-0.5) K/μL (p=0.003, Table II). Compared to the reactions before ESS, reactions to aspirin after ESS were less severe. In response to aspirin challenge, FEV1 decrease was significantly greater before than after ESS: −10.0% (IQR −19.0 to −3.8) vs. −8.6% (IQR −14.0 to −2.4), respectively (p=0.02, Table II). NPF decrease was significantly greater before than after ESS: −26.0% (IQR −45.0 to −16.0) vs. −13.0 (−20.0 to 4.0), respectively (p=0.001, Table II). FeNO decrease was significantly greater before ESS than following ESS: −23.0% (IQR −41.0 to −17.0) vs. −13.0% (IQR −32.0 to −2.0), respectively (p=0.04, Table II). There was no significant difference in time to onset of reaction after provoking aspirin dose: 43.0 (±5.7) min before vs. 38.0 (±5.0) min after ESS in those who reacted (p=0.5, Table II), or in the provoking dose: 80 (IQR 40-80) mg before ESS vs. 80 (IQR 40-80) mg post-ESS (p=0.5, Table II).
There were no significant differences in age, gender, or racial background between patients who reacted to aspirin after ESS and those who did not (Table III), although those who had no clinically overt reaction to aspirin appeared somewhat older (50.6 ±4.4 years vs. 42.5 ±3.1 years for those who reacted, p=0.1, Table III). The decrease in FEV1 %-predicted during the aspirin challenge was less in those who had no clinically obvious reaction to aspirin than in those who reacted: −5.3% (IQR −7.3 to 0) vs. −10.7% (IQR −18.0 to −3.0), respectively, p=0.04, Table III. NPF was not significantly different between those who had no clinical reaction to aspirin compared to those who reacted: 125.0 (±17.0) L/min vs. 148.0 (±9.1) L/min, respectively (p=0.2, Table III). However, the decrease in NPF was significantly less in those who did not react vs. those who reacted: 0 L/min (IQR −7.1 to 6.3) vs. −14.0 L/min (IQR −21.0 to −2.3), respectively, p=0.02, Table III. Peripheral blood eosinophil counts were lower in those who did not react compared to those who reacted: 0.1 K/μL (IQR 0.1-0.3) vs. 0.4 K/μL (0.2-0.8), respectively, p=0.006, Table III.
Table III.
Characteristics of aspirin reactors post-ESS versus aspirin non-reactors.
| No reaction, N=12 | Reacted to challenge, N=16 | P-value | |
|---|---|---|---|
| Age (y), mean ±SE | 50.6 ± 4.4 | 42.5 ± 3.1 | 0.1 |
| Female patients, N (%) | 9 (75) | 9 (56) | 0.4 |
| Race, N (%) | 0.99 | ||
| African American | 6 (50) | 8 (50) | |
| Latino | 3 (25) | 4 (25) | |
| White | 3 (25) | 64 (25) | |
| Baseline FEV1, % predicted median (IQR) | 95 (76–109) | 75 (64–95) | 0.1 |
| Decrease in FEV1 % during hypersensitivity reaction to aspirin, % median (IQR) | −5.3 (−7.3 to 0) | −10.7 (−18 to −3) | 0.04 |
| Minimal FEV1 % value during hypersensitivity reaction to aspirin, % median (IQR) | 82 (71–100) | 67 (54–88) | 0.08 |
| Baseline nasal peak flow, L/min (±SEM) | 125 ± 17 | 148 ± 9 | 0.2 |
| Decrease in nasal peak flow, % median (IQR) | 0 (−7.1 to 6.3) | −14.0 (−21.0 to −2.3) | 0.02 |
| Minimal nasal peak flow during hypersensitivity reaction to aspirin, L/min, median (IQR) | 120 (70–170) | 130 (100–160) | 0.8 |
| FeNO*, ppb median (IQR) | 18 (15–34) | 31 (27–51) | 0.06 |
| FeNO decrease during aspirin challenge, %, median (IQR) | −4 (−21 to 18) | −16 (−33 to −7) | 0.07 |
| Baseline peripheral blood eosinophil counts, K/μL, median (IQR) | 0.1 (0.1–0.3) | 0.4 (0.2–0.8) | 0.006 |
| Last aspirin dose before the 2nd, post-ESS challenge, weeks (IQR) | 15 (a8–19) | 12 (9–26) | 0.8 |
| Number of surgeries before enrollment, median (IQR) | 1 (0–1) | 1 (0–2) | 0.6 |
| Lund-Mackay score* prior to surgery, median (IQR) | 15 (14–22) | 19 (19–21) | 0.3 |
| Perioperative oral corticosteroid taper completion, days before aspirin challenge (±SEM) | 16.2 ± 0.7 | 15.6 ± 0.7 | 0.6 |
| Lund-Mackay score – method used for radiologic staging of chronic rhinosinusitis severity. Higher score corresponds to worse disease (range 0-24). | |||
Eicosanoid changes during aspirin challenge before and after ESS
Baseline urinary leukotriene E4 (LTE4) levels were higher before ESS than after ESS: 164.0 pg/mg creatinine (IQR 93.0-267.0) vs. 71.0 pg/mg creatinine (IQR 61.0-124.0), respectively (p=0.001, Figure 1A). Urinary LTE4 levels significantly increased after aspirin challenges both before and after ESS. Before ESS, urinary LTE4 levels increased from 164.0 pg/mg creatinine (IQR 93.0-267.0) to 1023.0 pg/mg creatinine (IQR 375.0-1933.0) (p<0.001, Figure 1B). After ESS, aspirin challenge induced a smaller increase in urinary LTE4: from 71.0 pg/mg creatinine (IQR 61.0-124.0) to 150.0 pg/mg creatinine (IQR 98.0-874.0) (p<0.001 for increase from baseline and p<0.01 for comparison of urinary LTE4 levels after challenges before and after ESS, Figure 1B).
Figure 1.
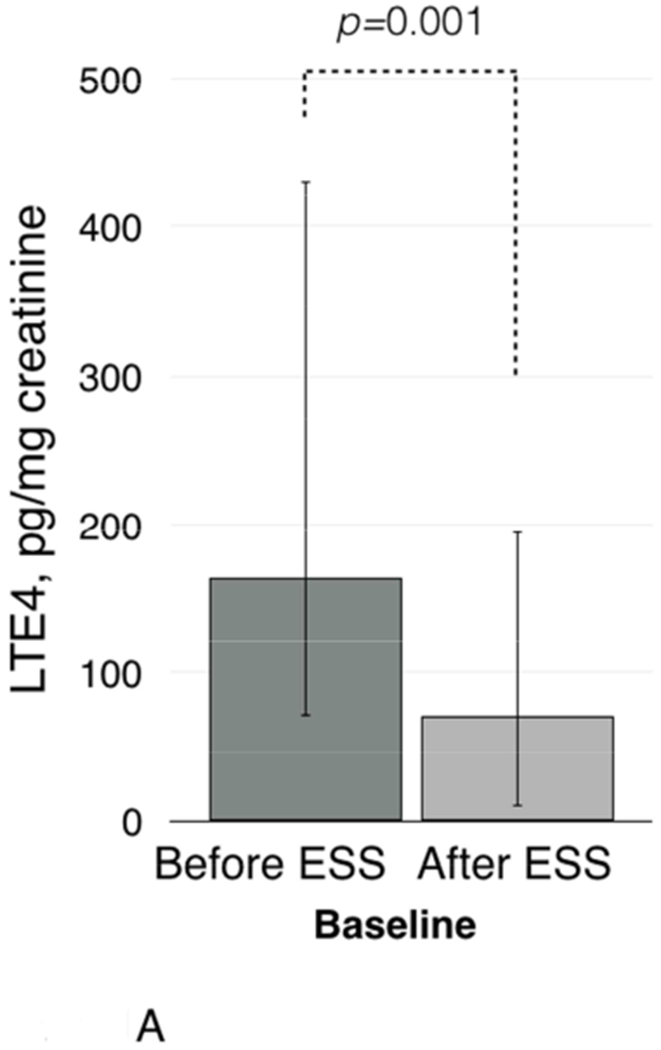
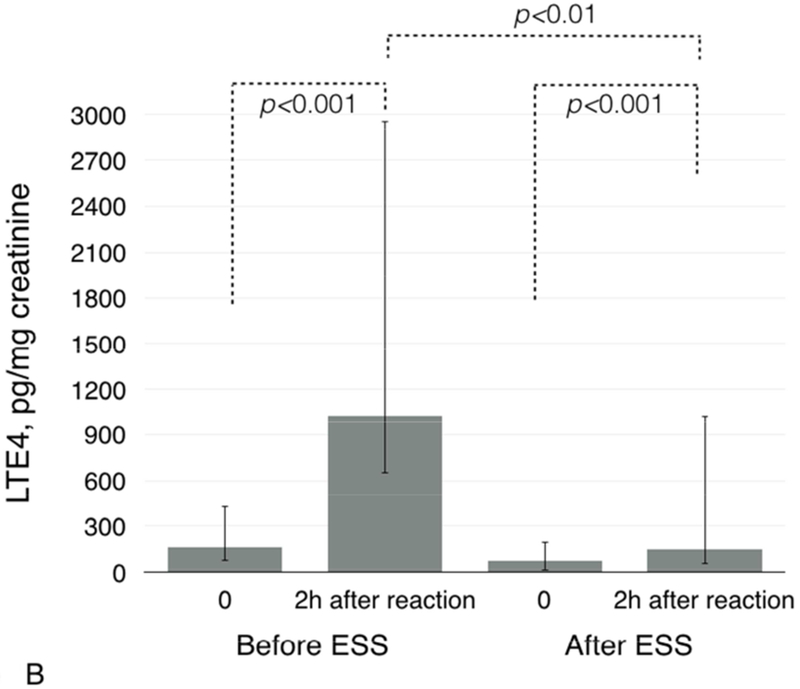
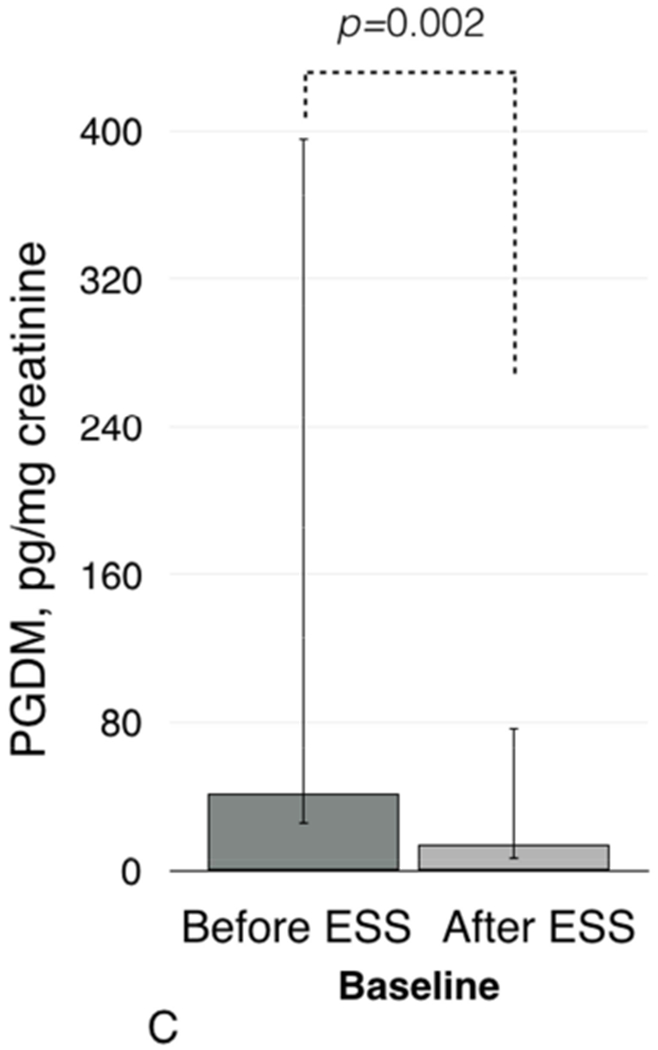
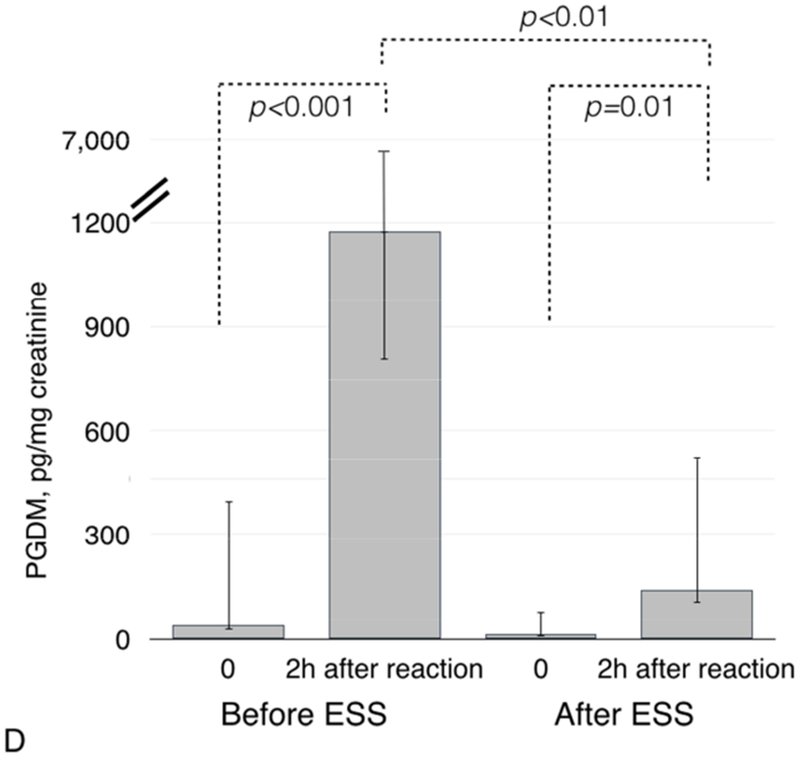
Baseline urinary LTE4 levels before and after ESS (1A) and after aspirin challenges (1B). Baseline urinary PGDM levels before and after ESS (1C) and after aspirin challenges (1D).
Baseline urinary prostaglandin D2 metabolite (PGDM) levels were also higher before ESS than after ESS: 41.0 pg/mg creatinine (IQR 16.0-355.0) vs. 14.0 pg/mg creatinine (IQR 8.0-63.0), respectively (p=0.002, Figure 1C). Before ESS, urinary PGDM levels increased after aspirin challenge from 41.0 pg/mg creatinine (IQR 16.0-355.0) to 1175.0 pg/mg creatinine (IQR 368.0-5579.0) (p<0.001, Figure 1D). After ESS, aspirin challenge induced a smaller increase in urinary PGDM: from 14.0 pg/mg creatinine (IQR 8.0-63.0) to 138.0 pg/mg creatinine (IQR 37.0-385.0) (p=0.01 and p<0.01 for comparison of urinary PGDM levels after challenges before and after ESS, Figure 1D).
After ESS, plasma lipoxin A4 plasma levels were significantly higher than before ESS: 44.0 pg/mL (IQR 31.0-62.0) vs. 28.0 pg/mL (IQR 23.0-40.0), respectively (p<0.01, Figure 2).
Figure 2.
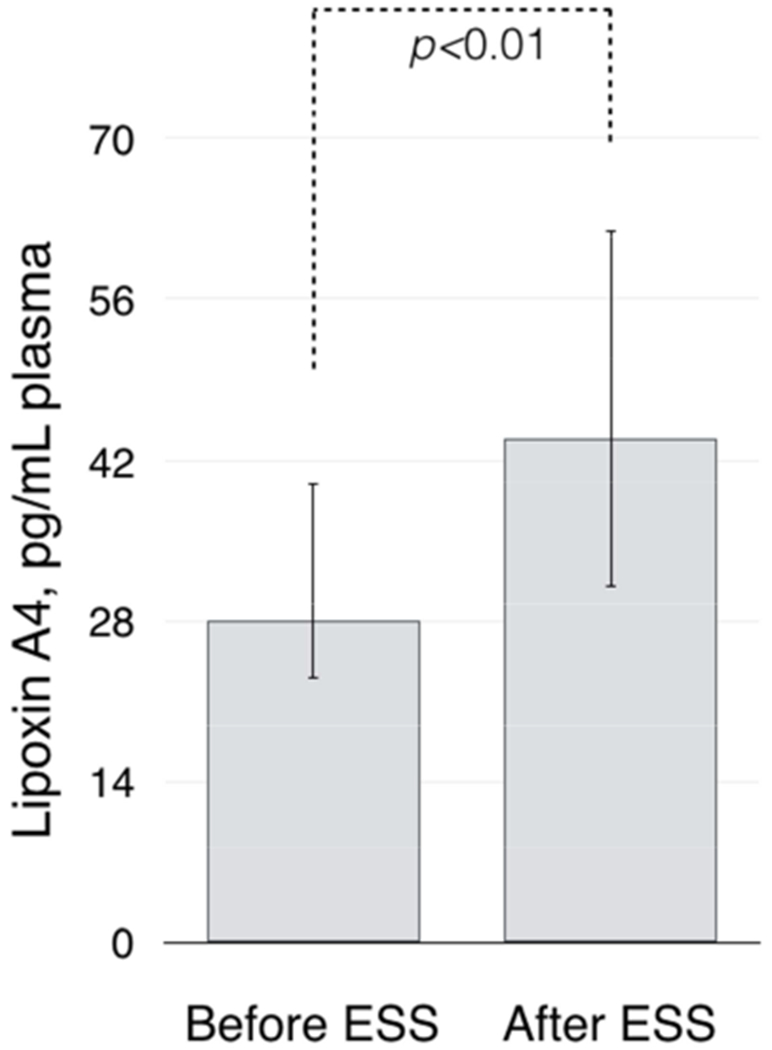
Baseline plasma lipoxin A4 levels before and after ESS.
Eicosanoid changes in aspirin reactors versus aspirin non-reactors
There was no significant difference in baseline urinary LTE4 levels after ESS between patients with clinical reactions to aspirin compared to patients without clinical reaction: 95.0 pg/mg creatinine (IQR 62.0-149.0) in those who reacted vs. 71.0 pg/mg creatinine (IQR 57.0-86.0) in those who did not react (p=0.2, Figure 3A). However, after aspirin challenge, patients who had a clinical reaction to aspirin had significantly higher urinary LTE4 levels than those who did not have a reaction: 459.0 pg/mg creatinine (IQR 141.0-1344.0) vs. 98.0 pg/mg creatinine (IQR 61.0-239.0), respectively (p=0.02, Figure 3A).
Figure 3.
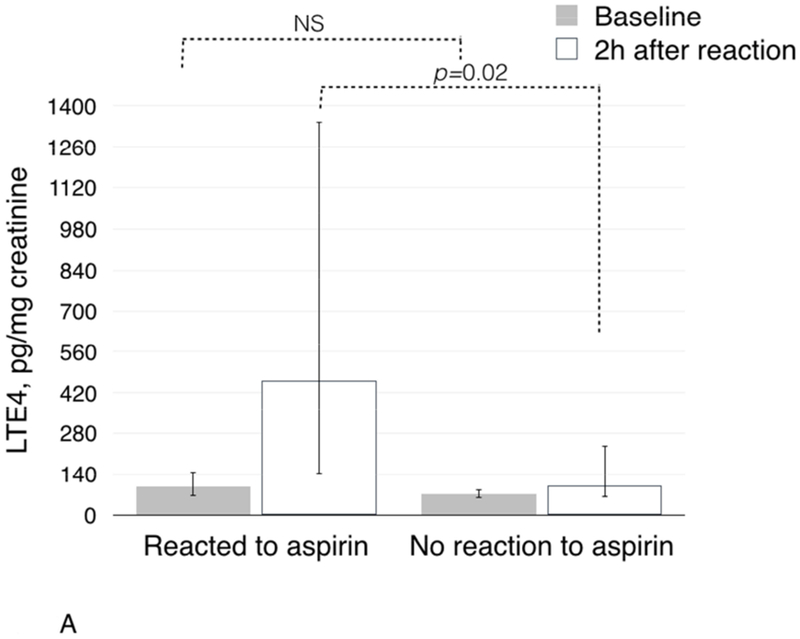
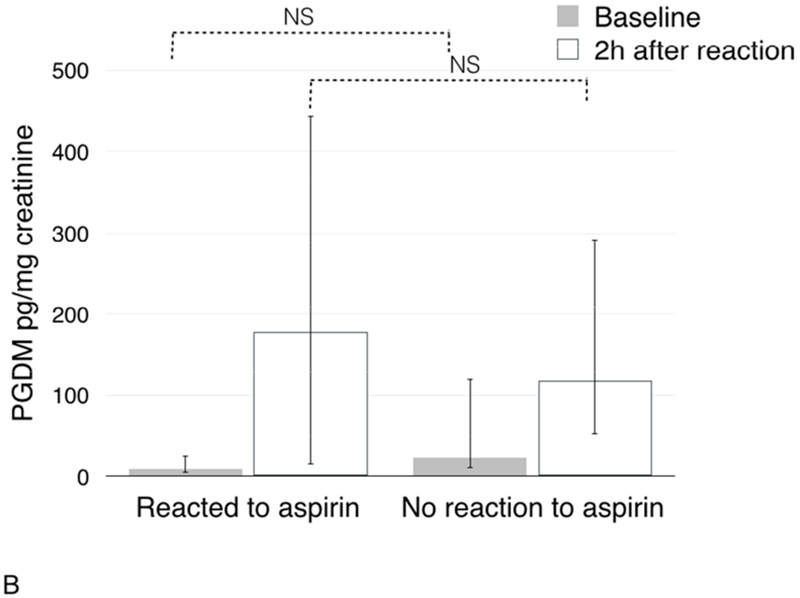
Changes in urinary LTE4 (3A) and PGDM (3B) in urine during aspirin challenges after ESS in patients with and without clinical reactions to aspirin challenges.
After ESS there was no significant difference in baseline urinary PGDM between patients who reacted and those who did not react to aspirin: 9.0 pg/mg creatinine (IQR 5.0-25.0) vs. 23.0 pg/mg creatinine (IQR 10.0-120.0), respectively (p=0.09, Figure 3B). Unlike urinary LTE4, there was no significant difference in urinary PGDM after the challenge between patients who reacted to aspirin challenge and those who did not react: 178.0 pg/mg creatinine (IQR 15.0-443.0) vs. 117.0 pg/mg creatinine (IQR 53.0-291.0), respectively (p=0.8, Figure 3B).
Since high PGD2/PGE2 ratio in nasal polyp tissue has been reported to be associated with NSAID hypersensitivity reactions, we analyzed plasma PGD2/PGE2 ratio before and after ESS. In the whole group, there was no significant change in levels of either prostaglandin after ESS compared to levels before ESS and no significant change in the plasma PGD2/PGE2 ratio (data not shown). However, patients who had a clinical reaction to aspirin after ESS (reactors) had significantly higher plasma PGD2/PGE2 ratio than those without reaction (non-reactors): 0.43 (±0.2) vs. 0 (±0), respectively, p=0.03 (Figure 4A). To investigate this finding further, we compared plasma levels of PGD2 and PGE2 between reactors and non-reactors. Plasma levels of PGD2 were significantly higher in reactors compared to non-reactors: 39.4 (±18.4) vs. 0 (±0), respectively, p=0.03 (Figure 4B). The plasma levels of PGE2 were also higher in reactors than in non-reactors: 177.8 (±44.0) vs. 70.7 (±14.5), respectively, p=0.04 (Figure 4C).
Figure 4.
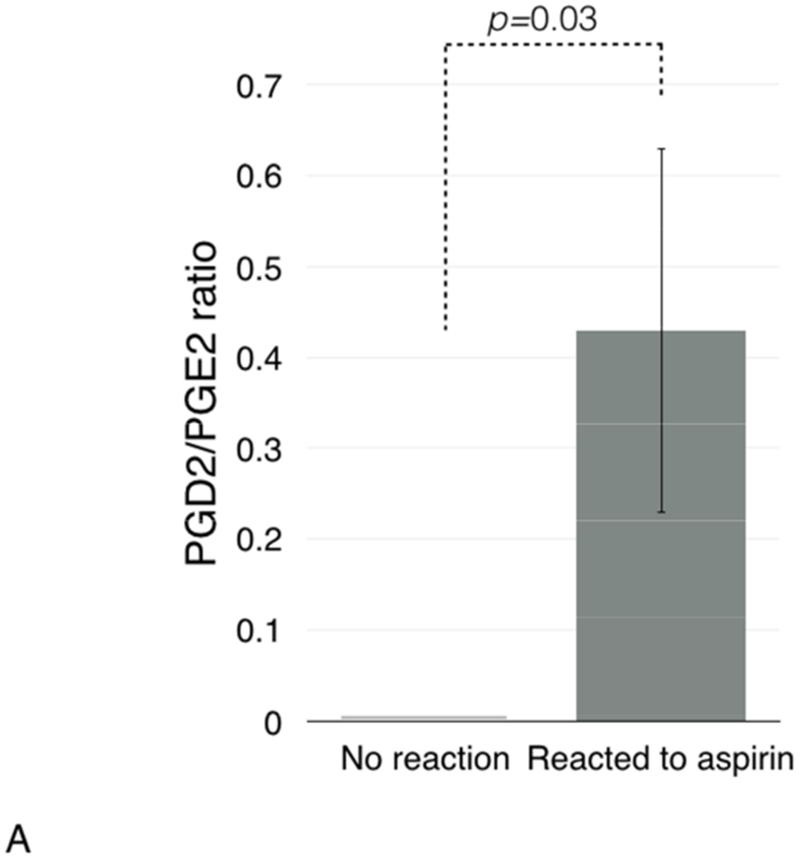
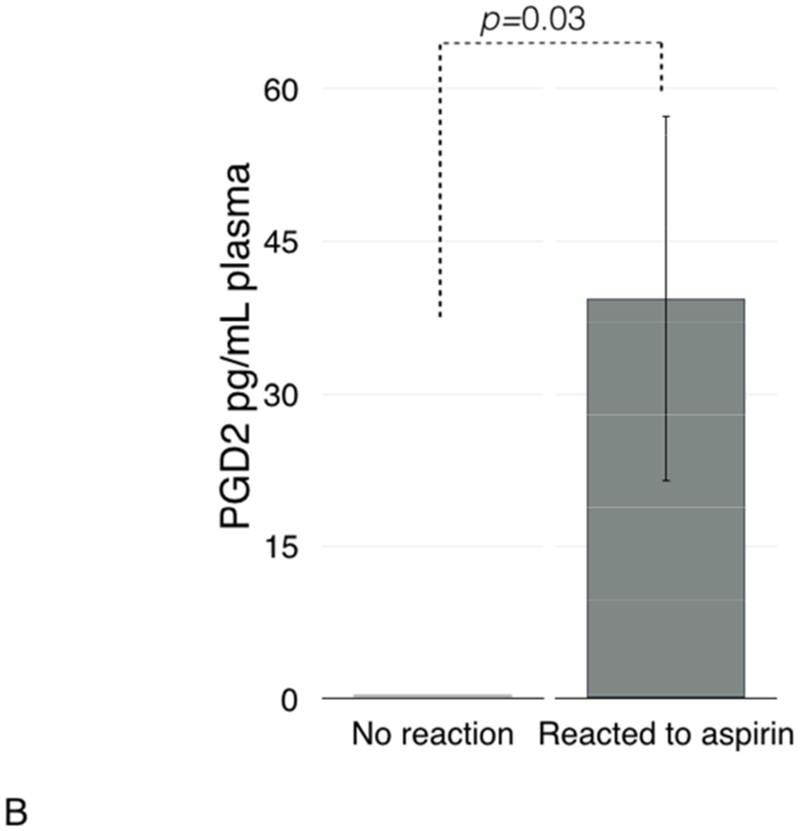
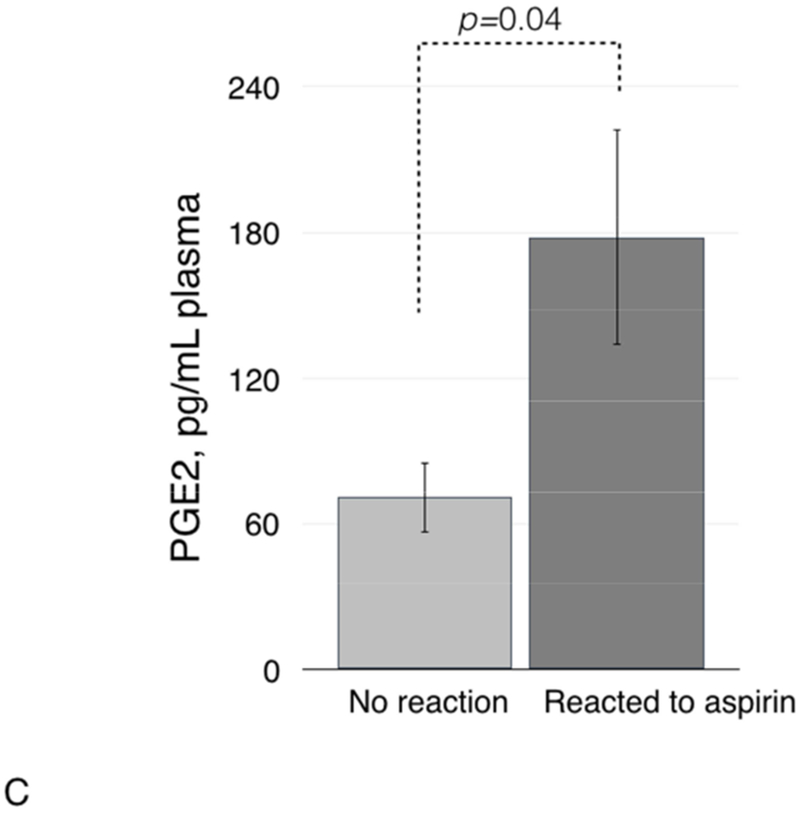
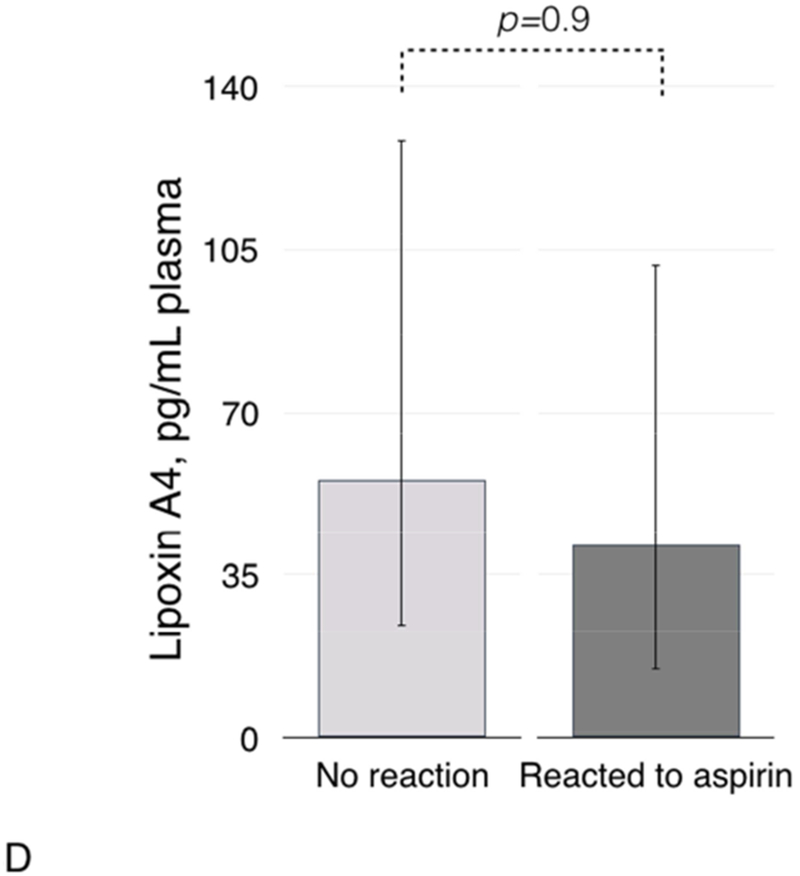
Plasma PGD2/PGE2 ratio (4A), and plasma levels of PGD2 (4B), PGE2 (4C), and lipoxin A4 (4D) in patients with and without clinical reactions to aspirin challenges.
Plasma lipoxin A4 was not significantly different in patients who did not react to aspirin after ESS vs. those who reacted (Figure 4D).
Discussion
NSAID hypersensitivity is the defining feature of AERD. This study suggests that 3-4 weeks after ESS, AERD patients become less reactive to aspirin and some patients become aspirin-tolerant.
Clinically, this study highlights the importance of establishing the diagnosis of AERD prior to ESS in patients with suspected but not confirmed AERD. On the other hand, in those with confirmed AERD aspirin desensitization would be safer and the hypersensitivity reactions less severe after ESS. Mechanistically, the study indicates an important role that the presence of the nasal polyp tissue has in defining AERD phenotype.
While asymptomatic (“silent”) challenges and desensitizations have been reported to occur after premedication with leukotriene inhibitors, systemic corticosteroids, and/or antihistamines,24, 31 to our knowledge this is the first report of silent challenges occurring after recent ESS. While AERD diagnosis could be made based on historic reactions,2,33 not all AERD patients take NSAIDs. Therefore, some patients could be unaware of their sensitivity to NSAIDs. If undiagnosed, such patients are at risk for severe, life-threatening reactions to NSAIDs and at risk for regrowth of the nasal polyps despite multiple polyp surgeries.24 Moreover, some patients take low-dose NSAIDs and identify themselves as non-allergic.32 For such patients, confirmatory aspirin challenge remains an important diagnostic procedure.32 Since more than a third of the patients in this study had silent challenges after ESS, we suggest that diagnostic aspirin challenge should be offered prior to the nasal polyp removal to increase its diagnostic accuracy in patients with suspected but not confirmed AERD. Aspirin challenges are an accepted diagnostic modality for AERD.24, 32 These are frequently followed by aspirin desensitization that is currently the only unique treatment available to patients with AERD.33
Urine and plasma levels of several biomarkers of AERD changed after ESS. There was a significant decrease in urinary LTE4 and PGD2 – the key eicosanoids of hypersensitivity reactions during aspirin challenges. Urinary LTE4 levels have been previously suggested as a biomarker of reaction severity19, 21 and it was observed that LTE4 levels decreased after ESS.22 Interestingly, urinary LTE4 was also proposed to be a diagnostic marker of AERD.34, 35 A concentration of 166 pg/mg creatinine was found to be a reliable threshold for diagnosing aspirin hypersensitivity.34 Notably, median LTE4 urinary levels in this study population at baseline (prior to ESS) were close to the proposed diagnostic value, 164 pg/mg creatinine. However, urinary LTE4 levels significantly decreased after surgery. It should be emphasized that the diagnostic value of LTE4 for identifying aspirin hypersensitivity has higher sensitivity before ESS. LTE4 levels between 67 and 80 pg/mg creatinine were reported as normal.36–38 The median urinary LTE4 concentration after ESS in this study was within the normal range (71 pg/mg creatinine), and was associated with either a less severe or a complete lack of a clinical reaction to aspirin during challenges. While urinary LTE4 is an accurate diagnostic marker for aspirin intolerance in asthma patients,35 it is important to note that LTE4 levels become less reliable after ESS. Thus, LTE4 urinary levels should be considered as a diagnostic marker for AERD in patients in whom nasal polyps are present and who do not have a recent ESS. Both higher peripheral blood eosinophil counts and a greater increase in urinary LTE4 levels during challenge were associated with symptomatic challenges after ESS. Thus, higher peripheral eosinophil counts could be predictive of a greater increase in urinary LTE4 levels and higher probability of experiencing a hypersensitivity reaction during aspirin challenge. Baseline median FeNO values after ESS were significantly lower than before ESS (Table II). This may imply that the eosinophilic inflammation in the lungs decreased after surgery and/or that nasal polyp eosinophilia contributes to higher FeNO values when polyps are present. Although it did not reach statistical significance, baseline FeNO value and the decrease in FeNO levels after aspirin challenges were somewhat lower in patients with silent challenges after ESS compared with patients who reacted (Table III). As reported previously, greater FeNO decrease during aspirin challenge in AERD patients was associated with a greater decrease in FEV1.20
After ESS, there was a significant increase in plasma lipoxin A4 levels. Higher lipoxin A4 levels and higher PGE2 levels are associated with less severe asthma.39, 40 This study also suggests that having a lower plasma PGD2/PGE2 ratio is associated with a lack of a clinical reactions to aspirin after nasal polyp removal, pointing towards a possible link between a high plasma PGD2/PGE2 ratio and NSAID hypersensitivity in AERD. Both eicosanoids are produced from a common precursor PGH2.41 In nasal polyp tissue, PGD2 is primarily produced by mast cells,13 and polyp removal may diminish this source of PGD2. However, some patients that remain aspirin-reactive maintain elevated PGD2/PGE2 ratios. It is possible that PGD2-synthase (PGDS) is available in other respiratory tissues after the excision of nasal polyps that are high in PGDS.13 Elevated PGD2 may be a causative factor in AERD reactivity, as higher PGD2 plasma levels are associated with reduced eosinophil apoptosis and with eosinophilic inflammation in patients with chronic rhinosinusitis.42
Limitations of this study are lack of placebo and randomization preceding aspirin challenges before and after ESS. In addition, repeating challenges several months after surgery have been of clinical interest to reconfirm reproducibility of the results, although a previous study reported 97.6% reproducibility of aspirin challenges in patients with AERD and intact nasal polyps.43 Moreover, correlating outcomes from aspirin challenges and the status of the nasal polyp recurrence might further confirm our hypothesis that the presence of nasal polyps influences aspirin-induced hypersensitivity reactions during challenges. This study suggests increased safety of aspirin challenges within 3-4 weeks after ESS (notably, some patients had evidence of polyp regrowth at this time). However, polyp recurrence could reduce safety of aspirin challenges and desensitizations at later times. Despite these limitations, our study has several strengths including each patient serving as his/her control, a unique representation of minority patients who constituted 75% of the study population, and correlation of the clinical findings and biomarker levels.
In summary, our study presents two novel findings in AERD: 1) Sinus surgery is associated with characteristic changes in plasma and urine eicosanoids and with a subsequent decrease in reaction severity to aspirin challenges; 2) Diagnostic accuracy of oral graded aspirin challenge for AERD is greater if attempted prior to ESS than three to four weeks after ESS. In confirmed AERD patients, aspirin desensitization would be safer and the hypersensitivity reaction less severe three to four weeks after ESS. It is important to emphasize that aspirin challenges and desensitizations should be conducted by physicians who have appropriate training in protocol administration and management of allergic reactions and anaphylaxis.
Clinical symptoms during challenges are strongly linked to changes in several plasma and urine eicosanoids and peripheral blood eosinophils. These associations appear to depend on the presence of the nasal polyp tissue in AERD patients. Therefore, nasal polyp tissue studies might be particularly informative in elucidating the mechanism of AERD.
Highlights Box.
1. What is already known about this topic?
Nasal polyps influence the burden of aspirin exacerbated respiratory disease (AERD) by contributing to eicosanoid production.
2. What does this article add to our knowledge?
In AERD patients, sinus surgery lessens aspirin reactivity during graded challenges. The loss of aspirin sensitivity is associated with characteristic changes in urine and plasma eicosanoid levels and in circulating eosinophils.
3. How does this study impact current management guidelines?
Aspirin challenges after ESS are more likely to be asymptomatic. Diagnostic aspirin challenges should be offered to patients with suspected AERD prior to ESS to increase diagnostic accuracy. Patients with established AERD should undergo aspirin desensitizations after ESS
Acknowledgement
The authors would like to thank Dr. Charles Serhan and Dr. Jeremy Winkler for their help in measuring 15-HETE chirality and for their thorough review and comments on the manuscript.
Declaration of funding sources:
This work was supported by CTSA grant number 5KL2TR001071 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Portions of this work were funded by the Stony Wold-Herbert Fund research grant and by Hiram and Jeanne Gray Funding (to EJ) and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025034 to DCZ). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations:
- AERD
aspirin-exacerbated respiratory disease
- COX
cyclooxygenase
- ESS
endoscopic sinus surgery
- FeNO
fraction of exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- HETE
hydroxyeicosatetraenoic acid
- IL
interleukin
- IQR
interquartile range
- LTE4
leukotriene E4
- LO
lipoxygenase
- NPF
nasal inspiratory peak flow
- NSAIDs
non-steroidal anti-inflammatory drugs
- OR
odds ratio
- PGDM
prostaglandin D2 metabolite
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
- SEM
standard error of the mean
- 95%CI
95% confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Elina Jerschow has research support from Stony-Wold Herbert Fund and Cumberland Pharmaceuticals, Inc. (not relevant to the present study). Waleed M. Abuzeid is a consultant for Medtronic, Inc. Other authors have no conflicts of interest to disclose.
References
- 1.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25. [DOI] [PubMed] [Google Scholar]
- 2.Dursun AB, Woessner KA, Simon RA, Karasoy D, Stevenson DD. Predicting outcomes of oral aspirin challenges in patients with asthma, nasal polyps, and chronic sinusitis. Ann Allergy Asthma Immunol. 2008;100(5):420–5. [DOI] [PubMed] [Google Scholar]
- 3.Macy E, Bernstein JA, Castells MC, Gawchik SM, Lee TH, Settipane RA, et al. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98(2):172–4. [DOI] [PubMed] [Google Scholar]
- 4.Laidlaw TM, Boyce JA. Aspirin-Exacerbated Respiratory Disease--New Prime Suspects. N Engl J Med. 2016;374(5):484–8. [DOI] [PubMed] [Google Scholar]
- 5.Cahill KN, Raby BA, Zhou X, Guo F, Thibault D, Baccarelli A, et al. Impaired E Prostanoid2 Expression and Resistance to Prostaglandin E2 in Nasal Polyp Fibroblasts from Subjects with Aspirin-Exacerbated Respiratory Disease. Am J Respir Cell Mol Biol. 2016;54(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, Perez-Gonzalez M, Pujols L, Alobid I, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin Immunol. 2011;128(1):66–72 e1. [DOI] [PubMed] [Google Scholar]
- 7.Machado-Carvalho L, Martin M, Torres R, Gabasa M, Alobid I, Mullol J, et al. Low E-prostanoid 2 receptor levels and deficient induction of the IL-1beta/IL-1 type I receptor/COX-2 pathway: Vicious circle in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137(1):99–107 e7. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–9. [DOI] [PubMed] [Google Scholar]
- 9.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92(21):9475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerschow E, Edin ML, Pelletier T, Abuzeid WM, Akbar NA, Gibber M, et al. Plasma 15-Hydroxyeicosatetraenoic Acid Predicts Treatment Outcomes in Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2017;5(4):998–1007 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16(1):44–9. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi H, Higashi N, Mita H, Ono E, Komase Y, Nakagawa T, et al. Urinary concentrations of 15-epimer of lipoxin A(4) are lower in patients with aspirin-intolerant compared with aspirin-tolerant asthma. Clin Exp Allergy. 2011;41(12):1711–8. [DOI] [PubMed] [Google Scholar]
- 13.Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado-Carvalho L, Roca-Ferrer J, Picado C. Prostaglandin E2 receptors in asthma and in chronic rhinosinusitis/nasal polyps with and without aspirin hypersensitivity. Respir Res. 2014;15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergol Int. 2008;57(4):429–36. [DOI] [PubMed] [Google Scholar]
- 16.Christie P, Tagari P, Ford-Hutchinson A, Charlesson S, Chee P, Arm J, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–9. [DOI] [PubMed] [Google Scholar]
- 17.Ferreri N, Howland W, Stevenson D, Spiegelberg H. Release of leukotrienes, prostaglandins, and histamine into nasal secretions of aspirin-sensitive asthmatics during reaction to aspirin. Am Rev Respir Dis. 1988;137(4):847–54. [DOI] [PubMed] [Google Scholar]
- 18.Picado C, Ramis I, Rosello J, Prat J, Bulbena O, Plaza V, et al. Release of peptide leukotriene into nasal secretions after local instillation of aspirin in aspirin-sensitive asthmatic patients. Am Rev Respir Dis. 1992;145(1):65–9. [DOI] [PubMed] [Google Scholar]
- 19.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D: A dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerschow E, Ren Z, Hudes G, Sanak M, Morales E, Schuster V, et al. Utility of low-dose oral aspirin challenges for diagnosis of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol. 1999;104(3 Pt 1):559–64. [DOI] [PubMed] [Google Scholar]
- 22.Higashi N, Taniguchi M, Mita H, Kawagishi Y, Ishii T, Higashi A, et al. Clinical features of asthmatic patients with increased urinary leukotriene E4 excretion (hyperleukotrienuria): Involvement of chronic hyperplastic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol. 2004;113(2):277–83. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier T, Roizen G, Ren Z, Hudes G, Rosenstreich D, Jerschow E. Comparable safety of 2 aspirin desensitization protocols for aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2018. [DOI] [PubMed] [Google Scholar]
- 24.White AA, Bosso JV, Stevenson DD. The clinical dilemma of “silent desensitization” in aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2013;34(4):378–82. [DOI] [PubMed] [Google Scholar]
- 25.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43(9):1563–78. [DOI] [PubMed] [Google Scholar]
- 26.Zha W, Edin ML, Vendrov KC, Schuck RN, Lih FB, Jat JL, et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J Lipid Res. 2014;55(10):2124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121(2):569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenfeld RM, Andes D, Bhattacharyya N, Cheung D, Eisenberg S, Ganiats TG, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 Suppl):S1–31. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 30.Han JK, Marple BF, Smith TL, Murr AH, Lanier BJ, Stambaugh JW, et al. Effect of steroid-releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta-analysis. Int Forum Allergy Rhinol. 2012;2(4):271–9. [DOI] [PubMed] [Google Scholar]
- 31.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135(3):676–81 e1. [DOI] [PubMed] [Google Scholar]
- 32.Lee-Sarwar K, Johns C, Laidlaw TM, Cahill KN. Tolerance of daily low-dose aspirin does not preclude aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2015;3(3):449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. N Engl J Med. 2018;379(11):1060–70. [DOI] [PubMed] [Google Scholar]
- 34.Divekar R, Hagan J, Rank M, Park M, Volcheck G, O’Brien E, et al. Diagnostic Utility of Urinary LTE4 in Asthma, Allergic Rhinitis, Chronic Rhinosinusitis, Nasal Polyps, and Aspirin Sensitivity. J Allergy Clin Immunol Pract. 2016;4(4):665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagan JB, Laidlaw TM, Divekar R, O’Brien EK, Kita H, Volcheck GW, et al. Urinary Leukotriene E4 to Determine Aspirin Intolerance in Asthma: A Systematic Review and Meta-Analysis. J Allergy Clin Immunol Pract. 2017;5(4):990–7 e1. [DOI] [PubMed] [Google Scholar]
- 36.Duffield-Lillico A, Boyle J, Zhou X, Ghosh A, Butala G, Subbaramaiah K, et al. Levels of Prostaglandin E Metabolite and Leukotriene E4 Are Increased in the Urine of Smokers: Evidence that Celecoxib Shunts Arachidonic Acid into the 5-Lipooxygenase Pathway. Cancer Prevention Research. 2009;2:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micheletto C, Visconti M, Tognella S, Facchini FM, Dal Negro RW. Aspirin induced asthma (AIA) with nasal polyps has the highest basal LTE4 excretion: a study vs AIA without polyps, mild topic asthma, and normal controls. Eur Ann Allergy Clin Immunol. 2006;38(1):20–3. [PubMed] [Google Scholar]
- 38.Westcott JY, Maxey KM, MacDonald J, Wenzel SE. Immunoaffinity resin for purification of urinary leukotriene E4. Prostaglandins Other Lipid Mediat. 1998;55(5-6):301–21. [DOI] [PubMed] [Google Scholar]
- 39.Pavord ID, Tattersfield AE. Bronchoprotective role for endogenous prostaglandin E2. The Lancet. 1995;345:436–8. [DOI] [PubMed] [Google Scholar]
- 40.Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178(6):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50(6):1015–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JH, Choi GE, Lee BJ, Kwon SW, Lee SH, Kim HS, et al. Natural killer cells regulate eosinophilic inflammation in chronic rhinosinusitis. Sci Rep. 2016;6:27615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller B, Mirakian R, Gane S, Larco J, Sannah AA, Darby Y, et al. Nasal lysine aspirin challenge in the diagnosis of aspirin – exacerbated respiratory disease: asthma and rhinitis. Clin Exp Allergy. 2013;43(8):874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


