Highlights
-
•
Risk factors for rhinovirus lower respiratory tract infection are not well characterized.
-
•
Several risk factors in hematopoietic cell transplant recipients were identified.
-
•
A risk score for progression to lower respiratory tract infection was developed.
Key Words: Rhinovirus, Viral pneumonia, Hematopoietic cell transplantation, Respiratory virus
Abstract
Human rhinovirus lower respiratory tract infection (LRTI) is associated with mortality after hematopoietic cell transplantation (HCT); however, risk factors for LRTI are not well characterized. We sought to develop a risk score for progression to LRTI from upper respiratory tract infection (URTI) in HCT recipients. Risk factors for LRTI within 90 days were analyzed using Cox regression among HCT recipients with rhinovirus URTI between January 2009 and March 2016. The final multivariable model included factors with a meaningful effect on the bootstrapped optimism corrected concordance statistic. Weighted score contributions based on hazard ratios were determined. Cumulative incidence curves estimated the probability of LRTI at various score cut-offs. Of 588 rhinovirus URTI events, 100 (17%) progressed to LRTI. In a final multivariable model allogeneic grafts, prior rhinovirus URTI, low lymphocyte count, low albumin, positive cytomegalovirus serostatus, recipient statin use, and steroid use ≥2 mg/kg/day were associated with progression to LRTI. A weighted risk score cut-off with the highest sensitivity and specificity was determined. Risk scores above this cut-off were associated with progression to LRTI (cumulative incidence 28% versus 11% below cut-off; P < .001). The weighted risk score for progression to rhinovirus LRTI can help identify and stratify patients for clinical management and for future clinical trials of therapeutics in HCT recipients.
Introduction
Human rhinovirus (HRV) is the most commonly detected respiratory virus after hematopoietic cell transplantation (HCT) in both the upper and lower respiratory tract 1, 2. Although rare, once virologically confirmed lower respiratory tract infection (LRTI) has developed, mortality rates due to HRV appear to be high (21% to 41%) and even similar to those seen with other respiratory viruses including influenza, respiratory syncytial virus (RSV), and parainfluenza viruses (PIVs) 3, 4, 5. Risk factors associated with LRTI in this population have been defined for RSV, PIVs, influenza, and human metapneumovirus (hMPV) 6, 7, 8, 9, 10, which include lymphopenia, use of high–dose steroids, and conditioning regimen; this has not been done systematically in HCT recipients presenting with HRV upper respiratory tract infection (URTI). In a study of HRV infection that included both HCT recipients and patients with hematologic malignancies, factors more likely to be present in patients with LRTI included inpatient status, lymphopenia, and hypoalbuminemia [11]. Of note, not all cases of LRTI were virologically confirmed in this study, and the analysis was not conducted in a time–dependent manner. Furthermore, several key factors including viral load were not evaluated in this study.
In the present study we aimed to identify specific risk factors for progression to LRTI in a cohort of patients presenting with URTI. We then developed a risk score for disease progression to help clinicians risk stratify patients who may benefit from more intensive monitoring and follow-up. Ultimately, identification of high–risk patients will be important for clinical trial design that will require defining an enriched cohort at high risk for LRTI that may benefit from potential interventions.
Methods
Patients and Data Collection
We retrospectively identified all HCT patients who underwent transplant between January 1, 2009 and April 1, 2016, at Fred Hutchinson Cancer Research Center. Subjects were eligible for inclusion in the study if positive for HRV from the upper respiratory tract post-transplant. Patients with HRV infection before transplant were excluded.
Clinical data were collected from databases and supplemental review of the medical record. This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center. Subjects signed informed consent permitting the use of data for research.
HRV Case Identification
Respiratory tract samples collected as part of routine clinical care from adult and pediatric patients with respiratory symptoms were tested for 12 respiratory viruses by real-time reverse-transcriptase PCR (RT-qPCR) assays, including RSV, hMPV, influenza viruses A and B, PIVs 1 to 4, adenovirus, human coronaviruses, HRV, and human bocavirus. RT-qPCR with this multiplex panel was performed on all respiratory samples during the time period of this study. The RT-qPCR cycle threshold (CT) was used as a proxy for viral load, with lower CT indicating a higher viral load. CT values were analyzed in quartiles as well as above and below the median.
Definitions
URTI was defined as a positive HRV PCR result from an upper tract sample (nasopharyngeal swab or wash) only from a symptomatic patient, without any radiographic abnormalities. LRTI was defined as a positive lower tract sample (bronchoalveolar lavage [BAL], lung biopsy, or autopsy specimen) with or without radiographic abnormality (proven or probable LRTI, respectively) or a positive upper tract sample with radiographic abnormality (possible LRTI). Both chest x-rays and chest computed tomography results were included if completed within 2 weeks of last positive HRV date. Lower tract sample results were included up to 3 months from URTI date. The day of LRTI diagnosis was defined as the date when HRV was detected in the lower tract specimen (in proven/probable LRTI) or the date the radiographic abnormality was detected (in possible LRTI). Patients who met criteria for LRTI within 2 days of URTI were considered to have LRTI at presentation and were not included in the progression analyses. An HRV illness event was considered to be a new event if ≥12 weeks elapsed between 2 positive samples or if there were ≥2 negative HRV samples between 2 HRV positive samples.
Statistical Analysis
The probability of progression from upper to lower HRV infection was estimated using cumulative incidence, treating death as a competing risk event. Factors associated with progression to LRTI were evaluated using Cox regression models.
The following variables were considered potential predictors of progression: patient age; sex; donor type; cell source; year transplant performed; conditioning regimen; most recent WBC count; neutrophil, lymphocyte, monocyte, and platelet counts before HRV infection (within 2 weeks); lowest albumin level in the 2 weeks before HRV infection; highest daily steroid dose in the 2 weeks before HRV infection; pretransplant lung function; HCT-specific comorbidity index (HCT-CI) [12]; time from transplant to HRV infection; HRV CT values, acute and chronic graft-versus-host-disease; and recipient and donor cytomegalovirus (CMV) serostatus. Recipient and donor statin use was defined as previously reported [13].
All covariates with P ≤ .2 in the univariable analyses were candidates for inclusion in the multivariable Cox regression models. Initially, any variable was retained in the multivariable model if its P ≤ .1 in the full model. The multivariable model was further reduced for constructing the risk score by evaluating the change in the bootstrapped optimism corrected concordance statistic (c-statistic) when each factor was removed from the full model [14]. The bootstrapped c-statistic was based on 100 replications. Those factors having little impact on the c-statistic were removed. Score point contributions were developed using the hazard ratios from the final multivariable model. Hazard ratios were rounded to the nearest integer and scaled such that the maximum achievable score was 100. The risk score for each subject was calculated based on their covariate values and the associated points given. Curves were constructed to examine impact of shifting cut points of the risk score on sensitivity and specificity rates. Cumulative incidence curves for progression to LRTI for score values above (positive predictive value) and below (negative predictive value) a series of binary cut-off values as well as within categorized ranges of scores were evaluated and compared using log-rank tests and Gray's test. Subjects with missing values for albumin, CMV serostatus, and recipient statin status were excluded from univariable and multivariable analyses.
Statistical significance was defined as 2-sided P < .05. SAS version 9.4 TS1M3 (SAS Institute, Inc., Cary, NC) and R version 3.3.0 [15] were used for statistical analyses.
Results
Cohort Description
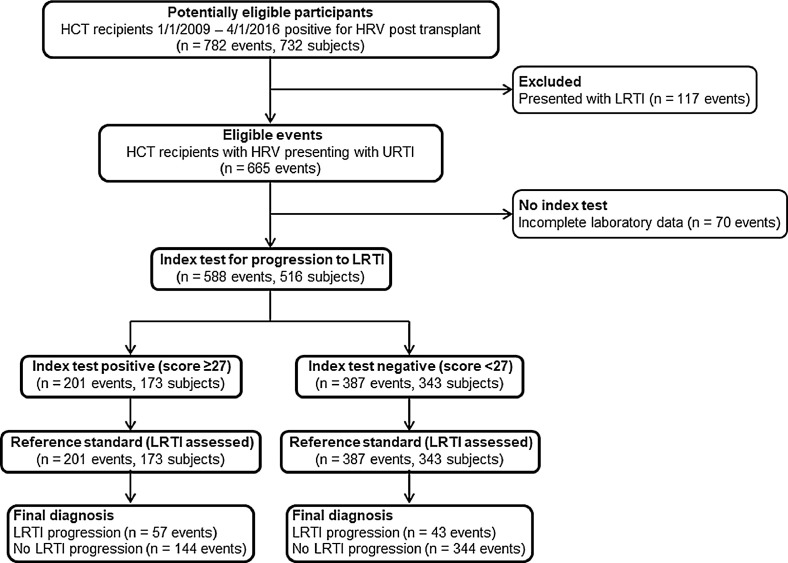
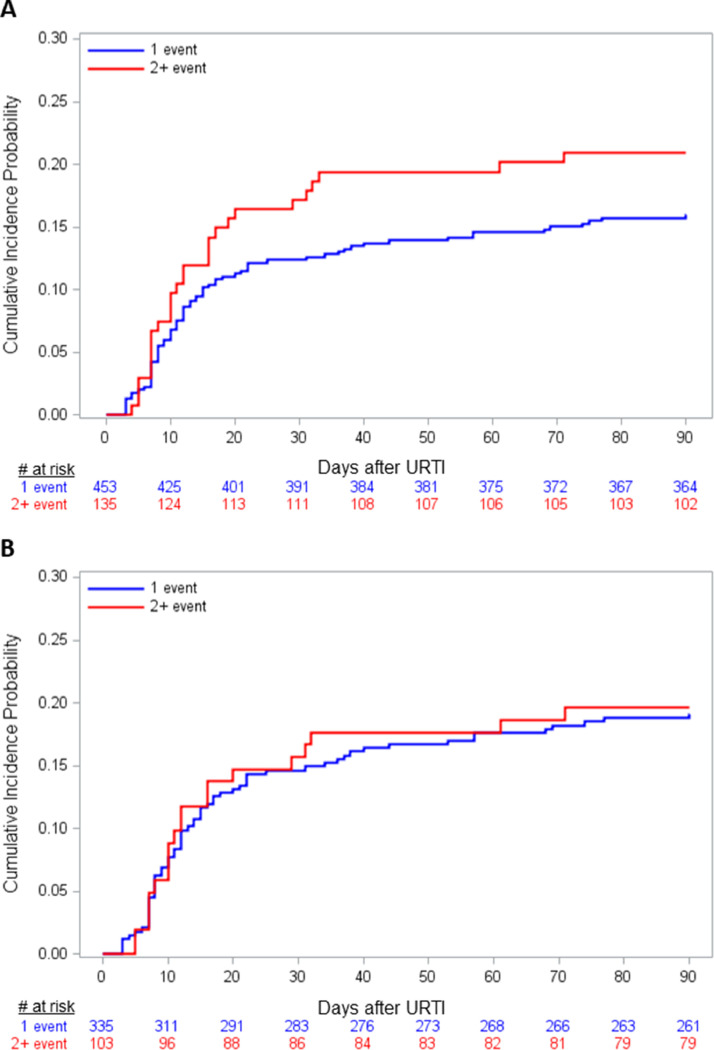
Among 3445 patients undergoing HCT during the study time period, 732 (21%) were positive for HRV from at least 1 respiratory site (Figure 1 ), resulting in 782 HRV illness events. Of these, 665 events (85%) represented URTI at presentation, whereas 15% of events involved presentation with LRTI. Of 665 URTI events, 588 events had complete data for all covariates and were included in the progression analyses. Among subjects with multiple HRV URTI events, the median time between events was 302 days (interquartile range [IQR], 140 to 515). In subjects with URTI at presentation, median age was 42.8 years (IQR, 22.2 to 57.7), and there was a slight male predominance (59%) (Table 1 ). Seventeen percent of URTI events (100/588) progressed to LRTI with 28 (28%) proven LRTI events and 72 (72%) possible LRTI events. No probable LRTI events occurred. Most HRV URTI events occurred in recipients of allogeneic HCT (438/588; 74%); 19% (84/438) of events progressed to LRTI in this group with 25 (30%) proven LRTI events and 59 (70%) possible LRTI events. Median time to progression from URTI to LRTI was 12 days for all events (IQR, 7 to 22) and 12 days for allogeneic subjects only (IQR, 8 to 24) (Figure 2 A,B). Of subjects with possible LRTI events, none had a BAL performed.
Figure 1.
Standards for Reporting of Diagnostic Accuracy (STARD) flowchart demonstrating the score performance on the study population.
Table 1.
Demographics (N = 588)
| Covariates | Categories | Value |
|---|---|---|
| Age at transplant, yr | Median (IQR) | 42.8 (22.2-57.7) |
| Age at transplant | <21 yr | 140 (24) |
| 21-60 yr | 330 (56) | |
| 61+ yr | 118 (20) | |
| Transplant year | 1992-2010 | 237 (40) |
| 2011-2015 | 351 (60) | |
| Sex | Female | 239 (41) |
| Male | 349 (59) | |
| Race | Nonwhite | 149 (25) |
| White | 397 (68) | |
| Unknown | 42 (7) | |
| Cell source | PBSC | 418 (71) |
| BM/cord | 170 (29) | |
| Donor type | Allogeneic/unrelated | 438 (74) |
| Autologous | 150 (26) | |
| Conditioning regimen | Myeloablative + high TBI | 158 (27) |
| Myeloablative ± low TBI | 287 (49) | |
| Non-myeloablative | 143 (24) | |
| % FEV1/FVC before HRV URTI | ≤70 | 89 (15) |
| >70 | 427 (73) | |
| Missing | 72 (12) | |
| % TLC before HRV URTI | ≤80 | 74 (13) |
| >80 | 363 (62) | |
| Missing | 151 (26) | |
| WBC count closest to HRV URTI, 106 cells/L | ≤1000 | 60 (10) |
| >1000 | 528 (90) | |
| Lymphocyte count closest to HRV URTI, 106 cells/L | ≤100 | 56 (10) |
| >100 | 532 (90) | |
| Neutrophil count closest to HRV URTI, 106 cells/L | ≤100 | 43 (7) |
| >100 | 545 (93) | |
| Monocyte count closest to HRV URTI, 106 cells/L | ≤100 | 74 (13) |
| >100 | 514 (87) | |
| Platelet count closest to HRV URTI, 106 cells/L | <10,000 | 8 (1) |
| ≥10,000 | 580 (99) | |
| IVIG given before HRV URTI | No | 545 (93) |
| Yes | 40 (7) | |
| Missing | 3 (1) | |
| Steroid use before HRV URTI, mg/kg/day | 0 | 305 (52) |
| >0 to <1 | 236 (40) | |
| ≥1 to <2 | 31 (5) | |
| ≥2 | 16 (3) | |
| Any previous HRV event | No | 453 (77) |
| Yes | 135 (23) | |
| Viral copathogens at time of HRV URTI | No | 531 (90) |
| Yes | 57 (10) | |
| Time to URTI from transplant, days | 0-100 | 257 (44) |
| 101-365 | 125 (21) | |
| 365+ | 206 (35) | |
| Albumin before HRV URTI, g/dL | ≤3 | 81 (14) |
| >3 | 507 (86) | |
| HCT-CI score | 0 | 131 (22) |
| 1-2 | 197 (34) | |
| ≥3 | 248 (42) | |
| Missing | 12 (2) | |
| Donor statin use | No | 124 (21) |
| Yes | 8 (1) | |
| Auto | 150 (26) | |
| Unknown | 306 (52) | |
| Recipient statin use | No | 528 (90) |
| Yes | 60 (10) | |
| Recipient CMV serostatus | – | 269 (46) |
| + | 319 (54) | |
| Donor CMV serostatus | – | 377 (64) |
| + | 209 (36) | |
| Missing | 2 (0) | |
| HRV CT values | Median (IQR) | 25.7 (22.1-30.7) |
| Acute GVHD | Grades 0-I | 78 (13) |
| Grades II-IV | 350 (59) | |
| None | 160 (27) | |
| Chronic GVHD | No | 177 (30) |
| Yes | 411 (70) |
Values are n (%) unless otherwise defined. PBSC indicates peripheral blood stem cells; BM, bone marrow; TBI, total body irradiation; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; TLC: total lung capacity; IVIG, intravenous immunoglobulin.
Figure 2.
Cumulative incidence curves for progression to LRTI in all subjects (A) (log rank test P = .18) and in allogeneic recipients only (B) (log rank test P = .91), stratified by event number.
Risk Factors for Progression to LRTI
In univariable analysis of all events, donor type, conditioning regimen, prior HRV events, steroid use, albumin level, recipient statin use, recipient CMV serostatus, and most recent WBC count, and neutrophil, lymphocyte, and monocyte counts were sufficiently associated with progression to LRTI to be considered for inclusion in a multivariable model (Table 2 ). Notably, the presence of viral co-pathogens, pulmonary function, or intravenous immunoglobulin, and HRV CT values in the upper tract sample were not associated with progression to LRTI. Univariate and multiple multivariate models for risk factors for progression to proven LRTI only are shown in Supplementary Tables 1 and 2.
Table 2.
Univariable Cox Regression Analysis of Risk Factors for Any LRTI (N = 588)
| Covariates | Categories | HR (95% CI) | P |
|---|---|---|---|
| Age at transplant | <21 yr | 1 | |
| 21-60 yr | .82 (.51-1.33) | .416 | |
| 61+ yr | 1.25 (.72-2.17) | .42 | |
| Transplant year | 1992-2010 | 1 | |
| 2011-2015 | .91 (.61-1.36) | .655 | |
| Sex | Female | 1 | |
| Male | 1.16 (.77-1.74) | .479 | |
| Race | Nonwhite | 1 | |
| White | 1.12 (.70-1.78) | .632 | |
| Unknown | .72 (.28-1.90) | .51 | |
| Cell source | PBSC | 1 | |
| BM/cord | 1.35 (.90-2.04) | .151 | |
| Donor type | Autologous | 1 | |
| Allogeneic/unrelated | 1.85 (1.08-3.16) | .024 | |
| Conditioning regimen | Myeloablative ± TBI | 1 | |
| Non-myeloablative | 1.68 (1.11-2.54) | .014 | |
| % FEV1/FVC before HRV URTI | ≤70 | 1 | |
| >70 | .85 (.49-1.47) | .559 | |
| % TLC before HRV URTI | ≤80 | 1 | |
| >80 | 1.24 (.63-2.41) | .536 | |
| WBC count closest to HRV URTI, 106 cells/L | >1000 | 1 | |
| ≤1000 | 1.39 (.78-2.49) | .266 | |
| Lymphocyte count closest to HRV URTI, 106 cells/L | >100 | 1 | |
| ≤100 | 2.13 (1.26-3.59) | .005 | |
| Neutrophil count closest to HRV URTI, 106 cells/L | >100 | 1 | |
| ≤100 | 1.91 (1.04-3.49) | .036 | |
| Monocyte count closest to HRV URTI, 106 cells/L | >100 | 1 | |
| ≤100 | 2.01 (1.24-3.26) | .004 | |
| Platelet count closest to HRV URTI, 106 cells/L | ≥10,000 | 1 | |
| <10,000 | 1.55 (.38-6.30) | .537 | |
| IVIG given before HRV URTI | No | 1 | |
| Yes | 1.42 (.72-2.82) | .315 | |
| Steroid use before HRV URTI, mg/kg/day | 0 | 1 | |
| >0 to <1 | 1.09 (.71-1.67) | .686 | |
| ≥1 to <2 | 1.78 (.84-3.77) | .132 | |
| ≥2 | 3.01 (1.29-7.04) | .011 | |
| Any previous HRV event | No | 1 | |
| Yes | 1.35 (.87-2.09) | .178 | |
| Viral copathogens at time of HRV URTI | No | 1 | |
| Yes | 1.28 (.70-2.35) | .416 | |
| Time to URTI from transplant, days | 0-100 | 1 | |
| 101-365 | 1.42 (.89-2.28) | .142 | |
| 365+ | .80 (.50-1.29) | .367 | |
| Albumin before HRV URTI, g/dL | ≤3 | 1 | |
| >3 | .50 (.31-.81) | .004 | |
| HCT-CI score | 0-2 | 1 | |
| ≥3 | .70 (.47-1.06) | .093 | |
| Missing | .41 (.06-2.94) | .374 | |
| Donor statin use | No | 1 | |
| Yes | 1.16 (.28-4.90) | .835 | |
| Unknown | .81 (.51-1.28) | .359 | |
| Recipient statin use | No | 1 | |
| Yes | 1.67 (.96-2.89) | .068 | |
| Acute GVHD as time-dependent | Grades 0-I | 1 | |
| Grades II-IV | 1.52 (1.01-2.28) | .046 | |
| Chronic GVHD as time-dependent | No | 1 | |
| Yes | 1.18 (.79-1.75) | .415 | |
| Recipient CMV serostatus | – | 1 | |
| + | 1.90 (1.25-2.89) | .003 | |
| Donor CMV serostatus | – | 1 | |
| + | 1.22 (.82-1.83) | .332 | |
| HRV CT values | Below lower quartile | 1 | |
| Lower quartile to median | 1.22 (.69-2.16) | .487 | |
| Median to upper quartile | 1.04 (.58-1.87) | .896 | |
| Above upper quartile | 1.09 (.61-1.95) | .77 | |
| HRV CT values | ≤ lowest 10th percentile | 1 | |
| > lowest 10th percentile | .97 (.50-1.86) | .923 |
HR indicates hazard ratio; CI, confidence interval.
*Autologous HCT recipients excluded from the analysis.
Candidate variables from univariable analysis were evaluated in a multivariate model. Covariates were added sequentially and c-statistic calculated for each model until the optimal model with the highest c-statistic was achieved (Table 3 ). In the final model the c-statistic was .676, whereas the optimism corrected c-statistic was .656.
Table 3.
Multivariable Analyses of Risk Factors for LRTI in All Subjects
| Covariates | Categories | HR (95% CI) | P | Score Weight |
|---|---|---|---|---|
| Albumin | >3 | 1 | ||
| ≤3 | 1.65 (.99-2.76) | .056 | 11 | |
| Recipient CMV serostatus | - | 1 | ||
| + | 1.79 (1.17-2.73) | .007 | 12 | |
| Donor type | Autologous | 1 | ||
| Allogeneic/unrelated | 2.08 (1.22-3.58) | .008 | 14 | |
| Any previous HRV events | No | 1 | ||
| Yes | 1.67 (1.05-2.65) | .03 | 12 | |
| Lymphocyte count closest to HRV URTI, 106 cells/L | >100 | 1 | ||
| ≤100 | 2.39 (1.33-4.29) | .004 | 16 | |
| Recipient statin use | No | 1 | ||
| Yes | 2.06 (1.16-3.64) | .013 | 14 | |
| Steroid use before HRV URTI, mg/kg/day | 0 to <1 | 1 | ||
| ≥1 to <2 | 1.73 (.84-3.58) | .139 | 12 | |
| ≥2 | 2.91 (1.27-6.69) | .012 | 20 |
Score Development and Performance
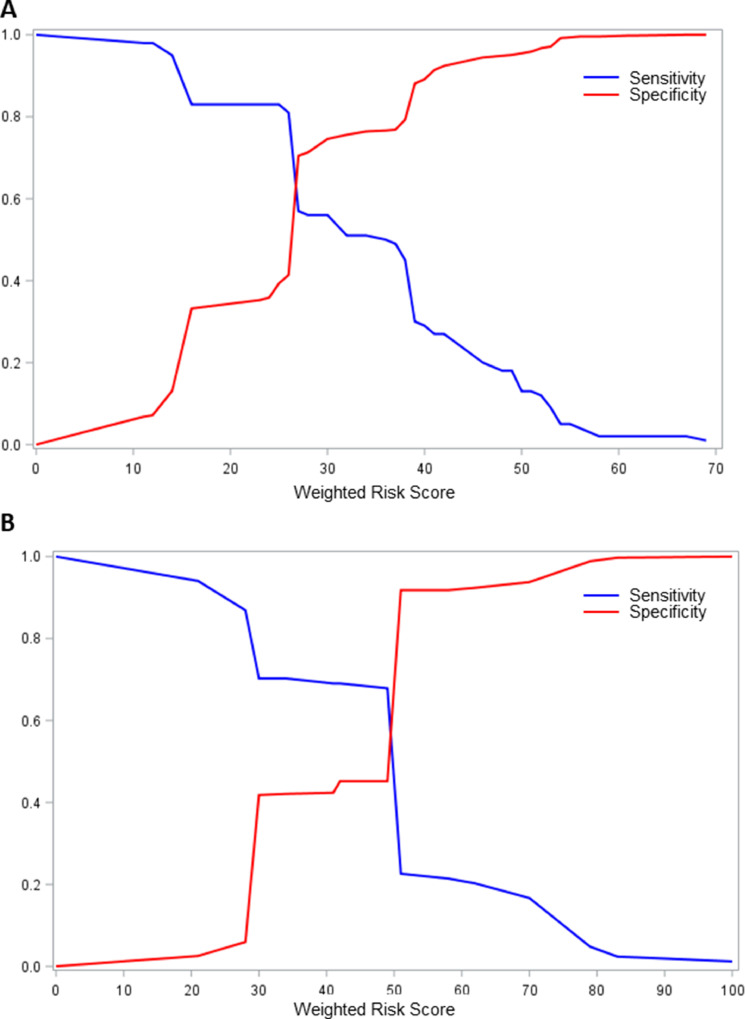
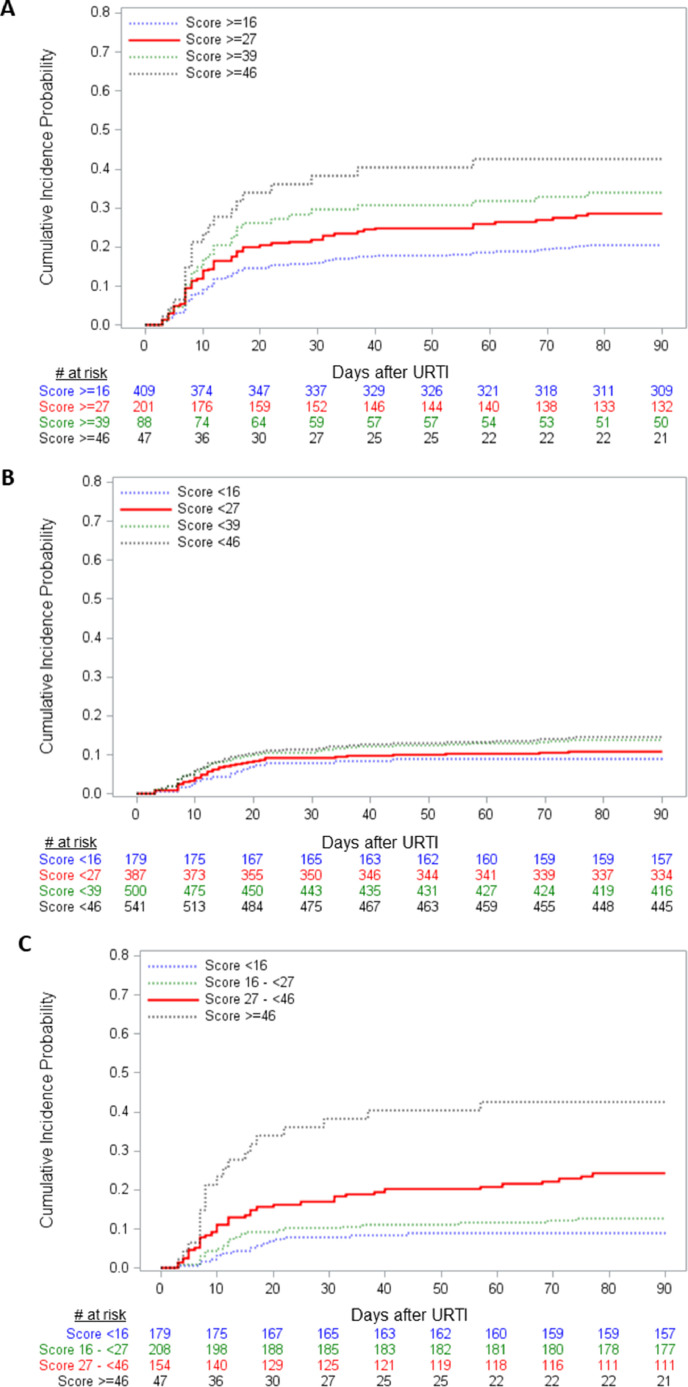
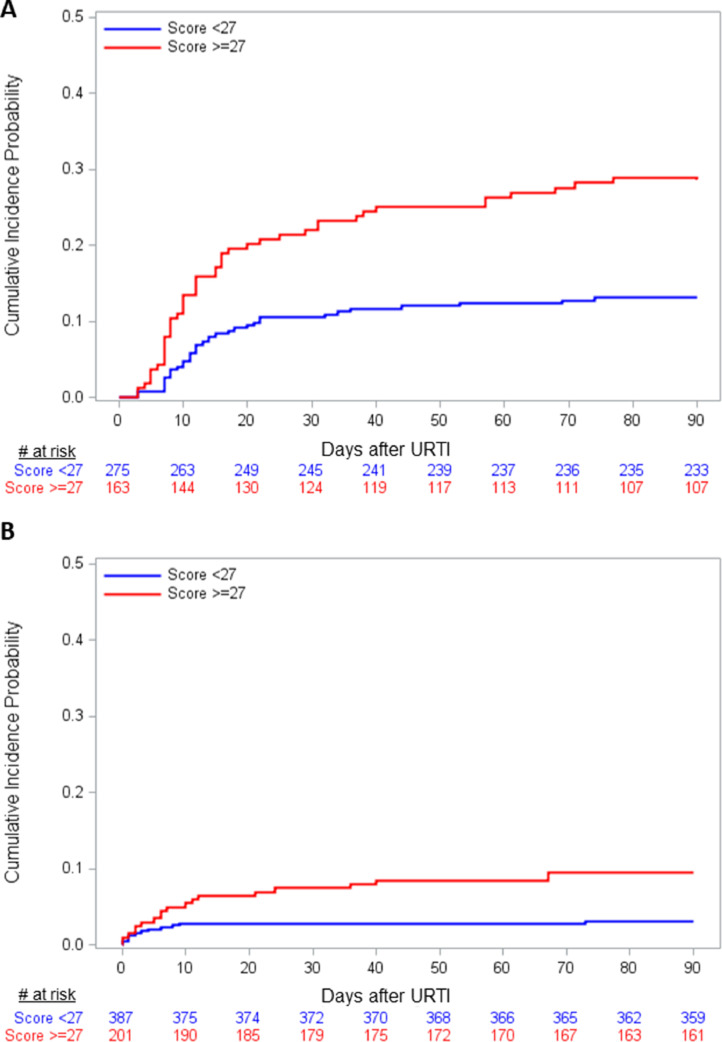
A risk score was developed as described and weighted contributions of each factor are shown in Table 3. Specificity and sensitivity of score cut–offs for development of LRTI in the entire cohort and the allogeneic cohort are shown in Figure 3 A and B, respectively. Among all subjects the score cut-off with the highest sensitivity (57%) and specificity (71%) was 27, corresponding to a positive predictive value of 28% and a negative predictive value of 89% in this population. Cumulative incidence curves for progression to LRTI in all subjects, stratified by above and below a range of binary score cut–offs are shown in Figure 4 A and B, representing positive and negative predictive values for a given cut–off, respectively. Cumulative incidence of progression to LRTI for the ≥27 group was 28% versus 11% in the <27 group (log-rank and Gray's test both P < .001). Figure 4C illustrates the ability of the score to stratify the population into 4 mutually exclusive levels of risk. Cumulative incidence of progression to LRTI was as follows: scores < 16 (9%), 16 to <27 (13%), 27 to <46 (24%), and ≥46 (43%) (log-rank test and Gray's test both P < .001). The weighted risk score was applied to the allogeneic subgroup only and cumulative incidence curves above and below the 27 cut–off are shown Figure 5 A. For scores ≥ 27 the cumulative incidence was 29% versus 13% for scores < 27 (log-rank test and Gray's test P < .001). The score was also applied to the whole cohort and evaluated with proven LRTI only as the outcome. Figure 5B shows the cumulative incidence curves above and below 27 for progression to proven LRTI only. For scores ≥ 27 the cumulative incidence was 9% versus 3% for scores < 27 (log-rank test and Gray's test P = .001).
Figure 3.
Specificity and sensitivity calculations for weighted score for progression to LRTI in all subjects (A) and in allogeneic subjects only (B).
Figure 4.
Cumulative incidence curves for progression to LRTI in all subjects, stratified by above (A) and below (B) a range of binary score cut-offs, representing positive and negative predictive values for a given cut-off, respectively. Log-rank and Gray's test comparing the ≥27 versus <27 groups both P < .001. Cumulative incidence curves for progression to LRTI in all subjects, stratified into 4 mutually exclusive levels of risk (C; log-rank test and Gray's test P < .001).
Figure 5.
Cumulative incidence curves of progression to LRTI in allogeneic recipients only (A) and of progression to proven LRTI in all subjects (B), stratified above and below score of 27. Log-rank test and Gray's test P < .001 for both curves.
Discussion
In this large retrospective study of HCT recipients infected with HRV in the upper respiratory tract, we established risk factors and developed a risk score for progression to LRTI. To our knowledge this is the largest and statistically most rigorous study to systematically examine patients specifically presenting with URTI to determine the risk of subsequent LRTI. In multivariable models for any LRTI event in all subjects, significant risk factors identified include allogeneic transplant, prior HRV URTI events, lymphocyte count < 100 × 106 cells/L, recipient statin use, recipient CMV serostatus, and steroid use ≥ 2 mg/kg/day. We further characterized risk factors for progression by calculating the change in the bootstrapped optimism corrected c-statistic for each risk factor and developed an optimized model for risk prediction. Using this model we developed a weighted score and describe the performance characteristics of the score at various cut–offs. The risk score presented in this article is a novel tool with potential to help risk stratify patients for clinical care and future clinical trials.
HRVs have recently been recognized as significant pathogens that can cause severe disease and poor outcomes in certain high risk groups, including infants, pregnant women, asthmatics, hospitalized adults, and immunocompromised children and adults 1, 3, 4, 11, 16, 17. Once HRV LRTI has developed in HCT recipients, mortality rates are comparable with those associated with RSV, influenza, and PIV [3]. In a study of HRV infection that included both HCT recipients and patients with hematologic malignancies, factors more likely to be present in patients with LRTI included inpatient status, lymphopenia, and hypoalbuminemia [11]. In HCT recipients alone from the same center, hypoalbuminemia, and isolation of respiratory co-pathogen were associated with LRTI in multivariable models [4]. These analyses, although not done in a time-dependent manner, are similar to findings in the present study, in which lymphopenia and hypoalbuminemia were associated with progression to LRTI. We evaluated co-pathogens in the upper respiratory tract as a risk factor and did not find an association with LRTI.
Lymphopenia has been associated with progression to LRTI for other respiratory viruses including PIVs, RSV, hMPV, and influenza 6, 7, 9, 10, 18, 19, and was also more common in HRV LRTI subjects in a prior study [4]. Monocytopenia has not been evaluated systematically in prior respiratory virus progression studies, although there was a trend toward significance for hMPV [8], and monocytopenia was associated with mortality in HCT recipients with HRV and RSV LRTI 3, 20. The relative role of monocytes over lymphocytes in progression of disease is not understood, and most studies have not evaluated both cell types concurrently. A potential mechanism of inflammatory and immune modulation via HRV induced monocytic cell directed CXL10 and IFN-γ release is plausible [21].
Steroid use is a well described risk factor for progression to LRTI for many respiratory viruses 6, 8, 9, 18, and higher doses of ≥2 mg/kg/day were associated with HRV LRTI in the present study. Of note, high–dose steroids are no longer frequently used for graft–versus–host disease treatment at our institution, as is reflected in the low frequency of use (3%) in this cohort. However, even lower dose steroids (≥1 to 2 mg/kg/day) showed a trend toward significance in the multivariable models, and this was included in the weighted score. Other centers may have higher rates of high–dose steroid use and should still be able to apply the score to their patient population.
Prior post-transplant HRV URTI infection was associated with increased risk of LRTI, an interesting and somewhat unexpected finding suggesting an effect of the cumulative burden of HRV infections, perhaps by mediating inflammatory responses that increase the likelihood of LRTI development. For parainfluenza viruses, URTI without LRTI is associated with late airflow decline, and a similar pathway may be at play for HRV infections [22]. Although it is possible we overestimated new events, our definition of >12 weeks between events was chosen given our recent surveillance data showing the median duration of HRV shedding in HCT recipients is 9.5 days (range, 2 to 89) [23]. Given the long duration between events in the present study (median, 302 days; IQR, 140 to 515), we do not believe we are capturing prolonged viral shedding but rather truly new viral events.
Recipient CMV serostatus was associated with increased risk of LRTI, a novel finding for respiratory virus disease severity. CMV has a profound impact on the immune system in both immunocompetent and immunosuppressed individuals and known to have immunosuppressive effects 24, 25, 26. It is well known to affect nonrelapse mortality in HCT patients [27]. CMV has also been shown to increase the risk of other viruses after HCT; however, the mechanisms are poorly understood. That CMV was associated with progression in this study is plausible but the results should be validated in a different cohort, and further studies are needed to elucidate the mechanism.
Recipient statin use as a risk factor for HRV LRTI is also an intriguing finding and corroborates prior results from a separate cohort at our center that demonstrated a trend toward association between recipient statin use and all respiratory viral infections and LRTIs [13]. Statins may act as immunosuppressants via direct inhibition of induction of MHC-II expression by IFN-γ and thus leading to reduced T cell activation [28] and may also have an effect of type I IFN receptor signaling in monocytes in the setting of HRV challenge, leading to reduced inflammatory response [29]. The role of statins on HRV progression should be validated in other cohorts and evaluated for other respiratory virus infections independently.
We evaluated the impact of HRV viral load and found no association with progression to LRTI. CT value as a surrogate for viral load has limitations given the diversity of HRV genotypes and the resulting differences in amplification efficiency using RT-qPCR assays with a consensus HRV primer and probe set. RT digital PCR has been recently shown to provide more precise quantification [30]. However, even when evaluating the extreme highest viral load group (CT values in the lowest 10th percentile), no effect was seen. In lung transplant recipients, higher HRV viral load was associated with increased symptoms associated with LRTI [31]. In immunocompetent pediatric patients, viral load was associated with disease severity in older but not younger children [32] and was also associated with higher rates of viremia and oxygen use [33]. The variable role of viral load in studies to date suggest both limitations in capturing accurate viral loads from clinical samples such as was done in our study and that other host factors including immune responses may be an important player in progression to LRTI and other clinical outcomes. Additionally, HRV species were not evaluated in the present study, and data have suggested HRV-C may be associated with worse outcomes, especially in children 34, 35. The presence of viral co–pathogens in the upper respiratory tract was not associated with progression to HRV LRTI.
To provide clinical applicability for our findings, we developed weighted risk scores for progression to LRTI using methodology that allows for multiple sampling within the dataset to develop an optimized model [14]. Our final model showed an acceptable but not robust c-statistic of .656. Using this model we developed a weighted score and show specificity and sensitivity calculations for a range of score cut–offs, as well as cumulative incidence curves stratified by score cut–offs and within mutually exclusive score ranges. Maximum sensitivity and specificity was demonstrated at a score cut–off of 27; however, optimal score cut–off depends on the ultimate application of the score. For example, if the risk score is used for patient risk stratification for interventional clinical trials, a score cut–off with higher sensitivity and negative predictive value may be desirable.
Sample size limited our ability to extensively evaluate proven LRTI, which for other respiratory viruses (RSV and PIV) has been associated with worse clinical outcomes 36, 37. In several separate multivariate models, however, risk factors for progression to proven LRTI were similar (low albumin, low monocyte count, and high–dose steroids). We applied our risk score to the proven LRTI outcome only, and subjects with a risk score higher than 27 were associated with proven LRTI. With a larger sample size a separate risk score for proven LRTI could be developed, although it is likely that risk factors would remain similar. Probable LRTI could represent contamination from the upper respiratory tract during the BAL procedure; however, there were no cases of probable LRTI in our cohort.
Although our analyses used an accepted resampling methodology (bootstrapping), ultimately a multicenter study is needed for validation of the score, especially given differences in clinical practices including the use of high–dose steroids. An immunodeficiency scoring index was developed for allogeneic HCT recipients with RSV infection to predict LRTI and RSV associated mortality [10]; the score was also applied to influenza [19]. Similar variables were evaluated in the present study and were not associated with the outcome with the exception of steroid use, suggesting rhinovirus progression may be determined by a unique set of risk factors compared with other respiratory viruses and a separate risk score is needed.
This study has strengths and limitations. First, this is a retrospective study in which data collection, including viral load determination, was dependent on clinical databases. However, at our institution data from transplant recipients is prospectively recorded in a central database, and we also conducted detailed chart review on every patient to obtain additional clinical data. Second, we captured both proven LRTI (HRV detected in the lower respiratory tract with abnormal imaging) and possible LRTI (HRV detected in the upper respiratory tract with abnormal imaging but no lower tract sampling). At our institution the use of BAL is based on standardized protocols outlining that BAL should be performed in HCT recipients with a respiratory virus detected in the upper respiratory tract and abnormal chest imaging. However, ultimately the need for BAL is determined by the attending physician, and thus some proven or probable cases (HRV detected in the lower respiratory tract without abnormal imaging) may have been missed. By including both proven and possible LRTI events (no probable events occurred), this effect is mitigated; however, proven LRTI may be the more relevant outcome given the mortality rates seen with proven HRV LRTI and the differences in outcomes observed in proven LRTI for PIV and RSV 3, 36, 37. Regardless, many institutions do not routinely use BAL for virologic confirmation, and thus our results for any LRTI events can be broadly applicable. Finally, our data include both allogeneic and autologous HCT recipients, and there may be a bias toward increased testing in the allogeneic group and therefore more LRTI events captured. However, the risk score performed reasonably well in the allogeneic subgroup.
We were not able to evaluate risk factors for HRV LRTI cases without any co–pathogens in the lower respiratory tract separately. In a prior study the presence of co–pathogens was not associated with mortality in HCT recipients with proven HRV LRTI, suggesting that HRV detection in the lower tract is pathogenic regardless of co–pathogens and therefore making any proven HRV LRTI an important endpoint [3]. Because BAL was not done in possible LRTI cases, it is plausible that other pathogens are contributing to the radiographic changes, and thus these are not true LRTI events. However, as stated above, in clinical practice BAL is often not attempted, and co–pathogen data are not available, yet patients are considered to have LRTI. Thus, we included possible LRTI as an endpoint. Several epidemiologic studies and clinical trials of respiratory viruses in HCT recipients have used definitions of LRTI that include possible cases 4, 10, 35, 38, 39, 40, 41, 42.
In summary, we describe the risk factors and development of a risk score for progression to LRTI in a large cohort of HCT recipients with HRV URTI. The risk score proposed may have implications for risk stratifying patients for more intense clinical monitoring. Additionally, risk stratification will help inform the design of future randomized clinical trials of novel therapeutics, including the growing field of virus specific T cells, which are currently being evaluated for other respiratory viruses including PIV and hMPV 43, 44.
Acknowledgments
Presented in part at the 2017 BMT Tandem Meetings, Orlando, Florida, February 22-26, 2017.
The authors thank Chris Davis and Zach Stednick for database services and Louise Kimball, Jennifer Schaeffer, Elizabeth Nguyen, Sonia Goyal, and Lisa Chung for chart review assistance.
Financial disclosure: Supported by grants from the National Institutes of Health (National Institute of Allergy and Infectious Diseases K23 AI114844 [to A.W.], National Heart, Lung, and Blood Institute K24 HL093294 [to M.B.], and National Cancer Institute P30 CA15704 [to W.L.]) and Vaxart, Inc. (to A.W. and M.B.). The study sponsors (National Institutes of Health and Vaxart, Inc.) did not contribute to the study design, data collection, analysis, interpretation of data, writing of the report, or to the decision to submit the paper for publication.
Conflict of interest statement: A.W. reports grants from Gilead Sciences and Vaxart, Inc. J.A.E. reports support from Gilead Sciences, Inc. M.B. reports grants and consulting fees from Gilead Sciences, Vaxart, Inc. and Ansun Biopharma.
Footnotes
Financial disclosure: See Acknowledgments on page 1020.
Supplementary data related to this article can be found online version at doi:10.1016/j.bbmt.2018.12.005.
Appendix. Supplementary materials
References
- 1.Milano F., Campbell A.P., Kuypers J. Human coronavirus (HCOV) and rhinovirus (HRHV) infection among hematopoietic stem cell transplantation (HCT) recipients. Biol Blood Marrow Transplant. 2009;15:88–89. [Google Scholar]
- 2.Boeckh M, Campbell AP, Xie H, et al. Progression, shedding patterns, and clinical disease associated with respiratory virus infections after allogeneic hematopoietic cell transplantation. Blood; 122:3278.
- 3.Seo S., Waghmare A., Scott E.M. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica. 2017;102:1120–1130. doi: 10.3324/haematol.2016.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs S.E., Soave R., Shore T.B. Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013;15:474–486. doi: 10.1111/tid.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan I.A., Chopra R., Swindell R., Mutton K.J. Respiratory viral infections after bone marrow/peripheral stem-cell transplantation: the Christie hospital experience. Bone Marrow Transplant. 2003;32:73–77. doi: 10.1038/sj.bmt.1704048. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.J., Guthrie K.A., Waghmare A. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis. 2014;209:1195–1204. doi: 10.1093/infdis/jit832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah D.P., Shah P.K., Azzi J.M., Chemaly R.F. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett. 2016;370:358–364. doi: 10.1016/j.canlet.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo S., Gooley T.A., Kuypers J.M. Human metapneumovirus infections following hematopoietic cell transplantation: factors associated with disease progression. Clin Infect Dis. 2016;63:178–185. doi: 10.1093/cid/ciw284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S.M., Boudreault A.A., Xie H., Englund J.A., Corey L., Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117:5050–5056. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah D.P., Ghantoji S.S., Ariza-Heredia E.J. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123:3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs S.E., Lamson D.M., Soave R. Clinical and molecular epidemiology of human rhinovirus infections in patients with hematologic malignancy. J Clin Virol. 2015;71:51–58. doi: 10.1016/j.jcv.2015.07.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorror M.L., Maris M.B., Storb R. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo S., Boeckh M., Storer B.E. The association between donor and recipient statin use and infections after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:444–448. doi: 10.1038/bmt.2014.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrell F.E.H., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.R Development Core Team . Austria; R Found Stat Comput; Vienna: 2016. R: a language and environment for statistical computing. [Google Scholar]
- 16.Marcone D.N.N., Carballal G., Irañeta M. Nosocomial transmission and genetic diversity of rhinovirus in a neonatal intensive care unit. J Pediatr. 2018;193:252–255.e1. doi: 10.1016/j.jpeds.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Visseaux B., Burdet C., Voiriot G. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS One. 2017;12(7):e0180888. doi: 10.1371/journal.pone.0180888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo S., Xie H., Karron R.A. Parainfluenza virus type 3 Ab in allogeneic hematopoietic cell transplant recipients: factors influencing post-transplant Ab titers and associated outcomes. Bone Marrow Transplant. 2014;49:1205–1211. doi: 10.1038/bmt.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kmeid J., Vanichanan J., Shah D.P. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant. 2016;22:542–548. doi: 10.1016/j.bbmt.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waghmare A., Campbell A.P., Xie H. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57:1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korpi-Steiner N.L., Valkenaar S.M., Bates M.E., Evans M.D., Gern J.E., Bertics P.J. Human monocytic cells direct the robust release of CXCL10 by bronchial epithelial cells during rhinovirus infection. Clin Exp Allergy. 2010;40:1203–1213. doi: 10.1111/j.1365-2222.2010.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erard V., Chien J.W., Kim H.W. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193:1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogimi C., Xie H., Leisenring W.M. Initial high viral load is associated with prolonged shedding of human rhinovirus in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2018;24(10):2160–2163. doi: 10.1016/j.bbmt.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeckh M., Nichols W.G., Leisenring W.M. Immunosuppressive effects of beta-herpesviruses. Herpes. 2003;10(1):12–16. [PubMed] [Google Scholar]
- 25.Brodin P., Jojic V., Gao T. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1-2):37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanakry C.G., Coffey D.G., Towlerton A.M.H. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight. 2016;1(5):e85252. doi: 10.1172/jci.insight.86252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teira P., Battiwalla M., Ramanathan M. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. doi: 10.1182/blood-2015-11-679639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mach F. Statins as immunomodulators. Transplant Immunol. 2002;9(2-4):197–200. doi: 10.1016/s0966-3274(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 29.Wickert L.E., Karta M.R., Audhya A. Simvastatin attenuates rhinovirus-induced interferon and CXCL10 secretion from monocytic cells in vitro. J Leuk Biol. 2014;95(6):951–959. doi: 10.1189/jlb.0713413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sedlak R.H., Nguyen T., Palileo I., Jerome K.R., Kuypers J. Superiority of digital reverse transcription-PCR (RT-PCR) over Real-time RT-PCR for quantitation of highly divergent human rhinoviruses. J Clin Microbiol. 2017;55:442–449. doi: 10.1128/JCM.01970-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambrosioni J., Bridevaux P.O., Aubert J.D., Soccal P., Wagner G., Kaiser L. Role of rhinovirus load in the upper respiratory tract and severity of symptoms in lung transplant recipients. J Clin Virol. 2015;64:1–5. doi: 10.1016/j.jcv.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Takeyama A., Hashimoto K., Sato M. Rhinovirus load and disease severity in children with lower respiratory tract infections. J Med Virol. 2012;84:1135–1142. doi: 10.1002/jmv.23306. [DOI] [PubMed] [Google Scholar]
- 33.Esposito S., Daleno C., Scala A. Impact of rhinovirus nasopharyngeal viral load and viremia on severity of respiratory infections in children. Eur J Clin Microbiol Infect Dis. 2014;33:41–48. doi: 10.1007/s10096-013-1926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piralla A., Rovida F., Campanini G. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol. 2009;45:311–317. doi: 10.1016/j.jcv.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson P.E., Gilroy N.M., Faux C.E. Human rhinovirus C in adult haematopoietic stem cell transplant recipients with respiratory illness. J Clin Virol. 2013;56:255–259. doi: 10.1016/j.jcv.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waghmare A., Xie H., Kimball L. Supplemental oxygen-free days in hematopoietic cell transplant recipients with respiratory syncytial virus. J Infect Dis. 2017;216:1227–1234. doi: 10.1093/infdis/jix390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo S., Xie H., Campbell A.P. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis. 2014;58:1357–1368. doi: 10.1093/cid/ciu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah D.P., Ghantoji S.S., Shah J.N. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68:1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chemaly R.F., Torres H.A., Munsell M.F. An adaptive randomized trial of an intermittent dosing schedule of aerosolized ribavirin in patients with cancer and respiratory syncytial virus infection. J Infect Dis. 2012;206:1367–1371. doi: 10.1093/infdis/jis516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sim S.A., Leung V.K.Y., Ritchie D., Slavin M.A., Sullivan S.G., Teh B.W. Viral respiratory tract infections in allogeneic hematopoietic stem cell transplantation recipients in the era of molecular testing. Biol Blood Marrow Transplant. 2018;24(7):1490–1496. doi: 10.1016/j.bbmt.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher B.T., Danziger-Isakov L., Sweet L.R. A multicenter consortium to define the epidemiology and outcomes of inpatient respiratory viral infections in pediatric hematopoietic stem cell transplant recipients. J Pediatr Infect Dis Soc. 2018;7(4):275–282. doi: 10.1093/jpids/pix051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansun Biopharma, Inc. A phase II, randomized, double-blind, placebo-controlled study to examine the effects of DAS181 in immunocompromised subjects with lower respiratory tract parainfluenza infection on supplemental oxygen (DAS181-2-05). In: ClinicalTria. https://clinicaltrials.gov/ct2/show/NCT01644877. Accessed November 26, 2018
- 43.Aguayo-Hiraldo P.I., Arasaratnam R.J., Tzannou I. Characterizing the cellular immune response to parainfluenza virus 3. J Infect Dis. 2017;216(2):153–161. doi: 10.1093/infdis/jix203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzannou I., Nicholas S.K., Lulla P. Immunologic profiling of human metapneumovirus for the development of targeted immunotherapy. J Infect Dis. 2017;216:678–687. doi: 10.1093/infdis/jix358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.