Abstract
The number of times a cell divides before irreversibly arresting is termed replicative lifespan. Despite discovery of many chemical, dietary and genetic interventions that extend replicative lifespan, usually first discovered in budding yeast and subsequently shown to apply to metazoans, there is still little understanding of the underlying molecular mechanisms involved. One unifying theme is that most, if not all, interventions that extend replicative lifespan induce “hormesis”, where a little inflicted damage makes cells more able to resist similar challenges in the future. One of the many cellular changes that occur during hormesis is a global reduction in protein synthesis, which has been linked to enhanced longevity in many organisms. Our recent study in budding yeast found that it was not the reduction in protein synthesis per se, but rather the subsequent induction of the conserved Gcn4 transcriptional regulator and its ability to induce autophagy that was responsible for extending replicative lifespan. We propose that Gcn4-dependent induction of autophagy occurring downstream of reduced global protein synthesis may be a unifying molecular mechanism for many interventions that extend replicative lifespan.
Keywords: aging, yeast, autophagy, hormesis, Gcn4
Introduction
Common interventions that extend lifespan in a broad range of eukaryotic species, including dietary restriction and inhibition of the nutrient sensor target of rapamycin (TOR) serine/threonine kinase, perturb regulation of mRNA translation, among their many other effects (Hansen et al., 2007). Taken together with their own discovery of numerous yeast mutants with both reduced protein synthesis and extended replicative lifespan (Steffen et al., 2008), Matt Kaeberlein and colleagues proposed regulation of mRNA translation as a conserved mechanism of longevity control (Mehta et al., 2010). The molecular mechanisms for how mRNA translation influenced aging remained unclear, but potential hypotheses included differential translation, improved protein homeostasis and improved energy balance (Mehta et al., 2010). Our recent work, which we discuss here, indicated that an intervention that reduced protein synthesis in a TOR-independent manner, extended lifespan via the induction of autophagy (Hu et al., 2018). We speculate that autophagy induction may be the ultimate molecular output of many common interventions that extend lifespan and perturb protein synthesis.
Where examined, protein synthesis is globally reduced during aging in all eukaryotic species (Gonskikh and Polacek, 2017), but the molecular mechanisms responsible for this down regulation during aging were unknown. Our characterization of reduced global mRNA translation during yeast replicative aging found that it was not accompanied by, or caused by, inhibition of Tor1. Another pathway that globally reduces Cap-dependent protein synthesis (in response to stress) is the integrated stress response and we found that this conserved pathway was partially activated in old yeast cells, as indicated by phosphorylation of eIF2α (Hu et al., 2018). Furthermore, deletion of the gene encoding the Gcn2 kinase that phosphorylates eIF2α in response to amino acid depletion (Castilho et al., 2014) negated the reduced protein synthesis in old cells (Hu et al., 2018), showing that Gcn2-mediated eIF2α phosphorylation contributes to reduced protein synthesis in old cells. We asked whether experimental activation of Gcn2 kinase in young cells under nutrient-rich conditions could extend replicative lifespan. To activate Gcn2, we overexpressed a tRNA to mimic the uncharged tRNAs that result from amino acid depletion normally responsible for Gcn2 activation (Castilho et al., 2014). Activation of Gcn2 in this way in young cells reduced protein synthesis globally and extended lifespan (Hu et al., 2018). These findings were consistent with the many other genetic manipulations, chemical and dietary interventions that extend replicative lifespan and also reduce protein synthesis.
Induction of Gcn4, not reduction of protein synthesis per se, extends replicative lifespan
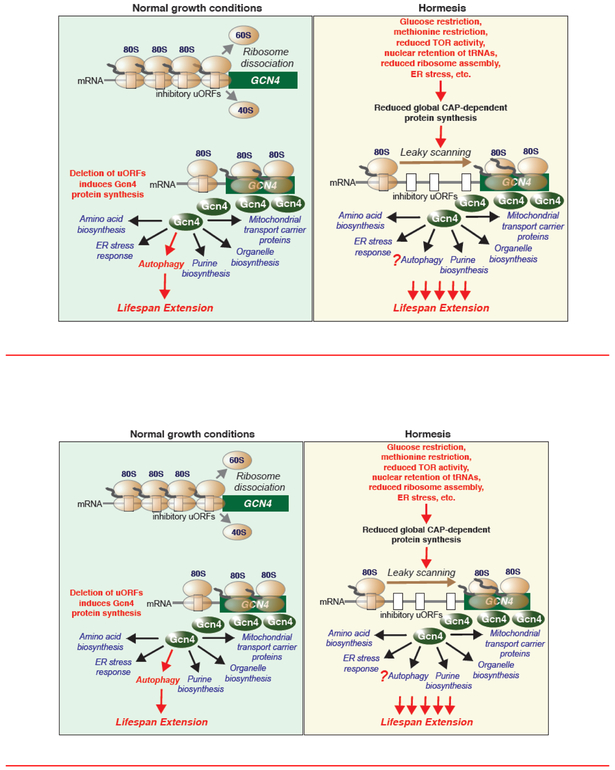
One consequence of the suppression of global protein synthesis is the paradoxical upregulation of translation of genes with inhibitory upstream open reading frames (uORFs), including GCN4. The uORFs negatively regulate translation of GCN4 under conditions of rapid growth, as they promote dissociation of the ribosome prior to its reaching the GCN4 ORF (Left side of Fig. 1) (Hinnebusch, 2005). However, in conditions that reduce the efficiency of the translation machinery, the ribosomes scan over the uORFs, leading to efficient translation of the Gcn4 transcriptional regulator protein. This happens during many hormetic interventions that extend lifespan (right side of Fig. 1). Induction of Gcn4 protein synthesis occurs downstream of Gcn2 activation, as a consequence of reduced global protein synthesis. Strikingly, the extension of replicative lifespan that resulted from Gcn2 activation was totally dependent on GCN4 (Hu et al., 2018).
Figure 1.
Left side: in normal growth conditions, yeast Gcn4 is not efficiently synthesized due to engagement of the ribosome with the inhibitory upstream open reading frames (uORFs), leading to ribosome dissociation by the time they reach the Gcn4 protein coding ORF. Upon deletion of the GCN4 uORFs (bottom), the ribosomes efficiently scan to the Gcn4 protein coding ORF, inducing Gcn4 production. Gcn4 induces transcription of genes important for key stress responses, some of which are indicated. Induction of Gcn4 in this way extended yeast replicative lifespan in a manner that was totally dependent on autophagy, but independent of Tor1 inhibition and independent of noticable effects on global protein synthesis. Right side: nutrient depletion or other stresses (indicated in red) that induce hormesis and extend replicative lifespan reduce global Cap-dependent protein synthesis, leading to less efficient translation machinery which promotes leaky scanning of the ribosomes along the mRNA to translate Gcn4. Whether autophagy is involved in the replicative lifespan extension that is stimulated by hormesis is unknown (hence the “?”).
Gcn4 also plays a role in other commonly used regimens that extend lifespan in multiple organisms. For example, deletion of GCN4 compromised the yeast replicative lifespan extension that is achieved by TOR1 deletion / Tor1 inhibition, glucose restriction (a yeast version of dietary restriction) and mutants of the large subunit of the ribosome (Kubota et al., 2003; Steffen et al., 2008; Yang et al., 2000). Defects in mitochondrial translation are known to extend yeast replicative lifespan and in the case of deletion of the ATF3 gene that encodes a protein required for maturation of a mitochondrial ribosome protein, the replicative lifespan extension was dependent on GCN4 (Delaney et al., 2013). Methionine restriction, another dietary intervention that extends lifespan in multiple species (Ables and Johnson, 2017) also induces Gcn4 in yeast (Zou et al., 2017). ER stress extends replicative lifespan and it also induces Gcn4 (Deloche et al., 2004). As such, many conserved lifespan extending regimens induce Gcn4, and where tested, their ability to extend replicative lifespan depends on GCN4.
We have found that induction of Gcn4, achieved by deletion of the uORFs from the GCN4 gene, was sufficient for replicative lifespan extension (right side of Fig. 1) (Hu et al., 2018). This result indicates that it is not the restoration of proteostasis or energy balance as a consequence of reduced amounts of proteins being made globally per se that is extending lifespan. Instead, it is the induction of Gcn4 that results from inefficient translation machinery that is the key to enhanced longevity (Hu et al., 2018). That overexpression of Gcn4 was sufficient to extend yeast replicative lifespan in the absence of any apparent reduction in protein synthesis (Hu et al., 2018) is reminiscent of the situation that occurs with a variety of yeast mutants that inhibit tRNA nuclear export. Deletion of NUP100, encoding a component of the nuclear pore complex, LOS1 or MSN5, encoding nuclear tRNA exporters, each upregulate Gcn4 protein levels, and extend replicative lifespan in a manner that is dependent on GCN4, in the absence of apparent changes to global protein synthesis (Lord et al., 2017; Lord et al., 2015; McCormick et al., 2015). Taken together, these studies suggest that it is not the reduction of global protein synthesis that is key to replicative lifespan extension, but rather the accompanying induction of Gcn4 is what is important.
Autophagy induction by Gcn4 overexpression extends yeast replicative lifespan
Having ruled out reduced global levels of proteins as the key longevity factor, the next question is how does Gcn4 influence longevity? The yeast transcriptional regulator Gcn4 (ATF4 in mammals) induces and represses many genes in response to stress, including amino acid depletion (Postnikoff et al., 2017). The outcome of Gcn4 induction is the transcriptional activation of multiple different stress response pathways including amino acid biosynthesis, organelle biosynthesis, purine biosynthesis, the ER stress response, and autophagy (Postnikoff et al., 2017). Strikingly, we found that the ability of Gcn4 induction to extend lifespan was fully dependent on autophagy, in a manner that did not involve Tor1 inhibition (Hu et al., 2018) (Fig. 1). As such, Gcn4-mediated induction of autophagy is the essential pathway downstream of protein synthesis reduction that promotes replicative lifespan extension.
Autophagy is the process literally of self-eating, which is used by the cell to remove damaged organelles or structures (Yu et al., 2018). The evidence supporting the contribution of autophagy to organismal lifespan extension induced by many interventions in drosophila, worms, mice, and during chronological aging in yeast is quite compelling (recently reviewed in (Nakamura and Yoshimori, 2018; Tyler and Johnson, 2018)). Moreover, induction of autophagy by overexpression of autophagy proteins or regulators in drosophila, worms or mice is sufficient to extend organismal lifespan (Nakamura and Yoshimori, 2018). By contrast, autophagy is not induced during the normal yeast replicative lifespan (Hu et al., 2018) and consequently deletion of genes required for autophagy, as expected, has no influence on replicative lifespan in normal growth conditions (Hu et al., 2018; Steffen et al., 2008). Other than our work during Gcn4 overexpression, only one study to date has shown a requirement for autophagy in replicative lifespan extension, and that was in response to ER stress (Ghavidel et al., 2015). However, other regimens that extend lifespan do induce autophagy. For example, the polyamine Spermidine can extend yeast replicative lifespan and is known to induce autophagy (Morselli et al., 2009), but whether autophagy is required for the replicative lifespan extension by Spermidine remains untested. Tor1 inhibition by rapamycin extends replicative lifespan and induces autophagy, but again, whether autophagy is required for the replicative lifespan extension by rapamycin remains untested. One influential study concluded that macroautophagy does not play a role in yeast replicative lifespan extension during glucose restriction (Tang et al., 2008). However, this was not surprising given that the authors performed their analyses at a glucose concentration (0.5%) that does not even induce autophagy, while they showed that autophagy was induced at 0.2% glucose concentration (Tang et al., 2008).
Given the induction of Gcn4 and the involvement of Gcn4 in yeast replicative lifespan extension during so many life common extending regimens, and given that Gcn4 induction activates autophagy, we predict that it is only a matter of time before Gcn4-induced autophagy is shown to be required for yeast replicative lifespan extension by Tor1 inhibition, glucose restriction, etc. It will be important in the future to discern whether it is generally inducing autophagy or one of the many selective types of autophagy that are key to replicative lifespan extension and to identify the relevant autophagy cargo(s). For example, mitophagy is required for stress-induced lifespan extension in worms (Palikaras et al., 2015). One of the next challenges is to show that induction of autophagy is sufficient to extend replicative lifespan. If this proves to be the case, therapeutic interventions that induce autophagy would be an attractive targeted approach to promote the division of progenitor and stem cells during human aging, to treat diseases associated with aging and ultimately enhance longevity.
References
- Ables GP, and Johnson JE (2017). Pleiotropic responses to methionine restriction. Experimental gerontology 94, 83–88. [DOI] [PubMed] [Google Scholar]
- Castilho BA, Shanmugam R, Silva RC, Ramesh R, Himme BM, and Sattlegger E (2014). Keeping the eIF2 alpha kinase Gcn2 in check. Biochim Biophys Acta 1843, 1948–1968. [DOI] [PubMed] [Google Scholar]
- Delaney JR, Ahmed U, Chou A, Sim S, Carr D, Murakami CJ, Schleit J, Sutphin GL, An EH, Castanza A, et al. (2013). Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell 12, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche O, de la Cruz J, Kressler D, Doere M, and Linder P (2004). A membrane transport defect leads to a rapid attenuation of translation initiation in Saccharomyces cerevisiae. Mol Cell 13, 357–366. [DOI] [PubMed] [Google Scholar]
- Ghavidel A, Baxi K, Ignatchenko V, Prusinkiewicz M, Arnason TG, Kislinger T, Carvalho CE, and Harkness TA (2015). A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan. PLoS genetics 11, e1005429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonskikh Y, and Polacek N (2017). Alterations of the translation apparatus during aging and stress response. Mech Ageing Dev 168, 30–36. [DOI] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, and Kenyon C (2007). Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6, 95–110. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG (2005). Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59, 407–450. [DOI] [PubMed] [Google Scholar]
- Hu Z, Xia B, Postnikoff SD, Shen ZJ, Tomoiaga AS, Harkness TA, Seol JH, Li W, Chen K, and Tyler JK (2018). Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Obata T, Ota K, Sasaki T, and Ito T (2003). Rapamycin-induced translational derepression of GCN4 mRNA involves a novel mechanism for activation of the eIF2 alpha kinase GCN2. J Biol Chem 278, 20457–20460. [DOI] [PubMed] [Google Scholar]
- Lord CL, Ospovat O, and Wente SR (2017). Nup100 regulates Saccharomyces cerevisiae replicative life span by mediating the nuclear export of specific tRNAs. Rna 23, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CL, Timney BL, Rout MP, and Wente SR (2015). Altering nuclear pore complex function impacts longevity and mitochondrial function in S. cerevisiae. J Cell Biol 208, 729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MA, Delaney JR, Tsuchiya M, Tsuchiyama S, Shemorry A, Sim S, Chou AC, Ahmed U, Carr D, Murakami CJ, et al. (2015). A Comprehensive Analysis of Replicative Lifespan in 4,698 Single-Gene Deletion Strains Uncovers Conserved Mechanisms of Aging. Cell metabolism 22, 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Chandler-Brown D, Ramos FJ, Shamieh LS, and Kaeberlein M (2010). Regulation of mRNA translation as a conserved mechanism of longevity control. Adv Exp Med Biol 694, 14–29. [DOI] [PubMed] [Google Scholar]
- Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, Madeo F, and Kroemer G (2009). Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging 1, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, and Yoshimori T (2018). Autophagy and Longevity. Molecules and cells 41, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, and Tavernarakis N (2015). Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell death and differentiation 22, 1399–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postnikoff SDL, Johnson JE, and Tyler JK (2017). The integrated stress response in budding yeast lifespan extension. Microb Cell 4, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, et al. (2008). Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell 133, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Watkins JW, Bermudez M, Gray R, Gaban A, Portie K, Grace S, Kleve M, and Craciun G (2008). A life-span extending form of autophagy employs the vacuole-vacuole fusion machinery. Autophagy 4, 874–886. [DOI] [PubMed] [Google Scholar]
- Tyler JK, and Johnson JE (2018). The role of autophagy in the regulation of yeast life span. Ann N Y Acad Sci 1418, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Wek SA, and Wek RC (2000). Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Mol Cell Biol 20, 2706–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Chen Y, and Tooze SA (2018). Autophagy pathway: Cellular and molecular mechanisms. Autophagy 14, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Ouyang Q, Li H, and Zheng J (2017). A global characterization of the translational and transcriptional programs induced by methionine restriction through ribosome profiling and RNA-seq. BMC genomics 18, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]