Abstract
Monocyte recovery following hematopoietic cell transplantation (HCT) has been correlated with overall survival (OS). However, monocytes are heterogeneous and consist of classical (CD14++CD16−), intermediate (CD14+CD16+) and non-classical (CD14+CD16++) subpopulations, with unique functional properties. We hypothesized that monocyte subpopulation reconstitution would vary based on allogeneic stem cell source and would be associated with outcomes. We studied monocyte subpopulation recovery at days 28, 60, 100, 180 and 365 post-HCT among 202 patients with hematologic malignancy. Significant differences in absolute monocyte count (AMC) and monocyte subpopulation counts at days 60 and 100 were identified based on stem cell source (all p<0.01), with more robust recovery in umbilical cord blood (UCB) recipients. Using two-fold cross validation, optimal cutpoints were calculated for day 28 AMC and monocyte subpopulations based on OS. These were used to calculate hazard ratios for OS, disease free survival (DFS), relapse, transplant related mortality (TRM), acute and chronic GVHD. OS and DFS were superior when AMC and classical monocyte recovery were above optimal cutpoints (all p<0.03). Relapse was reduced for those with AMC (p<0.01) and classical (p=0.05) monocyte counts above optimal cutpoints. TRM was also reduced when classical (p=0.02) monocyte count exceeded optimal cutpoint. Intermediate and non-classical monocyte recovery were not associated with outcomes. In summary, hematopoietic cell source is associated with monocyte subpopulation recovery, with the early robust recovery in UCB recipients. Recovery of AMC and classical monocytes were prognostic for survival, relapse and TRM. These indicators may identify patients at increased risk for post-HCT failure and guide therapeutic interventions.
Introduction
Rapid monocyte recovery following hematopoietic cell transplantation (HCT) has been correlated with improved overall survival (OS) 1–4. However, monocytes are heterogeneous and can be separated into three distinct phenotypic and functional subpopulations based on CD14 and CD16 surface expression, including: CD14++CD16−(classical), CD14+CD16+ (intermediate), and CD14+CD16++ (non-classical) monocytes 5, 6. In healthy individuals, classical monocytes are most abundant in the peripheral blood (PB) and account for approximately 70–90% of the absolute monocyte count (AMC) 7, 8. Each subpopulation has unique functionality, although there are some inconsistencies across studies in the precise behavior of each sub-population 9–17. Classical monocytes have phagocytic activity, are less inflammatory and are skewed toward the production of counter-regulatory cytokines, including IL-10 10, 15, 18, 19. In contrast, non-classical monocytes have greater capacity to produce inflammatory cytokines upon activation, including TNF-α and IL-1β, whereas, intermediate monocytes are a transitional population, sharing features of both classical and non-classical monocytes, but with an inflammatory cytokine profile that is closer to non-classical monocytes13.
Monocyte sub-populations have been associated with unique states of health and disease. Classical monocytes have been described in the setting of tissue repair and innate immune functions 15, 20, while non-classical monocytes are important for immune patrolling and inflammatory functions. Both intermediate and non-classical monocytes are expanded in states of acute inflammation, such as sepsis 11, or in chronic inflammation such as systemic lupus erythematous 11 or obesity 9. The recovery of monocyte subpopulations after HCT has not been thoroughly examined. A previous pilot study of a small population of pediatric and young adult patients (N=30) undergoing HCT showed that the proportion of classical monocytes decreased during acute graft versus host disease (aGVHD), whereas, the non-classical and intermediate monocytes were increased. However, whether significant changes in absolute values of each of these populations occurred was not presented, and the small sample size limited the conclusions that could be drawn 21. Monocytes isolated from umbilical cord blood (UCB) and adult blood have distinct transcriptional profiles and response to cytokine stimulation, correlating with differences between the functionality between these two cell sources 22. To date, it is unclear whether monocytes differ in their reconstitution based on the source of the HCT graft and whether this, in turn, is associated with transplant outcomes. Here we compare HCT graft source in terms of monocyte recovery, as well as perform an analysis of monocyte subpopulation recovery and HCT outcomes.
Methods
Patients included in this study underwent their first allogeneic HCT for any malignant diagnosis at the University of Minnesota between 2010–2014 and enrolled onto an institutional immune reconstitution protocol (Figure 1). Demographic and clinical data were prospectively collected and managed in the University of Minnesota Bone Marrow Transplant (BMT) Database. All participants and/or legal guardians provided informed consent according to the principles of the Declaration of Helsinki for inclusion in the immune reconstitution study and the University of Minnesota BMT Database prior to transplantation. The University of Minnesota Institutional Review Board approved the prospective collection of blood for immune reconstitution and clinical HCT data.
Figure 1.
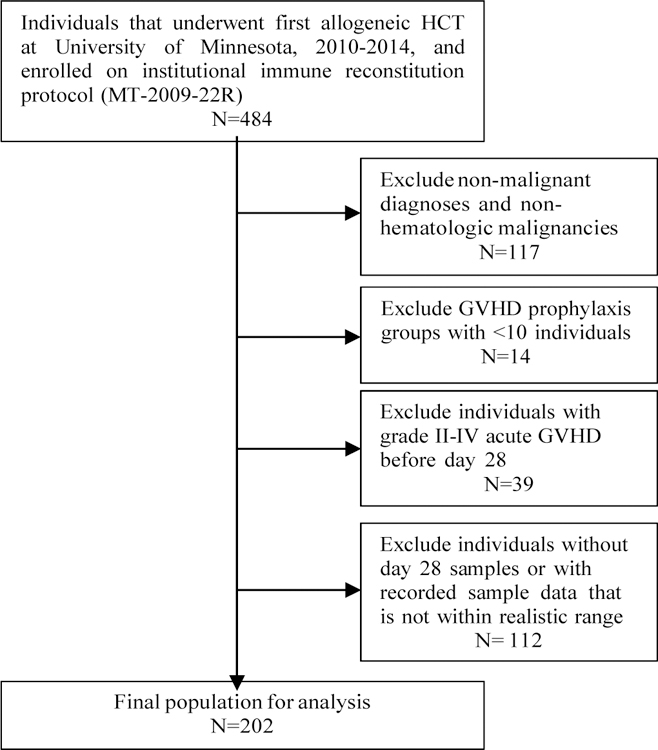
Study population. Summary of individuals included in analysis, with inclusion and exclusion criteria.
Pediatric and adult transplant recipients were included. Individuals who experienced grade II or greater aGVHD prior to day 28 were excluded due to potential effects of GVHD treatment on monocyte recovery. Individuals with missing day 28 measurements, who relapsed, or who died before day 28 were excluded. Absolute monocyte count (AMC), as well as absolute counts of classical, intermediate and non-classical monocyte subpopulations were assessed at days 28, 60, 100 and 365 following HCT. Monocytes and subpopulations were identified based on their relative surface expression of CD14 and CD16. The gating strategy is shown in Supplemental Figure 1. Patient blood was processed in real time using ficolled samples. Cells were stained on the day they were received, fixed and acquired by flow cytometry within 2 days of staining. At the time of each acquisition on the flow cytometer, an identically stained sample from third party cryopreserved healthy donor cells was thawed, stained and also acquired and analyzed to ensure that population frequencies remained constant, demonstrating antibody quality assurance over time. Descriptive statistics for patient demographic and clinical data were performed for the overall sample and based on stem cell source, including bone marrow (BM), PB, single UCB (SUCB) and double UCB (DUCB). Differences in the recovery of the AMC and absolute numbers of monocyte subpopulations (median and interquartile range) were evaluated between stem cell sources for each time point using nonparametric tests. To assess monocyte recovery and transplant outcomes, optimal cutpoints were calculated for the day 28 AMC and each monocyte subpopulation based on the Cox regression for the primary outcome, 2-year OS, adjusted for sex, conditioning intensity (myeloablative vs. reduced intensity), and age group (<18, 18–44.9 and ≥45 years) for the entire sample. Adjusted variables were selected for inclusion in the regression models if significant (p<0.05) in univariate analyses; variables tested included: stem cell source, conditioning intensity, sex, GVHD prophylaxis regimen, recipient CMV status, CMV reactivation at day 100, disease risk, age group, and body mass index (BMI) group (<25 kg/m2 or ≥25 kg/m2). For OS, the inference for the binary group variable (< optimal cutpoint vs. ≥ optimal cutpoint) was obtained by using the two-fold cross validation method23 to avoid the inflated type-I error caused by the multiple tests when searching for the optimal cutpoint. Specifically, we randomly selected half of the sample as the training set to determine the cutpoint, which was associated with the highest significance of the binary variable in the multivariable Cox regression, to be used for defining the high/low groups for the other half of the sample; then we repeated this process by switching the two subsets until the binary variable for the entire sample was determined; finally, the hazard ratio (HR; for high vs. low) and p-value for the binary variable were calculated using the multivariable Cox regression stratified by the subset. The advantage of the two-fold cross validation method is to include all sample in both cutpoint searching and effect estimation without scarifying type-I error. Cox regression or Fine-Gray regression was performed for other clinical endpoints including the 1-year transplant related mortality (TRM), 2-year disease free survival (DFS), 2-year relapse, 100-day aGVHD and 1-year chronic GVHD, using the same binary monocyte variables and covariates as for the OS. All regression analyses started at day 28, the landmark time point. All tests were two-sided and p-values <0.05 were considered statistically significant. Statistical analyses were performed in R 3.3.2 (R Core Team, 2016).
Results
Patient characteristics
Among 202 participants, median age at the time of transplant was 50.9 years (range 1.3–72.8), 36% were female and 40% received myeloablative conditioning. Sixteen percent received BM grafts, 37% received PB, 10% received SUCB and 37% received DUCB. Additional patient, treatment and disease characteristics are presented in Table 1.
Table 1.
Patient and treatment characteristics.
| All Groups | BM | PBSC | SUCB | DUCB | P-value | |
|---|---|---|---|---|---|---|
| N=202 | N=32 | N=75 | N=21 | N=74 | ||
| Patient sex | 0.77 | |||||
| Female | 73 (36.1%) | 14 (43.8%) | 25 (33.3%) | 8 (38.1%) | 26 (35.1%) | |
| Age at HCT (yrs) | <0.01* | |||||
| Median (range) | 50.9 (1.3– 72.8) | 36.1 (1.6–69.4) | 57.0 (21.2–72.8) | 9.4 (2.3–70.7) | 53.6 (1.3–71.9) | |
| Age group (yrs) | <0.01* | |||||
| 0–18 | 40 (19.8%) | 11 (34.4%) | 0 | 18 (85.7%) | 11 (14.9%) | |
| 18–45 | 41 (20.3%) | 7 (21.9%) | 16 (21.3%) | 1 (4.8%) | 17 (23.0%) | |
| >=45 | 121 (59.9%) | 14 (43.8%) | 59 (78.7%) | 2 (9.5%) | 46 (62.2%) | |
| Follow up time (days) | 0.81 | |||||
| Median (range) | 741.0 (33.0–2229.0) | 737.5 (39.0–2159.0) | 736.0 (33.0–1974.0) | 728.0 (37.0–2229.0) | 762.5 (34.0–1967.0) | |
| Disease | <0.01* | |||||
| ALL | 46 (22.8%) | 7 (21.9%) | 9 (12.0%) | 15 (71.4%) | 15 (20.3%) | |
| AML | 66 (32.7%) | 5 (15.6%) | 29 (38.7%) | 0 | 32 (43.2%) | |
| CML | 10 (5.0%) | 8 (25.0%) | 1 (1.3%) | 0 | 1 (1.4%) | |
| Other Leukemia | 10 (5.0%) | 1 (3.1%) | 4 (5.3%) | 0 | 5 (6.8%) | |
| Myelodysplasia | 32 (15.8%) | 2 (6.3%) | 15 (20.0%) | 3 (14.3%) | 12 (16.2%) | |
| Non-Hodgkin Lymphoma | 17 (8.4%) | 3 (9.4%) | 8 (10.7%) | 3 (14.3%) | 3 (4.1%) | |
| Hodgkin Lymphoma | 10 (5.0%) | 2 (6.3%) | 5 (6.7%) | 0 | 3 (4.1%) | |
| Myeloproliferative Disease | 1 (0.5%) | 1 (3.1%) | 0 | 0 | 0 | |
| Multiple Myeloma | 10 (5.0%) | 3 (9.4%) | 4 (5.3%) | 0 | 3 (4.1%) | |
| Disease risk group | 0.37 | |||||
| Standard | 109 (54.0%) | 14 (43.8%) | 38 (50.7%) | 12 (57.1%) | 45 (60.8%) | |
| High | 93 (46.0%) | 18 (56.3%) | 37 (49.3%) | 9 (42.9%) | 29 (39.2%) | |
| Timefrom diagnosis to HCT(months) | 0.02* | |||||
| Median (range) | 6.5 (2.0–376.7) | 13.2 (3.5–76.6) | 6.1 (2.0–125.9) | 25.1 (3.0–76.3) | 4.9 (2.3–376.7) | |
| Recipient CMV status | 0.22 | |||||
| Positive | 105 (52.0%) | 16 (50.0%) | 33 (44.0%) | 13 (61.9%) | 43 (58.1%) | |
| Negative | 96 (47.5%) | 16 (50.0%) | 42 (56.0%) | 7 (33.3%) | 31 (41.9%) | |
| Missing | 1(0.5%) | 0 | 0 | 1 (4.8%) | 0 | |
| Conditioning intensity | <0.01* | |||||
| Myeloablative | 81 (40.1%) | 16 (50.0%) | 20 (26.7%) | 19 (90.5%) | 26 (35.1%) | |
| Reduced intensity | 121 (59.9%) | 16 (50.0%) | 55 (73.3%) | 2 (9.5%) | 48 (64.9%) | |
| GVHD prophylaxis | <0.01* | |||||
| CSA/MMF | 112 (55.4%) | 9 (28.1%) | 38 (50.7%) | 17 (81.0%) | 48 (64.9%) | |
| CSA/MMF+ATG | 32 (15.8%) | 7 (21.9%) | 14 (18.7%) | 3 (14.3%) | 8 (10.8%) | |
| CSA/MTX | 41 (20.3%) | 16 (50.0%) | 23 (30.7%) | 1 (4.8%) | 1 (1.4%) | |
| SIRO/MMF | 17 (8.4%) | 0 | 0 | 0 | 17 (23.0%) | |
| ATG | 0.43 | |||||
| Yes | 32 (15.8%) | 7 (21.9%) | 14 (18.7%) | 3 (14.3%) | 8 (10.8%) | |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; BM, bone marrow; CML, chronic myeloid leukemia; CMV, cytomegalovirus; CSA, cyclosporine; DUCB, double umbilical cord blood; GVHD, graft versus host disease; HCT, hematopoietic cell transplantation; MMF, mycophenolate mofetil; MTX, methotrexate; PBSC, peripheral blood stem cells; SIRO, sirolimus; SUCB, single umbilical cord blood.
P-value < 0.05
Stem cell source and monocyte recovery
Monocyte subpopulation recovery was evaluated separately for BM, PB, SUCB and DUCB at days 28, 60, 100 and 365 following HCT (Table 2 and Figure 2). The recovery of the classical monocyte subpopulation was statistically significantly different between stem cell sources at day 28 (p=0.02), with the most robust recovery seen in SUCB recipients, but AMC and other subpopulations did not differ based on stem cell source (all p > 0.05). By day 60, along with significant differences in the recovery of the classical monocyte subpopulation (p<0.01), there were also differences in the recovery of the intermediate monocyte population (p<0.01) and AMC (p<0.01), with SUCB and DUCB recipients showing significantly greater absolute numbers compared to BM or PBSC. These differences persisted at day 100 (p<0.01 for AMC, classical and intermediate monocytes), although AMC and subpopulation values were higher at day 60 compared to day 100. By day 365, only intermediate monocytes remained significantly different across stem cell sources, with SUCB recipients at least 2.5-fold greater than BM, PBSC or DUCB recipients (p< 0.01).
Table 2.
Monocyte subpopulation recovery (×109/L) post-HCT, by stem cell source, presented as median (inter-quartile range).
| Monocyte type | BM | PBSC | SUCB | DUCB | P-value | |
|---|---|---|---|---|---|---|
| N=32 | N=75 | N=20 | N=74 | |||
| Day 28 | AMC | 0.63 (0.41–0.96) | 0.53 (0.33–0.86) | 0.66 (0.48–1.12) | 0.51 (0.27–0.83) | 0.10 |
| Non-classical | 0.03 (0.02–0.05) | 0.02 (0.01–0.04) | 0.04 (0.02–0.08) | 0.03 (0.01–0.07) | 0.08 | |
| Intermediate | 0.08 (0.04–0.14) | 0.06 (0.04–0.09) | 0.08 (0.06–0.11) | 0.08 (0.04–0.16) | 0.20 | |
| Classical | 0.47 (0.24–0.69) | 0.38 (0.23–0.60) | 0.48 (0.32–0.75) | 0.27 (0.16–0.53) | 0.02* | |
| N=20 | N=59 | N=8 | N=42 | |||
| Day 60 | AMC | 0.40 (0.18–0.68) | 0.45 (0.24–0.62) | 1.27 (0.59–1.46) | 0.95 (0.55–1.27) | <0.01* |
| Non-classical | 0.02 (0.01–0.04) | 0.03 (0.01–0.04) | 0.04 (0.01–0.08) | 0.04 (0.02–0.08) | 0.05 | |
| Intermediate | 0.03 (0.02–0.06) | 0.04 (0.03–0.06) | 0.06 (0.05–0.19) | 0.08 (0.05–0.17) | <0.01* | |
| Classical | 0.30 (0.11–0.49) | 0.31 (0.16–0.45) | 0.92 (0.48–1.22) | 0.70 (0.43–0.96) | <0.01* | |
| N=17 | N=45 | N=9 | N=37 | |||
| Day 100 | AMC | 0.44 (0.20–0.53) | 0.26 (0.17–0.38) | 0.9 (0.68–0.95) | 0.52 (0.34–0.80) | <0.01* |
| Non-classical | 0.01 (0.0–0.05) | 0.02 (0.01–0.03) | 0.03 (0.01–0.03) | 0.02 (0.01–0.05) | 0.23 | |
| Intermediate | 0.04 (0.02–0.06) | 0.03 (0.02–0.06) | 0.06 (0.05–0.09) | 0.07 (0.04–0.10) | <0.01* | |
| Classical | 0.33 (0.16–0.38) | 0.19 (0.10–0.29) | 0.69 (0.58–0.78) | 0.40 (0.23–0.70) | <0.01* | |
| N=16 | N=27 | N=6 | N=32 | |||
| Day 365 | AMC | 0.43 (0.30–0.65) | 0.48 (0.29–0.68) | 0.39 (0.24–0.55) | 0.51 (0.33–0.83) | 0.58 |
| Non-classical | 0.01 (0.01–0.03) | 0.02 (0.01–0.04) | 0.02 (0.01–0.03) | 0.03 (0.01–0.04) | 0.24 | |
| Intermediate | 0.03 (0.02–0.04) | 0.03 (0.02–0.08) | 0.09 (0.06–0.13) | 0.06 (0.04–0.09) | <0.01* | |
| Classical | 0.33 (0.21–0.52) | 0.35 (0.19–0.46) | 0.30 (0.17–0.41) | 0.41 (0.23–0.64) | 0.54 | |
Abbreviations: AMC, absolute monocyte count; BM, bone marrow; DUCB, double umbilical cord blood; HCT, hematopoietic cell transplantation; PBSC, peripheral blood stem cells; SUCB, single umbilical cord blood.
P-value < 0.05
Figure 2.
Monocyte subpopulation recovery (×109/L) post-HCT, by stem cell source and day.1
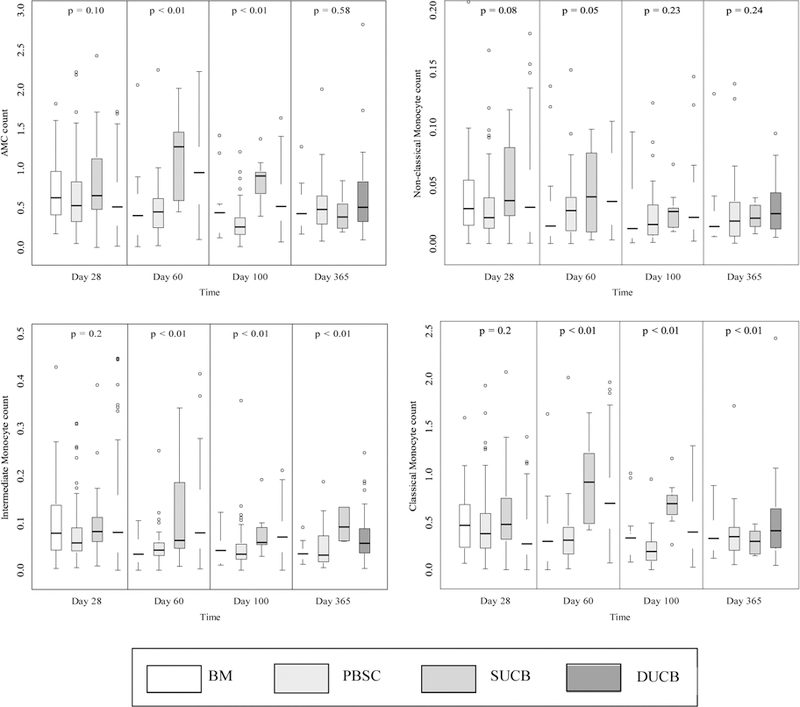
1The box shows the interquartile range with the bottom and top indicating the 25th and 75th percentiles; the thick line inside the box indicating the median; the whiskers representing 1.5 times of the height of the box (or minimum/maximum values if there is no value in that range); and the circle indicating the outliers.
Monocyte Recovery and Clinical Outcomes
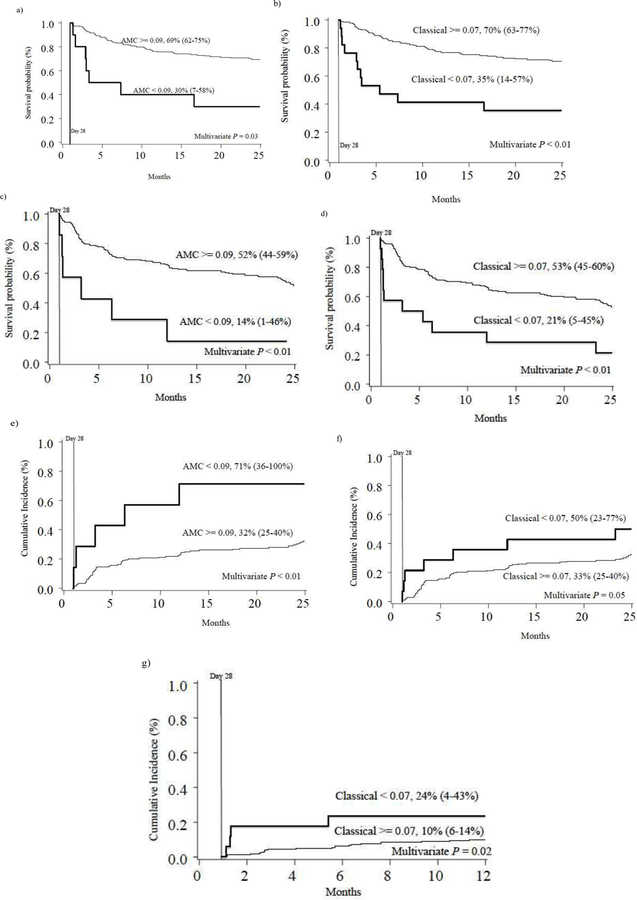
Using day 28 post-HCT values, the optimal cutpoint for AMC was 0.09 × 109/L, and was 0.02 × 109/L, 0.03 × 109/L, and 0.07 × 109/L for non-classical, intermediate and classical monocytes, respectively. The distribution of individuals falling above and below the cutpoints is shown in Table 3. In multivariable regression analyses, adjusted for sex, conditioning intensity, and age at transplant, multiple significant associations were identified between monocyte recovery and post-HCT outcomes. For 2-year OS, following two-fold cross validation, high day 28 AMC (HR=0.44, 95% CI, 0.21–0.92, p=0.03) and high classical monocyte count (HR=0.30, 95% CI, 0.14–0.65, p<0.01) were significantly associated with decreased risk of death, (Table 3 and Figure 3). Similarly, for 2-year DFS, AMC (HR=0.30, 95% CI, 0.13–0.69, p<0.01) and classical monocyte (HR=0.29, 95% CI, 0.15–0.56, p<0.01) recovery above the optimal cutpoint at day 28 were associated with superior DFS (Figure 3). Intermediate and non-classical monocyte counts were not associated with OS or DFS. Risk of relapse at 2 years was less for individuals with AMC (HR=0.29, 95% CI, 0.12–0.74, p<0.01) and classical monocyte (HR=0.46, 95% CI, 0.21–1.00, p=0.05) recovery above specified optimal cutpoints at day 28 (Figure 3). Non-classical and intermediate monocyte recovery was not associated with relapse. One-year TRM was associated with classical monocyte subpopulation recovery (HR=0.24, 95% CI, 0.07–0.80, p=0.02; Figure 3), but not with AMC or the recovery of other monocyte subpopulations. Day 100 grade II-IV and III-IV acute GVHD and 2-year chronic GVHD were not associated with day 28 AMC or subpopulation recovery in adjusted models.
Table 3.
Overall survival analysis of individuals falling above and below the optimal cutpoints of monocyte subpopulation
| Monocyte population | Optimal cutpoint (× 109/L) | N (< : ≥ cutpoint) | Estimated hazard ratio for high vs. low (95% CI) | P-value |
|---|---|---|---|---|
| AMC | 0.09 | 10:188 | 0.44 (0.21–0.92) | 0.03* |
| Non-classical | 0.02 | 73:125 | 0.93 (0.56–1.54) | 0.77 |
| Intermediate | 0.03 | 27:171 | 0.60 (0.33–1.10) | 0.10 |
| Classical | 0.07 | 17:181 | 0.30 (0.14–0.65) | <0.01* |
Abbreviation: AMC, absolute monocyte count.
P-value < 0.05
Figure 3.
Two-year overall survival (a and b),1 disease-free survival (c and d),1 relapse (e and f),2 and one-year transplant related mortality (g)2 of individuals above and below the optimal cutpoints3 (×109/L), based on absolute monocyte count (AMC), and classical monocyte count.
1Kaplan-Meier estimates
2Cumulative incidence estimates
3The optimal cutpoints were selected based on the whole sample.
P-values were calculated from the two-fold cross-validation method based on the multivariable regression adjusting for sex, conditioning intensity, and age at transplant.
Discussion
Prior studies demonstrate that absolute monocyte recovery is associated with transplant outcomes 1–4. However, monocytes are heterogeneous and to date, no study has investigated the recovery of monocyte subpopulations and determined whether they are associated with HCT outcomes. Here we used a standard approach of classifying monocyte subpopulations based on CD14 and CD16 expression to understand whether reconstitution of these subpopulations vary based on stem cell source and whether the kinetics of recovery was associated with transplant outcomes. Stem cell source was associated with monocyte subpopulation recovery; however, in univariate analyses stem cell source was not significantly associated with clinical outcomes and was not included in multivariate models. Through use of two-fold cross-validation, optimal cutpoints were identified for AMC and for monocyte subpopulations, and recipients with cell counts above those cutpoints were found to have improved survival and decreased relapse and TRM.
There was considerable variability in AMC and monocyte subpopulation recovery within each stem cell source. Differences appeared to be most clinically relevant, in terms of absolute differences, at days 60 and 100. Interestingly, individuals who received UCB transplantation showed more robust recovery of the total monocyte population and for all sub-populations, at day 60 and day 100; although these differences between UCB and other stem cell sources were mostly resolved by day 365 and differences in HCT outcomes were not observed based on stem cell source within univariate analyses. It is possible that higher numbers of monocytes after UCB is due to delayed T-cell recovery and proportionately higher monocytes or due to inherent differences in the hematopoietic stem cells that are present in the fetal sources (i.e. UCB) compared to adult stem cell sources (i.e. PBSCs or BM). The AMC and monocyte subpopulation absolute values peaked at day 60 and remained relatively stable thereafter. Interestingly, the recipients of SUCB and DUCB transplantation showed more robust monocyte recovery relative to other stem cell sources, despite having distinct clinical demographic characteristics; notably recipient age at the time of HCT and underlying disease, suggesting that host factors are perhaps less important than stem cell source for monocyte recovery.
For the entire cohort, we observed significantly different transplant outcomes based on AMC and classical monocyte subpopulation recovery. Patients with recovery of these populations below the optimal cutpoints experienced inferior OS and DFS; furthermore, those with AMC and classical monocyte recovery below the optimal cutpoint also showed increased relapse and those with classical monocyte recovery below the optimal cutpoint experienced increased TRM. These findings are consistent with observations made in previous studies1–4, although, the calculated AMC cutpoint in the present analysis is lower than what has been used in previous analyses based on differences in populations and methodology. It is somewhat anticipated that individuals with more robust bone marrow recovery would have superior outcomes, but we did not see this for all outcomes within the analysis. This supports the assertion that these cells play important roles in immune recovery, perhaps through their cytokine production or antigen presentation, which may lead to protection from relapse. Interestingly, non-classical monocyte recovery, which would be expected to enhance inflammatory capacity and immune patrolling, was not associated with any post-HCT outcomes. Given the increased systemic inflammation expected with post-HCT conditions like sepsis, GVHD or veno-occlusive disease (VOD), which may lead to subsequent mortality, we had hypothesized potential associations between non-classical monocyte recovery and end-points such as TRM, DFS or OS; however, this was not observed. One potential explanation for this finding is that that this study used circulating, peripheral blood monocyte analysis and changes in the frequency of monocyte populations associated with GVHD may be occurring at the tissue level and not in the peripheral blood.
The lack of differences in the incidence of aGVHD between the groups may be multifactorial; the most feasible explanation is that individuals who developed grade III-IV aGVHD prior to day 28 (N=19) were excluded from the analysis because it was felt their inclusion and the effect of GVHD therapies on monocyte recovery would alter results. While these same concerns can be raised for patients who developed grade III-IV aGVHD after this time point, individuals fitting this description were relatively minimal (n=30). The lack of association between AMC or monocyte subpopulation recovery and cGVHD may in part be related to the exclusion of individuals who developed early aGVHD (i.e., prior to day 28). In fact, Moon and coworkers compared patients that developed aGVHD prior to and after day 28 and found significantly more cGVHD in the former group.24 It is also possible that the latency between day 28 monocyte recovery and the development of cGVHD is too distant to see a meaningful relationship or that there is no relationship.
Although we did not have data on other sources of inflammation, such as lifestyle factors or underlying health conditions such as autoimmune disease or type 2 diabetes, we did have the BMI at the time of transplant. Chronic inflammation is well-described in the setting of overweight and obesity 25–27. We hypothesized that individuals with increased BMI may have more inflammatory monocyte subpopulation recovery compared to those who are non-overweight/obese (BMI<25kg/m2) and possibly inferior clinical outcomes; however, we did not see significant associations between BMI group and clinical outcomes in univariate analyses, nor did we see associations between monocyte subpopulation recovery and BMI group (data not shown), even when the analysis was restricted to adults only, possibly consistent with the reports suggesting no impact of recipient obesity on transplant outcomes 28–30.
This analysis provides valuable data regarding the prognostic role of monocyte subpopulation recovery following HCT; however, there are limitations to this study. We do not report concurrent results of other white blood cell lineages, which may be similarly predictive of HCT outcomes. This approach was taken because the inclusion of additional white blood cell lineages would have diminished our power to draw conclusions on our primary hypotheses regarding the importance of monocyte subpopulation recovery. In addition, the monocyte counts were obtained at pre-determined time points and may not be reflective of other time points or clinical events in the post-HCT course; thus, not fully capturing the monocyte-associated predictive value or response to transplant-related health effects. Furthermore, we do not have functional data to interrogate the behavior of these monocyte subpopulations at the defined post-HCT time points. Although we observed numerical differences in UCB and adult stem cell source monocyte recovery, it has previously been demonstrated that UCB monocytes are also functionally unique; they respond differently to cytokine stimulation and they exhibit increased sensitivity to activation of key signaling pathways 22. Additionally, previous work has shown that among PBSC recipients, despite numerical monocyte reconstitution early in the post-HCT course, functional recovery, as evidenced by oxidative burst following stimulation with PMA, was impaired until day 90 post-HCT 31. Thus, a potential future direction for study would be to understand whether the monocyte phenotype and function are similar post-engraftment and beyond across donor sources and at what point function is appreciably recovered. Of note, the methodology adopted in this study for identifying cutpoints for a continuous monocyte recovery marker focused on the marker itself while adjusting for important patient and treatment characteristics. However, if the overall predictive power of the combined covariates including the marker were of interest, the survival ROC method could be used32. Despite the stated limitations, this study presents a single institution HCT population, with standardized supportive care guidelines, GVHD treatment approaches and relatively homogenous conditioning regimens that can be associated with comprehensive monocyte subpopulation immune reconstitution data,33 which is a notable strength and sets this study apart from others which have reported on absolute monocyte count and HCT outcomes.
Herein, we have shown that hematopoietic cell source is associated with monocyte subpopulation recovery following HCT, in that recipients of UCB demonstrate more robust monocyte recovery in the first 100 days post-HCT compared to BM and PBSCs. We have also shown that optimal cutpoints based on early (day 28) monocyte subpopulation recovery following HCT are prognostic using the cross-validation method. If further validated, these simple and easily accessible indicators could be used prospectively to identify patients at increased risk for adverse outcomes and may direct supportive care efforts. Additional functional analyses of monocyte subpopulations could further define the etiology of the demonstrated outcome advantages.
Supplementary Material
Supplemental Figure 1. Monocyte gating strategy. Sequential gating to identify monocyte subpopulations based on CD14 and CD16 expression.
Highlights.
Robust monocyte subpopulation recovery was seen in UCB recipients at day 60 and 100
OS, DFS superior when AMC and classical monocyte recovery above optimal cutpoints
Relapse reduced when AMC and classical monocyte counts above optimal cutpoints
TRM reduced when classical monocyte count exceeded optimal cutpoint
Acknowledgements:
This work was supported in part by NIH P30 CA77598 utilizing the Translational Therapy Laboratory Shared Resource of the Masonic Cancer Center, University of Minnesota; the Translational Therapy Lab processed specimens, stained and acquired cells on an LSRII flow cytometer (BD Biosciences) and analyzed data using FlowJo (FlowJo LLC). Additional support was provided by NIH/NCI 5P01CA065493 (QC, SAC, JC, XL, DJW, BRB, JSM, JEW, MRV), NIH R01 HL56067, the Children’s Cancer Research Fund (Minneapolis, MN) and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award (UL1TR000114) (LMT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.DeCook LJ, Thoma M, Huneke T, et al. Impact of lymphocyte and monocyte recovery on the outcomes of allogeneic hematopoietic SCT with fludarabine and melphalan conditioning. Bone marrow transplantation 2013;48:708–714. [DOI] [PubMed] [Google Scholar]
- 2.Dhakal B, Brazauskas R, Lara CA, Hari P, Pasquini M, D’Souza A. Monocyte recovery at day 100 is associated with improved survival in multiple myeloma patients who undergo allogeneic hematopoietic cell transplantation. Bone marrow transplantation 2016;51:297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Bourgeois A, Peterlin P, Guillaume T, et al. Higher Early Monocyte and Total Lymphocyte Counts Are Associated with Better Overall Survival after Standard Total Body Irradiation, Cyclophosphamide, and Fludarabine Reduced-Intensity Conditioning Double Umbilical Cord Blood Allogeneic Stem Cell Transplantation in Adults. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2016;22:1473–1479. [DOI] [PubMed] [Google Scholar]
- 4.Thoma MD, Huneke TJ, DeCook LJ, et al. Peripheral blood lymphocyte and monocyte recovery and survival in acute leukemia postmyeloablative allogeneic hematopoietic stem cell transplant. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2012;18:600–607. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Frontiers in immunology 2013;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010;116:e74–80. [DOI] [PubMed] [Google Scholar]
- 7.Sen A, Chowdhury IH, Mukhopadhyay D, et al. Increased Toll-like receptor-2 expression on nonclassic CD16+ monocytes from patients with inflammatory stage of Eales’ disease. Invest Ophthalmol Vis Sci 2011;52:6940–6948. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler-Heitbrock L Monocyte subsets in man and other species. Cellular immunology 2014;289:135–139. [DOI] [PubMed] [Google Scholar]
- 9.Devevre EF, Renovato-Martins M, Clement K, Sautes-Fridman C, Cremer I, Poitou C. Profiling of the three circulating monocyte subpopulations in human obesity. Journal of immunology (Baltimore, Md. : 1950) 2015;194:3917–3923. [DOI] [PubMed] [Google Scholar]
- 10.Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood 1996;87:373–377. [PubMed] [Google Scholar]
- 11.Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non- Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Scientific reports 2015;5:13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989;74:2527–2534. [PubMed] [Google Scholar]
- 13.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunologic research 2012;53:41–57. [DOI] [PubMed] [Google Scholar]
- 14.Zhao C, Zhang H, Wong WC, et al. Identification of novel functional differences in monocyte subsets using proteomic and transcriptomic methods. Journal of proteome research 2009;8:4028–4038. [DOI] [PubMed] [Google Scholar]
- 15.Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011;118:e16–31. [DOI] [PubMed] [Google Scholar]
- 16.Zawada AM, Rogacev KS, Rotter B, et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 2011;118:e50–61. [DOI] [PubMed] [Google Scholar]
- 17.Skrzeczynska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scandinavian journal of immunology 2008;67:152–159. [DOI] [PubMed] [Google Scholar]
- 18.Smedman C, Ernemar T, Gudmundsdotter L, et al. FluoroSpot Analysis of TLR-Activated Monocytes Reveals Several Distinct Cytokine-Secreting Subpopulations. Scandinavian journal ofimmunology 2012;75:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010;33:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stansfield BK, Ingram DA. Clinical significance of monocyte heterogeneity. Clinical and translational medicine 2015;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doring M, Cabanillas Stanchi KM, Haufe S, et al. Patterns of monocyte subpopulations and their surface expression of HLA-DR during adverse events after hematopoietic stem cell transplantation. Annals of hematology 2015;94:825–836. [DOI] [PubMed] [Google Scholar]
- 22.Krow-Lucal ER, Kim CC, Burt TD, McCune JM. Distinct functional programming of human fetal and adult monocytes. Blood 2014;123:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Statistics in medicine 1996;15:2203–2213. [DOI] [PubMed] [Google Scholar]
- 24.Moon JH, Kim SN, Kang BW, et al. Early onset of acute GVHD indicates worse outcome in terms of severity of chronic GVHD compared with late onset. Bone marrow transplantation 2010;45:1540–1545. [DOI] [PubMed] [Google Scholar]
- 25.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology 2011;29:415–445. [DOI] [PubMed] [Google Scholar]
- 26.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. The Journal of clinical investigation 2017;127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro WH, Agovi MA, Logan BR, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2010;16:1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pine M, Wang L, Harrell FE Jr., et al. The effect of obesity on outcome of unrelated cord blood transplant in children with malignant diseases. Bone marrow transplantation 2011;46:1309–1313. [DOI] [PubMed] [Google Scholar]
- 30.Navarro WH, Loberiza FR Jr., Bajorunaite R, et al. Effect of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2006;12:541–551. [DOI] [PubMed] [Google Scholar]
- 31.Rommeley M, Spies-Weisshart B, Schilling K, Hochhaus A, Sayer HG, Scholl S. Reconstitution and functional analyses of neutrophils and distinct subsets of monocytes after allogeneic stem cell transplantation. Journal of cancer research and clinical oncology 2011;137:1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005;61:92–105. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 2002;100:1611–1618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Monocyte gating strategy. Sequential gating to identify monocyte subpopulations based on CD14 and CD16 expression.