Abstract
Introduction:
In utero particulate matter exposure produces oxidative stress that impacts cellular processes that include telomere biology. Newborn telomere length is likely critical to an individual’s telomere biology; reduction in this initial telomere setting may signal increased susceptibility to adverse outcomes later in life. We examined associations between prenatal particulate matter with diameter ≤ 2.5 μm (PM2.5) and relative leukocyte telomere length (LTL) measured in cord blood using a data-driven approach to characterize sensitive windows of prenatal PM2.5 effects and explore sex differences.
Methods:
Women who were residents of Mexico City and affiliated with the Mexican Social Security System were recruited during pregnancy (n=423 for analyses). Mothers’ prenatal exposure to PM2.5 was estimated based on residence during pregnancy using a validated satellite-based spatio-temporally resolved prediction model. Leukocyte DNA was extracted from cord blood obtained at delivery. Duplex quantitative polymerase chain reaction was used to compare the relative amplification of the telomere repeat copy number to single gene (albumin) copy number. A distributed lag model incorporating weekly averages for PM2.5 over gestation was used in order to explore sensitive windows. Sex-specific associations were examined using Bayesian distributed lag interaction models.
Results:
In models that included child’s sex, mother’s age at delivery, prenatal environmental tobacco smoke exposure, pre-pregnancy BMI, gestational age, birth season and assay batch, we found significant associations between higher PM2.5 exposure during early pregnancy (4–9 weeks) and shorter LTL in cord blood. We also identified two more windows at 14–19 and 34–36 weeks in which increased PM2.5 exposure was associated with longer LTL. In stratified analyses, the mean and cumulative associations between PM2.5 and shortened LTL were stronger in girls when compared to boys.
Conclusions:
Increased PM2.5 during specific prenatal windows was associated with shorter LTL and longer LTL. PM2.5 was more strongly associated with shortened LTL in girls when compared to boys. Understanding sex and temporal differences in response to air pollution may provide unique insight into mechanisms.
Keywords: particulate matter, leukocyte telomere length, distributive lag models, prenatal exposure, Bayesian distributed lag interaction models
Introduction
Environmental exposures during prenatal life may lead to alterations in the normal maturation of multiple organ systems impacting their developmental trajectories in pregnancy and throughout childhood. Evidence underscores oxidative stress as a key contributor to toxicant-elicited disruption of fetal development, and highlights the importance of optimal oxidant balance at the maternal-fetal interface in normal development (Herrera et al., 2014). Oxidative stress impacts telomere biology, which in turn has been implicated in fetal programming (Cameron and Demerath, 2002; Entinger et al., 2012; Janssen et al., 2014). Telomeres, the repetitive nucleotide sequences at the distal end of chromosomes that protect coding DNA from deterioration, play a critical role in mitosis and are sensitive to reactive oxygen species (ROS) damage. Telomeres are fundamental for cell division and shorten as the number of cell divisions increases and this shortening serves as a biomarker for cellular and biologic aging and longevity. Pregnancy may be a time of heightened susceptibility to risk factors that may cause telomere shortening because of the enormous number of cell divisions in the embryo and the fetus. Shortening of telomere length may also help assess premature aging in newborns (Menon et al., 2016; Menon et al., 2012).
Emerging evidence, the majority from occupational or adult cohorts, has shown that leukocyte telomere length (LTL) shortening can be accelerated by exposure to environmental factors like particulate matter (PM) (Hou et al., 2012; Pieters et al., 2016), elemental carbon (EC) (Hou et al., 2012) and black carbon (BC) (McCracken et al., 2010). In a study of drivers and office workers in China, average ambient PM10 over the 14 days before subject evaluation was significantly associated with shorter LTL (Hou et al., 2012). In a cohort of elderly people in Belgium, a 5μg/m3 increment in annual PM2.5 concentration was associated with a relative decrease in LTL (Pieters et al., 2016). In the Normative Aging Study, an IQR increase in annual BC was associated with a 7.6% shortening of LTL in elderly men (McCracken et al., 2010).
While measurements of telomere length have traditionally been used in adult or aging cohorts, newborn LTL is increasingly recognized as being critical to an individual’s telomere biology (Sabharwal et al., 2018) and reduction in this initial telomere setting is a potential biomarker of the effects of maternal-fetal processes on offspring long-term health. Also, there is emerging evidence linking pro-oxidant exposures in pregnancy to changes in LTL in newborns. For example, Salihu and colleagues reported a dose response association between smoking status and shortened LTL in cord blood, with women who reported active smoking having shorter TL than former smokers and never smokers (Salihu et al., 2015). Another study reported that higher pregnancy-related stress was associated with shorter LTL in cord blood (Entringer et al., 2013). In a prospective birth cohort in Belgium, prenatal PM2.5 was associated with shortened TL in cord blood and placenta (Martens et al., 2017). Thus, LTL may serve as a biomarker capable of identifying newborns at higher risk for PM health effects (Pinkerton and Joad, 2006) and early identification of children at increased risk allows for intervention to promote more optimal development going forward.
The objective of this study was to leverage daily prenatal PM2.5 measures available over pregnancy to more precisely identify sensitive windows in relation to LTL measured in cord blood. We combined these approaches with distributive lag models (DLM) that allow us to statistically model and visualize the exposure timing-dependent pattern of associations. Because there is evidence to suggest sex-specific effects of air pollution exposure during pregnancy (Chiu et al., 2016; Hsu et al., 2015; Lakshmanan et al., 2015) we also examined sex stratified associations using Bayesian distributed lag interaction models (BDLIMs).
Methods
Study population
Between July 2007 and February 2011, pregnant women who were receiving prenatal care through the Mexican Social Security System (Instituto Mexicano del Seguro Social –IMSS) were recruited into the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study. The IMSS provides healthcare to affiliated private sector employees, the majority low- to middle-income workers and their families. Women were eligible to participate if they were less than 20 weeks gestation, were at least18 years old, had completed primary education, planned to stay in Mexico City for the next 3 years, had access to a telephone, had no medical history of heart or kidney disease, did not consume alcohol daily, and did not use any steroid or anti-epilepsy medications. Procedures were approved by institutional review boards at the Harvard School of Public Health, Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health. Women provided written informed consent.
Prenatal PM2.5 levels
Daily residential exposure to PM2.5 was estimated during pregnancy using a previously described spatio-temporal model that incorporates Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived Aerosol Optical Depth (AOD) measurements at a 1× 1 km spatial resolution (Just et al., 2015). In brief, remote sensing data were calibrated with 12 municipal ground level monitors of PM2.5, meteorological data and land use regression (LUR) variables (roadway density, temperature, relative humidity, planetary boundary layer and daily precipitation) to yield estimates of daily residential PM2.5 levels for each participant. Mixed effect models with spatial and temporal predictors and day-specific random effects were used to account for temporal variations in the PM2.5−AOD relationship. The model was fit with a seasonal smooth function of latitude and longitude and time-varying average incorporating local monitoring for days without AOD data. Model performance was assessed using monitor-level leave one-out cross-validation; the model performed well with an R2 of 0.724. Due to day-to-day variation, daily PM2.5 measures were averaged into weekly measurements as in prior work to reduce potential noise caused by day-to day variation in PM2.5 levels. (Chiu et al., 2016; Hsu et al., 2015; Rosa et al., 2017). To compare the DLM approach to traditional windows, we also calculated the average PM over clinically defined trimesters (1st trimester: 1–13 weeks, 2nd trimester: 14–27 weeks, 3rd trimester: 28 weeks-delivery).
Leukocyte telomere length assay
DNA was obtained from venous umbilical cord blood as described previously (Rosa et al., 2017). The first 260 whole blood samples were stored in PAXgene™ Blood DNA Tubes (PreAnalytiX GmbH, Hombrechtikon Switzerland) and extracted using a QIAamp DNA Blood Kit (QIAGEN). The DNA was then stored at −80°C prior to analysis. The next 271 samples were extracted by conventional phenol–chloroform method after red cell lysis by a second laboratory. The second laboratory stored the DNA at 4°C. Some samples in the original sample list could not be used due to insufficient volume or low concentration of stock DNA. After normalization and quantification, 503 samples had enough DNA to measure LTL.
LTL was measured using the qPCR method developed by Cawthon (Cawthon, 2002) and modified by Pavanello et al. (Pavanello et al., 2011). DNA samples were normalized to 2 ng/μl and concentrations were confirmed using PicoGreen quantification prior to amplification. Duplex quantitative polymerase chain reaction (qPCR) was used to determine relative telomere length. This was done comparing the relative amplification of telomere repeat copy number to single gene (albumin) copy number. The primers used for telomere length were Telc 5’-TGT TAG GTA TCC CTA TCC CTA TCC CTA TCC CTA TCC CTA ACA-3’ and Telg 5’-ACA CTA AGG TTT GGG TTT GGG TTT GGG TTT GGG TTA GTG T-3’. Additionally, iQ SYBR Green Supermix was used. This reagent contains an antibody-mediated hot-start iTaq DNA polymerase as well as a passive reference dye fluorescein. The qPCR conditions were set up as follows: the thermal cycling profile started with a 95°C incubation for 3 min to activate the hot-start iTaq DNA polymerase, then 2 cycles of 15 sec at 94°C, 15 sec at 49°C, and 32 cycles of 15 sec at 94°C, 10 sec at 62°C, 10 sec at 74°C with signal acquisition, 10 sec at 84°C, 10 sec at 88°C with signal acquisition At the end of each real-time PCR reaction to verify the specificity of amplified, a melting curve was added from 72 to 95°C with an increment 0.5°C per step. Each sample was run in triplicate and a pooled quality control sample was run on each plate made with equal mass of total DNA from all samples normalized to 2 ng/μl. Samples were assayed in 6 batches/plates. Each plate contained a standard curve as a reference. The standard curve was made from a study-specific pooled sample comprising equal mass of total DNA from all samples normalized to 30 ng/μl. The telomere/single copy gene ratio (T/S ratio) was calculated as the ratio of telomere copy number relative to albumin copy number, both of which were estimated by the Bio-Rad software using the study-specific standard curve (Cq= slope*Log10(Sq)+ intercept). The ratio was calculated by dividing the starting quantity of telomere copy number by the starting quantity of albumin copy number. The coefficient of variation (CV) for triplicate samples was calculated and a threshold of 16.3 was determined using the inter-quartile range. If the CV was outside of this range, the samples were re-run and a second LTL measure was recorded. If both runs of a sample resulted in a CV outside of the acceptable range, the sample was flagged as potentially unreliable. Samples where the variation between triplicates fell outside the threshold for the CV after multiple runs were flagged, deemed potentially not reliable and excluded from analysis (n=26). Because DNA was extracted using two different methods, we tested for differences in their variance. Variance was not significantly different (Levene test p>0.3) between the two methods. Supplemental figure S1 shows the distribution of values by lab procedure.
Covariates
In Mexico, ultrasounds are not routinely performed as standard of care, therefore gestational age was based on last menstrual period (LMP) and by a standardized physical examination to determine gestational age at birth (Capurro et al., 1978). If the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP, the physical exam gestational age was used instead of the gestational age determined by LMP. Only 7 mothers in the original cohort (n=948) reported smoking during pregnancy, and only 1 mother reported smoking in pregnancy in our current sample, therefore prenatal exposure to environmental tobacco smoke was included in the models and was defined as report of any smoker in the home during the second or third trimester of pregnancy. Assay batch was included as a covariate in the analysis in order to account for potential batch effects. Maternal pre-pregnancy height and weight were determined via self-report at enrollment; body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). Season was defined according to weather patterns in Mexico City as dry cold (January-February; November-December), dry warm (March-April) and rainy (May-October).
Statistical Analyses
Out of the 477 samples with reliable LTL measures, 47 were excluded for analysis based on gestational age <37 weeks. 1 participant had missing gestational age data, 1 participant was missing PM data and 4 samples were removed as outliers based on Q-Q plot (final N=423). We fitted distributive lag models (DLMs) to estimate the time-varying association between relative leukocyte telomere length in cord blood and estimated PM2.5 level during a given week in pregnancy as previously described (Chiu et al., 2016; Hsu et al., 2015; Rosa et al., 2017). This method incorporates40 data from all time points simultaneously and assumes that the association between the outcome and exposure at a given time point, controlling for exposure at all other time points, varies smoothly as a function of time. We fitted the linear DLM , where APij is the estimated PM2.5 level in week j of pregnancy and x1i, …, xpi are the additional covariates for subject I and Yi is the value for relative telomere length. A maximum lag of 40 weeks was included in the DLM starting from the date of estimated conception. For participants with gestational age 37–39 weeks, postnatal exposure was used for missing weeks. Covariates included sex, maternal age at delivery, prenatal exposure to environmental tobacco smoke, assay batch, self-reported pre-pregnancy BMI, birth season and gestational age. Inclusion of a variable accounting for lab extraction method did not noticeably alter our results.
DLMs that modeled a smooth function using B-splines with 5 degrees of freedom were fit (Gasparrini, 2011; Gasparrini et al., 2010) chosen due to its parsimony and best AIC value; additional smoothing did not significantly improve the model. A sensitive window was identified when the pointwise 95% confidence bands did not contain zero. DLMs were implemented using the dlnm package version 2.3.2 (Gasparrini, 2011) in RStudio Version 1.0.136 (Boston, MA) and other analyses were performed in SPSS version 23 (Chicago, IL). Traditional linear regression models with adjustment for the same covariates were also run to compare DLM results to clinically defined trimesters and average PM2.5 over pregnancy. In order to avoid bias in estimates (Wilson et al., 2017b), trimester exposures were included in a single model.
Bayesian distributed lag interaction models (BDLIM) were employed to determine effect modification by fetal sex. BDLIM estimates the cumulative effect of PM2.5- exposure over the entire pregnancy for each sex-specific subgroup, accounting for identified sensitive windows and within-window effects, as previously described (Wilson et al., 2017a). Each stratum can have either the same, or different, sensitive windows (weights) and the same or different within-window effects (effects) (Wilson et al., 2017a). The model quantifies the likelihood of each pattern of heterogeneity and estimates the association between exposure and outcome under the effect modification pattern that is best supported by the data. In addition to sensitive windows, BDLIM estimates the mean effects over the identified windows and the cumulative effect of PM2.5 exposure over pregnancy for each stratum accounting for sensitive windows and within-window effects.
3. Results
The majority of participants did not have more than 12 years of schooling (77%) and half were classified as having low socioeconomic status. More than a third of participants reported exposure to a smoker in the home during pregnancy. Other relevant cohort characteristics are shown in Table 1. These baseline characteristics did not differ significantly between those included in these analyses when compared to the remainder of the base cohort, except for maternal age at delivery (see supplemental material, Table S1).
Table 1.
PROGRESS cohort characteristics
| Characteristic | N=423 |
|---|---|
| Child’s sex, n (% male) | 229 (54) |
| Prenatal ETS exposure, n (%) | 148 (35) |
| Maternal education at enrollment | |
| Less than high school, n (%) | 165 (39) |
| Some high school or high school graduate, n (%) | 160 (38) |
| More than high school, n (%) | 98 (23) |
| Maternal age delivery, median (25th–75th) | 28.1 (24.2–32.5) |
| Pre-pregnancy BMI, median (25th–75th) | 24.6 (22.1, 27.8) |
| Average prenatal PM2.5 μg/m3, median (25th–75th) | 22.8 (20.5, 24.5) |
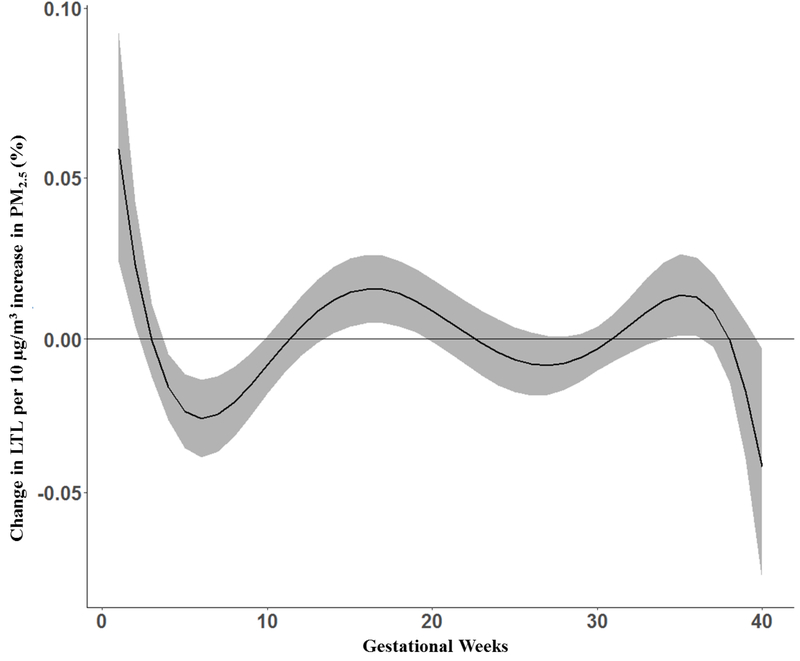
Figure 1 shows the association between a 10 μg/m3 increase in prenatal PM2.5 and cord blood LTL in the sample as a whole, adjusting for maternal age, child’s sex, maternal education, prenatal tobacco smoke exposure, gestational age, birth season and assay batch. The DLM identified a significant association between increased PM2.5 exposure in early pregnancy, specifically 4–9 weeks gestation, and shortened LTL in cord blood. The DLM also identified 2 more windows in which increased PM2.5 exposure was associated with longer LTL at 14–19 and 34–36 weeks. We did not find a significant cumulative effect in the overall population (1.99% 95%CI [−9.91%, 15.45%]).
Figure 1.
Associations between weekly prenatal PM2.5 and cord blood LTL in the entire sample (n=423). Adjusted for sex, maternal age at delivery, prenatal exposure to environmental tobacco smoke, pre-pregnancy BMI, gestational age, birth season and batch. The y-axis represents the change in LTL associated with a 10 μg/m3 increase in PM2.5; the x-axis is gestational age in weeks. Solid lines show the predicted change in LTL. Gray areas indicate 95% CIs. A sensitive window is identified for the weeks where the estimated pointwise 95% CI (shaded area) does not include zero.
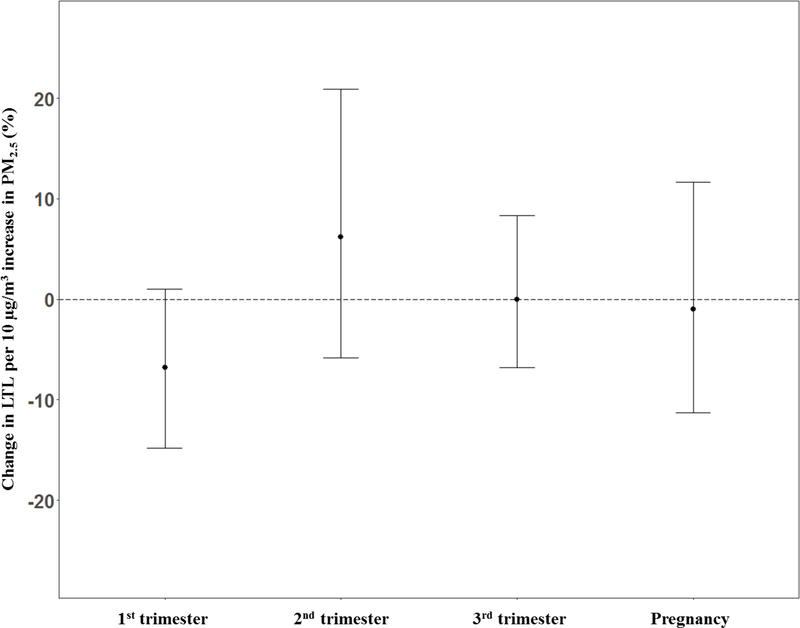
We fitted linear regression models between PM2.5 averaged over clinically defined trimesters and over all of pregnancy. Figure 2 shows a forest plot comparing the difference in LTL for a 10 μg/m3 increment in PM2.5 averaged the 1st, 2nd and 3rd trimester and averaged over the entire pregnancy. When using the trimester averages, we did not find any statistically significant associations between PM2.5 averaged over trimesters or pregnancy and LTL.
Figure 2.
Linear model estimates for percent change in LTL per 10 μg/m3 higher PM2.5 concentration averaged clinically defined trimesters and the entire pregnancy. All models adjusted for sex, maternal age at delivery, prenatal exposure to environmental tobacco smoke, pre-pregnancy BMI, gestational age, birth season and batch. Trimester results are from a single model that included all three trimesters.
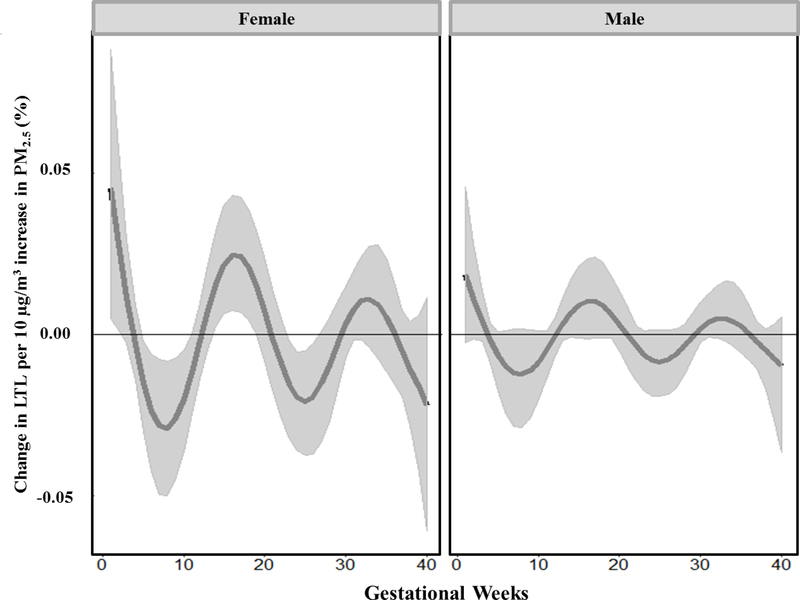
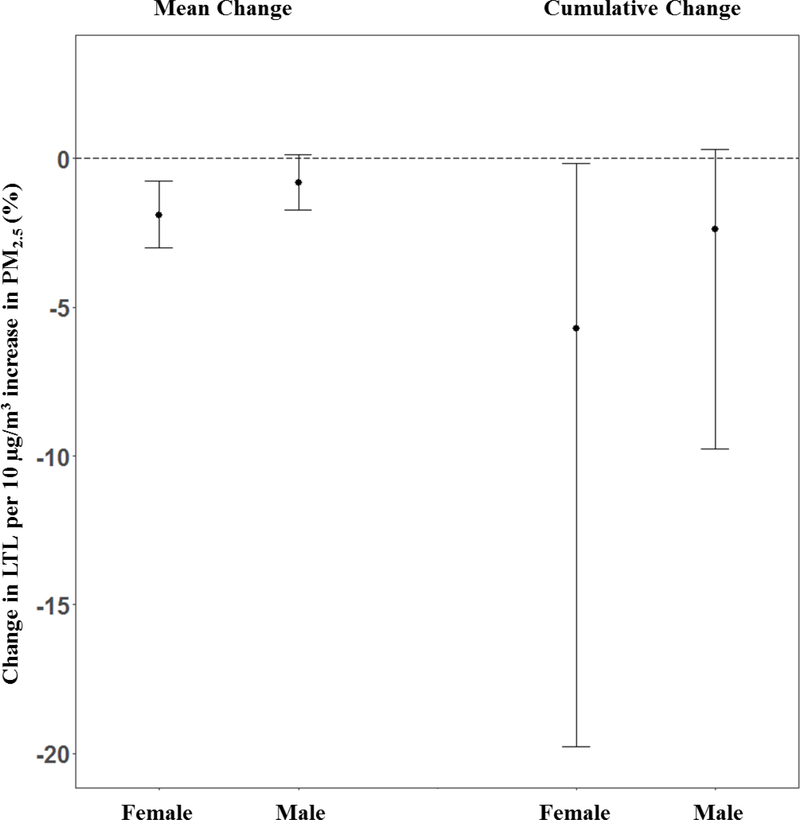
Figure 3 demonstrates results from a BDLIM analysis of the association of prenatal PM2.5 exposure and LTL, accounting for effect modification by child sex. We found that two of the sensitive windows suggested in the overall population were more pronounced in girls, with a significant window at 5–10 and 14–18 weeks of gestation. We additionally saw window at 23–26 weeks gestation in which increased PM2.5 was associated with shorter LTL in girls. No sensitive window was found between prenatal PM2.5 and LTL in boys. The BDLIM analysis suggested that effect modification by sex was attributable to the difference in the magnitude of the within-window association, while the window was not different between the two sexes (the normalized posterior density was 0.938, interpreted as the probability that this was the best fitting pattern of effect modification). Figure 4 shows BDLIM analyses for sex differences showing both boys and girls have negative mean within-window effect and cumulative effects, meaning PM2.5 is associated with shortened LTL but the effect is stronger and statistically significant only in the girls when compared to boys. Estimated cumulative effects across pregnancy, accounting for sensitive windows and within-window effects, were −5.72% (95%CI: −19.78%, −0.17%) for girls and −2.38% (95%CI: −9.78%, 0.32%) for males.
Figure 3.
Sex-stratified association between weekly ambient PM2.5 over gestation and cord blood LTL. Bayesian distributed lag interaction models (BDLIM) were used to estimate week-specific effects and interaction by sex. Models were adjusted for sex, maternal age at delivery, prenatal exposure to environmental tobacco smoke, pre-pregnancy BMI, gestational age, birth season and batch. The x-axis demarcates the gestational age in weeks. The y-axis represents the percent change in LTL per 10 μg/m3 increase in PM2.5. Solid lines represent the predicted time–varying percent change in LTL and gray areas indicates the 95% confidence intervals (CI). A sensitive window is identified when the estimated point-wise 95% CI does not include zero.
Figure 4.
Bayesian distributed lag interaction models (BDLIM) were used to estimate mean within –window effect and cumulative changes in LTL per 10 μg/m3 higher PM2.5 by sex. All models adjusted for sex, maternal age at delivery, prenatal exposure to environmental tobacco smoke, pre-pregnancy BMI, gestational age, birth season and batch.
4. Discussion
Our findings suggest that prenatal exposure to PM2.5 during a specific window in early pregnancy is associated with shortened LTL in cord blood while PM2.5 later in pregnancy is associated with elongated LTL. In addition, PM2.5 was more strongly associated with shorter LTL in girls when compared to boys. The observed associations remained significant after adjustment for a number of important potential confounders and covariates. While there is evidence that pro-oxidant exposures like smoking (Salihu et al., 2015) and maternal stress (Entringer et al., 2013) are associated with changes in newborn LTL, only a couple of studies have examined the association between ambient pollution exposure during pregnancy and newborn LTL. In a study of twins in East Flanders, Belgium, distance to road was used as a proxy measure of ambient air pollution exposure and increasing proximity to a major road was associated with shorter LTL in placenta while increased level of greenness was associated with longer LTL (Bijnens et al., 2015). A recently published study, found that increasing polycyclic aromatic hydrocarbons (PAH)-adducts in cord blood was associated with shorter LTL in the cord blood of children born in Tongliang, China (Perera et al., 2018). The authors also reported longer LTL in children who were born after the local coal burning power plant, hypothesized as the primary source of PAHs, was closed when compared to the children who were born before it closed (Perera et al., 2018). The ENVIRONAGE cohort in Belgium, also used DLMs to examine the association between PM2.5 and cord blood and placenta LTL and they identified a sensitive window during mid-pregnancy at 12–25 weeks for cord blood and 15–27 weeks for placenta (Martens et al., 2017). They also reported an association between PM2.5 exposure during the entire pregnancy and shorter LTL in both placenta and cord blood.
Evidence underscores the central role of oxidative stress (Wells et al., 2009) and the importance of optimal oxidant balance at the maternal-fetal interface in normal development (Herrera et al., 2014). Telomeres are highly sensitive to DNA damage induced by oxidative stress due to their high guanine content (Kawanishi and Oikawa, 2004). Particulate matter may induce systemic oxidative stress (Donaldson and MacNee, 2001) and production of reactive oxygen species (ROS) induced DNA damage (Blackburn et al., 2015; Kawanishi and Oikawa, 2004), leading to increased telomere shortening. Furthermore, ROS- produced single strand breaks have a high frequency in telomeric DNA due to an inefficient repair of these breaks (Petersen et al., 1998) which might lead to accumulation of oxidative damage. Particulate matter may also affect the fetus directly potentially through the translocation of particles into the placenta or fetus. Particles enter the mother’s circulation, and may eventually reach the placenta where particles with a diameter less than 240 nm may be able to cross the transplacental barrier (Wick et al., 2010).
We identified early pregnancy as a critical period for the association between PM2.5 and decreased LTL in the overall cohort. This might be a sensitive period to PM2.5 exposure due to accelerated cell division as the fetus develops. Early pregnancy is also a critical period for exposure to environmental insults and exposure to ambient air pollution during this period has been associated with adverse pregnancy and birth outcomes (Lavigne et al., 2018; Lee et al., 2013; Pedersen et al., 2017).Our window did differ from the mid-pregnancy window identified by Martens and colleagues in which PM2.5 was associated with shorter TL in both placenta and cord blood. This might be due to differences in exposure with the average exposure in Mexico being higher or differences in the underlying population’s response to air pollution. We also identified windows in the second and third trimester of exposure to PM2.5 which were associated with elongated telomere length in cord blood in the overall cohort. Previous work in adult populations has also shown positive associations between exposure to ambient air pollution and longer telomeres (Dioni et al., 2011; Hou et al., 2012; Pieters et al., 2016). Martens et al also reported an association between increasing PM2.5 in the third trimester and longer LTL in cord blood but not in placenta (Martens et al., 2017). These associations may be reflective of a compensatory mechanism after an environmental insult. LTL attrition due to oxidative insults is counteracted by the enzymatic activity of telomerase, which works to stabilize telomeres by adding hexameric (TTAGGG) repeats to the telomeric ends of the chromosomes (Hug and Lingner, 2006) which aims to preserve telomere length and cell viability (Shore and Bianchi, 2009). Data are limited on the regulation of telomerase expression in cord blood mononuclear cells but it has been shown that cells with shortened telomeres lose their ability to divide and become senescent or undergo apoptosis (Blackburn, 2001) while cells with longer telomeres can maintain cell capacity for rapid proliferation (Hodes et al., 2002). Therefore, increased telomerase activity in lymphocytes in response to these exposures may result in the clonal expansion of subpopulations of lymphocytes with longer telomeres (Dioni et al., 2011).
Evidence from both animal and human studies underscores the importance of the initial telomere setting and early life attrition (Asghar et al., 2015; Bateson et al., 2015; Benetos et al., 2013; Bijnens et al., 2017; Entringer et al., 2018; Heidinger et al., 2012; Hjelmborg et al., 2015). In a study of zebra finches, TL was measured at 6 different time points during their lifespan and TL measured at the earliest time point of 25 days, was the best predictor of lifespan (Heidinger et al., 2012). In another study of European starlings, developmental telomere attrition was associated with more impulsive decision making while foraging (Bateson et al., 2015).In humans, an analysis of LTL rankings in adulthood conducted in 4 cohorts in Israel, France, United States and Denmark, the authors concluded that the variation between participants arose in early life, given that their LTL rankings remained unchanged during their 60 year follow-up (Benetos et al., 2013). In the East Flanders Prospective Twin Survey LTL was measured at birth in placenta and in buccal cells in adulthood to examine their association with air pollution exposure(Bijnens et al., 2017). While the two time points were moderately correlated, the authors reported that participants with longer TL at birth had the greatest downward shift in ranking in relation to air pollution exposure (Bijnens et al., 2017).
Previous studies have suggested sex-specific differences in response to in utero exposure to particulate matter, including differences in fetal outcomes (Ghosh et al., 2007), body composition (Chiu et al., 2017) and other markers of oxidative stress (Rosa et al., 2017). The majority of studies that have reported on prenatal ambient air pollution exposures and telomere length at birth did not examine potential sex-differences. Only Martens and colleagues reported on sex difference and they did not find evidence of effect modification by sex in their analyses in either placenta or cord blood LTL (Martens et al., 2017). In our analyses, we saw stronger associations between prenatal PM2.5 and telomere shortening in girls when compared to boys. Recent data from the Shanghai Allergy Cohort showed an association between concentrations of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in cord blood and telomere shortening only in female newborns and the association being partly mediated by ROS (Liu et al., 2018). The authors posited that the sex-specific effect might be the result of PFASs acting as endocrine disruptors and impairing the protective effect of estrogen against ROS-induced DNA damage (Liu et al., 2018) and there is some evidence that ambient air pollutants have endocrine disrupting properties (Darbre, 2018; Huang et al., 2017). Other pro-oxidant exposures and conditions have shown to have sex-specific effects in adults (Cheng et al., 2017; Enokido et al., 2014). For example perceived lower maternal care during the first 16 years of life was associated with shorter LTL in young adult women in Japan, while perceived lower paternal care was associated with shorter LTL in men (Enokido et al., 2014). In an analysis of metabolic syndrome and LTL collected in the National Health and Nutrition Examination Survey (NHANES), that an increase in metabolic syndrome components was associated with shorter LTL especially in women (Cheng et al., 2017).
Our study has several strengths. First, we were able to leverage highly spatial and temporally resolved ambient air pollution data reflecting each individual participant’s exposure during the entire pregnancy. We used data-driven statistical methods that can better identify sensitive windows to air pollution exposure and better assess potential sex-effects. We were also able to adjust for important confounders. We also acknowledge some limitations. Exposure to air pollution in other microenvironments outside of the home might lead to personal exposure levels that differed from our ambient exposure estimated at the home. We did not have complete cell count data in our population which may influence LTL. However, it has been shown that telomere length is synchronized across leukocyte cell types and even when the synchrony is not absolute, the variation across individuals is much greater than the variation in telomere length among different cell types within an individual (Kimura et al., 2010).We cannot rule out potential residual confounding due to unmeasured host and environmental factors that may influence LTL in cord blood. Nevertheless, our findings were limited to specific time periods within pregnancy suggesting any unmeasured confounders would likely need to vary in time along with PM2.5 and would not be explained by more time-invariant characteristics. Finally, the generalizability of our findings may be limited due the composition of our cohort which consisted mostly of Mexican women belonging mostly to low-income families living in a megacity with substantial PM2.5 exposure.
In our study, increased PM2.5 was associated with changes in LTL at 3 specific windows during pregnancy, suggesting heightened sensitivity to PM during these particular time periods. We also found evidence that the effect was stronger in girls when compared to boys. Understanding of sex-specific effects and refined determination of time windows in which PM has the greatest magnitude of effect can enhance insight into underlying mechanisms and may better inform future interventions.
Supplementary Material
Highlights.
Examined sensitive windows of prenatal PM2.5 exposure on LTL in cord blood.
Sensitive window identified at gestational weeks 4–9, 14–19 and 34–36
Findings suggest sex-specific associations.
PM2.5 more strongly associated with shortened LTL in girls compared to boys
Acknowledgements:
The (PROGRESS) project has been funded by grants R01 ES014930 and R01 ES013744 (Wright RO, PI). Phenotyping and biostatistical support was funded by P30 ES023515. During preparation of this manuscript, MJR was supported by K99 ES027496. This study was supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico and we thank the ABC (American British Cowdray Medical Center) in Mexico for providing research facilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asghar M, et al. , 2015. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science. 347, 436–438. [DOI] [PubMed] [Google Scholar]
- Bateson M, et al. , 2015. Developmental telomere attrition predicts impulsive decision-making in adult starlings. Proceedings of the Royal Society B-Biological Sciences. 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, et al. , 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 12, 615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnens E, et al. , 2015. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environment International. 79, 1–7. [DOI] [PubMed] [Google Scholar]
- Bijnens EM, et al. , 2017. Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med. 15, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, 2001. Switching and signaling at the telomere. Cell. 106, 661–673. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, et al. , 2015. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 350, 1193–8. [DOI] [PubMed] [Google Scholar]
- Cameron N, Demerath EW, 2002. Critical periods in human growth and their relationship to diesases of aging. American Journal Physical Anthropology. 35, 159–84. [DOI] [PubMed] [Google Scholar]
- Capurro H, et al. , 1978. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 93, 120–2. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, 2002. Telomere measurement by quantitative PCR. Nucleic Acids Research. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YY, et al. , 2017. Examining the gender difference in the association between metabolic syndrome and the mean leukocyte telomere length. Plos One. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, et al. , 2016. Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int. 87, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YHM, et al. , 2017. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environmental Research. 158, 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre PD, 2018. Overview of air pollution and endocrine disorders. Int J Gen Med. 11, 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioni L, et al. , 2011. Effects of short-term exposure to inhalable particulate matter on telomere length, telomerase expression, and telomerase methylation in steel workers. Environ Health Perspect. 119, 622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, MacNee W, 2001. Potential mechanisms of adverse pulmonary and cardiovascular effects of particulate air pollution (PM10). Int J Hyg Environ Health. 203, 411–5. [DOI] [PubMed] [Google Scholar]
- Enokido M, et al. , 2014. Parental care influences leukocyte telomere length with gender specificity in parents and offsprings. BMC Psychiatry. 14, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entinger S, et al. , 2012. Prenatal stress, telomere biology, and fetal programming of health disease risk. Science Signaling. 5, 112. [DOI] [PubMed] [Google Scholar]
- Entringer S, et al. , 2018. The fetal programming of telomere biology hypothesis: an update. Philosophical Transactions of the Royal Society B-Biological Sciences. 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, et al. , 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. American Journal of Obstetrics and Gynecology. 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, 2011. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. Journal of Statistical Software. 43, 1–20. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, et al. , 2010. Distributed lag non-linear models. Statistics in Medicine. 29, 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, et al. , 2007. Does the effect of air pollution on pregnancy outcomes differ by gender? A systematic review. Environmental Research. 105, 400–408. [DOI] [PubMed] [Google Scholar]
- Heidinger BJ, et al. , 2012. Telomere length in early life predicts lifespan. Proceedings of the National Academy of Sciences of the United States of America. 109, 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, et al. , 2014. The placental pursuit for an adequate oxidant balance between the mother and the fetus. Frontiers in Pharmacology. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmborg JB, et al. , 2015. The heritability of leucocyte telomere length dynamics. Journal of Medical Genetics. 52, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes RJ, et al. , 2002. Telomeres in T and B cells. Nature Reviews Immunology. 2, 699–706. [DOI] [PubMed] [Google Scholar]
- Hou L, et al. , 2012. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ Int. 48, 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HHL, et al. , 2015. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children Identifying Sensitive Windows and Sex Differences. American Journal of Respiratory and Critical Care Medicine. 192, 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JV, et al. , 2017. The Association of Air Pollution With Pubertal Development: Evidence From Hong Kong’s “Children of 1997” Birth Cohort. Am J Epidemiol. 185, 914–923. [DOI] [PubMed] [Google Scholar]
- Hug N, Lingner J, 2006. Telomere length homeostasis. Chromosoma. 115, 413–25. [DOI] [PubMed] [Google Scholar]
- Janssen BG, et al. , 2014. Variation of DNA methylation in candidate age-related targets on the mitochondrial-telomere axis in cord blood and placenta. Placenta. 35, 665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AC, et al. , 2015. Using High-Resolution Satellite Aerosol Optical Depth To Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environ Sci Technol. 49, 8576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi S, Oikawa S, 2004. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 1019, 278–84. [DOI] [PubMed] [Google Scholar]
- Kimura M, et al. , 2010. Synchrony of telomere length among hematopoietic cells. Experimental Hematology. 38, 854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, et al. , 2015. Associations between prenatal traffic-related air pollution exposure and birth weight: Modification by sex and maternal pre-pregnancy body mass index. Environmental Research. 137, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, et al. , 2018. Fine Particulate Air Pollution and Adverse Birth Outcomes: Effect Modification by Regional Nonvolatile Oxidative Potential. Environ Health Perspect. 126, 077012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, et al. , 2013. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern Child Health J. 17, 545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. , 2018. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances affects leukocyte telomere length in female newborns. Environ Pollut. 235, 446–452. [DOI] [PubMed] [Google Scholar]
- Martens DS, et al. , 2017. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA Pediatr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken J, et al. , 2010. Annual ambient black carbon associated with shorter telomeres in elderly men: Veterans Affairs Normative Aging Study. Environ Health Perspect. 118, 1564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, et al. , 2016. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY). 8, 216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, et al. , 2012. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 7, e31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, et al. , 2011. Shortened telomeres in individuals with abuse in alcohol consumption. International Journal of Cancer. 129, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, et al. , 2017. Impact of Road Traffic Pollution on Pre-eclampsia and Pregnancyinduced Hypertensive Disorders. Epidemiology. 28, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, et al. , 2018. Shorter telomere length in cord blood associated with prenatal air pollution exposure: Benefits of intervention. Environ Int. 113, 335–340. [DOI] [PubMed] [Google Scholar]
- Petersen S, et al. , 1998. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res. 239, 152–60. [DOI] [PubMed] [Google Scholar]
- Pieters N, et al. , 2016. Biomolecular Markers within the Core Axis of Aging and Particulate Air Pollution Exposure in the Elderly: A Cross-Sectional Study. Environmental Health Perspectives. 124, 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton KE, Joad JP, 2006. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmacol Physiol. 33, 269–72. [DOI] [PubMed] [Google Scholar]
- Rosa MJ, et al. , 2017. Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ Int. 98, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharwal S, et al. , 2018. Telomere length dynamics in early life: the blood-and-muscle model. FASEB J. 32, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu HM, et al. , 2015. Impact of intrauterine tobacco exposure on fetal telomere length. Am J Obstet Gynecol. 212, 205 e1–8. [DOI] [PubMed] [Google Scholar]
- Shore D, Bianchi A, 2009. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 28, 2309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, et al. , 2009. Oxidative Stress in Developmental Origins of Disease: Teratogenesis, Neurodevelopmental Deficits, and Cancer. Toxicological Sciences. 108, 4–18. [DOI] [PubMed] [Google Scholar]
- Wick P, et al. , 2010. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect. 118, 432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, et al. , 2017a. Bayesian distributed lag interaction models to identify perinatal windows of vulnerability in children’s health. Biostatistics. 18, 537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, et al. , 2017b. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am J Epidemiol. 186, 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.