Abstract
Non-graft-versus-host disease (non-GVHD) ocular complications are generally uncommon after hematopoietic cell transplantation (HCT), but can cause prolonged morbidity affecting activities of daily living and quality of life. Here we provide an expert review of non-GVHD ocular complications in a collaboration between transplant physicians and ophthalmologists through the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and the Transplant Complications Working Party of the European Society of Blood and Marrow Transplantation. Complications discussed in this review include cataracts, glaucoma, ocular infections, ocular involvement with malignancy, ischemic microvascular retinopathy, central retinal vein occlusion, retinal hemorrhage, retinal detachment and ocular toxicities associated with medications. We have summarized incidence, risk factors, screening, prevention and treatment of individual complications and generated evidence-based recommendations. Baseline ocular evaluation before HCT should be considered in all patients who undergo HCT. Follow-up evaluations should be considered according to clinical symptoms, signs and risk factors. Better preventive strategies and treatments remain to be investigated for individual ocular complications after HCT. Both transplant physicians and ophthalmologists should be knowledgeable of non-GVHD ocular complications and provide comprehensive collaborative team care.
Keywords: hematopoietic cell transplantation, complication, eye, review, prevention, treatment
Introduction
Hematopoietic cell transplantation (HCT) is a curative treatment for many hematologic malignancies and nonmalignant disorders, although a variety of complications and late effects may occur.1-3 Non-graft-versus-host disease (non-GVHD) ocular complications are generally uncommon after HCT, but can cause prolonged morbidity affecting activities of daily living and quality of life. It is important for all health professionals taking care of HCT recipients to have adequate knowledge about ocular complications.
This review summarizes recent updates in non-GVHD ocular complications after HCT in a collaborative effort between transplant physicians and ophthalmologists through the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research (CIBMTR) and the Transplant Complications Working Party of the European Society of Blood and Marrow Transplantation (EBMT). We aim to provide an expert review of non-GVHD ocular complications after HCT with evidence-based recommendations for clinical practice and future research. The most frequent ocular complication after allogeneic HCT is ocular GVHD, which is addressed in a companion review.
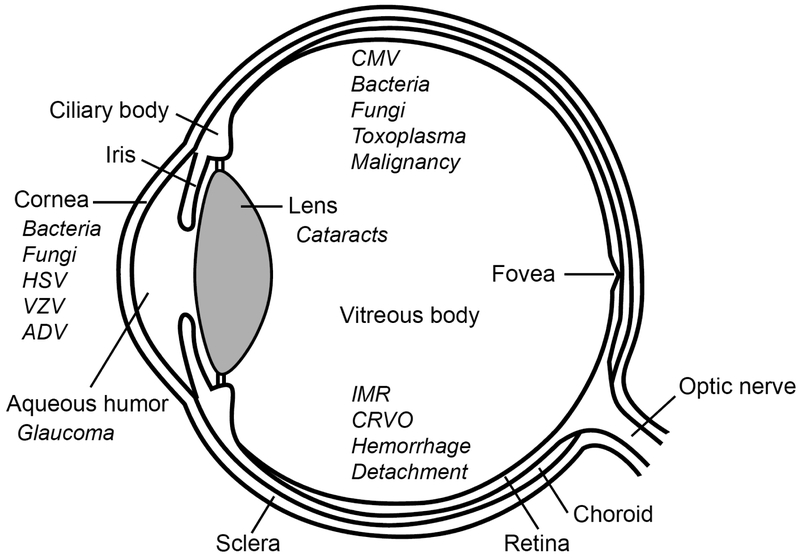
Structures of the eye are shown in Figure 1. The anterior segment of the eye is formed by the cornea, aqueous humor, iris, ciliary body and crystalline lens. The posterior segment is formed by the vitreous body, retina, choroid and optic nerve.
Figure 1.
Structures of the eye
Methods
We searched the Medline (PubMed) database using a broad search strategy to identify studies related to ocular complications after HCT. The primary search was conducted using the terms “hematopoietic transplantation AND (eye OR ocular),” and 552 articles were identified as of March 31, 2018. Relevant articles were also reviewed as needed. Recommendations are organized according to an evidence-based system described previously4 to reflect the strength of recommendations and the quality of evidence supporting them (Table 1).
Table 1.
Evidence-based rating system used in this review
| Category Definition | |
|---|---|
| Strength of the Recommendation: | |
| A | Should always be offered. |
| B | Should generally be offered. |
| C | Evidence for efficacy is insufficient to support a recommendation for or against, or evidence for efficacy might not outweigh adverse consequences, or cost of the approach. Optional. |
| D | Moderate evidence for lack of efficacy or for adverse outcome supports a recommendation against use. Should generally not be offered. |
| E | Good evidence for lack of efficacy or for adverse outcome supports a recommendation against use. Should never be offered. |
| Quality of Evidence Supporting the Recommendation: | |
| I | Evidence from at least one properly randomized, controlled trial. |
| II | Evidence for at least one well-designed clinical trial without randomization, from cohort or case-controlled analytic studies (preferable from more than one center) or from multiple time-series or dramatic results from uncontrolled experiments. |
| III | Evidence from opinions of respected authorities based on clinical experience, descriptive. |
Cataracts
Incidence and risk factors
Cataracts, defined clinically as progressive opacification of the lens, are one of the most frequent late ocular complications after HCT. The reported incidence of cataracts in HCT survivors varies among studies, ranging from 11% to 100% in adults5, 6 and from 4% to 76% in children (supplementary Table S1).7, 8 This large variability is attributable to heterogeneity in patient populations, conditioning regimens, supportive care, and length of follow-up.
Among patients who undergo allogeneic HCT, total body irradiation (TBI) as part of the conditioning regimen has been identified as a major risk factor for cataract formation. Posterior subcapsular cataracts are the most common subtype associated with exposure to ionizing radiation. The risk of cataracts is much higher after single dose TBI compared to hyper-fractionated TBI.9 A higher dose rate (>0.04 Gy/min) has also been identified as a risk factor.10 Lens-shielding in patients receiving TBI has been tested, mostly in pediatric patients, and has shown to reduce the incidence and severity of cataracts,11 although controversy exists regarding a potentially increased risk of extramedullary disease relapse in the shielded area. One pediatric study showed that fractionated TBI was assocated with cataracts requiring surgery, while chemotherapy-based conditioning caused less severe cataracts usually not requiring surgery.12
In addition to TBI, prolonged steroid therapy, such as for treatment of GVHD, has been identified as an independent risk factor for cataracts after HCT.13, 14 A population-based study showed that even inhaled corticosteroids were associated with the development of posterior subcapsular and nuclear cataracts.15 Survivors of allogeneic HCT are more likely to develop cataracts, when compared with those of autologous HCT.10
Screening, prevention and treatment
Comprehensive evaluation by ophthalmologists before HCT and then annually after HCT is warranted to diagnose and determine the appropriate timing for surgical intervention of cataracts (supplementary Table S2).16 In adults, decreased visual acuity reported by the patients should initiate an eye examination. Pediatric patients require regular follow-up given that they may not complain of visual changes. Cataract prevention with use of fractionated TBI or use of non-TBI conditioning regimens and corticosteroids sparing, when clinically feasible, is the most important for minimizing cataract development.9, 12
Surgery is the standard treatment for cataracts. Early diagnosis and appropriate timing for surgery are important in young children, especially those under 7 years old, in order to prevent irreversible amblyopia.17 Cataract surgery is usually indicated for those with bilateral cataracts and best corrected visual acuity of 20/40 or worse, although the visual threshold for performing surgery should be tailored according to the needs of the patient. In both adult and pediatric patients, cataracts may present with dry eye and other manifestations of ocular GVHD. The concurrent ocular surface problems and postoperative complications should be controlled to obtain good surgical outcomes in these patients.18
Ocular infections
Ocular infections can be severe after HCT. The types of infectious organisms, involved ocular structures, symptoms and diagnostic tests are summarized in Table 2.
Table 2.
Involved structures, symptoms and diagnostic tests of ocular infections following HCT
| Organism | Involved structures | Symptoms and clinical findings | Diagnostic tests |
|---|---|---|---|
| Bacteria21 Staphylococci, Haemophilus, Streptococci, Pseudomonas, and others |
Lids, conjunctiva, cornea, intraocular | Pain or irritation, erythema, discharge, photophobia, impaired vision | Conjunctival culture, culture of corneal scrapings or vitreal fluid |
| Fungi21 | Cornea, intraocular | Pain, erythema, photophobia, excessive tearing or discharge, blurred vision, vision loss | Corneal infiltrates with feathery edges, culture of corneal scrapings or vitreal fluid |
| Toxoplasma23, 24 | Retina | Pain, photophobia, scotoma, blurred vision, vision loss | Raised, yellow/white, cottony retinal lesions in a non-vascular distribution and vitreal inflammation, serum PCR or serology, PCR of aqueous humor, vitreal fluid and tissue |
| Virus | |||
| Cytomegalovirus25-28 | Retina | May be asymptomatic early, visual loss, floaters, progressive visual loss | Dilated pupil examination showing creamcolored lesions with hemorrhage and perivascular cuffing of retinal vessels, PCR of vitreous fluid |
| Herpes simplex virus29 Varicella zoster virus30, 31 |
Conjunctiva, cornea | May be asymptomatic, periorbital pain or rash, conjunctival erythema, blurred vision, vision loss | Swelling and cloudiness of cornea on ophthalmologic exam, PCR, DFA from base of skin lesion scrapings, viral culture |
| Adenovirus32, 41, 97 | Conjunctiva, cornea | Erythema, chemosis, discharge | Culture, PCR |
PCR, polymerase chain reaction; DFA, direct fluorescent assay.
Incidence and risk factors
The incidence of ocular infections has decreased over time with advances in prophylaxis, screening guidelines and effective medications.19, 20 In a large series of 620 patients undergoing allogeneic HCT, 18 (3%) had serious ocular infections due to Gram-positive and negative bacteria (1.6%), fungi (0.16%), and virus (0.8%).21 Candida and Aspergillus species are the most frequently isolated organisms in fungal keratitis and endophthalmitis.22 The most common ocular protozoan infection is Toxoplasma gondii, although ocular toxoplasmosis is rare (0.3-0.5% of autologous and allogeneic HCT recipients).23, 24 Viruses that cause ocular infection after HCT include cytomegalovirus (CMV),25-28 herpes simplex virus (HSV),29 varicella zoster virus (VZV),30, 31 and adenovirus (ADV).32 The most common virus that affects the eyes is CMV, with the incidence of retinitis ranging from <1% to 5% in all HCT recipients, and from 5% to 23% in patients with CMV viremia.25-28 Ocular infections by HSV, VZV and ADV are rare.29-31 In one study, ocular infection accounted for 2% of all VZV infections.30
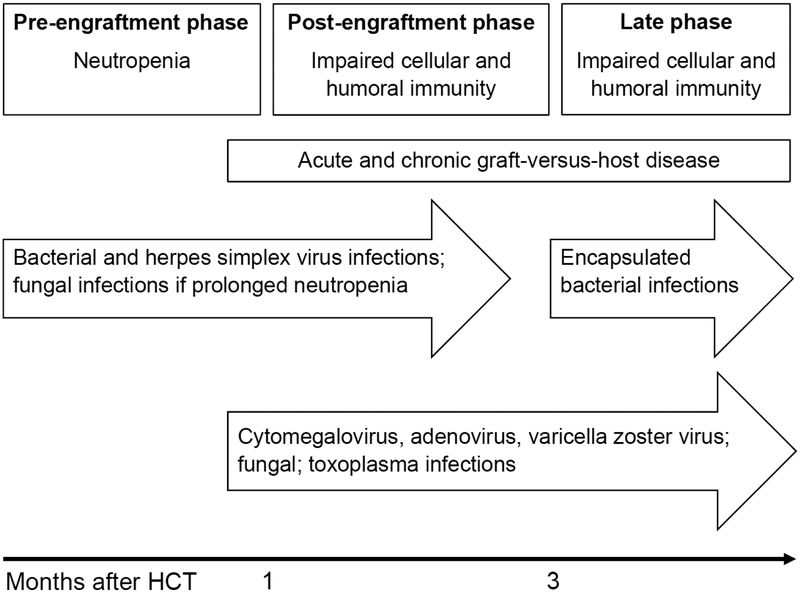
Since ocular infections are uncommon, risk factors are often extrapolated from those for infections at other sites and include pre-HCT latent infections and post-HCT neutropenia, impaired cellular and humoral immunity and development of GVHD (Figure 2).19, 21 Patients with neutropenia or humoral deficiency are susceptible to developing bacterial infections, including corneal ulcers and periorbital cellulitis.21 Risk factors for ocular fungal infection include fungemia and immunosuppression.33 Risk factors for ocular toxoplasmosis include toxoplasma infection before HCT and impaired cellular immunity.23 Risk factors for viral infections include positive serostatus before HCT, history of viremia before HCT, low lymphocyte counts, chronic GVHD, HLA mismatching, and unrelated donor.25-27, 30 Candidates for HCT should undergo serologic testing for latent viral infections and toxoplasmosis.19, 20
Figure 2.
Ocular infections after allogeneic hematopoietic cell transplantation
Screening, prevention and treatment
Eye examination should be performed before HCT and at any time when patients have ocular symptoms after HCT. Early detection and prompt treatment are required to prevent visual loss. Prevention and treatment of ocular infections is similar to that of other systemic infections except where ocular penetration of the therapeutic agent is poor.
Bacterial infections are treated with intravenous antibiotics based on culture and sensitivity results. Topical antibiotics are also used for bacterial keratitis.34 Oral or intravenous antifungal therapy is considered for patients with ocular fungal infection depending on the causative pathogen. Intravitreal administration can be performed with most antifungal agents, including amphotericin B, echinocandins and azoles, as penetration into eyes may not be sufficient with intravenous administration.35 To treat fungal keratitis, antifungal agents such as voriconazole can be used topically.36 There are no randomized trials comparing the efficacy of different prophylactic agents to prevent toxoplasmosis after HCT, although observational studies have demonstrated efficacy of oral trimethoprim-sulfamethoxazole from engraftment until immunosuppressive therapy is discontinued.37 Treatment of active toxoplasmosis consists of oral antimicrobial agents such as trimethoprim-sulfamethoxazole, pyrimethamine/sulfadiazine, clindamycin, atovaquone, or azithromycin.38
Systemic therapy (e.g. intravenous ganciclovir, oral valganciclovir and intravenous foscarnet) should be used for treatment of CMV ocular diseases.28 When patients do not respond sufficiently to systemic therapy, intravitreous therapy is considered.28 Third-party donor-derived CMV pp65-specific T-cells have resulted in resolution of refractory CMV retinitis.39 Prophylactic oral acyclovir has reduced the risk of HSV infection.40 A combination of systemic therapy and topical therapies is recommended for treatment of HSV ocular infection. Intravenous cidofovir is recommended for treatment of ADV infection.41
Ocular involvement by malignancy
Incidence and diagnosis
There is very limited information regarding ocular manifestations of malignancy after HCT. The incidence of ocular involvement by malignancy is rare. Relapsed disease after HCT may involve eyes, either de novo or in association with central nervous system or systemic relapse.42, 43
Clinical presentations of intraocular neoplasms after HCT can include the anterior and posterior segments and orbital manifestations, amongst others. Despite the infrequent incidence of de novo or recurrent ocular neoplasms, a high index of suspicion necessitates proper clinical assessment and evaluation by an experienced ophthalmologist.44 Computed tomography or magnetic resonance imaging may be helpful. Cytology, immunohistochemistry, flow cytometry, cytogenetics and molecular studies on aspirated fluid or tissue biopsy are necessary for definitive diagnosis.44-46
Screening, prevention and treatment
No evidence is available for screening and prevention of malignancies with ocular involvement. As a general recommendation, ocular evaluation may be necessary in patients with relapsed primary disease and ocular symptoms, in those with previous ocular involvement, and in those with new malignancy involving the central nervous system. Treatment approaches are extrapolated from literature on treatment of intraocular neoplasms in general and are not validated in HCT settings. These include intravitreal chemotherapy, radiation, photodynamic laser therapy, and disease-specific interventions based on site and histology of neoplasm.47-49
Ischemic microvascular retinopathy
Incidence and risk factors
Ischemic microvascular retinopathy (IMR) after HCT is a complication of the posterior segment that was first described in 1983.50 IMR presents with retinal cotton-wool patches, vitreous hemorrhage and optic disc edema. The clinical presentation varies from asymptomatic to sight-threatening forms. Symptomatic patients often complain of blurred vision or color vision abnormality which can occur abruptly, gradually or progressively. One or both eyes can be affected.
There are at least 8 cohort studies examining complications of the posterior segment after HCT (supplementary Table S3).21, 22, 51-56 IMR has been reported after both autologous and allogeneic HCT, and the incidence of IMR ranges from 0% to 10%,21, 22, 51-56 although the incidence is likely to be underestimated due to the asymptomatic presentation in many patients. IMR usually occurs within 6 months of allogeneic HCT,52 although atypically late onset around 50 months after allogeneic HCT has been described in 4 patients.54 Potential risk factors for IMR include TBI conditioning,22, 52, 54 cyclosporine prophylaxis,22, 52 and conditioning regimens including busulfan or carmustine.53, 54
Pathogenesis
Although the pathogenesis of HCT-associated IMR has not yet been elucidated, multifactorial processes are likely to cause capillary damage in the ocular fundus. IMR after HCT is morphologically similar to fundus changes associated with malignant hypertension in the general population,57 but most HCT patients with IMR do not have malignant hypertension, suggesting a different pathogenesis. Although radiation has been associated with vascular retinopathy,58 IMR also occurs among adult patients who have had autologous HCT using non-TBI conditioning regimens.53 In an experimental rat model using retinal imaging, the combination of cyclosporine and dexamethasone led to progressive degenerative changes of the fundus with histologic thinning of the outer nuclear layer of the retina, suggesting that calcineurin inhibitors may contribute to ocular vascular endothelial injury.59
Screening, prevention and treatment
Data are lacking for the management of IMR after HCT. Based on the potential risk factors, it is important to avoid radiation to ocular areas, to monitor levels of calcineurin inhibitors, and to treat cardiovascular risk factors such as hypertension, diabetes, and hyperlipidemia. Importantly, spontaneous regression is frequent and permanent loss of visual acuity is rare. Withdrawal or reduction of immunosuppression can lead to resolution of the retinal lesions in many cases,60 although proliferative retinopathy may have a poor visual prognosis.61
Future research should focus on the biology of IMR and identification of risk factors. Novel findings in retinopathy due to diabetes and sickle cell disease are worth investigating in HCT patients with IMR. For example, new sensitive technologies of ultra-wide field fluorescein angiography, spectral-domain optical coherence tomography and optical coherence tomography angiography may help diagnosis.62, 63 Vascular endothelial growth factor (VEGF) may serve as a diagnostic biomarker and a treatment target, since tissue ischemia increases VEGF that can promote abnormal neovascularization.64
Other ocular complications
Glaucoma
Glaucoma was diagnosed in 1.7% of HCT survivors, but this frequency was similar to 1.9% in their siblings.65 In a study of 218 patients with chronic GVHD, 33 (15%) had increased intraocular pressure, 8 (3.6%) had suspicion of glaucoma, and 1 (0.4%) had glaucoma.66 Classically, glaucoma is known as a late complication of irradiation used in conditioning regimens, with a median interval of 22 months to onset of glaucoma.67 Long-term use of systemic corticosteroids for chronic GVHD can elevate intraocular pressure in susceptible patients.66, 68, 69 The risk of early intraocular pressure rise and amount of elevation are greater in children than adults.70 Intraocular pressure may be elevated even after topical steroid use such as eye drops, ointment and inhalation. No correlation has been demonstrated between stem cell source and glaucoma.21
Patients who are receiving systemic or topical steroids should have periodic monitoring of intraocular pressure. A rise in intraocular pressure is often transient and resolves within 4 weeks of tapering or cessation of systemic steroid therapy.66 If steroid use exceeds 18 months, the intraocular pressure may be permanently elevated. First-line treatment of glaucoma is topical aqueous humor suppressants, including beta-blockers, alpha 2 agonists, or carbonic anhydrase inhibitors.71, 72 For the 1-5% of patients with intractable glaucoma not adequately responsive to eye drop treatment, glaucoma surgery with trabeculectomy or other technique may be necessary to prevent vision loss.73 Laser trabeculoplasty has shown efficacy in small retrospective studies.74
Central retinal vein occlusion (CRVO)
CRVO is a vision-threatening retinal vascular disease, with an incidence of 0.2%-0.4% in the general population.75 The pathophysiology of CRVO is not yet fully understood, but systemic diseases including hypertension, hyperlipidemia and diabetes mellitus, hypercoagulability and hyperviscosity may be associated with its pathophysiology. There are several reports of CRVO in patients with hematologic malignancies, but only two cases of CRVO have been reported at 2 and 5 months after HCT.76
The central retinal vein is compressed in patients with CRVO by the adjacent central retinal artery at the lamina cribrosa, leading to increased venous and capillary pressures, endothelial damage, permeability changes, and extravasation of blood and serous fluid in the retina. Capillary perfusion is reduced due to increased interstitial pressures, causing tissue ischemia. Interleukin-6 and VEGF are secreted in response to the ischemia, which promotes retinal edema, neovascularization and other complications including vitreous hemorrhage, tractional retinal detachment, iris neovascularization and neovascular glaucoma.77
Intravitreal injections of anti-VEGF agents are the first-line treatment for symptomatic macular edema due to CRVO, and have shown significant reduction in central retinal thickness from macular edema, significant improvement in best corrected visual acuity and a delay in development of neovascular complications in randomized studies.78, 79 Intravitreal steroid injections also have improved macular edema after CRVO in a randomized study.80 A retrospective study showed that macular grid and panretinal laser photocoagulation are also effective for CRVO,81 and these treatments are considered as second line or in cases with extensively ischemic retina.
Retinal hemorrhage and retinal detachment
Retinal hemorrhage and detachment usually occur as a consequence of other pathologies such as CMV retinitis and neovascularization due to ischemic retinopathy. A retrospective study of 635 patients who had allogeneic HCT showed that the most common posterior segment complication after allogeneic HCT was retinal hemorrhage (3.2%).76 Standard treatments include platelet transfusion to maintain the platelet count over 50,000/μl and correction of coagulopathy.
Retinal detachment is a rare posterior segment complication and accounts for <1% of ocular complications after HCT.22, 76 It can be seen in chronic GVHD, CMV retinitis, and rarely in acute GVHD. Most of the cases manifest as rhegmatogenous retinal detachment,76, 82 but 2 cases of vision-threatening exudative bullous retinal detachment have been reported in the literature.83 Tractional retinal detachment due to proliferative retinopathy has also been reported after HCT.84 In one study, retinal detachment occurred in 13% patients with CMV retinitis, and the visual prognosis was often poor.76 Treatment depends on underlying disease and includes laser photocoagulation, cryotherapy, and various surgical techniques.
Medications with ocular toxicities
A variety of medications can cause ocular toxicities after HCT (Table 3). The most commonly reported ocular toxicity of anti-cancer drugs is keratoconjunctivitis. Systemic corticosteroids accelerate cataract formation13, 14, 85 and glaucoma development.66 Topical corticosteroids are commonly used for treatment of ocular GVHD, but their long-term use is associated with elevated intraocular pressure, decreased epithelial healing, infection and cataracts.18 High-potency steroid eye drops should be avoided, and intraocular pressure should be monitored in patients who continue steroid eye drops for more than 2 weeks.66 Topical use of tacrolimus and cyclosporine for treatment of ocular GVHD can cause conjunctival injection, burning and stinging sensation, which may be controlled with lubricating eye drops.86-88 Oral or intravenous voriconazole can cause blurred vision, changes in visual acuity and color perception, photophobia and visual hallucinations.89 Other medications such as oral antihistamines90 (e.g., diphenhydramine, loratadine) and oral anticholinergics91 (e.g., sedatives, sleep aids, cold preparations, antidiarrheal and nasal decongestants) can inhibit lacrimal glandular secretions and cause dry eyes. Scopolamine patches used as antiemetic after high-dose chemotherapy can lead to anisocoria or mydriasis if the eye is contaminated with scopolamine.92 Oral selective serotonin reuptake inhibitors can result in reduced accommodation.93 Oral antipsychotics including phenothiazines such as haloperidol, chlorpromazine and thioridazine can cause mydriasis, cycloplegia,93 and pigmentation of conjunctiva, cornea, eyelids and retina with their long-term use.94 Oral sildenafil used for erectile dysfunction after HCT can cause a blue tinge to vision or an increased brightness of lights.95 Topical nonsteroidal anti-inflammatory eye drops can cause stromal necrosis in dry eyes, and should be avoided in patients with ocular GVHD.96
Table 3.
Medications with ocular toxicities
| Drug | Ocular toxicity | Frequency | Management and prevention recommendation |
Strength |
|---|---|---|---|---|
| Cytosine arabinoside | Keratoconjunctivitis98 | Common | Prophylactic glucocorticoid eye drops,98 2-deoxycytidine eye drops applied prior to therapy98 | A-II |
| Ocular pain, tearing, foreign body sensation, photophobia, epiphora, blurred vision with evidence of bilateral conjunctival hyperemia and fine corneal punctate opacities98 | Occasional | Eye washing with physiologic saline, 0.1% sodium betamethasone eye drops applied prior to therapy | B-III | |
| Imatinib | Peri-orbital edema, sometimes leading to visual obstruction99 | Common | Warm compress | A-III |
| Corticosteroids (systemic) | Cataracts,13, 14, 85 increased IOP, glaucoma66, 68, 69 | Common | Limit use | A-III |
| Screening for cataracts and glaucoma | C-III | |||
| Corticosteroids (topical) | Increased IOP, glaucomatous optic atrophy, visual field defects, cataracts, infectious keratitis | Occasional to rare | Monitor IOP within 1 month of initiating treatment, restrict extended use of high potency steroids | A-III |
| Regular screening for cataracts, surgery as needed18 | B-III | |||
| Discontinue steroids,66 topical aqueous humor suppressants,71, 72 trabeculectomy73 | A-I | |||
| Cyclosporine (systemic) | Microvascular retinopathy60 | Rare | Improve with drug discontinuation60 | A-II |
| Cyclosporine (topical) | Redness, stinging, burning | Occasional | Lubricating drops,88 chill bottle87 before instilling cyclosporine | A-II |
| Tacrolimus (topical) | Burning sensation | Common | Symptoms typically improve in 1 month after drug discontinuation.86 | A-II |
| Phenothiazines(chlorpromazine, thioridazine) | Abnormal pigmentation of the eyelids, interpalpebral conjunctiva and cornea with long-term use94 | Common | Improve with drug discontinuation | B-III |
| Decreased accommodation and deposits in the lens | Common | Topical cholinergic agent | B-III | |
| Tricyclic antidepressants (amitriptyline) | Mydriasis, cycloplegia, blurred vision, presbyopia, mild and transient visual disturbances93 | Common | Improve with time as tolerance develops.93 Topical cholinergic agents (bethanechol or pilocarpine) | B-II |
| Decreased lacrimation, dry eyes, glaucoma attacks (anticholinergic) | Rare to common | Artificial tears, avoid in patients with narrow angles93 | B-II | |
| Selective serotonin reuptake inhibitors (sertraline, paroxetine, fluoxetine, citalopram) | Mydriasis, reduced accommodation, increased IOP, glaucoma,93 blurred vision | Common | Improve with drug discontinuation | B-III |
| Antihistamines (chlorpheniramine, meclizine, promethazine, diphenhydramine) | Mydriasis (anticholinergic)90, dry eyes, decreased accommodation | Common | Transient, reversible | B-III |
| Angle closure glaucoma (anticholinergic) | Rare | Avoid in patients with angle closure glaucoma | B-III | |
| Scopolamine patch | Anisocoria, mydriasis, dry eyes92 | Common | Avoid rubbing eyes with fingers after application of the patch92 | A-II |
| Voriconazole | Blurred vision, changes in visual acuity, color perception, photophobia, visual hallucinations89 | Common | Consider alternative drugs | A-III |
| Erectile dysfunction drugs(e.g., sildenafil) | Blue vision95 | Common | Improve with drug discontinuation | B-II |
| Non-steroidal anti-inflammatory drugs (topical) | Redness, stinging, corneal epithelial toxicity, stromal necrosis (corneal melts) especially in dry eyes | Occasional | Limit use for dry eye or GVHD, drug discontinuation96 | B-II |
IOP, intraocular pressure; GVHD, graft-versus-host disease.
Summary and future recommendations
The incidence, risk factors, screening and prevention recommendations for non-GVHD ocular complications after HCT are summarized in Table 4. Treatment recommendations are summarized in Table 5. Although non-GVHD ocular complications after HCT are rare, baseline ocular evaluation before HCT should be considered in all patients who undergo HCT. Follow-up ocular evaluations should be considered according to clinical findings and risk factors. Better preventive strategies and treatments remain to be investigated for individual ocular complications after HCT, particularly in high-risk patients. There is significant overlap in the clinical presentation between infectious and non-infectious ocular complications and better diagnostic techniques need to be developed. There remains a paucity of data on the long-term visual outcomes, particularly in pediatric patients, as most studies have short follow up of less than 2 years. Better data collection is necessary to address these remaining questions. Both transplant physicians and ophthalmologists should be knowledgeable about non-GVHD ocular complications and provide a comprehensive collaborative team care.
Table 4.
Incidence, risk factor, screening and prevention recommendations of non-GVHD ocular complications after HCT
| Complication | Incidence | Risk factor | Prevention and screening recommendation (strength) |
|---|---|---|---|
| Cataracts | 11%-100% in adults,5, 6 4%-76% in children7, 8 | TBI (particularly single dose or dose rate>0.04 Gy/min),9, 10 corticosteroids,13-15 allogeneic HCT10 | Hyper-fractionated TBI instead of single-dose TBI9 (A-I), non-TBI conditioning12 (B-II), lens shielding11 (C-II) |
| Bacterial infection | <2%21 | Neutropenia,21 impaired humoral immunity21 | By clinical presentation (A-II) |
| Fungal infection | <2%21, 22 | Fungemia33, immunosuppression33 | By clinical presentation or fungemia33 (A-III) |
| Toxoplasma infection | 0.3-0.5%23, 24 | Pre-HCT infection,23 impaired cellular immunity23 | Pre-HCT screening examination for retina scar and serologic testing37 (B-III), by clinical presentation |
| Viral infection | |||
| Cytomegalovirus (CMV) | Retinitis <1%-5% in all HCT patients, 5%-23% in patients with CMV viremia25-28 | Positive serostatus,25 viremia pre-HCT,27 reactivation by day+100,25 high peak DNA levels,26 delayed lymphocyte engraftment,25 chronic GVHD,25, 27 HLA mismatching,26, 27 unrelated donor26 | Screening fundiscopic examination in patients at risk every 6-8 weeks (B-III) |
| Herpes simplex virus (HSV), | Rare,29, 31 2% of VZV infection30 | Extensive chronic GVHD30 | By clinical presentation (A-III), prophylactic acyclovir40 (A-I) |
| Varicella zoster virus (VZV) | |||
| Adenovirus | Rare | No data | PCR surveillance for systemic reactivation in high risk patients (B-III) |
| Involvement with malignancy | Rare | No data | Evaluation of ocular involvement in patients with relapsed primary disease and ocular symptoms42, 43 (C-III), and in asymptomatic high-risk patients (previous ocular involvement, new malignancy involving central nervous system)44 (C-III) |
| Ischemic microvascular retinopathy | 0-10%21, 22, 51-56 | TBI, 22, 52, 54 cyclosporine,22, 52 conditioning regimens including busulfan or carmustine53, 54 | Avoid radiation to ocular areas, monitor levels of calcineurin inhibitors (B-III) |
| Glaucoma | 0.4-1.7%65, 66 | Irradiation,67 topical or systemic corticosteroids66, 68, 69 | Monitor intraocular pressure in patients with risk factors (A-II) |
| Central retinal vein occlusion | Rare | Hypertension, hyperlipidemia, diabetes mellitus, hypercoagulability, hyperviscosity | Funduscopic testing if visual acuity impairment (A-III) |
| Retinal hemorrhage | 3.2%76 | Cytopenia, particularly thrombocytopenia | Funduscopic testing if visual acuity impairment (A-III) |
| Retinal detachment | <1%.27, 76 | CMV retinitis76 | Funduscopic testing if visual acuity impairment (A-III) |
TBI, total body irradiation; HCT, hematopoietic cell transplantation; GVHD, graft-versus-host disease.
Table 5.
Treatment recommendations of non-GVHD ocular complications after HCT
| Complication | Treatment recommendation | Strength |
|---|---|---|
| Cataracts | Surgery17 | A-II |
| Bacterial infection | Antibiotic eye drops,34 oral or intravenous antibiotic agents, intravitreal injections | A-I |
| Fungal infection | Antifungal eye drops,36 oral or intravenous antifungal agents, intravitreal injections35 | A-I |
| Toxoplasma infection | Trimethoprim-sulfamethoxazole-pyrimethamine, clindamycin, atovaquone, azithromycin38 | A-I |
| Viral infection | ||
| Cytomegalovirus | Intravenous ganciclovir, oral valganciclovir, intravenous foscarnet28 | A-II |
| Intravitreal ganciclovir or foscarnet for resistant infections28 | B-II | |
| Virus-specific T-cell infusion39 | C-II | |
| Herpes simplex virus | Oral acyclovir/valacyclovir/famciclovir, intravenous acyclovir | A-II |
| Varicella zoster virus | Oral acyclovir/valacyclovir/famciclovir, intravenous acyclovir for opthalmicus | A-II |
| Adenovirus | Decrease immunosuppression, intravenous cidofovir41 | A-II |
| Involvement with malignancy | Intravitreal chemotherapy, radiation therapy, photodynamic laser therapy, disease-specific interventions based on site and histology of neoplasm47-49 | C-III |
| Ischemic microvascular retinopathy | Reduction or withdrawal of calcineurin inhibitors,60 treatment for hypertension, GVHD and transplant-associated microangiopathy | B-III |
| Glaucoma | Discontinue steroids,66 topical aqueous humor suppressants,71, 72 trabeculectomy73 | A-I |
| Central retinal vein occlusion | Intravitreal anti-VEGF injections78, 79 | A-I |
| Intravitreal glucocorticoids80 | B-I | |
| Macular grid and panretinal laser photocoagulation81 | C-II | |
| Retinal hemorrhage | Maintain the platelet count over 50,000/μl | B-III |
| Retinal detachment | Photocoagulation, cryotherapy and surgical techniques | B-III |
VEGF, vascular endothelial growth factor.
Highlights.
Non-GVHD ocular complications are reviewed by expert transplant physicians and ophthalmologists.
Cataracts, ischemic microvascular retinopathy and CMV retinitis are relatively common.
A variety of medications can cause ocular toxicities after HCT.
Baseline ocular evaluation before HCT should be considered in all patients who undergo HCT.
Better preventive strategies and treatments remain to be investigated.
Acknowledgments
Financial disclosure: The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; * Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. - Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
* Corporate Members
Footnotes
Conflict-of-interest statement: There are no conflicts of interest to discloset.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Socie G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–85. [DOI] [PubMed] [Google Scholar]
- 2.Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battiwalla M, Hashmi S, Majhail N, Pavletic S, Savani BN, Shelburne N. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: Developing Recommendations to Improve Survivorship and Long-Term Outcomes. Biol Blood Marrow Transplant. 2017;23:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couriel D, Carpenter PA, Cutler C, Bolanos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–96. [DOI] [PubMed] [Google Scholar]
- 5.Dunn JP, Jabs DA, Wingard J, Enger C, Vogelsang G, Santos G. Bone marrow transplantation and cataract development. Arch Ophthalmol. 1993;111:1367–73. [DOI] [PubMed] [Google Scholar]
- 6.Tichelli A, Gratwohl A, Egger T, Roth J, Prunte A, Nissen C, et al. Cataract formation after bone marrow transplantation. Ann Intern Med. 1993;119:1175–80. [DOI] [PubMed] [Google Scholar]
- 7.Kinori M, Bielorai B, Souroujon D, Hutt D, Ben-Bassat Mizrachi I, Huna-Baron R. Ocular complications in children after hematopoietic stem cell transplantation without total body irradiation. Graefes Arch Clin Exp Ophthalmol. 2015;253:1397–402. [DOI] [PubMed] [Google Scholar]
- 8.Frisk P, Hagberg H, Mandahl A, Soderberg P, Lonnerholm G. Cataracts after autologous bone marrow transplantation in children. Acta Paediatr. 2000;89:814–9. [PubMed] [Google Scholar]
- 9.Benyunes MC, Sullivan KM, Deeg HJ, Mori M, Meyer W, Fisher L, et al. Cataracts after bone marrow transplantation: long-term follow-up of adults treated with fractionated total body irradiation. Int J Radiat Oncol Biol Phys. 1995;32:661–70. [DOI] [PubMed] [Google Scholar]
- 10.Belkacemi Y, Labopin M, Vernant JP, Prentice HG, Tichelli A, Schattenberg A, et al. Cataracts after total body irradiation and bone marrow transplantation in patients with acute leukemia in complete remission: a study of the European Group for Blood and Marrow Transplantation. Int J Radiat Oncol Biol Phys. 1998;41:659–68. [DOI] [PubMed] [Google Scholar]
- 11.van Kempen-Harteveld ML, van Weel-Sipman MH, Emmens C, Noordijk EM, van der Tweel I, Revesz T, et al. Eye shielding during total body irradiation for bone marrow transplantation in children transplanted for a hematological disorder: risks and benefits. Bone Marrow Transplant. 2003;31:1151–6. [DOI] [PubMed] [Google Scholar]
- 12.Fahnehjelm KT, Tornquist AL, Olsson M, Winiarski J. Visual outcome and cataract development after allogeneic stem-cell transplantation in children. Acta Ophthalmol Scand. 2007;85:724–33. [DOI] [PubMed] [Google Scholar]
- 13.van Kempen-Harteveld ML, Struikmans H, Kal HB, van der Tweel I, Mourits MP, Verdonck LF, et al. Cataract-free interval and severity of cataract after total body irradiation and bone marrow transplantation: influence of treatment parameters. Int J Radiat Oncol Biol Phys. 2000;48:807–15. [DOI] [PubMed] [Google Scholar]
- 14.Najima Y, Kakihana K, Ohashi K, Yamamoto N, Kobayashi T, Yamashita T, et al. Incidence, risk factors, and clinical outcomes of cataracts following hematopoietic stem cell transplantation. Am J Hematol. 2011;86:508–10. [DOI] [PubMed] [Google Scholar]
- 15.Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337:8–14. [DOI] [PubMed] [Google Scholar]
- 16.Bernard F, Auquier P, Herrmann I, Contet A, Poiree M, Demeocq F, et al. Health status of childhood leukemia survivors who received hematopoietic cell transplantation after BU or TBI: an LEA study. Bone Marrow Transplant. 2014;49:709–16. [DOI] [PubMed] [Google Scholar]
- 17.Tear Fahnehjelm K, Tomquist AL, Olsson M, Backstrom I, Andersson Gronlund M, Winiarski J. Cataract after allogeneic hematopoietic stem cell transplantation in childhood. Acta Paediatr. 2016;105:82–9. [DOI] [PubMed] [Google Scholar]
- 18.de Melo Franco R, Kron-Gray MM, De la Parra-Colin P, He Y, Musch DC, Mian SI, et al. Outcomes of cataract surgery in graft-versus-host disease. Cornea. 2015;34:506–11. [DOI] [PubMed] [Google Scholar]
- 19.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullmann AJ, Schmidt-Hieber M, Bertz H, Heinz WJ, Kiehl M, Kruger W, et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95:1435–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabbara KF, Al-Ghamdi A, Al-Mohareb F, Ayas M, Chaudhri N, Al-Sharif F, et al. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology. 2009; 116:1624–9. [DOI] [PubMed] [Google Scholar]
- 22.Coskuncan NM, Jabs DA, Dunn JP, Haller JA, Green WR, Vogelsang GB, et al. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol. 1994;112:372–9. [DOI] [PubMed] [Google Scholar]
- 23.Sumi M, Aosai F, Norose K, Takeda W, Kirihara T, Sato K, et al. Acute exacerbation of Toxoplasma gondii infection after hematopoietic stem cell transplantation: five case reports among 279 recipients. Int J Hematol. 2013;98:214–22. [DOI] [PubMed] [Google Scholar]
- 24.Decembrino N, Comelli A, Genco F, Vitullo A, Recupero S, Zecca M, et al. Toxoplasmosis disease in paediatric hematopoietic stem cell transplantation: do not forget it still exists. Bone Marrow Transplant. 2017;52:1326–9. [DOI] [PubMed] [Google Scholar]
- 25.Crippa F, Corey L, Chuang EL, Sale G, Boeckh M. Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32:214–9. [DOI] [PubMed] [Google Scholar]
- 26.Jeon S, Lee WK, Lee Y, Lee DG, Lee JW. Risk factors for cytomegalovirus retinitis in patients with cytomegalovirus viremia after hematopoietic stem cell transplantation. Ophthalmology. 2012;119:1892–8. [DOI] [PubMed] [Google Scholar]
- 27.Hiwarkar P, Gajdosova E, Qasim W, Worth A, Breuer J, Chiesa R, et al. Frequent occurrence of cytomegalovirus retinitis during immune reconstitution warrants regular ophthalmic screening in high-risk pediatric allogeneic hematopoietic stem cell transplant recipients. Clin Infect Dis. 2014;58:1700–6. [DOI] [PubMed] [Google Scholar]
- 28.Larochelle MB, Phan R, Craddock J, Abzug MJ, Curtis D, Robinson CC, et al. Cytomegalovirus Retinitis in Pediatric Stem Cell Transplants: Report of a Recent Cluster and the Development of a Screening Protocol. Am J Ophthalmol. 2017;175:8–15. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Scieux C, Garrait V, Socie G, Rocha V, Molina JM, et al. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin Infect Dis. 2000;31:927–35. [DOI] [PubMed] [Google Scholar]
- 30.Koc Y, Miller KB, Schenkein DP, Griffith J, Akhtar M, DesJardin J, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6:44–9. [DOI] [PubMed] [Google Scholar]
- 31.Walton RC, Reed KL. Herpes zoster ophthalmicus following bone marrow transplantation in children. Bone Marrow Transplant. 1999;23:1317–20. [DOI] [PubMed] [Google Scholar]
- 32.Castleton A, Kottaridis PD. A case of 'red eye' post allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:241–2. [DOI] [PubMed] [Google Scholar]
- 33.Donahue SP, Greven CM, Zuravleff JJ, Eller AW, Nguyen MH, Peacock JE Jr., et al. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophthalmology. 1994;101:1302–9. [DOI] [PubMed] [Google Scholar]
- 34.Shah VM, Tandon R, Satpathy G, Nayak N, Chawla B, Agarwal T, et al. Randomized clinical study for comparative evaluation of fourth-generation fluoroquinolones with the combination of fortified antibiotics in the treatment of bacterial corneal ulcers. Cornea. 2010;29:751–7. [DOI] [PubMed] [Google Scholar]
- 35.Kramer M, Kramer MR, Blau H, Bishara J, Axer-Siegel R, Weinberger D. Intravitreal voriconazole for the treatment of endogenous Aspergillus endophthalmitis. Ophthalmology. 2006;113:1184–6. [DOI] [PubMed] [Google Scholar]
- 36.Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martino R, Bretagne S, Einsele H, Maertens J, Ullmann AJ, Parody R, et al. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin Infect Dis. 2005;40:67–78. [DOI] [PubMed] [Google Scholar]
- 38.Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MS, van Ruyven RL, Klok AM, Hoyng CB, et al. A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol. 2002;134:34–40. [DOI] [PubMed] [Google Scholar]
- 39.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saral R, Burns WH, Laskin OL, Santos GW, Lietman PS. Acyclovir prophylaxis of herpes-simplex-virus infections. N Engl J Med. 1981;305:63–7. [DOI] [PubMed] [Google Scholar]
- 41.Matthes-Martin S, Feuchtinger T, Shaw PJ, Engelhard D, Hirsch HH, Cordonnier C, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis. 2012;14:555–63. [DOI] [PubMed] [Google Scholar]
- 42.Green W, Rao PK, Harocopos GJ. Extramedullary Relapse of Acute Myelogenous Leukemia Presenting as a Large Serous Retinal Detachment. Ocul Oncol Pathol. 2017;3:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuo T, Tanaka T, Ichimura K, Meguri Y. Intraocular Relapse with Hypopyon and Retinal Infiltrates after Chemotherapy and Peripheral Blood Stem Cell Transplantation for Extranodal NK/T-Cell Lymphoma. J Clin Exp Hematop. 2015;55:157–61. [DOI] [PubMed] [Google Scholar]
- 44.Rothova A, Ooijman F, Kerkhoff F, Van Der Lelij A, Lokhorst HM. Uveitis masquerade syndromes. Ophthalmology. 2001;108:386–99. [DOI] [PubMed] [Google Scholar]
- 45.Cohen VM, Dinakaran S, Parsons MA, Rennie IG. Transvitreal fine needle aspiration biopsy: the influence of intraocular lesion size on diagnostic biopsy result. Eye (Lond). 2001;15:143–7. [DOI] [PubMed] [Google Scholar]
- 46.Hormigo A, Abrey L, Heinemann MH, DeAngelis LM. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol. 2004;126:202–8. [DOI] [PubMed] [Google Scholar]
- 47.Smith JR, Rosenbaum JT, Wilson DJ, Doolittle ND, Siegal T, Neuwelt EA, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709–16. [DOI] [PubMed] [Google Scholar]
- 48.Wiegel T, Bottke D, Kreusel KM, Schmidt S, Bornfeld N, Foerster MH, et al. External beam radiotherapy of choroidal metastases--final results of a prospective study of the German Cancer Society (ARO 95-08). Radiother Oncol. 2002;64:13–8. [DOI] [PubMed] [Google Scholar]
- 49.Kaliki S, Shields CL, Al-Dahmash SA, Mashayekhi A, Shields JA. Photodynamic therapy for choroidal metastasis in 8 cases. Ophthalmology. 2012;119:1218–22. [DOI] [PubMed] [Google Scholar]
- 50.Gratwohl A, Gloor B, Hahn H, Speck B. Retinal cotton-wool patches in bone-marrow-transplant recipients. N Engl J Med. 1983;308:1101. [DOI] [PubMed] [Google Scholar]
- 51.Hirst LW, Jabs DA, Tutschka PJ, Green WR, Santos GW. The eye in bone marrow transplantation. I.Clinical study. Arch Ophthalmol. 1983;101:580–4. [DOI] [PubMed] [Google Scholar]
- 52.Bernauer W, Gratwohl A, Keller A, Daicker B. Microvasculopathy in the ocular fundus after bone marrow transplantation. Ann Intern Med. 1991;115:925–30. [DOI] [PubMed] [Google Scholar]
- 53.Johnson DW, Cagnoni PJ, Schossau TM, Stemmer SM, Grayeb DE, Baron AE, et al. Optic disc and retinal microvasculopathy after high-dose chemotherapy and autologous hematopoietic progenitor cell support. Bone Marrow Transplant. 1999;24:785–92. [DOI] [PubMed] [Google Scholar]
- 54.Bylsma GW, Hall AJ, Szer J, West R. Atypical retinal microvasculopathy after bone marrow transplantation. Clin Exp Ophthalmol. 2001;29:225–9. [DOI] [PubMed] [Google Scholar]
- 55.Westeneng AC, Hettinga Y, Lokhorst H, Verdonck L, van Dorp S, Rothova A. Ocular graft-versus-host disease after allogeneic stem cell transplantation. Cornea. 2010;29:758–63. [DOI] [PubMed] [Google Scholar]
- 56.Ivanir Y, Shimoni A, Ezra-Nimni O, Barequet IS. Prevalence of dry eye syndrome after allogeneic hematopoietic stem cell transplantation. Cornea. 2013;32:e97–101. [DOI] [PubMed] [Google Scholar]
- 57.Aissopou EK, Papathanassiou M, Nasothimiou EG, Konstantonis GD, Tentolouris N, Theodossiadis PG, et al. The Keith-Wagener-Barker and Mitchell-Wong grading systems for hypertensive retinopathy: association with target organ damage in individuals below 55 years. J Hypertens. 2015;33:2303–9. [DOI] [PubMed] [Google Scholar]
- 58.Kaushik M, Pulido JS, Schild SE, Stafford S. Risk of radiation retinopathy in patients with orbital and ocular lymphoma. Int J Radiat Oncol Biol Phys. 2012;84:1145–50. [DOI] [PubMed] [Google Scholar]
- 59.Cooper AE, Cho JH, Menges S, Masood S, Xie J, Yang J, et al. Immunosuppressive Treatment Can Alter Visual Performance in the Royal College of Surgeons Rat. J Ocul Pharmacol Ther. 2016;32:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avery R, Jabs DA, Wingard JR, Vogelsang G, Saral R, Santos G. Optic disc edema after bone marrow transplantation. Possible role of cyclosporine toxicity. Ophthalmology. 1991;98:1294–301. [DOI] [PubMed] [Google Scholar]
- 61.Lopez PF, Sternberg P Jr., Dabbs CK, Vogler WR, Crocker I, Kalin NS. Bone marrow transplant retinopathy. Am J Ophthalmol. 1991;112:635–46. [DOI] [PubMed] [Google Scholar]
- 62.Pahl DA, Green NS, Bhatia M, Lee MT, Chang JS, Licursi M, et al. Optical Coherence Tomography Angiography and Ultra-widefield Fluorescein Angiography for Early Detection of Adolescent Sickle Retinopathy. Am J Ophthalmol. 2017;183:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joltikov KA, de Castro VM, Davila JR, Anand R, Khan SM, Farbman N, et al. Multidimensional Functional and Structural Evaluation Reveals Neuroretinal Impairment in Early Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017;58:BIO277–BIO90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hillier RJ, Ojaimi E, Wong DT, Mak MYK, Berger AR, Kohly RP, et al. Aqueous Humor Cytokine Levels and Anatomic Response to Intravitreal Ranibizumab in Diabetic Macular Edema. JAMA Ophthalmol. 2018;136:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker KS, Ness KK, Weisdorf D, Francisco L, Sun CL, Forman S, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Leukemia. 2010;24:2039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saboo US, Amparo F, Shikari H, Dana R. Prevalence of ocular hypertension and glaucoma in patients with chronic ocular graft-versus-host disease. Graefes Arch Clin Exp Ophthalmol. 2016;254:923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda A, Shigematsu N, Suzuki S, Fujii M, Kawata T, Kawaguchi O, et al. Late retinal complications of radiation therapy for nasal and paranasal malignancies: relationship between irradiated-dose area and severity. Int J Radiat Oncol Biol Phys. 1999;44:599–605. [DOI] [PubMed] [Google Scholar]
- 68.Tatematsu Y, Ogawa Y, Abe T, Kamoi M, Uchino M, Saijo-Ban Y, et al. Grading criteria for chronic ocular graft-versus-host disease: comparing the NIH eye score, Japanese dry eye score, and DEWS 2007 score. Sci Rep. 2014;4:6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tripathi RC, Parapuram SK, Tripathi BJ, Zhong Y, Chalam KV. Corticosteroids and glaucoma risk. Drugs Aging. 1999;15:439–50. [DOI] [PubMed] [Google Scholar]
- 70.Kwok AK, Lam DS, Ng JS, Fan DS, Chew SJ, Tso MO. Ocular-hypertensive response to topical steroids in children. Ophthalmology. 1997;104:2112–6. [DOI] [PubMed] [Google Scholar]
- 71.Katz G, Dubiner H, Samples J, Vold S, Sall K. Three-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%. JAMA Ophthalmol. 2013;131:724–30. [DOI] [PubMed] [Google Scholar]
- 72.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S, Low-Pressure Glaucoma Study G. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671–81. [DOI] [PubMed] [Google Scholar]
- 73.Im L, Allingham RR, Singh I, Stinnett S, Fekrat S. A prospective study of early intraocular pressure changes after a single intravitreal triamcinolone injection. J Glaucoma. 2008;17:128–32. [DOI] [PubMed] [Google Scholar]
- 74.Rubin B, Taglienti A, Rothman RF, Marcus CH, Serle JB. The effect of selective laser trabeculoplasty on intraocular pressure in patients with intravitreal steroid-induced elevated intraocular pressure. J Glaucoma. 2008;17:287–92. [DOI] [PubMed] [Google Scholar]
- 75.Zhou JQ, Xu L, Wang S, Wang YX, You QS, Tu Y, et al. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing eye study. Ophthalmology. 2013;120:803–8. [DOI] [PubMed] [Google Scholar]
- 76.Yoo YS, Na KS, Shin JA, Park YH, Lee JW. Posterior Eye Segment Complications Related to Allogeneic Hematopoietic Stem Cell Transplantation. Retina. 2017;37:135–43. [DOI] [PubMed] [Google Scholar]
- 77.Campa C, Alivernini G, Bolletta E, Parodi MB, Perri P. Anti-VEGF Therapy for Retinal Vein Occlusions. Curr Drug Targets. 2016;17:328–36. [DOI] [PubMed] [Google Scholar]
- 78.Epstein DL, Algvere PV, von Wendt G, Seregard S, Kvanta A. Benefit from bevacizumab for macular edema in central retinal vein occlusion: twelve-month results of a prospective, randomized study. Ophthalmology. 2012;119:2587–91. [DOI] [PubMed] [Google Scholar]
- 79.Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119:802–9. [DOI] [PubMed] [Google Scholar]
- 80.Haller JA, Bandello F, Belfort R Jr., Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–60. [DOI] [PubMed] [Google Scholar]
- 81.Pikkel YY, Sharabi-Nov A, Beiran I, Pikkel J. Comparison of anti-vascular endothelial growth factors, laser treatments and a combination of the both for treatment of central retinal vein occlusion. Int J Ophthalmol. 2016;9:431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eid AJ, Bakri SJ, Kijpittayarit S, Razonable RR. Clinical features and outcomes of cytomegalovirus retinitis after transplantation. Transpl Infect Dis. 2008;10:13–8. [DOI] [PubMed] [Google Scholar]
- 83.Peng KL, Chen SJ, Lin PY, Hsu WM, Yang MH, Tzeng CH, et al. Exudative bullous retinal detachment after peripheral blood stem cell transplantation. Eye (Lond). 2005;19:603–6. [DOI] [PubMed] [Google Scholar]
- 84.Wiznia RA, Rose A, Levy AL. Occlusive microvascular retinopathy with optic disc and retinal neovascularization in acute lymphocytic leukemia. Retina. 1994;14:253–5. [DOI] [PubMed] [Google Scholar]
- 85.Alloin AL, Barlogis V, Auquier P, Contet A, Poiree M, Demeocq F, et al. Prevalence and risk factors of cataract after chemotherapy with or without central nervous system irradiation for childhood acute lymphoblastic leukaemia: an LEA study. Br J Haematol. 2014;164:94–100. [DOI] [PubMed] [Google Scholar]
- 86.Abud TB, Amparo F, Saboo US, Di Zazzo A, Dohlman TH, Ciolino JB, et al. A Clinical Trial Comparing the Safety and Efficacy of Topical Tacrolimus versus Methylprednisolone in Ocular Graft-versus-Host Disease. Ophthalmology. 2016;123:1449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28:1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dietrich-Ntoukas T, Cursiefen C, Westekemper H, Eberwein P, Reinhard T, Bertz H, et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss Consensus Conference on Clinical Practice in chronic GVHD. Cornea. 2012;31:299–310. [DOI] [PubMed] [Google Scholar]
- 89.Boucher HW, Groll AH, Chiou CC, Walsh TJ. Newer systemic antifungal agents : pharmacokinetics, safety and efficacy. Drugs. 2004;64:1997–2020. [DOI] [PubMed] [Google Scholar]
- 90.Slattery A, Liebelt E, Gaines LA. Common ocular effects reported to a poison control center after systemic absorption of drugs in therapeutic and toxic doses. Curr Opin Ophthalmol. 2014;25:519–23. [DOI] [PubMed] [Google Scholar]
- 91.Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications : recognition and management. Drugs. 2007;67:75–93. [DOI] [PubMed] [Google Scholar]
- 92.Jaanus SD. Ocular side effects of selected systemic drugs. Optom Clin. 1992;2:73–96. [PubMed] [Google Scholar]
- 93.Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24:501–26. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008;31:127–41. [DOI] [PubMed] [Google Scholar]
- 95.Laties AM, Fraunfelder FT. Ocular safety of Viagra, (sildenafil citrate). Trans Am Ophthalmol Soc. 1999;97:115–25; discussion 25-8. [PMC free article] [PubMed] [Google Scholar]
- 96.Gaynes BI, Fiscella R. Topical nonsteroidal anti-inflammatory drugs for ophthalmic use: a safety review. Drug Saf. 2002;25:233–50. [DOI] [PubMed] [Google Scholar]
- 97.Mynarek M, Ganzenmueller T, Mueller-Heine A, Mielke C, Gonnermann A, Beier R, et al. Patient, virus, and treatment-related risk factors in pediatric adenovirus infection after stem cell transplantation: results of a routine monitoring program. Biol Blood Marrow Transplant. 2014;20:250–6. [DOI] [PubMed] [Google Scholar]
- 98.Lass JH, Lazarus HM, Reed MD, Herzig RH. Topical corticosteroid therapy for corneal toxicity from systemically administered cytarabine. Am J Ophthalmol. 1982;94:617–21. [DOI] [PubMed] [Google Scholar]
- 99.Fraunfelder FW, Solomon J, Druker BJ, Esmaeli B, Kuyl J. Ocular side-effects associated with imatinib mesylate (Gleevec). J Ocul Pharmacol Ther. 2003;19:371–5. [DOI] [PubMed] [Google Scholar]