Abstract
Type 1 diabetes (T1D) is an autoimmune disease caused by immune-mediated pancreatic β-cell destruction. The endocrine disrupting chemical bisphenol A (BPA) has widespread human exposure and can modulate immune function and the gut microbiome (GMB), which may contribute to the increasing T1D incidence worldwide. It was hypothesized that BPA had sex-dependent effects on T1D by modulating immune homeostasis and GMB. Adult female and male non-obese diabetic (NOD) mice were orally administered BPA at environmentally relevant doses (30 or 300 μg/kg). Antibiotic-treated adult NOD females were exposed to 0 or 30 μg/kg BPA. BPA accelerated T1D development in females, but delayed males from T1D. Consistently, females had a shift towards pro-inflammation (e.g. increased macrophages and Bacteroidetes), while males had increases in anti-inflammatory immune factors and a decrease in both anti- and pro-inflammatory GMB. Although bacteria altered during sub-acute BPA exposure differed from bacteria altered from chronic BPA exposure in both sexes, the GMB profile was consistently pro-inflammatory in females, while males had a general decrease of both anti- and pro-inflammatory gut microbes. However, treatment of females with the antibiotic vancomycin failed to prevent BPA-induced glucose intolerance, suggesting changes in Gram-positive bacteria were not a primary mechanism. In conclusion, BPA exposure was found to have sex dimorphic effects on T1D with detrimental effects in females, and immunomodulation was identified as the primary mechanism.
Keywords: Bisphenol A, type 1 diabetes, NOD mice, immunomodulation, microbiome, vancomycin
Introduction
Bisphenol A (BPA), an endocrine disrupting chemical (EDC) used widely in a variety of polycarbonate plastics and epoxy resins (e.g., water bottles and the lining of food cans), has been identified in more than 90% of human urine samples (Lang et al. 2008). Worldwide, there has been a parallel increase in type 1 diabetes (T1D) and EDC exposure (Bergman et al. 2013; Pitkäniemi et al. 2004). While there are still uncertainties about the involvement of BPA in T1D risk, animal studies have shown that BPA can increase T1D and/or autoimmune responses in non-obese diabetic (NOD) female mice (Bodin et al. 2014; Bodin et al. 2013) and streptozotocin-treated C57BL/6 male mice (Cetkovic-Cvrlje et al. 2017). Furthermore, human studies demonstrate a correlation of increased diabetes with increased BPA exposure (Lang et al. 2008).
T1D, once afflicting mainly juveniles, is increasing in incidence among US adults with a 40% increased risk of death in women compared to men with T1D (Chiang et al. 2014; Huxley et al. 2015). Various studies have established the association between T1D and gut microbiome (GMB) (Brown et al. 2011; de Goffau et al. 2014). Immune function and development are influenced by GMB and the composition and localization of GMB are modulated by immunity (Belkaid and Hand 2014). Studies have shown that BPA can alter GMB and cause dysbiosis (Lai et al. 2016; Liu et al. 2016). However, whether alteration of GMB by BPA can increase disease risk for T1D has yet to be studied.
Differences in hormone levels in different sexes affect T1D susceptibility (Markle et al. 2013). Sex-biased changes from BPA exposure have been reported for human glucose homeostasis (Beydoun et al. 2014). NOD mice, a spontaneous T1D model that has similarities to human T1D, have also been known for their sex-dependent dysregulation of immune system and T1D development (Young et al. 2009). BPA has sex-related effects on sex hormone levels (Scinicariello and Buser 2016), and various hormones can regulate insulin secretion and metabolic function in T1D patients (Fu et al. 2013). The overall hypothesis of this study was that BPA would differentially affect T1D susceptibility depending on sex through alteration of immune responses in NOD mice in which changes in immune cell populations and cytokine/chemokine expressions lead to the destruction of pancreatic β-cells (Jörns et al. 2014). We have evaluated the effects of BPA on T1D development and the roles of the immune system and GMB using NOD mice exposed during adulthood. This is the first study to determine the role of BPA’s immunomodulatory mechanisms on T1D based on sex.
Materials and Methods
Animal Husbandry
Specific pathogen free (SPF) NOD mice were initially obtained from Taconic Biosciences (Hudson, NY). NOD mice were housed in polysulfone cages with irradiated laboratory animal bedding and Bed-r’Nest for enrichment (The Andersons Inc., Maumee, Ohio). Recent studies have reported that a negligible amount of BPA leaches from new or used polysulfone cages maintained at room temperature (Johnson et al. 2016). The animal room was maintained at 22–25°C with relative humidity 50±20 and 12-h light/dark cycle (7:00AM lights on). The mice had access to water and food ad libitum. Only mice that were receiving the same treatment were housed together to prevent coprophagy between groups. All procedures were conducted under an approved animal protocol by the University of Georgia Institutional Animal Care and Use Committee (IACUC). Animals were treated humanely with regard for alleviating suffering.
Bisphenol A Exposure
Exposure of NOD mice to BPA.
Mice (8–12 wks old; 10/group/sex) were randomized into vehicle (VH) or BPA groups, and initial body weight (BW) and blood glucose levels (BGLs) were not significantly different before treatment started. All mice fed with PicoLab diet (LabDiet, St. Louis, MO) containing ~ 237–655 ppm isoflavone (Huang et al. 2017) were dosed daily with VH, 30 or 300 μg BPA/kg BW by gavage. BPA was dissolved in 100% ethanol and added to corn oil at a final concentration of 0.05% ethanol. VH mice received the same volume of corn oil with 0.05% ethanol. The doses used in this study have previously been shown to alter the immune system (Yoshino et al. 2003); 30 μg/kg is within estimated human exposure levels, and 300 μg/kg is also relevant to human exposure based on median human blood levels (Calafat et al. 2005; Taylor et al. 2011). Females and males were euthanized at different time points (after 155 and 363 days of treatment, respectively) because female NOD mice developed T1D faster.
Exposure of NOD females to BPA in combination with antibiotics.
To determine the role of GMB alteration from BPA exposure, female NOD mice (10–12 wks old; 6/group) were randomized into VH or BPA groups according to BW and BGLs. They were dosed with either 0 or 30 μg BPA/kg BW as described above. Water bottles containing 0.5 g/L vancomycin were provided starting one week before dosing to ensure bacteria depletion and continuing throughout the experiment. Water bottles were changed once a week. This antibiotic and amount were chosen based on previous studies that determined their effects on GMB in female NOD mice (Hansen et al. 2012).
Measurement of BW, BGL And Diabetic Incidence
BW and non-fasting BGLs were measured every 1–2 weeks. BGLs were measured from a small sample of venous blood (tail nick) using Accu-Chek Diabetes monitoring kit (Roche Diagnostics, Indianapolis, IN) or Contour Blood Glucose Meter (Ascensia Diabetes Care, Parsippany, NJ). Mice were considered diabetic if 2 consecutive BGL measurements were ≥250 mg/dL. Mice were humanely euthanized using CO2 asphyxiation once a BGL ≥ 600 mg/dL was detected twice, or at the end of the study.
Tolerance Tests And Insulin Measurement
For the glucose tolerance test (GTT), mice were fasted overnight for 15 h and then injected with glucose (2 g/kg BW; i.p.; (Susiarjo et al. 2015)). For the insulin tolerance test (ITT), mice were injected with 1.5 IU/kg BW insulin (i.p.; (Cui et al. 2015)). Baseline BW and BGLs, and BGLs after injection at 15, 30, 60 and 120 minutes were measured. Glucose and insulin were obtained from Sigma. For insulin measurement, the Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem, Downers Grove, IL) was performed following manufacturer’s protocol.
Organ Collection, Flow Cytometry And Histopathology
At euthanization, pancreas, spleen, kidneys with adrenals, liver and thymus were removed and cleaned of connective tissue before weighing. Histopathology is included in the supplementary methods. For flow cytometry, the spleens were mashed in 3 mL PBS solution on ice. Leukocyte populations were characterized by flow cytometric analysis with different combinations of fluorochrome-labeled antibodies (diluted 1:80–1:100; BD PharMingen, San Diego, CA) including CD4-CD8-CD25 (PE-PerCP-FITC), CD40L-B220 (PE-FITC) and Mac3-Gr1 (PE-FITC) in females. For males, we used CD4-CD8-CD25 (PE-PerCP-FITC), CD40-LB220 (PE-FITC) and F4/80-Gr1 (PE-FITC). CD4-CD8-CD25-Mac3-CD45R (V450-APCH7-APCA-FITC-PE), CD40L-B220 (PE-FITC), CD5-CD24 (PE-FITC) and CD44-CD40 (PE-FITC) were used in antibiotic treated females. Isotype matched irrelevant antibodies were used as controls. Following addition of the antibodies, the cells were incubated at 4°C in the dark for 30 min. The cells were washed and enumeration performed on a Becton Dickinson LSRII Flow Cytometer (BD Biosciences, San Jose, CA) in which log fluorescence intensity was read and a forward scatter threshold high enough to eliminate red blood cells. Ten thousand cells were counted for each sample. Analysis was done using FlowJo software (FlowJo LLC).
Cytokine/chemokine Measurement
Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore, Billerica, MA) was used for measuring cytokines and chemokines from sera collected at euthanasia according to manufacturer protocols along with the quality controls provided in the kit. Bio-Plex MAGPIX™ Multiplex Reader with Bio-Plex Manager™ MP Software (Luminex, Austin, TX) was used to run the plates, and concentration was measured as pg/ml.
Antibody Measurement
ELISA kits (Bethyl Laboratories, Montgomery, TX) were used to measure IgG subclasses (IgG1, IgG2a, IgG2b) and IgM levels in sera obtained at euthanasia as previously described (Huang et al. 2017). IgG2a ELISA kits have been shown to be a measure of IgG2c in NOD mice (Martin et al. 1998). IgG2a and IgG2c are generally assumed identical in function (Collins 2016), so we have referred to IgG2a results as IgG2a/IgG2c. Plates were read using the Synergy 4 Hybrid Microplate Reader (BioTek, Winooski, VT) either at a wavelength of 405 nm or at 450 nm following adding 100 μL/well stop solution (2N sulfuric acid).
GMB, Bioinformatics And Metabolomics And Bioinformatics Analysis
Feces were collected from individual mice immediately after defecating and kept at −20°C until use. DNA was extracted using QIAamp DNA stool mini kits (Qiagen, Valencia, CA) following manufacturer protocols. For library preparation, DNA was normalized and the V3–V4 region of 16S rRNA was targeted using locus-specific primers for the first round of PCR. Illumina-specific iTru_R1_5’_fusion and iTru_R2_5’_fusion Read 1 and Read 2 sequencing primers (forward: 16S_341_F, and reverse: 16S_785_R) were used with 20 internal tags (8 forward fusion primers and 12 reverse fusion primers) ranging from 5 nucleotides (NT) to 8 NTs long (Glenn et al. 2016). For antibiotic treated females, the forward primer S-D-Bact-0564-a-S-15 and the reverse primer S-D-Bact-0785-a-A-21 were used. The PCR mix was from Kapa Biosystems, Inc. (Boston, MA). Next, the PCR amplicon aliquot was purified and quantified using Speedbeads and Qubit, respectively, or for the antibiotic treated females was purified with AMPure beads (Amplicon et al. 2013). The second round PCR was run using Illumina i5 and i7 primers, and sequencing was done on Illumina Miseq (Illumina Inc., San Diego, US). Bioinformatics analysis was performed as previously described (Lefever et al. 2016). Linear Discriminant Analysis (LDA) Effect Size (LEfSe) analysis was used to identify significantly different taxa following the conditions on the website: http://huttenhower.sph.harvard.edu/galaxy and increasing the LDA score to > 3 (Segata et al. 2011). Metabolomics analysis can be found in the supplementary methods.
Statistical Analysis
Likelihood ratio and Logrank test were performed to analyze the rate of T1D development and total T1D incidence over time, respectively. Correlational analysis was conducted using Spearman’s correlation test. Two-way ANOVA and Tukey’s test were used for sex × treatment interactions. For all other data sets, one-way ANOVA and Dunnett’s test (VH as the reference group) were used for comparisons when the equal variance assumption was met; otherwise, Wilcoxon test was performed to compare the means. A group was considered statistically significant if p < 0.05. JMP Pro 12 (SAS Inc., Cary, NC) and GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA) were used for statistical analysis and data visualization.
Results
Effect of BPA Exposure on BGLs and T1D Incidence.
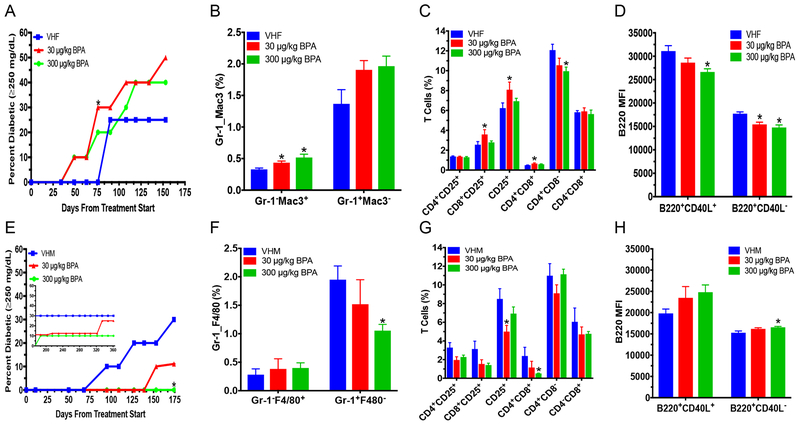
First, we determined BPA’s effect on BGLs and T1D outcome in NOD mice. Due to NOD males having a delayed T1D development compared to females, males were tested longer for T1D effects. BPA induced early onset of T1D in females with a significant change observed on day 76 after the initial dosing in the low dose group, although Logrank test analysis indicated total T1D incidence over time was not significantly altered (Fig. 1a). Consistently, increased BGLs in the time-course study were observed with a significant increase in the low dose group on day 76 in females; a similar increase, albeit not statistically significant (e.g. P = 0.07 at time 15 min for low dose), was produced in the day 119 (4 mo.) GTT, but no significant difference on day 84 serum insulin concentration was observed (Fig. S1a–c). Additionally, histopathology of the pancreas in females showed no difference in insulitis score (Table S1). Low dose non-diabetic female mice also had increased absolute spleen weight, and the high dose non-diabetic females had increased absolute kidney weight (Table S2).
Figure 1.
Diabetic incidence and flow cytometric analysis of spleen cells from adult NOD mice. T1D incidence in female (A) and (E) male mice. Blood glucose ≥250 mg/dL was considered diabetic. (B) % macrophages (Gr-1−Mac3+ or F4/80−Mac3+) and neutrophils (Gr-1+Mac3− or F4/80+Mac3−) in adult females (B) or males (F), respectively. % T cell populations in females (C) and males (G). B220 mean fluorescence intensity (MFI) in females (D) and males (H). The values are presented as mean ± SEM. *, p< 0.05 using Dunnett’s test or Wilcoxon based on whether equal variance assumption was met as compared to the respective vehicle (VH) control group. N = 6–10. VHF, vehicle females; and VHM, vehicle males
In contrast, BPA treated males at the high dose had a significant delay in T1D with a significant decrease in incidence compared to the control on day 174 (5 mo.), but not total diabetes incidence over time (Fig. 1e). No statistically significant effects on the BGLs in the time-course study or in GTTs were found, but the day 361 ITT showed the BGLs at 120 min and the area under the curve after insulin injection (Fig. S1d–h) in the low dose group was significantly increased. Additionally, both the absolute and % weights of spleen were increased by the low dose BPA in non-diabetic males (Table S3).
Effect of BPA on Immune Cells.
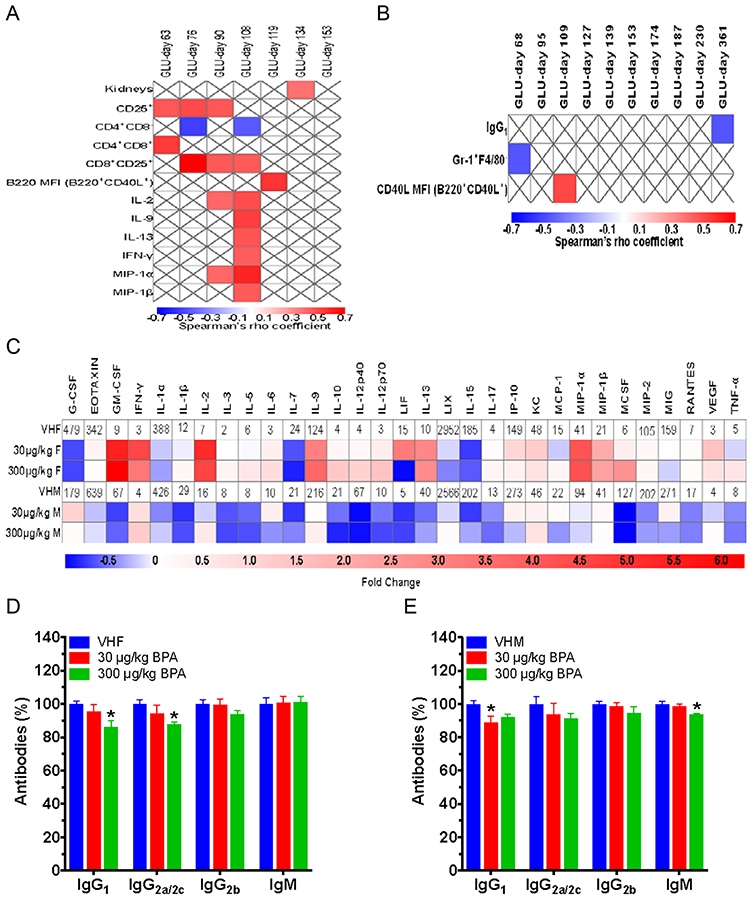
To determine the mechanisms of T1D modulation by BPA, splenic immune cells were analyzed. BPA exposed females had increased %Gr-1–Mac-3+ (macrophages) in both low and high dose groups, and %CD8+CD25+ cells, %CD4+CD8+ cells and %CD25+ cells in the low dose group (Fig. 1b–c). In contrast, %CD4+CD8– cells and B220 mean fluorescence intensity (MFI; expression levels of B220 on cells) of B220+CD40L+ and B220+CD40L– cells (Fig. 1c–d) were decreased by BPA. However, no changes were found for neutrophils (Gr-1+Mac-3–), %B cells or CD40L MFI in females (Fig. 1b and S2a–b). BGLs from days 63–119 were found to significantly correlate with immune endpoints, and BGLs from day 134 correlated with absolute kidney weight (Fig. 2a).
Figure 2.
Effect of BPA exposure on serum cytokine/chemokine and antibody levels, and their correlations with BGLs. Correlation between significant phenotypic endpoints and different timepoints of BGLs in females (A) and males (B) with red showing significantly positive and blue significantly negative correlation using Spearman correlation test (p < 0.05). Blank boxes with an X were not significantly different. (C) Heat map of serum cytokine/chemokine changes at time of euthanization for adult BPA exposed female (VHF) and male (VHM) NOD mice. Values of VHF and VHM are shown as mean pg/mL. (D) IgG1, IgG2a/2c, IgG2b and IgM in NOD females, which were measured at dilutions of 1:2,000, 1:50, 1:25 and 1:500, respectively, following titration by serial dilution. (E) IgG1, IgG2a/2c, IgG2b and IgM in NOD males, which were measured at dilutions of 1:1000, 1:50, 1:25 and 1:500, respectively, following titration by serial dilution. The values are presented as mean ± SEM. *, p< 0.05 using Dunnett’s test or Wilcoxon based on whether equal variance assumption was met as compared to the respective vehicle (VH) control group. N = 6–9
Unlike the females, immune cells in the spleen of males had significantly decreased neutrophils (Gr-1+F4/80–) in the high dose group, while macrophages were not significantly changed (Fig. 1f). As for splenic T cell populations, the low dose BPA decreased %CD25+ cells, whereas the high dose decreased %CD4+CD8+ cells. The %CD4+CD25+ cells were also decreased by the low dose, but this did not reach the level of statistical significance (p=0.053; Fig. 1g). The low dose group had a decrease in activated B cells (%B220+CD40L+), while in the high dose group, B220 MFI of naïve B cells (B220+CD40L−) was increased (Fig. S2c and 1h). Furthermore, both BPA treatment groups in males had increased CD40L MFI of B220+CD40L+ (Fig. S2d). BGLs on days 68 and 109 correlated with neutrophils and CD40L MFI of B220+CD40L+, respectively (Fig. 2b).
Effects of BPA on Cytokine/Chemokine and Antibody Levels.
Cytokine, chemokine and antibody levels in sera were measured to establish if the alterations in immune cells resulted in pro-inflammatory or anti-inflammatory changes. In general, the cytokines/chemokines were increased in females and decreased in males by BPA showing a sex-dependent alteration (Fig. 2c). In the high dose female group, significant increases included IL-2, IL-9, IL-13, granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, macrophage inflammatory protein (MIP)-1α and MIP-1β; however, decreased IL-7 was observed as compared to the VH control (Table S4). In the low dose female group, significant increases included IL-2, IL-9 and IP-10. In males, the high dose BPA significantly decreased pro-inflammatory cytokines IL-1β and IL-5, and the low dose BPA also decreased IL-1β compared to the VH control (Table S4). As for antibody changes, high dose females had decreased IgG1 and IgG2a/IgG2c, while males had decreased IgG1 and IgM levels in the low and high dose groups, respectively (Fig. 2d–e).
BPA Alteration of GMB.
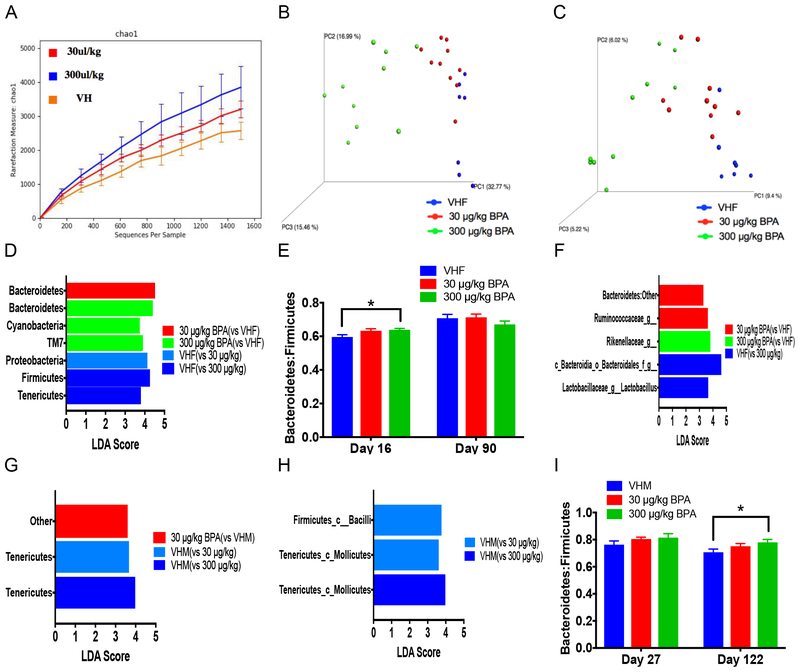
Next, BPA alteration of GMB composition was determined as a possible mechanism of immunomodulation and T1D development. Sub-acute (16 days) BPA exposure significantly altered the alpha diversity between all groups and the weighted and unweighted beta diversity in the high dose groups when compared to the VH (Fig. 3a–c). At the phylum level, both BPA doses increased Bacteroidetes, and the high dose increased Cyanobacteria and TM7. In contrast, Proteobacteria was decreased from the low dose, and Firmicutes and Tenericutes were decreased from the high dose (Fig. 3d; Fig. S3a). The Bacteroidetes to firmicutes (B:F) ratio was increased in the high dose from sub-acute exposure (Fig. 3e). Bacteria significantly altered at the class level from both BPA doses including increased Bacteroidia and decreased Betaproteobacteria. At the genus level, Bacteroidales (Other), Odoribacter and unclassified bacteria from Rikenellacaea were increased, and Bacteroides, Clostridiales (Other), Ruminococcaceae (Other), Sutterella and Turicibacter were decreased (Fig. S3b–c). Additionally, these bacteria correlated with significant phenotypic endpoints (e.g. macrophages) and with day 63 BGLs (Fig. S4).
Figure 3.
Gut microbiome composition based on 16S rRNA sequencing in female (A-F) and male (G-I) mice after sub-acute and chronic exposure. The alpha diversity (chao1; A), weighted (B) and unweighted (C) UniFrac beta diversity from sub-acute exposure in females. (D) LEfse results of gut microbiome shown at the phylum level from female sub-acute exposure. (E) Female bacteroidetes to firmicutes (B:F) ratio alteration after sub-acute (16 days) and chronic exposure (90 days). (F) LEfse results for the genus level from female chronic exposure. LEfse results of gut microbiome shown at the phylum (G) and class (H) levels from male sub-acute exposure. (I) B:F ratio alteration after sub-acute (27 days) and chronic exposure (122 days) in males. N = 6–10. VHF, vehicle females; VHM, vehicle males
After chronic BPA exposure (90 days), the B:F ratio was no longer significant (Fig. 3e), and no significant differences were found at the phylum level (Fig. S5a). However, the low dose NOD females had significantly increased Bacteroidetes (listed as Bacteroidetes:Other) at the class and genus levels and unclassified bacteria Ruminococcaceae at the genus level when compared to the VH control (Fig. 3f; Fig. S5b). At the genus level in the high dose, unclassified bacteria from Rikenellacaea was increased, while unclassified bacteria from Bacteroidales and Lactobacillus were decreased when compared to VH control (Fig. 3f). No significant difference was seen in alpha or beta diversity in BPA exposed females (Fig. S5c–e).
As for males after sub-acute exposure (27 days), bacteria classified as other at the phylum level was significantly increased from the low dose, while Tenericutes was significantly decreased from both BPA doses (Fig. 3g; Fig. S6a). At the class level, Bacilli was decreased from the low dose, and Mollicutes was decreased by both BPA doses (Fig. 3h). At the genus level, the high dose increased Odoribacter and unclassified Campylobacterales and decreased Anaeroplasma and Camplybacter, while Anaeroplasma, unclassified RF32 and Lactobacillus were decreased from the low dose (Fig. S6b). The weighted beta diversity was significantly different between the low dose and VH, but no significant effect was found for the alpha diversity or unweighted beta diversity (Fig. S6c–e).
In males after chronic BPA treatment (122 days), bacteria at the phylum level was not significantly altered, but the B:F ratio was significantly increased (Fig. 3i; Fig. S7a). The various bacteria significantly altered at the class level in the high dose group included increases in Bacteroidia and decreases in Erysipelotrichi, Verrucomicrobiae, YS2 and Bacilli compared to the VH group (Fig. S7b). At the genus level, the high dose BPA increased Bacteroides and decreased Akkermansia, Ruminococcus, Betaproteobacteria listed as other, unclassified bacteria from YS2 and unclassified bacteria from Erysipelotrichaceae (from the class Bacilli) compared to VH control. Additionally, both doses significantly reduced abundances of unclassified bacteria from Lachnospiraceae and Erysipelotrichaceae (from the class Erysipelotrichi) when compared to the VH (Fig. S7c). No significant difference was found in alpha or weighted beta diversity, but unweighted beta diversity was significantly different between the low dose and VH (Fig. S7d–f). Several bacteria were found to correlate with changes in spleen weight, cytokines and immune cells, and with different timepoints of BGLs (Fig. S8).
Analysis of Urine Metabolic Profiles.
BPA’s effect on the metabolites in urine at 4 mo. after initial dose was next determined. Our results showed that BPA did not significantly alter the global urinary metabolomics profile compared to control in either females or males (Fig. S9).
Effects of BPA in Antibiotic Treated Females.
Our data in Figure 3 suggested that BPA increased unclassified Ruminococcaceae. To further examine the role of GMB in T1D exacerbation from BPA exposure, female mice were exposed to BPA in combination with vancomycin since vancomycin decreases microbes from Bacteroidetes and Firmicutes, including unclassified Ruminococcaceae (Hansen et al. 2012). No statistically significant difference was observed in T1D incidence and little effect on the time course of BGLs; however, the 3 mo. GTT and ITT (day 100 and 103, respectively) showed increased BGLs (Fig. 4a–b and Fig. S10a–b). Additionally, %macrophages (Mac3+CD45R−), %CD5−CD24+, %CD40+CD44+ and %CD40−CD44+ splenic immune cells were increased, while no changes were observed in %T cell populations (Fig. 4c–d and Fig. S10c–d). Macrophages also positively correlated with BGLs on days 96 (Spearman ρ = 0.63; P = 0.03) and 106 (Spearman ρ = 0.61; P = 0.04). BPA exposure in antibiotic treated mice increased several bacteria (e.g. Actinobacteria) compared to the antibiotic treated VH control at the phylum, class and genus levels that were not altered in females without antibiotics (Fig. 4e–f and Fig. S11a–b). No significant difference was observed in alpha or beta diversity (Fig. S11c–e).
Figure 4.
Glucose tolerance test (GTT), insulin tolerance test (ITT), splenocyte differentials and microbiota alterations in BPA exposed female NOD mice treated with antibiotics. GTT (A) and ITT (B) of non-diabetic adult females were performed at 3 mo. after initial dose. % macrophages (C; Mac3+CD45R−) and % T cells (D) were measured using flow cytometric analysis. Phylum level taxonomy of gut microbiome excluding bacteria less than 0.05% (E) and LEfse results (F) are shown. The values are presented as mean ± SEM. *, p < 0.05 using Dunnett’s test or Wilcoxon based on whether equal variance assumption was met as compared to the respective vehicle (VH) control group. N = 4–6. VHF, vehicle females
Discussion
Currently, there are no epidemiological studies correlating BPA exposure with T1D and only a few animal studies have been published concerning BPA’s impact on T1D risk (Xu et al. 2016). This is the first study to determine the role of BPA alteration of GMB in T1D pathogenesis and examine immunity after chronic BPA exposure. Although total T1D incidence was not increased as further evidenced from no significant difference in the insulitis score at the end of study, exposure to BPA in female NOD mice accelerated T1D development with a greater effect in the low dose group. It was possible that insulitis was higher in this group during early BPA exposure (i.e. 76 days). Similarly, the only other study to determine T1D alteration after BPA exposure during adulthood in NOD mice did not find a significant difference in total T1D incidence, but saw increased insulitis at 8 weeks of treatment from their low dose (about 150 μg/kg; (Bodin et al. 2013)). This previous study and our results showing accelerated T1D development and increased BGLs at day 76 indicate that low dose BPA exposure during adulthood has detrimental effects to accelerate T1D development. While it is still uncertain why low dose BPA exposure can have a greater effect than higher doses, this non-monotonic dose effect of BPA has been previously described in different tissues including pancreas, and possibly from low dose activation of ER-extranuclear signaling pathways (Nadal et al. 2017).
Changes seen in immune cells (e.g. an increase in macrophages) corroborated the finding of accelerated T1D incidence from BPA exposed females. We also found decreased CD4+CD8− cells, which might be the Th2 phenotype that has been previously indicated to decrease in female NOD mice with an increased amount of inflammation (Young et al. 2009). CD8+CD25+ cells decreased CD4+CD8− cell proliferation and might be a mechanism for the decreased CD4+CD8− levels found (Bisikirska et al. 2005). Additionally, autoreactive cells that contributed to autoimmune diseases including CD4+CD8+ T cells and B cells with low B220 expression (Abo et al. 2012) were increased by BPA. Activated B cells could increase inflammation and activate autoreactive T cells to accelerate T1D development (Leeth et al. 2016). We also found that BPA altered B cell antibody production. BPA-exposed females had decreases in IgG1, a Th2 supporting antibody, and IgG2a/IgG2c, which delayed T1D in NOD mice. These decreases could also promote a pro-inflammatory response by increasing Th1 cytokines including IFN-γ (Kaplan et al. 2001; Todd et al. 1998).
This immunomodulation towards a pro-inflammatory state in females was further confirmed by the increase of several pro-inflammatory cytokines/chemokines that could exacerbate T1D, including IFN-γ (Barthson et al. 2011), GM-CSF (Alnek et al. 2015), IP-10 (Devaraj et al. 2011), MIP-1α (Cardozo et al. 2003), MIP-1β (Codella et al. 2015) and IL-9 (Ryba-Stanislawowska et al. 2016). We found many of these changes in the T cell populations (e.g. CD4+CD8+ cells) significantly correlated with BGLs on or before the day T1D was significantly accelerated in the low dose group. On the other hand, many of the cytokines/chemokine and B220 MFI of B220+CD40L+ correlated with BGLs after the significant effect in T1D from BPA exposure was observed. This suggests BPA’s alteration of T cell populations were important for initiating the acceleration of T1D and should be further investigated in future experiments. BPA’s immunomodulation towards pro-inflammation was further suggested as having a primary role in accelerating T1D by the increased macrophages in both BPA exposed females with and without antibiotics.
Sub-acute BPA exposure in female mice resulted in an overall pro-inflammatory GMB profile. The increased Bacteroidetes from sub-acute BPA exposure characterizes preclinical T1D and is involved in the progression of β-cell autoimmunity, which usually coincides with a decrease in Firmicutes in T1D patients compared to controls (Knip and Siljander 2016). Consistent with our results, Bacteroidetes and Cyanobacteria have been found increased, while Firmicutes, Tenericutes and Proteobacteria were decreased in T1D patients compared to controls and in NOD mice compared to NOD mice on an anti-diabetogenic diet (Brown et al. 2011; Hansen et al. 2014). Conversely, TM7, which was increased from high dose BPA, was previously found increased in NOD mice receiving an anti-diabetogenic diet (Hansen et al. 2014). Chronically BPA exposed female’s GMB continued to be pro-inflammatory (e.g. decreased T1D protecting Bacteroidales and Lactobacillus; see Supplemental Material, Supplementary Discussion; (Krych et al. 2015)). However, BGLs did not significantly correlate with GMB except a few bacteria altered from sub-acute exposure on day 63. Since females receiving antibiotics still had increased BGLs from BPA exposure in the tolerance tests with a different set of bacteria altered from BPA exposure, the later GMB changes were unlikely to be the primary contributor of the accelerated T1D development. The increased B:F ratio, BGL correlations and clear separation in diversity between the control and high dose BPA treated females after sub-acute exposure suggests that early changes in GMB drove the changes in the immune system and led to an accelerated T1D.
As for the males, BPA exposure protected them from T1D development as demonstrated by a decreased T1D incidence in mice treated with the high dose of BPA. This was also seen from immune cell changes including decreased neutrophils (Guo et al. 2014) along with increased B cells with high B220 expression indicating less autoreactivity (Abo et al. 2012). Males given the high BPA dose also had decreased IgM, which indicated that there were less activated B cells. BPA modulation towards anti-inflammation in high dose males was further confirmed from the overall decrease of cytokines/chemokines. Specifically, the pro-inflammatory IL-1β, which exacerbates T1D (Tuller et al. 2013), was decreased by BPA. On the other hand, while IL-1β was also decreased in low dose males, no effect was observed in T1D incidence; long term low dose BPA treatment resulted in insulin resistance and decreased Th2 supporting IgG1.
The GMB profile after sub-acute BPA exposure in males showed decreased Tenericutes, which was similar to what was found in females, and was inconsistent with the delayed T1D in males. However, at the class level, decreased Bacilli was consistent with our T1D results (de Goffau et al. 2014). Currently, there is a lack of information for the effect of bacteria labeled as other and Mollicutes on T1D. The male GMB changes following chronic BPA exposure were a mix of protective (e.g. decreased Lachnospiraceae; (Krych et al. 2015)) and exacerbating effects (e.g. decreased Betaproteobacteria; (Guo et al. 2016)). These results, together with the increased B:F ratio in chronically high dose exposed males, metabolomics and female results indicated that the later alterations in the GMB were not driving the changes in T1D incidence. Additionally, other studies that have examined the GMB changes share little commonalities with this study and each other on what bacteria BPA alters (Javurek et al. 2016; Lai et al. 2016; Malaisé et al. 2017). This could be from differences in timepoints used or other study parameters, and further suggests BPA impacts on later GMB may be from secondary effects.
BPA doses in this study are relevant for human exposure since the current US EPA reference dose is 50 μg/kg/day. Other studies in mice have found 250 μg/kg and 400 μg/kg doses of BPA provide environmentally relevant levels of total and unconjugated serum BPA that are within the range reported in humans (Rubin et al. 2017; Taylor et al. 2011). However, future studies using a wider range of BPA doses, fecal transfer and/or a phytoestrogen-free diet would help elucidate the causal relationship of GMB, immunity and T1D modulation from BPA exposure. More time points examining the effects of BPA on the GMB, fecal metabolites, cytokine/chemokine and antibody levels will benefit further studies in examining the immune and GMB changes leading to T1D pathogenesis. Additional studies also are needed to determine the cause of these sex-related differences (see Supplemental Material, Supplementary Discussion).
Conclusions
In summary, this study has shown sex plays an important role in BPA altering T1D risk. BPA accelerated T1D development in adult NOD females, but delayed male mice from T1D development. The alteration of immune homeostasis was found to be the most likely mechanism of altering T1D risk. BPA’s promotion of autoimmunity related to T1D should be further examined with more mechanistic experiments, so biomarkers of risk factors for early T1D development from BPA exposure can be determined. Thus, the potential health consequences from BPA and other EDC exposure on T1D should further be evaluated in adult females of different species and mouse strains.
Supplementary Material
Acknowledgements
The authors would like to thank Daniel E. Lefever, Dr. Travis Glenn and his lab members, and the Georgia Genomics and Bioinformatics Core of UGA for their help with the 16S rRNA library preparation, sequencing and bioinformatics analysis, and CVM Cytometry Core Facility (the College of Veterinary Medicine, UGA) for assisting flow cytometric analysis. This study was supported by NIH R21ES24487, and in part by NIH R41AT009523 and Interdisciplinary Toxicology Program at University of Georgia (UGA).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: “All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.”
References
- Abo T, Tomiyama C, Watanabe H (2012) Biology of autoreactive extrathymic T cells and B-1 cells of the innate immune system Immunologic research 52:224–230 [DOI] [PubMed] [Google Scholar]
- Alnek K, Kisand K, Heilman K, Peet A, Varik K, Uibo R (2015) Increased blood levels of growth factors, proinflammatory cytokines, and Th17 cytokines in patients with newly diagnosed type 1 diabetes PloS one 10:e0142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amplicon P, Clean‐Up P, Index P (2013) 16S Metagenomic Sequencing Library Preparation.
- Barthson J et al. (2011) Cytokines tumor necrosis factor-α and interferon-γ induce pancreatic β-cell apoptosis through STAT1-mediated Bim protein activation Journal of Biological Chemistry 286:39632–39643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation Cell 157:121–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman A et al. (2013) The impact of endocrine disruption: a consensus statement on the state of the science Environ Health Perspect 121:A104–106 doi: 10.1289/ehp.1205448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun HA, Khanal S, Zonderman AB, Beydoun MA (2014) Sex differences in the association of urinary bisphenol-A concentration with selected indices of glucose homeostasis among US adults Annals of epidemiology 24:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC (2005) TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+ CD25+ Tregs The Journal of clinical investigation 115:2904–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin J, Bolling AK, Becher R, Kuper F, Lovik M, Nygaard UC (2014) Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice Toxicol Sci 137:311–323 doi: 10.1093/toxsci/kft242 [DOI] [PubMed] [Google Scholar]
- Bodin J, Bolling AK, Samuelsen M, Becher R, Lovik M, Nygaard UC (2013) Long-term bisphenol A exposure accelerates insulitis development in diabetes-prone NOD mice Immunopharmacol Immunotoxicol 35:349–358 doi: 10.3109/08923973.2013.772195 [DOI] [PubMed] [Google Scholar]
- Brown CT et al. (2011) Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes PloS one 6:e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL (2005) Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population Environmental health perspectives:391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL (2003) IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice Diabetologia 46:255–266 doi: 10.1007/s00125-002-1017-0 [DOI] [PubMed] [Google Scholar]
- Cetkovic-Cvrlje M, Thinamany S, Bruner KA (2017) Bisphenol A (BPA) aggravates multiple low-dose streptozotocin-induced Type 1 diabetes in C57BL/6 mice Journal of immunotoxicology 14:160–168 [DOI] [PubMed] [Google Scholar]
- Chiang JL, Kirkman MS, Laffel LM, Peters AL (2014) Type 1 diabetes through the life span: a position statement of the American Diabetes Association Diabetes care 37:2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codella R et al. (2015) Moderate Intensity Training Impact on the Inflammatory Status and Glycemic Profiles in NOD Mice Journal of diabetes research 2015:737586 doi: 10.1155/2015/737586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AM (2016) IgG subclass co‐expression brings harmony to the quartet model of murine IgG function Immunology and cell biology 94:949–954 [DOI] [PubMed] [Google Scholar]
- Cui X-B, Luan J-N, Ye J, Chen S-Y (2015) RGC32 deficiency protects against high-fat diet-induced obesity and insulin resistance in mice Journal of Endocrinology 224:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goffau MC et al. (2014) Aberrant gut microbiota composition at the onset of type 1 diabetes in young children Diabetologia 57:1569–1577 [DOI] [PubMed] [Google Scholar]
- Devaraj S, Tobias P, Jialal I (2011) Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes Cytokine 55:441–445 [DOI] [PubMed] [Google Scholar]
- Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes Current diabetes reviews 9:25–53 [PMC free article] [PubMed] [Google Scholar]
- Glenn TC et al. (2016) Adapterama I: universal stubs and primers for thousands of dual-indexed Illumina libraries (iTru & iNext) BioRxiv:049114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Wang Y, Xiong T, Ling X, Zheng J (2014) Genistein modulation of streptozotocin diabetes in male B6C3F1 mice can be induced by diet Toxicology and applied pharmacology 280:455–466 doi: 10.1016/j.taap.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Liu CQ, Shan CX, Chen Y, Li HH, Huang ZP, Zou DJ (2016) Gut microbiota after Roux‐en‐Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes Diabetes/Metabolism Research and Reviews [DOI] [PubMed] [Google Scholar]
- Hansen CH et al. (2014) A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice Diabetes:DB_131612 [DOI] [PubMed] [Google Scholar]
- Hansen CHF et al. (2012) Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse Diabetologia 55:2285–2294 [DOI] [PubMed] [Google Scholar]
- Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, Guo TL (2017) Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis Toxicology and applied pharmacology doi: 10.1016/j.taap.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley RR, Peters SA, Mishra GD, Woodward M (2015) Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis The lancet Diabetes & endocrinology 3:198–206 doi: 10.1016/s2213-8587(14)70248-7 [DOI] [PubMed] [Google Scholar]
- Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA, Rosenfeld CS (2016) Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model Gut Microbes 7:471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA et al. (2016) Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: A CLARITY-BPA study Hormones and behavior 80:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörns A et al. (2014) Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW. 1AR1-iddm rat and humans with type 1 diabetes Diabetologia 57:512–521 [DOI] [PubMed] [Google Scholar]
- Kaplan C, Valdez JC, Chandrasekaran R, Eibel H, Mikecz K, Glant TT, Finnegan A (2001) Th1 and Th2 cytokines regulate proteoglycan-specific autoantibody isotypes and arthritis Arthritis Research & Therapy 4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knip M, Siljander H (2016) The role of the intestinal microbiota in type 1 diabetes mellitus Nature Reviews Endocrinology 12:154. [DOI] [PubMed] [Google Scholar]
- Krych Ł, Nielsen DS, Hansen AK, Hansen CHF (2015) Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN-γ level in NOD Mice Gut microbes 6:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K-P, Chung Y-T, Li R, Wan H-T, Wong CK-C (2016) Bisphenol A alters gut microbiome: Comparative metagenomics analysis Environmental Pollution 218:923–930 [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults Jama 300:1303–1310 [DOI] [PubMed] [Google Scholar]
- Leeth CM et al. (2016) B-Lymphocytes Expressing an Ig Specificity Recognizing the Pancreatic β-Cell Autoantigen Peripherin Are Potent Contributors to Type 1 Diabetes Development in NOD Mice Diabetes 65:1977–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever DE, Xu J, Chen Y, Huang G, Tamas N, Guo TL (2016) TCDD modulation of gut microbiome correlated with liver and immune toxicity in streptozotocin (STZ)-induced hyperglycemic mice Toxicology and applied pharmacology 304:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yao Y, Li H, Qiao F, Wu J, Du Z-y, Zhang M (2016) Influence of Endogenous and Exogenous Estrogenic Endocrine on Intestinal Microbiota in Zebrafish PloS one 11:e0163895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisé Y et al. (2017) Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to Bisphenol A precede obese phenotype development Scientific reports 7:14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG et al. (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity Science 339:1084–1088 [DOI] [PubMed] [Google Scholar]
- Martin RM, Brady JL, Lew AM (1998) The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice Journal of immunological methods 212:187–192 [DOI] [PubMed] [Google Scholar]
- Nadal A et al. (2017) Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus? The Journal of steroid biochemistry and molecular biology doi: 10.1016/j.jsbmb.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Pitkäniemi J, Onkamo P, Tuomilehto J, Arjas E (2004) Increasing incidence of Type 1 diabetes–role for genes? BMC genetics 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Paranjpe M, DaFonte T, Schaeberle C, Soto AM, Obin M, Greenberg AS (2017) Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: The addition of peripubertal exposure exacerbates adverse effects in female mice Reproductive Toxicology 68:130–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba-Stanislawowska M, Werner P, Brandt A, Mysliwiec M, Mysliwska J (2016) Th9 and Th22 immune response in young patients with type 1 diabetes Immunologic research 64:730–735 doi: 10.1007/s12026-015-8765-7 [DOI] [PubMed] [Google Scholar]
- Scinicariello F, Buser MC (2016) Serum testosterone concentrations and urinary bisphenol A, benzophenone-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011–2012 Environmental Health Perspectives 124:1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation Genome biology 12:R60 doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, Bartolomei MS (2015) Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse Endocrinology 156:2049–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA et al. (2011) Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure Environmental health perspectives 119:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd I, Davenport C, Topping JH, Wood PJ (1998) IgG2a antibodies non-specifically delay the onset of diabetes in NOD mice Autoimmunity 27:209–211 [DOI] [PubMed] [Google Scholar]
- Tuller T, Atar S, Ruppin E, Gurevich M, Achiron A (2013) Common and specific signatures of gene expression and protein–protein interactions in autoimmune diseases Genes and immunity 14:67–82 [DOI] [PubMed] [Google Scholar]
- Xu J, Huang G, Guo TL (2016) Developmental Bisphenol A Exposure Modulates Immune-Related Diseases Toxics 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y (2003) Effects of bisphenol A on antigen‐specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice British journal of pharmacology 138:1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EF, Hess PR, Arnold LW, Tisch R, Frelinger JA (2009) Islet lymphocyte subsets in male and female NOD mice are qualitatively similar but quantitatively distinct Autoimmunity 42:678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.