Abstract
Background
Quinolinic acid (QA), a neuroactive metabolite of the Kynurenine Pathway (KP), is an excitotoxin that is implicated in the pathogenesis of many neurological disorders. KP is the main tryptophan degradation pathway. Phthalates can structurally mimic tryptophan metabolites and diets containing phthalates in rats enhanced the production and excretion of QA. However, there are no human studies that have examined the association between phthalates and QA.
Objectives
Taking advantage of different mesalamine formulations with/without dibutyl phthalate (DBP), we assessed whether DBP from mesalamine (>1000x background) altered the urinary concentrations of QA.
Methods
Men with inflammatory bowel disease participated in a prospective crossover pilot study. 15 Men were on non-DBP mesalamine (background) at baseline crossed-over for 4 months to high-DBP mesalamine (high) (B1H-Arm) and vice versa for 15 men who were on high-DBP mesalamine at baseline (H1B-Arm). Men provided 60 urine samples (2/man). We estimated crossover and cross-sectional changes in the creatinine normalized-QA using multivariable linear mixed effect models with random intercepts.
Results
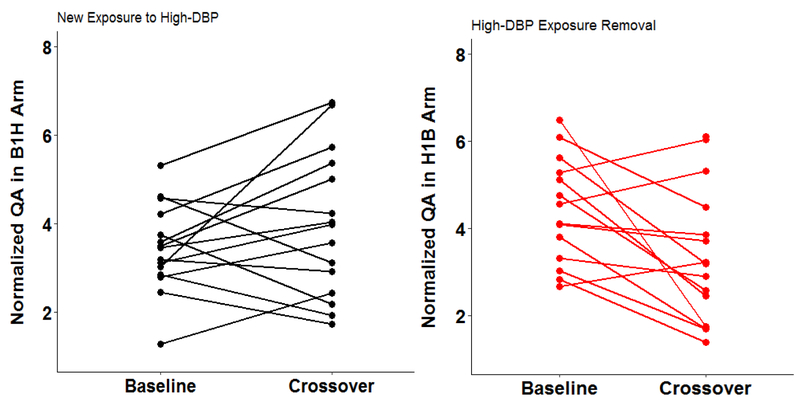
At baseline, men who were on high-DBP mesalamine (H1B-Arm) had 72%, (95% confidence interval (CI): 18, 151) higher normalized-QA than men who were on background exposure and when high-DBP mesalamine was removed for four months, normalized-QA decreased with 32%, (95% CI: −45.0, −15.1). Consistently, when men in B1H-Arm were newly-exposed to high-DBP mesalamine, normalized-QA increased with 11%, (95% CI: −11, 38).
Conclusions
High-DBP exposure from mesalamine increased the urinary concentrations of QA, which was largely reversed after removal of the high-DBP exposure for four months. This novel hypothesis should warrant new promising research considering the KP and QA concentrations as a plausible mediator for the neurotoxicity possibly linked with phthalate exposures.
Keywords: Phthalate, endocrine disruptor, Kynurenine Pathway (KP), quinolinic acid (QA), crossover study, men
1. Introduction
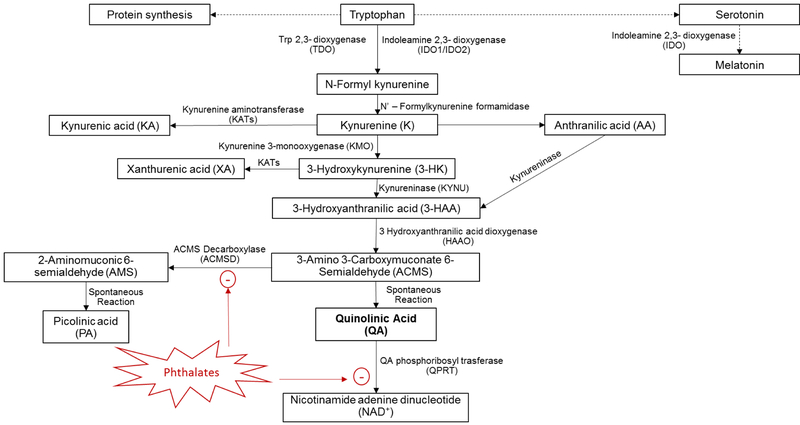
Quinolinic acid (QA) is a neuroactive metabolite of the Kynurenine Pathway (KP) (Figure 1). The KP is the primary route for tryptophan catabolism and accounts for the degradation of approximately 95% of dietary tryptophan in humans3. Tryptophan is an essential amino acid and is present in most protein-based foods e.g., chocolate, oats, dairy products, red meat, eggs, fish, poultry, and nuts. Tryptophan is used to build protein and is a precursor to numerous neurologically active compounds. It is mostly known as the starting point for serotonin and melatonin biosynthesis1. Synthesis of QA from dietary tryptophan is essential as it serves as a precursor to the redox cofactor nicotinamide adenine dinucleotide (NAD+), which is a crucial co-factor for a wide range of enzymes2. However, the excitotoxic effects of QA accumulation due to overproduction or impaired clearance have been implicated in the pathogenesis of several neurological disorders that have increasing prevalence3,4,5,6,7,8,9,10,11,12,13,14.
Fig 1:
The Kynurenine Pathway (KP).
The role of environmental exposures in neurological disorders has received considerable scientific attention as the incidence and prevalence of neurodevelopmental and neurodegenerative disorders has increased15,16. More recently, epidemiologic studies have suggested links between phthalate exposure and neurodevelopmental and neurodegenerative outcomes17. Ortho-phthalates (hereto referred to as phthalates) are high production volume chemicals18 used widely in many consumer and personal care products, leading to ubiquitous exposure in the general population19. Some phthalates have long been suspected of being environmental endocrine disruptors even though mechanisms of toxicity have yet to be fully elucidated. It has been shown that diets containing phthalates (di-(2 ethylhexyl) phthalate (DEHP)) in rats after oral administration strongly enhanced the production and excretion of QA20. Authors concluded that phthalates can perturb tryptophan metabolism and hypothesized that phthalates may exhibit toxicity via metabolic disruption when intake of a tryptophan-rich diet and exposure to phthalates occur coincidentally20. Furthermore, phthalates can structurally mimic tryptophan metabolites which can lead to accumulation of QA21.
However, as far as we are aware, there are no human studies that have examined the association between phthalates and QA, a potential missing link between phthalate exposure and adverse neurological outcomes. Given their structural similarities between phthalates and QA and the compelling preliminary evidence that some phthalates can perturb QA homeostasis, we aimed to determine whether dibutyl phthalate (DBP) was associated with increased formation and excretion of QA. We hypothesized that very high human exposure to DBP from some mesalamine medications increases the urinary concentrations of the QA. To examine this hypothesis, we took advantage of a unique crossover study we recently completed on the effects of high-DBP-exposure from specific mesalamine formulations.
2. Materials and methods
2.1. Participants
As previously described22–24, 73 men enrolled in the Mesalamine And Reproductive health Study (MARS), between 2010–2016, from gastroenterology clinics at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH) in Boston, Massachusetts. Eligible participants were 18 to 55 years old men, with mild severity inflammatory bowel disease (IBD), and on mesalamine for at least three months at the time of enrollment as maintenance therapy for their IBD condition. Men were not eligible if they had a history of steroid medication use in the last three months, vasectomy, diabetes mellitus, hepatic, or renal diseases. MARS was approved by the institutional review boards of Harvard T.H. Chan School of Public Health, BIDMC, BWH and MGH. All men signed informed consents.
1.1. Study design
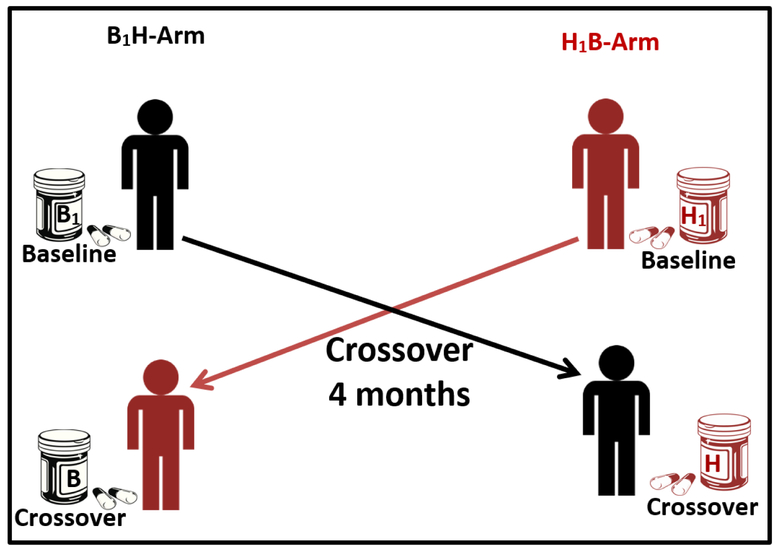
Men participated in MARS as a crossover-crossback study and contributed up to six visits (v) (baseline or enrollment: v1&v2, crossover: v3&v4 and crossback: v5&v6). Men who started on non-DBP mesalamine (background-DBP exposure from other sources) crossed-over to high-DBP mesalamine (high-DBP exposure) then crossed-back to non-DBP mesalamine (background) and vice versa in men who started on high-DBP mesalamine. Crossover periods were designed to be four months. We a priori chose the four month periods to be longer than the spermatogenesis cycle (around 70 days)25, as one of our primary aims in the main study was semen parameters22. At baseline, men reported demographic, lifestyle and health information on questionnaires and height and weight were measured. At each visit, men reported health history in the previous three months and provided blood, semen, and urine samples. For the present pilot study, we randomly selected a sub-set of the men to examine our current hypothesis related to QA. Specifically, among all men who crossed-over in the main study, we randomly selected 30 men, 15 men from each arm (here referred as B1H-arm and H1B-arm). We sent one urine sample at baseline and one sample after crossover per participant for QA analysis i.e., 60 urine samples (Figure 2 & Supplemental Figure1).
Fig 2: Design of the Pilot Mesalamine And Reproductive health Study (MARS).
Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate. B1H-arm: B1 represents background-exposure of DBP at baseline and H represents high-DBP exposure after crossover. H1B-arm: H1 represents high-DBP exposure at baseline and B represents background-exposure after crossover.
Each arm included 15 men with total; 30 men and 60 urine samples.
1.2. Exposure assessment
High-DBP exposure was defined by medication type i.e., DBP-containing mesalamine versus non-DBP mesalamine. The enteric coating of Asacol®, and Asacol®HD contains DBP as an excipient26,27, whereas it is not used in other mesalamine formulations such as Lialda®, Pentasa®, Apriso®, and Delzicol® 28. Asacol® and Asacol®HD use leads to high-DBP exposure as measured by urinary monobutyl phthalate (MBP) concentrations, the primary DBP metabolite29,30, that are approximately 1000 times higher than the median reported for men in the U.S. general population (National Health and Nutrition Examination Survey (NHANES))19. Therefore, we used a priori medication type as a proxy for high-DBP exposure22–24 rather than measuring urinary MBP concentrations which represent only exposure over the past several hours due to the short DBP half-life31.
1.3. Urinary QA measurement
At each visit, men provided a urine sample in a sterile polypropylene cup using standard procedures. Study staff recorded the time of collection (between 7:30 am and 2 pm). Urine samples were divided into aliquots, frozen, and stored at −80°C before shipment on dry ice to the analyzing lab. All 60 samples were shipped in a single batch for analysis to the laboratories at Ethos Research & Development, Newport KY for quantifying QA in urine.
Calibrators and internal standards for quinolinic acid (QA) and creatinine were created from solid standards. Bio Rad Lyphocheck Quantitative Urine Levels 1 and 2 were used as quality control samples. Calibrators, quality control samples, and patient samples were all prepared in the same manner. Level 1 was reconstituted according to manufacturer instructions in 10 mL of Type 1 water. Level 2 was reconstituted in only 5 mL of Type 1 water instead of 10 mL in order to achieve a greater variation between the two QC concentrations. QA and creatinine were analyzed in separate LC-MS/MS assays.
Samples were analyzed using Agilent 1260 Infinity HPLC and Agilent 6410B triple quadrupole mass spectrometer. QA samples were resolved on Agilent Poroshell 120 EC-C18, 2.7 μm, 3.0 × 100 mm column. Creatinine samples were resolved on Agilent Poroshell 120 HILIC, 2.7 μm, 2.1 × 100 mm column. Data analysis was performed using Mass Hunter Quantitative Analysis Version B.08.00 Software.
Normalized QA concentrations were corrected for urinary dilution using urinary creatinine and reported as μg/mg creatinine.
1.4. Statistical Analysis
We performed descriptive statistics and tested for differences in demographics between men in the two arms using Pearson’s chi-square test (or Fisher’s exact test when appropriate) for categorical variables and Kruskal-Wallis test for continuous variables. Initially we defined exposure as a binary variable (high-DBP versus background-DBP) regardless of the order of exposure in the arms. Then, for the main analysis, we defined exposure as a four-level indicator variable cross-classifying each observation based on the medication type (high-versus background-DBP) at each period (baseline versus crossover) for the two study arms (H1B and B1H). We modeled the continuous outcome as the natural log-transformed normalized-QA concentrations due to skewness. We selected the covariates a priori based on directed acyclic graphs from previous knowledge32 and statistical considerations (>10% change in the effect estimate). The final model included age (continuous), race (Caucasian or not), body mass index (BMI) (continuous), and duration on high-DBP mesalamine at baseline (continuous). In preliminary models, we also considered adjustment for IBD severity, IBD diagnosis (ulcerative colitis (UC) or Crohn’s disease (CD)), duration since diagnosis, education, alcohol consumption, fever and illness in the previous three months, but these additional factors were not confounders and thus not retained in the final models.
We used linear mixed effect models (LMEM) with a random intercept for each man to account for the within-man correlation among longitudinal measures of the outcome arising from man-to-man heterogeneity across the study participants. We estimated the crossover percent changes (crossover versus baseline) in the normalized-QA within each arm, as well as the cross-sectional percent changes at baseline between arms (Baseline while on H1 versus baseline while on B1). As a sensitivity analysis, we assessed LMEM model sensitivity by further adjusting for season of the sample collection (warm versus cold season). Because men in H1B-arm were on their high-DBP mesalamine at baseline for a wide range of durations, we explored whether the duration on high-DBP mesalamine at baseline modified associations. Thus, we subdivided men in the H1B-arm based on the median duration of four years on high-DBP mesalamine at baseline i.e., (H1B-arm, H1<4 yrs) and (H1B-arm, H1≥4 yrs) and conducted analyses based on the resulting three arms using the same method as the two-arm analyses above. Finally, we conducted sensitivity analyses using fixed effect models (FEMs) containing fixed subject effects, which isolated the longitudinal within-person effect of exposure by fully adjusting for both observed and unobserved covariates that do not change within person across visits33. FEMs estimate the ordinary fixed regression coefficients in the model, rather than assuming a random distribution for the person-specific intercepts. We also assessed the LMEM model sensitivity to the covariance structure implied by the random intercept model by remodeling the LMEM using robust empirical standard errors33. We considered two-sided alpha <0.05 as statistically significant. We conducted all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC) and used the R package ggplot for generation of graphical output.
2. Results
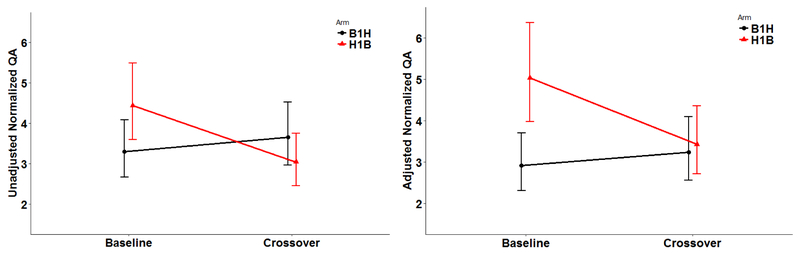
Of the 30 men in this pilot study, those in B1H-Arm (15 men, 30 samples) were on non-DBP mesalamine medication at baseline for a median of 1 year (range 0.5 to 6) whereas those in H1B-Arm (15 men, 30 samples) were on their high-DBP mesalamine medication at baseline (H1) for a median duration of 4 years (range 0.3 to 15). The median crossover duration was 119 days. Men in the two arms had comparable demographics. Men were mainly Caucasian (87%), never smokers (93%), and had college or higher degrees (93%) (Table 1). All urine samples had detectable concentrations for QA, with a normalized-QA geometric mean of 3.56 μg/mg and a range of (1.29, 8.15). When men were not on high-DBP mesalamine, the geometric mean was 3.2 μg/mg with a range of (1.3, 6.1) while it was 4.0 (1.7, 8.2) μg/mg when men were on high-DBP mesalamine. The normal reference range is between 0 and 6.30 μg/mg (Supplemental Table 1). In the adjusted analyses overall for both arms (Table 2 & Figure 3, 4), on average, exposure to high-DBP from mesalamine was associated with higher concentrations of urinary normalized-QA of 27%, 95% confidence interval (95% CI): (8.6, 49). Furthermore, cross-sectionally at baseline, men who were on high-DBP mesalamine had 72%, 95% CI (18, 151) higher concentration of urinary normalized-QA than men with background DBP exposure. Duration on high-DBP mesalamine at baseline was the most important covariate. When we investigated the longitudinal change within each arm, there was a decrease of 32% (95% CI: −45, −15) when men in H1B-Arm were crossed over to background-DBP exposure for four months. On average, although not statistically significant, the concentration of urinary normalized-QA increased 11% (95% CI: −11, 37.7) when men in B1H-Arm were newly-exposed to high-DBP mesalamine. Results remained the same in all sensitivity analyses with stronger results among men who were on high-DBP mesalamine for longer duration (≥ 4 years) and in the unadjusted analyses (Supplemental Tables 2–4, Supplemental Figures 2 and 3).
Table 1:
Demographic characteristics of the 30 men contributing 60 urine samples in MARS by crossover arm.
| Baseline characteristics, men (N)a | B1H-arm (15 men, 30 samples) | H1B-arm (15 men, 30 samples) | Total (30 men, 60 samples) | P-value b |
|---|---|---|---|---|
| Age (Years) | 35.2 (8.63) | 34.5 (10.8) | 34.8 (9.60) | 0.63 |
| Race | 0.35 | |||
| Caucasian | 14 (93) | 12 (80) | 26 (87) | - |
| Black/African American | 1 (7) | 0 | 1 (3) | - |
| Asian | 0 | 1 (7) | 1 (3) | - |
| Other | 0 | 2 (13) | 2 (7) | - |
| BMI (Kg/m2) | 26.6 (3.68) | 24.3 (3.23) | 25.5 (3.59) | 0.08 |
| BMI-categories | 0.15 | |||
| Normal weight | 5 (33) | 9 (60) | 14 (47) | - |
| Overweight | 7 (47) | 6 (40) | 13 (43) | - |
| Obese | 3 (20) | 0 | 3 (10) | - |
| Education | 0.26 | |||
| Below college | 0 | 2 (14) | 2 (7) | - |
| College graduate | 9 (69) | 6 (43) | 15 (56) | - |
| Graduate degree | 4 (31) | 6 (43) | 10 (37) | - |
| Smoking status | 0.99 | |||
| Never smoker | 14 (93) | 14 (93) | 28 (93) | - |
| Former smoker | 1 (7) | 1 (7) | 2 (7) | - |
| Current smoker | 0 | 0 | 0 | - |
| IBD diagnosis | 0.99 | |||
| Ulcerative colitis | 11 (73) | 10 (67) | 21 (70) | - |
| Crohn’s disease | 4 (27) | 5 (33) | 9 (30) | - |
| IBD scored | 1.8 (1.42) | 0.93 (1.44) | 1.37 (1.47) | 0.07 |
| Duration since IBD diagnosis (years) | 10.5 (8.15) | 10.5 (7.50) | 10.5 (7.70) | 0.95 |
| Alcohole | 0.07 | |||
| < 1 days/week | 5 (36) | 8 (57) | 13 (46) | - |
| 1–2 days/week | 8 (57) | 2 (14) | 10 (36) | - |
| ≥2 days/week | 1 (7) | 4 (29) | 5 (18) | - |
| Time-varying characteristics, Samples (n)a | ||||
| Warm season of urine sample c | 11 (37) | 17 (57) | 28 (47) | 0.12 |
| Fever in last 3 months | 1 (3) | 0 | 1 (2) | 0.99 |
| Illness in last 3 months | 9 (33) | 4 (15) | 13 (25) | 0.20 |
Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate; BMI, body mass index; Kg, Kilogram; m, meter; SD, standard deviation; IBD, Inflammatory Bowel Disease; UC, ulcerative colitis; CD, Crohn’s disease; N; number of men, CI; confidence interval.
B1H-arm: B1 represents background-exposure of DBP at baseline and H represents high-DBP exposure after crossover. H1B-arm: H1 represents high-DBP exposure at baseline and B represents background-exposure after crossover.
B1H arm: men at baseline were on Liald (N=10), Pentasa (N=4) and Apriso (N=1).
H1B arm: men at baseline were on Asacol (N=7), and Asacol-HD (N=8).
IBD score: included bowel frequency and urgency, presence of blood in the stool and general wellbeing. Mild IBD score: 5 or less on the simple clinical colitis activity index for UC and 4 or less on the Harvey-Bradshaw index for CD.
Season of sample collection, Warm: April through September.
3 missing education, 2 missing alcohol, 1 missing alcohol, and 7 missing illness in the last 3 months.
N (%) for categorical/binary variables and mean (SD) for continuous variables.
P-values are based on Fisher exact test for categorical variables and Kruskal Wallis test for continuous variables.
Table 2:
The adjusted geometric means (95%CI) and the percent change of urinary quinolinic acid (QA) at baseline and crossover for the 2 arms (B1H- arm, H1B-arm) in MARS.
| Adjusteda Geometric Means (95%CI) of Normalized-QA |
Adjusted Comparisons | |||||
|---|---|---|---|---|---|---|
| Arm | Baseline | Crossover | Crossover % Change (95%CI) Crossover- Baseline |
P value | Cross-sectional % Change (95%CI) Baseline (H1- B1) |
P value |
| H1B | 5.03 (3.97, 6.36) | 3.43 (2.71, 4.35) | −31.7% (−45.0, −15.1) | 0.001 | 72.1% (18.2, 151) | 0.006 |
| B1H | 2.92 (2.31, 3.70) | 3.24 (2.56, 4.10) | 10.8% (−10.8, 37.7) | 0.34 | ||
Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate; N; number of men, CI; confidence interval.
B1H-arm: B1 represents background-exposure of DBP at baseline and H represents high-DBP exposure after crossover. H1B-arm: H1 represents high-DBP exposure at baseline and B represents background-exposure after crossover.
The model was adjusted for age (continuous), race (Caucasian or not), body mass index (BMI) (continuous), and duration on high-DBP mesalamine at baseline (continuous).
Fig 3: Concentrations for the urinary Quinolinic Acid (QA) in 30 Men (60 blood samples) in the MARS-pilot Study.
Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate.
B1H-arm: B1 represents background-exposure of DBP at baseline and H represents high-DBP exposure after crossover. H1B-arm: H1 represents high-DBP exposure at baseline and B represents background-exposure after crossover. Each arm included 15 men with total; 30 men and 60 urine samples.
The adjusted model was adjusted for age (continuous), race (Caucasian or not), body mass index (BMI) (continuous), and duration on high-DBP mesalamine at baseline (continuous).
Fig 4: The unadjusted and adjusted geometric means (95%CI) of urinary Quinolinic acid (QA) at baseline and crossover for the 2 arms (B1H- arm, H1B-arm) in MARS.
Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate.
B1H-arm: B1 represents background-exposure of DBP at baseline and H represents high-DBP exposure after crossover. H1B-arm: H1 represents high-DBP exposure at baseline and B represents background-exposure after crossover. Each arm included 15 men with total; 30 men and 60 urine samples.
The adjusted model was adjusted for age (continuous), race (Caucasian or not), body mass index (BMI) (continuous), and duration on high-DBP mesalamine at baseline (continuous).
3. Discussion
In our pilot study, we found that on average men on high-DBP mesalamine (H1B-Arm) had significantly higher urinary concentration of QA compared to men on the non-DBP mesalamine (B1H-Arm). Furthermore, when exposure to the high-DBP mesalamine was removed for four months, the urinary concentrations of QA significantly decreased among men in the H1B-Arm suggesting reversibility of the increased QA. Alternatively, when men were newly-exposed to high-DBP, urine concentrations of QA increased although not statistically significantly.
QA is produced during the KP as a neuroactive metabolite and as it serves as a precursor to NAD+ 2 (Figure 1). Tryptophan degradation along the KP produces various biologically active metabolites in addition to QA including the NMDA antagonist Kynurenic acid (KA), the immunosuppressive compounds 3-hydroxykynurenine (3-HK) and 3-hydroxyanthranilic acid (3-HAA), the Zinc-binding compound picolinic acid (PA), and the redox cofactor nicotinamide adenine dinucleotide (NAD+) 2 (Figure 1). As an agonist of the N-methyl-D-Aspartate (NMDA) receptor, QA exerts much of its potency as an excitotoxin leading to neuronal dysfunction and apoptotic death. In addition to NMDA receptor mediated excitotoxicity, QA accumulation also induces lipid peroxidation and cytoskeletal destabilization34. Therefore, QA accumulation due to overproduction or impaired clearance have been implicated in the pathogenesis of many neurological disorders including autism3, epilepsy4, Huntington disease5, Parkinson’s disease6, Multiple Sclerosis7, Amyotrophic lateral sclerosis (ALS)8, Alzheimer’s disease9,10, familial cortical myoclonic tremor and epilepsy11, major depressive disorder12 and suicidality13,14. The formation of QA is controlled by several enzymes of the KP including indoleamine 2,3-dioxygenase (IDO1) which is responsible for the first and rate-limiting step in the pathway (Figure 1). IDO1 is induced by pro-inflammatory cytokines meaning that inflammation-mediated IDO1 expression increases the activity of the KP and thus the catabolic degradation of tryptophan. Activation of the KP by proinflammatory mediators increases the flux of tryptophan through this pathway and can lead to tryptophan depletion and decreased synthesis of serotonin. Increased flux through the KP as a result of systemic inflammation results in higher circulating concentrations of all KP metabolites and hence requires optimal functioning of downstream enzymes to ensure adequate control of QA concentrations. QA is produced by the spontaneous conversion of its precursor 2-amino-3-carboxymuconate-6-semialdehyde (ACMS), however, the ACMS precursor can also be enzymatically processed by amino-β-carboxymuconate-semialdehyde-decarboxylase (ACMSD) to form the neuroprotective compound Picolinic acid (PA). As a result of this branch in the KP, ACMSD limits QA formation by competitively converting the ACMS precursor into PA before it can undergo spontaneous cyclization into QA. Therefore, decreased ACMSD activity can lead to excessive QA formation leading to excitotoxicity and neuroinflammation14. Once formed, QA is catabolized to the essential coenzyme NAD+ and carbon dioxide by the action of quinolinate phosphoribosyl transferase (QPRT) in the final step of the KP. QPRT is therefore essential for maintaining the homeostasis of QA concentrations and decreased QPRT activity results in accumulation of this excitotoxin. Proinflammatory cytokine mediated induction of IDO1 is necessary for activation of the KP but the degree of QA accumulation and excitotoxicity is determined by the activities of ACMSD and QPRT which control the formation and clearance of QA. Another major factor that contributes to the potent neurotoxicity of QA is the fact that neuronal QPRT is saturated at QA concentrations that exceed 500nM. This saturation results in a rate of QA formation that exceeds its rate of conversion to NAD+, causing accumulation of toxic QA and NMDA-mediated excitotoxicity35,36.
Fukuwatari and colleagues investigated the effect of diets containing phthalates on tryptophan metabolism in rats and found that orally administered phthalates strongly enhanced the production and excretion of QA. After confirming that mono (2-ethylhexyl) phthalate (MEHP)-a metabolite of di-(2-ethylhexyl) phthalate (DEHP)-reversibly inhibited amino-β-carboxymuconate-semialdehyde-decarboxylase (ACMSD) from rat liver, mouse kidney and the recombinant human enzyme, authors concluded that phthalates perturb tryptophan metabolism by inhibiting ACMSD. Based on these findings the researchers hypothesized that phthalates may exhibit toxicity via metabolic disruption when intake of a tryptophan-rich diet and exposure to phthalates occur coincidentally20. They also reported that DBP also increased the urinary excretion of QA in rats but to a lesser extent20. Singh and colleagues recently evaluated the structure based inhibitory potential of various phthalates and their metabolites on human (ACMSD). By docking each of the phthalates and their metabolites into the active site of ACMSD and determining dynamics and stability of the phthalates-ACMSD complexes their group was able to show that several phthalates, including DBP, can structurally mimic tryptophan metabolites which leads to the inhibition of ACMSD activity and the accumulation of QA21. In addition, phthalic acid has long been recognized as a potent inhibitor of QPRT due to its structural similarities to QA(Malik, Patterson et al. 2014). Therefore the structural similarities of the phthalates and QA and the compelling evidence that phthalates can perturb QA homeostasis through ACMSD/QPRT inhibition may explain our pilot results (Figure 1).
To our knowledge the link between phthalate exposure and QA was never investigated in humans. This potential link is a compelling hypothesis given the accumulating evidence of associations between phthalate exposure and neurodevelopmental and neurodegenerative disorders. For instance, a recent systematic review that included 11 original articles has reported that prenatal exposure to phthalates including DBP was associated with adverse cognitive and behavioral outcomes in children, including lower IQ, and problems with attention, hyperactivity, and poorer social communication37. In a more recent meta-analysis that included ten studies from Taiwan, Korea, Mexico, Poland, Japan, and USA has reported a statistically significant association between the urinary concentrations of DEHP metabolites and the neurodevelopment outcomes of children, and found significant association between DEHP exposure measured in prenatal period and the psychomotor development outcomes measured later in childhood38. Our pilot study had several potential limitations including the lack of randomization to mesalamine formulations at baseline. However, the cross-sectional results were consistent with the longitudinal associations. In addition, physicians did not prescribe mesalamine based on DBP in the enteric coating or in relation to QA concentrations, arguing against potential confounding by medication at baseline. Although our sample size of the pilot was limited, the power of the study is derived from the innovative design and the use of participants as their own control. This avoids the purely cross-sectional analysis that may be confounded by inter-individual variability and lacks temporality. We also acknowledge that we did not have available information about dietary tryptophan intake at baseline or during follow-up that could have contributed to QA concentrations. In addition, this was a pilot study to explore this novel hypothesis with larger future studies planned. Although there may be a concern of generalizing results from men with IBD, all men were classified as having mild IBD. In addition, because of the very high-DBP exposure in this pilot, results also may not be generalized to background (low) exposure. Therefore, in the future, we plan to test the same hypothesis in another population who are exposed to low background DBP and phthalates. Our study had several important strengths including that this is the first study to investigate the hypothesis of the association between phthalate exposure and QA as a neurotoxin precursor. In addition, the innovative prospective design enabled us to compare, within the same men, their QA concentrations during periods of high-DBP to background-DBP exposure and vice versa accounting for confounding by measured and unmeasured non-time-varying characteristics22–24. This is a major strength compared to cross-sectional studies. We also had repeated measures at two time points per man which enabled us to assess the longitudinal change over time. The different orders of exposure and non-exposure in the different arms gave us an additional opportunity to observe the reversibility of the changes under the study period. Confounding by indication was unlikely because mesalamine medications (with or without DBP coating) prescribed for treating IBD have the same active ingredient (mesalamine). We had the opportunity to examine high-DBP exposure (1000 times background) compared to environmental background exposure. Finally, this novel hypothesis should warrant new promising research considering the KP and QA concentration as plausible mediators for the neurotoxicity possibly linked with phthalate exposures.
Conclusions
Our results suggest that high-DBP exposure from mesalamine increased the urinary concentrations of QA which was largely reversed after removal of the high-DBP exposure for four months. Although the health implications of increased QA concentrations of the magnitude observed in our study is unclear, we anticipate that our novel results will lead to future research studies that may ultimately identify QA in the KP as a potential missing link between phthalate (and other environmental chemical exposures) and adverse neurological outcomes.. Our hypothesis needs to be further explored in large epidemiologic studies Specific additional research questions include determining whether low background human exposure to DBP, as well as other phthalates, impact the KP and alter urinary concentrations of QA.
Supplementary Material
Highlights.
Quinolinic acid (QA) is a metabolite in the Kynurenine Pathway (KP) which is the main tryptophan degradation pathway.
QA is an excitotoxin that is implicated in the pathogenesis of neurological disorders.
Phthalates and QA are structurally similar.
Dibutyl phthalate exposure increased the urinary QA concentrations.
This is a novel hypothesis that could be the missing link between phthalates and neurological disorders.
Acknowledgments
The authors gratefully thank the study participants, all members of the MARS team especially Jennifer Ford, Ramace Dadd, Pat Morey, and Myra Keller and the clinical staff.
Sources of funding
The study was supported by the grant R01ES017285 to investigator Russ Hauser from the National Institute of Environmental Health Sciences (NIEHS).
This work was also supported by grant P30ES000002 from the National Institute of Environmental Health Sciences (NIEHS).
Abbreviations:
- QA
quinolinic acid
- KP
Kynurenine Pathway
- DBP
dibutyl phthalate
- IBD
Inflammatory Bowel Disease
- UC
ulcerative colitis
- CD
Crohn’s disease
- MBP
monobutyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- MARS
Mesalamine And Reproductive health Study
- BIDMC
Beth Israel Deaconess Medical Center
- BWH
Brigham and Women’s Hospital
- MGH
Massachusetts General Hospital
- B1HB2
Background1-High-Background2 DBP exposure
- H1BH2
High1-Background-High2 DBP exposure
- BMI
body mass index
- Kg
Kilogram; m, meter
- SD
standard deviation
- LMEM
mixed effects models
- N
number of men
- 95% CI
95% Confidence Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
All authors have nothing to declare.
References
- 1.Davis I, Liu A. What is the tryptophan kynurenine pathway and why is it important to neurotherapy? Expert review of neurotherapeutics. 2015;15(7):719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawy AAB. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. International Journal of Tryptophan Research : IJTR. 2017;10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim CK, Essa MM, de Paula Martins R, et al. Altered kynurenine pathway metabolism in autism: Implication for immune-induced glutamatergic activity. Autism research : official journal of the International Society for Autism Research. 2016;9(6):621–631. [DOI] [PubMed] [Google Scholar]
- 4.Heyes MP, Saito K, Devinsky O, Nadi NS. Kynurenine pathway metabolites in cerebrospinal fluid and serum in complex partial seizures. Epilepsia. 1994;35(2):251–257. [DOI] [PubMed] [Google Scholar]
- 5.Schwarcz R, Okuno E, White RJ, Bird ED, Whetsell WO. 3-Hydroxyanthranilate oxygenase activity is increased in the brains of Huntington disease victims. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(11):4079–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang KH, Cheng ML, Tang HY, Huang CY, Wu YR, Chen CM. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Molecular neurobiology. 2018;55(8):6319–6328. [DOI] [PubMed] [Google Scholar]
- 7.Lim CK, Bilgin A, Lovejoy DB, et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Scientific reports. 2017;7:41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Stankovic R, Cullen KM, et al. The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotoxicity research. 2010;18(2):132–142. [DOI] [PubMed] [Google Scholar]
- 9.Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox report : communications in free radical research. 2002;7(4):199–206. [DOI] [PubMed] [Google Scholar]
- 10.Guillemin GJ, Williams KR, Smith DG, Smythe GA, Croitoru-Lamoury J, Brew BJ. QUINOLINIC ACID IN THE PATHOGENESIS OF ALZHEIMER’S DISEASE In: Allegri G, Costa CVL, Ragazzi E, Steinhart H, Varesio L, eds. Developments in Tryptophan and Serotonin Metabolism. Boston, MA: Springer US; 2003:167–176. [DOI] [PubMed] [Google Scholar]
- 11.Marti-Masso JF, Bergareche A, Makarov V, et al. The ACMSD gene, involved in tryptophan metabolism, is mutated in a family with cortical myoclonus, epilepsy, and parkinsonism. Journal of molecular medicine (Berlin, Germany). 2013;91(12):1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savitz J, Drevets WC, Wurfel BE, et al. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain, behavior, and immunity. 2015;46:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serafini G, Adavastro G, Canepa G, et al. Abnormalities in Kynurenine Pathway Metabolism in Treatment-Resistant Depression and Suicidality: A Systematic Review. CNS & neurological disorders drug targets. 2017;16(4):440–453. [DOI] [PubMed] [Google Scholar]
- 14.Brundin L, Sellgren CM, Lim CK, et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Translational psychiatry. 2016;6(8):e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. The Lancet Neurology. 2014;13(3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. [DOI] [PubMed] [Google Scholar]
- 17.Miodovnik A, Edwards A, Bellinger DC, Hauser R. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology. 2014;41:112–122. [DOI] [PubMed] [Google Scholar]
- 18.Phthalates and Their Alternatives: Health and Environmental Concerns. Lowell Center for Subtainable Production: University of Massachusetts Lowell;2011. [Google Scholar]
- 19.CDC. (Centers for Disease Control and Prevention).National Report on Human Exposure to Environmental Chemicals. 2018; https://www.cdc.gov/exposurereport/. Accessed July 2018.
- 20.Fukuwatari T, Ohsaki S, Fukuoka S, Sasaki R, Shibata K. Phthalate esters enhance quinolinate production by inhibiting alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase (ACMSD), a key enzyme of the tryptophan pathway. Toxicological sciences : an official journal of the Society of Toxicology. 2004;81(2):302–308. [DOI] [PubMed] [Google Scholar]
- 21.Singh N, Dalal V, Kumar P. Structure based mimicking of Phthalic acid esters (PAEs) and inhibition of hACMSD, an important enzyme of the tryptophan kynurenine metabolism pathway. International journal of biological macromolecules. 2018;108:214–224. [DOI] [PubMed] [Google Scholar]
- 22.Nassan FL, Coull BA, Skakkebaek NE, et al. A crossover-crossback prospective study of dibutylphthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environment international. 2016;95:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nassan FL, Coull BA, Skakkebaek NE, et al. A crossover-crossback prospective study of dibutylphthalate exposure from mesalamine medications and serum reproductive hormones in men. Environmental research. 2018;160:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassan FL, Korevaar TIM, Coull BA, et al. Dibutyl-phthalate exposure from mesalamine medications and serum thyroid hormones in men. International journal of hygiene and environmental health. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science (New York, NY). 1963;140(3563):184–186. [DOI] [PubMed] [Google Scholar]
- 26.FDA. ASACOL® (mesalamine) delayed-release tablets, for oral use - HIGHLIGHTS OF PRESCRIBING INFORMATION. 2015; http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm215476.htm, http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/019651s025lbl.pdf. Accessed April, 2017.
- 27.FDA. Asacol® HD (mesalamine) delayed-release tablet for oral administration -HIGHLIGHTS OF PRESCRIBING INFORMATION. 2010; http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021830s005lbl.pdf. Accessed April, 2017.
- 28.Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environmental health perspectives. 2012;120(3):379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environmental health perspectives. 2004;112(6):751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hait EJ, Calafat AM, Hauser R. Urinary phthalate metabolite concentrations among men with inflammatory bowel disease on mesalamine therapy. Endocr Disruptors (Austin). 2014;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebaek NE, Wulf HC. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environmental science & technology. 2007;41(15):5564–5570. [DOI] [PubMed] [Google Scholar]
- 32.Cho HJ, Savitz J, Dantzer R, Teague TK, Drevets WC, Irwin MR. Sleep Disturbance and Kynurenine Metabolism in Depression. Journal of psychosomatic research. 2017;99:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed 2011. [Google Scholar]
- 34.Pierozan P, Goncalves Fernandes C, Ferreira F, Pessoa-Pureur R. Acute intrastriatal injection of quinolinic acid provokes long-lasting misregulation of the cytoskeleton in the striatum, cerebral cortex and hippocampus of young rats. Brain research. 2014;1577:1–10. [DOI] [PubMed] [Google Scholar]
- 35.Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. The FEBS journal. 2012;279(8):1356–1365. [DOI] [PubMed] [Google Scholar]
- 36.Braidy N, Guillemin GJ, Grant R. Effects of Kynurenine Pathway Inhibition on NAD(+) Metabolism and Cell Viability in Human Primary Astrocytes and Neurons. International Journal of Tryptophan Research : IJTR. 2011;4:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environmental research. 2015;142:51–60. [DOI] [PubMed] [Google Scholar]
- 38.Lee D-W, Kim M-S, Lim Y-H, Lee N, Hong Y-C. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environmental research. 2018;167:558–566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.