Abstract
A growing body of evidence suggests that network-based interventions to reduce HIV transmission and/or improve HIV-related health outcomes have an important place in public health efforts to move towards 90-90-90 goals. However, the social processes involved in network-based recruitment may pose a risk to participants of increasing HIV-related stigma if network recruitment causes HIV status to be assumed, inferred, or disclosed. On the other hand, the social processes involved in network-based recruitment to HIV testing may also encourage HIV-related social support. Yet despite the relevance of these processes to both network-based interventions and to other more common interventions (e.g., partner services), there is a dearth of literature that directly examines them among participants of such interventions. Furthermore, both HIV-related stigma and social support may influence participants’ willingness and ability to recruit their network members to the study. This paper examines 1) the extent to which stigma and support were experienced by participants in the Transmission Reduction Intervention Project (TRIP), a risk network-tracing intervention aimed at locating recently HIV-infected and/or undiagnosed HIV-infected people and linking them to care in Athens, Greece; Odessa, Ukraine; and Chicago, Illinois; and 2) whether stigma and support predicted participant engagement in the intervention.
Overall, experiences of stigma were infrequent and experiences of support frequent, with significant variation between study sites. Experiences and perceptions of HIV-related stigma did not change significantly between baseline and six-month follow-up for the full TRIP sample, and significantly decreased during the course of the study at the Chicago site. Experiences of HIV-related support significantly increased among recently-HIV-infected participants at all sites, and among all participants at the Odessa site. Both stigma and support were found to predict participants’ recruitment of network members to the study at the Athens site, and to predict participants’ interviewer-rated enthusiasm for naming and recruiting their network members at both the Athens and Odessa sites. These findings suggest that network-based interventions like TRIP which aim to reduce HIV transmission likely do not increase stigma-related risks to participants, and may even encourage increased social support among network members. However, the present study is limited by its associational design and by some variation in implementation by study site. Future research should directly assess contextual differences to improve understanding of the implications of site-level variation in stigma and support for the implementation of network-based interventions, given the finding that these constructs predict participants’ recruitment of network members and engagement in the intervention, and thereby could limit network-based interventions’ abilities to reach those most in need of HIV testing and care.
Introduction
Undiagnosed infections (Hall, Holtgrave, & Maulsby, 2012) and recent infections (Brenner et al., 2007; Marzel et al., 2016; Vasylyeva et al., 2016) both account for a large share of HIV transmission events. Locating undiagnosed positives and finding people with recent HIV infection (PwRI) and linking them to care should therefore be a public health priority. However, there is limited evidence about how best to locate them, and about how best to extend the reach of current common case-finding methods (e.g., clinic testing; self-testing). Improving our ability to do so is a crucial part of the global 90-90-90 strategy to limit transmission of HIV and reduce morbidity and mortality by diagnosing 90% of all HIV-positive persons, providing antiretroviral treatment for 90% of those diagnosed, and achieving viral suppression for 90% of those treated by 2020 (UNAIDS, 2014).
Empirical evidence suggests that interventions aiming to prevent HIV transmission by working with HIV-infected people and their social or risk networks can be effective not only at reducing HIV risk behaviors and/or promoting HIV-preventive behaviors (e.g., Amirkhanian et al., 2003; Latkin et al., 2013; Sherman et al., 2009), but also at locating undiagnosed (Smyrnov et al., 2018) and/or recent HIV infections (Nikolopoulos et al., 2016) to link them to HIV testing and care. Our Transmission Reduction Intervention Project (TRIP) study traced the sexual and injection networks of recently-infected and longer-term HIV+ “seeds” (original participants) in order to locate, test, and link previously undiagnosed and/or recently infected HIV-positive individuals to care. This TRIP case-finding intervention was able to successfully find such new cases (Morgan et al., 2018; Nikolopoulos et al., 2016) and link them to treatment at higher rates (i.e., more efficiently) than were other local HIV testing programs (specifically, a respondent-driven sampling1 based program for people who inject drugs, and a community-based harm reduction site program; Smyrnov et al., 2018; Williams et al., under review). The TRIP intervention study was therefore an important part of the growing body of evidence on the utility of network-based interventions for reducing HIV transmission.
However, network-based interventions may pose some potential risks to participants. Specifically, when network-tracing is used to recruit people for HIV testing and other HIV-related health promotive activities, participants’ risk of experiencing HIV-related stigmatizing events (i.e., negative, discriminatory, exclusionary, or hostile treatment from others based on others’ thinking or knowing that one has HIV) might be potentially heightened, as HIV infection statuses may be inferred by and/or disclosed to other network members during the process of participants’ recruiting their own network members (Friedman et al., 2014). This is true not only for HIV-positive participants, but also for those who are HIV-negative, since people may make incorrect assumptions about the HIV status of negatives.
On the other hand, in addition to their benefits to participants’ and network members’ HIV-related health, network-based interventions may also provide other benefits to participants as a function of the network-based recruitment method itself. The social processes of recruiting or being recruited by one’s social network members for HIV testing or other HIV-related health-promotive activities may increase HIV treatment-related discussion and support among participants. (HIV-related social support is also relevant to HIV-negative participants, as people may offer them various forms of social support if they incorrectly assume they are infected, or offer them support related to HIV testing.) Since social support has been found to have its own benefits to HIV-related outcomes among HIV-positives, such as significantly predicting both treatment adherence (Gonzalez et al., 2004; Ncama et al., 2008; Power et al., 2003) and HIV-status disclosure (Kalichman et al., 2003), it is useful to understand whether a network-based intervention such as TRIP has any social support-related benefits to participants.
Finally, the potential inverse relationship between stigma and participation in a network intervention may be important. It is possible that participants’ perceptions and/or experiences of stigma in their environments and/or within their social networks may affect their engagement in the intervention (and therefore intervention efficacy). Both setting-level (i.e., contextual) and individual-level variation in participants’ perceptions of stigma may affect their willingness or ability to recruit people they know to participate in an HIV-related study. Participants who view their environments as stigmatizing may be afraid to recruit people because they fear being suspected of being HIV-positive. Variation in perceptions and experiences of stigma has, to our knowledge, never been studied among participants of a network-tracing intervention. Yet, understanding the degree to which stigma impedes full engagement in network-based interventions could help us improve intervention efficacy and could shed light on best practices for future implementation of interventions like TRIP (e.g., whether a stigma-reducing module preceding network-based interventions may be useful in some contexts or for some individuals).
Social network interventions are potentially important tools for preventing HIV transmission. Furthermore, there is a wealth of extant literature on the pervasiveness of HIV-related stigma as both a barrier to participation in preventive interventions (e.g., Pulerwitz et al., 2008; Turan & Nyblade, 2013) and as an important risk or potential iatrogenic effect of other kinds of HIV interventions and services (e.g., partner services – Passin et al., 2006). However, to our knowledge, no studies to date have examined the degree of stigma experienced among participants of a network-recruitment intervention aiming to reduce HIV transmission and link previously undiagnosed and/or recently-infected HIV-positives to care. (One study did examine stigma among participants in an HIV treatment-outcome intervention which also contained a social support component specifically designed to reduce stigma (Hickey et al., 2015); and Vasylyeva et al. (2015) reported that there were no cases of stigmatization reported by participants of Project Protect, our pilot project that preceded TRIP in Ukraine.) The present study therefore examines, in three diverse contexts with relatively large concentrated epidemics, the extent to which participants in a network intervention aimed primarily at locating and linking new HIV cases to care to reduce transmission (i.e., the TRIP study) experienced HIV-related stigma and/or support during or after their participation in the program. It will also examine the extent to which stigma and/or support predicted participant engagement in TRIP. It will use TRIP data from Athens, Greece; Odessa, Ukraine; and Chicago, Illinois to address the following research questions:
-
1)
To what degree did individuals participating in the TRIP intervention experience HIV-related stigma and support?
-
2)
Did experiences of stigma and support vary by study site, study arm (intervention and control arms), HIV status, gender, and drug injection status?
-
3)
Did reported experiences of stigma and support change significantly between baseline and post-intervention follow-up?
-
4)
Did participants’ experiences of stigma and support and/or their perceptions of stigma and support within their social networks predict participant engagement in the TRIP intervention (as measured by the number of network members each participant recruited)?
Methods
Recruitment and Procedures
Details on TRIP recruitment and procedures for each TRIP site are reported elsewhere (Nikolopoulos et al., 2016; Morgan et al., 2018; Symrnov et al., 2018). Here we provide a summary of key information. The TRIP intervention recruited recently-HIV-infected (i.e., PwRI) and longer-term HIV-positive (LTP) “seeds” (original participants) via clinic- and other treatment-based referrals in Athens, Odessa, and Chicago. Referred seeds were eligible to participate if they fell into one of these HIV status categories, were at least 18 years of age, and were able to complete interviews in English (Chicago), Greek (Athens), or Russian (Odessa). Members of PwRI’s and LTP’s expanded risk networks (i.e., people who they reported having sex with and/or injecting drugs with, or who were present with them while they were having sex and/or injecting drugs) were named by participants during interviews, and participants were asked to recruit them to the study. Participants were given coupons with which to recruit the network members that they named during the interviews. These coupons contained alphanumeric codes that were used to confidentially link network members to the participant who recruited them (and to the seed for each network). Participants were also recruited by TRIP project staff from venues named by seeds as places they frequently met risk partners and/or engaged in sex and/or injection. These “venue-recruited” participants were included as part of the expanded risk networks of the seeds who named the venue from which they were recruited. Network- and venue-recruited individuals were eligible to participate if they presented a recruitment coupon to project staff, and met the same age and language criteria described above for seeds. All enrolled study participants (including seeds) were tested for HIV antibodies, received pre- and post-test counseling, and were interviewed about their socio-demographic characteristics; drug use; risky sex and injection behaviors; access to drug treatment, HIV-related care, and other healthcare; experiences and perceptions of stigma and support; and their risk network members. HIV-positive participants were tested for recency of infection (see next paragraph) and their viral load was measured. All HIV-positive participants were referred to treatment. This process was repeated for two network “steps.” In other words, all individuals with direct risk connections to seeds (i.e., Step 1 network members) were recruited, and were then asked to recruit their own direct risk connections (i.e., Step 2 network members). All recent infections found within networks were then assigned recent seed status, and their networks were correspondingly traced and recruited for two additional steps, regardless of their distance from the original seed. HIV-negative control “seeds” were also recruited to the study as a comparison group; but their risk networks were not traced or recruited.
Participants in Chicago received $50 for completing interviews and $20 for each person who came to the study site with a coupon that had been assigned to them (i.e., for each person they “recruited”). Likewise, participants in Odessa received 50 hryvnia (approximately $6 US in 2013) for completing interviews and 20 hryvnia (approximately $2.50 US in 2013) for each person who came to the study site with a coupon that had been assigned to them; and participants in Athens received 10 Euros for completing interviews and 5 Euros for each person who came to the study site with a coupon that had been assigned to them. These incentives were intended to compensate participants modestly for their time and recruitment efforts, without influencing their decisions to participate.
In Athens and Odessa, recent HIV infections were identified using Limiting Antigen (LAg)-Avidity Assays, using a median optical density (ODn) score ≤ 1.5 (Nikolopoulos et al., 2017), corresponding to a window of infection within 130 days (Duong et al., 2012). In Odessa, having viral load ≥ 1,000 copies/mL was an additional criterion for classification as a recent infection. Participants with documented positive HIV test results within the last six months were also classified as “recently infected” in both Athens and Odessa. “Borderline” recent infections were also identified (at the Chicago and Athens sites only), operationalized in Athens as newly HIV-diagnosed individuals with very reliable recorded negative HIV test results in the last 9 months, or with a very high viral load (> 100,000 copies/mL) typical of recent infection and an absence of AIDS-identifying illnesses. In Chicago, recent and borderline-recent HIV infections were identified using testing history. Newly HIV-diagnosed individuals with laboratory-confirmed negative HIV test results in the last 6 months were categorized as “recently infected,” while those with laboratory-confirmed negative HIV test results in the last 9 months, or with a very high viral load (> 100,000 copies/mL) typical of recent infection and an absence of AIDS-identifying illnesses, were categorized as borderline recent infections. For the present analyses, recent infections and borderline recent infections are grouped together and all considered to be “PwRI,” as they were all treated as such by TRIP protocol and procedures. All study protocols and procedures were approved by Institutional Review Boards in New York; Athens (for Athens study site only); Odessa (for Odessa study site only); and Chicago (for Chicago study site only).
Sample
A total of 130 PwRI seeds, 57 LTP seeds, and 115 negative controls were recruited to the study; and 1,251 and 610 network members of PwRI and of LT positive seeds, respectively, were successfully recruited and interviewed at baseline across all three sites (total baseline N = 2,163). Follow-up interviews were completed by 71 PwRI seeds, 34 LTP seeds, 83 negative controls, 397 network members of recent seeds, and 179 network members of LTP seeds (total follow-up N = 764). Of these, 737 participants completed stigma-related measures at follow-up. Retention rates varied by study site (Athens – 67.7%; Chicago – 53.2%; Odessa – 23.9%). Table 1 presents a comparison of demographic characteristics and stigma and support variables for the baseline and follow-up samples (for participants who completed the interview questions on the constructs of interest for the present study). Participants who did and who did not complete follow-up were not significantly different in terms of gender, age, or reported number of sex partners. On other characteristics, participants with more “vulnerable” statuses (e.g., HIV-positive; recently HIV-infected; unemployed; did not complete high school; people who inject drugs – i.e., PWID; non-heterosexual) were more likely to be retained for follow-up. Homelessness was an exception to this trend, as homeless participants completed follow-up assessments at significantly lower rates than non-homeless participants. Figure 1 presents N’s for the present analyses by study site, sex, recent drug injection history, and HIV status.
Table 1.
Sample Sociodemographic Characteristics at Baseline
| Baseline Sample (N = 2,163) | Participants who Completed Follow-up (with follow-up data on stigma/support) (N = 737) | Participants who Did Not Complete Follow-up (N = 1,426) | Significant Differencei between participants who did and did not complete follow-up? | |
|---|---|---|---|---|
| Male | 1,713 (79.2%) | 553 (75.0%) | 1,160 (81.3%) | No (p = .20) |
| HIV-Positive | 637 (29.4%) | 257 (34.9%) | 380 (26.6%) | Yes (p = .003) |
| Recently HIV-Infected | 106 (4.9%) | 64 (8.7%) | 42 (2.9%) | Yes (p < .0005) |
| PWID | 1,039 (48.0%) | 393 (53.3%) | 646 (45.3%) | Yes (p < .0005) |
| Completed High School | 1,589 (73.5%) | 478 (64.9%) | 1,111 (77.9%) | Yes (p < .0005) |
| Unemployed | 924 (42.7%) | 340 (46.1%) | 584 (41.0%) | Yes (p = .003) |
| Identified as “Straight” | 1,912 (88.4%) | 600 (81.4%) | 1,312 (92.0%) | Yes (p < .0005) |
| Homeless | 310 (14.3%) | 86 (11.7%) | 224 (15.7%) | Yes (p = .02) |
| Mean (SD) Age | 34.9 (9.9) | 34.7 (9.4) | 35.1 (10.1) | No (p = .67) |
| Mean (SD) Reported Number of Sex and Injection Partners | 6.0 (19.3) | 6.2 (14.1) | 5.8 (21.5) | No (p = .33) |
Results from Chi-square (categorical variables) or independent samples t-test (continuous variables).
Figure 1.
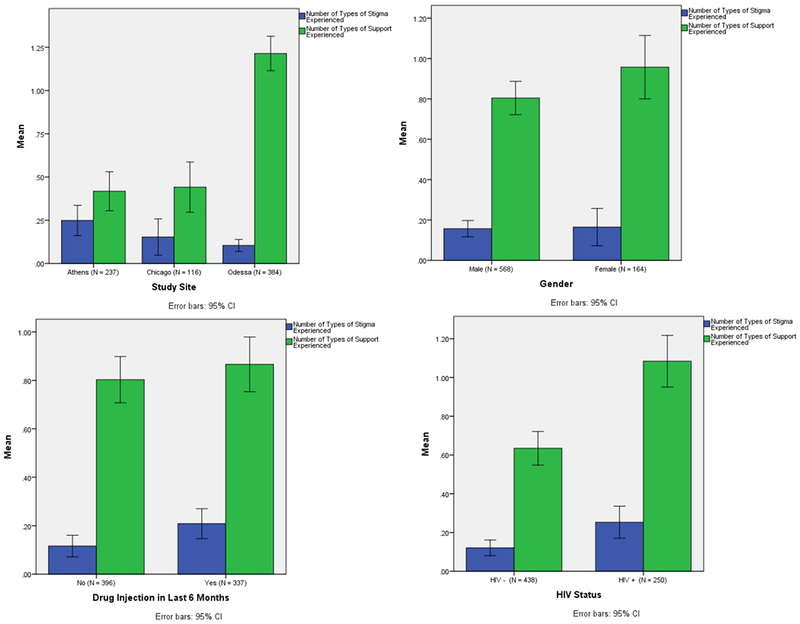
Comparisons of Mean HIV-related Stigma and Support by Study Site, Gender, Drug Injection History, and HIV Status
Measures
All study participants were interviewed about their experiences of stigma and discrimination both at baseline and in follow-up interviews six months after entering the study. Stigma items were developed by the TRIP team and asked participants about their own experiences of negative, stigmatizing behaviors including nasty comments, threats/attacks, or exclusions from social gatherings (4 items, Cronbach’s α =.60 at follow-up) based on others thinking that they were recently infected. Experiences of support were measured using items developed by the TRIP team, asking participants about helpful behavior related to HIV (i.e., being offered emotional support, assistance, or information on HIV services) from others who thought they were recently infected (3 items, α = .64 at follow-up). Both experiences of stigma and of support were operationalized as the number of items to which participants responded affirmatively (i.e., as the number of “types” of stigma or support participants reported experiencing). Responses were compared by intervention site, by sex, by HIV status, and by study arm. Perceptions of stigma and support in participants’ social networks were measured using items developed by the TRIP team, asking participants what proportion of their friends and family members (i.e., their social network members - rated using a Likert scale from 0-4, where 0 = none; 1 = few; 2 = about half; 3 = most; and 4 = all) they believed would engage in (or had observed engaging in) stigmatizing behavior towards or would make (had made) stigmatizing comments about people with HIV (3 items, α = .69 at follow-up); or would engage (or had engaged) in supportive behavior towards or would offer (had offered) assistance to people with HIV (3 items, α = .82 at follow-up).
Participant engagement in the intervention was operationalized as number of network members recruited, and as interviewer-rated enthusiasm for naming names and for recruiting network members. Number of network members recruited was measured as a count of the number of coupon-recruited (as opposed to venue-recruited), network members that each individual directly recruited who enrolled in the study. Enthusiasm for naming names (of network members) and enthusiasm for recruiting network members were measured using a 10-point Likert scale with which interviewers rated (at the end of baseline interviews) their perceptions of each participant’s reaction to being asked to engage in these components of the intervention. Higher responses indicated greater enthusiasm. These measures of participant engagement are applicable only to PwRI seeds, LTP seeds, and their Step 1 network members (as Step 2 network members and negative control seeds were not asked to recruit their network members to the study).
Analyses
All statistical analyses were conducted in IBM SPSS Statistics 21. Statistical tests to address study questions included simple descriptive analyses (Questions 1 and 2), independent samples t-tests (Question 2), one-way analyses of variance (ANOVAs – Question 2), paired samples t-tests (Question 3), and multiple ordinary least squares regression analyses (Question 4).
Results
Analyses addressing Question 1 assess the degree to which participants in the TRIP intervention study experienced HIV-related stigma and support. Reports of stigmatizing experiences were relatively infrequent for the full sample of TRIP participants. Frequencies of HIV-related supportive experiences were higher. Mean number of types of stigma experienced was less than 0.2 for all participants, with 11.3% of participants reporting at least one stigmatizing experience. Mean number of types of HIV-related support experienced was 0.8 for all participants, with 43.9% reporting at least one supportive/helping event.
Variation in Stigma and Support by Site, Study Arm, and Key Participant Characteristics
Analyses addressing Question 2 assess the extent to which experiences of stigma and support varied by study site, study arm (intervention and control arms), HIV status, gender, and drug injection status, and are depicted in Figures 1 and 2. Additional details on these analyses (including standard deviations and ranges for each subgroup, and the proportion of participants in each subgroup who reported at least one stigmatizing experience and at least one support experience) are presented in Tables S1, S2, and S3 in the online supplement for this paper.
Figure 2.
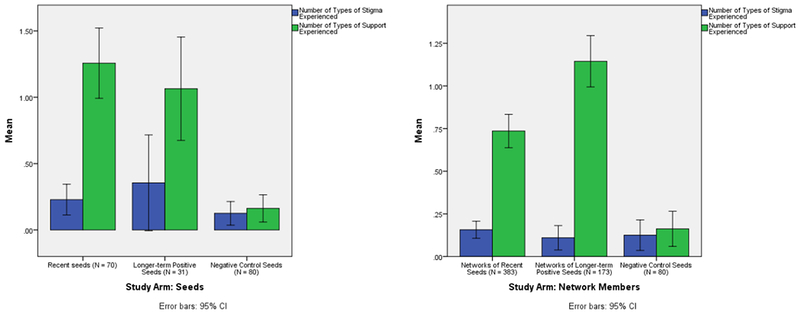
Comparisons of Mean HIV-related Stigma and Support by Study Arm
Participants’ reported experiences of both stigma and support differed significantly by study site (Figure 1; Table S1 in the online supplement). Athens participants reported significantly more stigmatizing experiences (F = 5.94, p = .003) than did either Chicago or Odessa participants. Odessa participants reported significantly more helping experiences (F = 67.90; p < .0005) than did either Athens or Chicago participants.
For the full sample of TRIP participants (across all sites) neither experiences of stigma nor of support differed significantly by gender (operationalized in the present analyses as dichotomous, cis-gender identification, given very low N’s of non-cis-gender participants; see Figure 1). But, there was a trend-level difference for experiences of support, with female participants reporting marginally more experiences of support than males (t = −1.72; p = .086). Participants who injected drugs in the last six months (PWID) reported significantly more experiences of stigma than did non-PWID participants (t = −2.36; p = .018); but there were no significant differences between PWID and non-PWID on reported experiences of HIV-related support. Unsurprisingly, HIV-positive participants reported significantly more experiences of stigma (t = −2.81; p = .005) and of HIV-related support (t = −5.79; p < .0005) than did HIV-negative participants.
Differences between study arms, and by seed vs. network member recruitment status, are presented in Figure 2 (and Table S2 in the online supplement) for the full sample (all study sites). No significant differences were found between study arm on reported stigmatizing experiences, either among seeds or among network members. Negative control seeds reported significantly fewer (F = 31.82; p < .0005) HIV-related support experiences (mean = 0.16) than did either PwRI seeds (mean = 1.11) or LTP seeds (mean = 1.06). Reported experiences of HIV-related support also varied significantly by study arm among network members (F = 31.01; p < .0005). LTP seeds’ network members reported significantly more support experiences (mean = 1.14) than did PwRI seeds’ network members (mean = 0.73); and network members from both groups reported significantly more such experiences than did negative control seeds.
Table S3 in the online supplement presents descriptive statistics and statistical comparisons by group (gender, PWID vs. non-PWID, study arm, and HIV status) for each study site, separately. Notably, reports of support were most frequent among LTP seeds and their network members in Odessa (79% reported at least one type of HIV-related support, with a mean of 1.6 types of support reported), and were least frequent among negative control seeds in Athens (3% reported at least one type of support, with a mean of 0.05 types of support reported). Reports of stigma were most frequent among HIV-positive participants in Athens (25% reported at least one stigmatizing experience, with a mean of 0.41 types of stigmatizing experiences reported), and were least frequent among female participants in Chicago (0 stigmatizing events reported) and among LTP seeds and their network members in Odessa (0.8% of participants reported at least one stigmatizing experience, with a mean of 0.01 types of stigmatizing experiences). Also, PWID experienced significantly more types of support than non-PWID (t = −3.06; p = .003) in Chicago, but not elsewhere.
Changes in Stigma and Support between Baseline and Follow-up
Analyses addressing Question 3 assess the extent to which reported experiences of stigma and support and/or perceptions of stigma and support changed significantly between baseline and follow-up interviews six months after recruitment to the study.2 Paired-samples t-tests were conducted to address Question 3, with only participants who had both baseline and follow-up data included in the analysis. Baseline stigma and support were compared between participants who did and did not complete follow-up interviews (Table 1), to better understand the degree to which the follow-up sample might be biased and affect Question 3 results. There were no significant differences between participants who did and did not complete follow-up on either experiences of stigma or experiences of support reported at baseline. Participants who completed follow-up reported perceptions of significantly greater stigma (t = 2.57; p = .010) and of significantly greater support (t = 2.94; p = .003) among their social networks at baseline than did participants who did not complete follow-up.
For the full sample of participants with follow-up data on stigma/support, reported stigmatizing experiences decreased slightly, but not significantly, between baseline and follow-up. This was also true among only HIV-positive participants, and among only recently-HIV-infected participants. For the full sample of participants with follow-up data, perceptions of stigma among family and friends decreased significantly (i.e., participants reported that they believed that fewer of their friends or family had stigmatizing beliefs or engaged in stigmatizing behaviors) between baseline-and follow-up (t = 2.98; p = .003). Decreases in mean value of perceptions of stigma were also observed among HIV-positives only and among recently-infected participants only, but these differences were not statistically significant (perhaps due to small N’s).
For the full sample of participants with follow-up data, and for the subsample of HIV-positive participants, experiences of support did not change significantly over time. However, for the subsample of recently infected participants, reported experiences of support increased between baseline and follow-up (from a mean of 0.85 to 1.20 types of HIV-related support reported), at a level of significance indicating a possible trend (t = −1.80; p = .08). Finally, for the full sample of participants with follow-up data, perceptions of HIV-related support among family and friends decreased significantly over time (i.e., participants reported that they believed fewer of their friends or family engaged in HIV-related helping behaviors) - from a mean value of 0.54 to a mean value of 0.37 - between baseline and follow-up (t = 5.07; p < .0005). A similar significant change was also observed among HIV-positive participants only, but not among recently-infected participants only.
Results of analyses assessing change over time varied somewhat by study site. Notably, among the full sample of Chicago participants with follow-up data, reported experiences of stigma significantly decreased (t = 2.02; p = 0.046). This decrease in mean reported types of stigma experienced was largest among PwRI (from 0.40 to 0.10). Among the full sample of Athens participants, perceptions of stigma significantly decreased between baseline and follow-up (from .50 to .34; t = 2.89; p = .004). This change over time was apparently driven by HIV-negative participants, as there was no significant change in perceptions of stigma among PwRI or among all HIV-positive participants. Among Odessa participants, support experiences significantly increased between baseline and follow-up. This was true among the full Odessa sample, as well as among all HIV-positive participants and among only PwRI, with the largest increase found among PwRI (from 0.72 to 1.89; t = −4.12; p = .001).
Stigma and Support as Predictors of Participant Engagement
Analyses addressing Question 4 assessed the extent to which participants’ experiences and/or perceptions of stigma and support predicted participant engagement in the TRIP intervention (as measured by the number of network members each participant recruited). Multiple regression models were used to regress participant engagement outcomes on either 1) experiences of stigma and support or 2) participants’ perceptions of stigma and support among their social networks. Both stigma and support were included in each model, given moderate significant correlations indicating that these variables share variance. Adjusted models were also used, which controlled for participants’ number of sex and/or injection partners, HIV status, drug injection history, homelessness status, reported sexual orientation, employment status, and education. Results varied as a function of HIV status and study site (Athens and Odessa), and are presented accordingly. (These analyses were not conducted for the Chicago site, because recruitment chain data were not available for a large enough proportion of TRIP Chicago participants.) Tables 2 and 3 present results from both unadjusted and adjusted models for Athens and for Odessa, respectively. For both sites, patterns of association produced by adjusted models were nearly identical to those produced by unadjusted models. Table S4 in the online supplement for this paper summarizes results for models conducted using subsamples by HIV status and site.
Table 2.
Multiple Regression Models Predicting Participant Engagement in TRIP from Stigma and Support Variables, in Athens (N = 157)
| Predictor | Outcome | |||||
|---|---|---|---|---|---|---|
| Number of Network Members Recruited | Number of Network Members Recruited | Interviewer-Rated Participant Enthusiasm for Naming Names | Interviewer-Rated Participant Enthusiasm for Naming Names | Interviewer-Rated Participant Enthusiasm for Recruiting Network Members | Interviewer-Rated Participant Enthusiasm for Recruiting Network Members | |
| Unstandardized Coefficient (Standard Error) | ||||||
| Unadjusted Model: | Model 1: | Model 2: | Model 3: | Model 4: | Model 5: | Model 6: |
| Experiences of Stigma | −0.04 (0.19) | −0.39 (0.23)† | −0.50 (0.24)* | |||
| Experiences of Support | 0.10 (0.15) | 0.27 (0.19) | 0.37 (0.21)† | |||
| Perceptions of Stigma among Social Network | −0.05 (0.23) | −0.04 (0.25) | 0.07 (0.27) | |||
| Perceptions of Support among Social Network | 0.48 (0.29) | 0.68 (0.31)* | 0.83 (0.33)* | |||
| Adjusted Model: | Model 1 Adjusted: | Model 2 Adjusted: | Model 3 Adjusted: | Model 4 Adjusted: | Model 5 Adjusted: | Model 6 Adjusted: |
| Experiences of Stigma | −0.12 (0.19) | −0.39 (0.23)† | −0.45 (0.24)† | |||
| Experiences of Support | 0.12 (0.16) | 0.33 (0.20)† | 0.42 (0.21)* | |||
| Perceptions of Stigma among Social Network | −0.16 (0.23) | −0.10 (0.25) | 0.09 (0.27) | |||
| Perceptions of Support among Social Network | 0.35 (0.30) | 0.53 (0.30)* | 0.68 (0.32)* | |||
| Number of Sex and/or Injection Partners | 0.002(0.004) | −0.003 (0.004) | −0.004 (0.004) | −0.01 (0.004) | −0.01 (0.01)† | −0.01 (0.01)* |
| HIV Positive | 0.05 (0.32) | 0.30 (0.32) | −0.76 (0.37)* | −0.51 (0.35) | −0.91 (0.39)* | −0.70 (0.38)† |
| PWID | 1.46 (0.48)** | 1.48 (0.50)** | 2.90 (0.56)*** | 2.82 (0.57)*** | 3.06 (0.60)*** | 2.65 (0.62)*** |
| Homeless | 0.19 (0.32) | 0.32 (0.35) | 0.13 (0.38) | 0.09 (0.39) | 0.29 (0.40) | 0.37 (0.42) |
| Straight | −0.44 (1.00) | −1.36 (1.20) | −1.27 (1.11) | −2.33 (1.17)* | −1.02 (1.18) | −1.91 (1.28) |
| Unemployed | −0.33 (0.30) | −0.16 (0.35) | −0.25 (0.35) | −0.12 (0.37) | −0.41 (0.37) | −0.29 (0.40) |
| Completed High School | 0.10 (0.28) | 0.26 (0.31) | 0.11 (0.34) | −0.01 (0.34) | −0.01 (0.36) | −0.17 (0.37) |
signifies p < .001;
signifies p < .01;
signifies p < .05;
signifies p < .10
Table 3.
Multiple Regression Models Predicting Participant Engagement in TRIP from Stigma and Support Variables, in Odessa (N = 143)
| Predictor | Outcome | |||||
|---|---|---|---|---|---|---|
| Number of Network Members Recruited | Number of Network Members Recruited | Interviewer-Rated Participant Enthusiasm for Naming Names | Interviewer-Rated Participant Enthusiasm for Naming Names | Interviewer-Rated Participant Enthusiasm for Recruiting Network Members | Interviewer-Rated Participant Enthusiasm for Recruiting Network Members | |
| Unstandardized Coefficient (Standard Error) | ||||||
| Unadjusted Model: | Model 1: | Model 2: | Model 3: | Model 4: | Model 5: | Model 6: |
| Experiences of Stigma | −0.68 (0.76) | 0.29 (0.22) | 1.53 (0.21)*** | |||
| Experiences of Support | −0.05 (0.16) | −0.21 (0.08)** | −1.25 (0.08)*** | |||
| Perceptions of Stigma among Social Network | −0.71 (0.83) | 1.28 (0.45)** | 2.23 (0.49)*** | |||
| Perceptions of Support among Social Network | 0.20 (0.48) | 0.61 (0.31)* | 1.58 (0.33)*** | |||
| Adjusted Model: | Model 1 Adjusted: | Model 2 Adjusted: | Model 3 Adjusted: | Model 4 Adjusted: | Model 5 Adjusted: | Model 6 Adjusted: |
| Experiences of Stigma | −0.56 (0.79) | 0.26 (0.22) | 1.51 (0.21)*** | |||
| Experiences of Support | −0.04 (0.17) | −0.24 (0.08)** | −1.27 (0.08)*** | |||
| Perceptions of Stigma among Social Network | −0.76 (0.91) | 1.20 (0.45)** | 2.13 (0.49)*** | |||
| Perceptions of Support among Social Network | 0.46 (0.55) | 0.36 (0.32) | 1.40 (0.35)*** | |||
| Number of Sex and/or Injection Partners | −0.02 (0.02) | 0.02 (0.08) | 0.02 (0.01) | 0.10 (0.03)** | 0.01 (0.01) | 0.04 (0.04) |
| HIV Positive | 0.36 (0.35) | 0.60 (0.52) | −0.29 (0.18) | −0.01 (0.30) | −0.28 (0.18) | 0.09 (0.33) |
| PWID | 0.14 (0.34) | −0.49 (0.62) | 0.76 (0.17)*** | 0.24 (0.30) | 0.67 (0.16)*** | 0.19 (0.33) |
| Homeless | 0.15 (0.56) | −0.11 (0.74) | −0.62 (0.23)** | −0.06 (0.35) | −0.35 (0.22) | −0.46 (0.39) |
| Straight | −0.56 (0.70) | 1.41 (1.99) | −0.52 (0.48) | −0.44 (0.96) | −0.25 (0.46) | 0.95 (1.03) |
| Unemployed | −0.67 (0.34)† | −0.18 (0.52) | −0.20 (0.16) | −0.23 (0.28) | −0.19 (0.16) | −0.25 (0.31) |
| Completed High School | 0.29 (0.38) | 0.29 (0.53) | −0.13 (0.20) | −0.33 (0.32) | −0.26 (0.19) | −0.51 (0.36) |
signifies p < .001;
signifies p < .01;
signifies p < .05;
signifies p < .10
Among the full Athens sample (Table 2), perceptions of greater HIV-related support among friends and/or family predicted greater interviewer-rated “enthusiasm for naming names” of network members during participant interviews (B = 0.68; p = .028) and greater “enthusiasm for recruiting others” to the study (B = 0.83; p = .014), as did more baseline-reported (i.e., previous) experiences of HIV-related support (for enthusiasm for recruiting others: B = 0.37; p = .073) and fewer previous experiences of HIV-related stigma (for enthusiasm for naming names: B = −0.39; p = .090; for enthusiasm for recruiting others: B = −0.50; p = .043).
Among recently-infected Athens participants (N = 45; Table S4), experiences of HIV-related stigma were unrelated to recruitment of network members, but having had more experiences of HIV-related support predicted recruiting a high number of people to the study (B = 0.53; p = .049). Also among PwRI, perceptions of HIV-related support were unrelated to recruitment of network members, but perceptions of more stigma among friends and/or family predicted (at a level of marginal significance) recruiting a lower number of people to the study (B = −0.64; p = .082).
Among HIV-negative participants in Athens, perceptions of greater HIV-related support among friends and/or family predicted recruiting a higher number of people to the study (B = 1.21; p = .047), but perceptions of stigma and experiences of both stigma and support were not significantly related to recruitment of network members. No relationships were found between stigma or support and network member recruitment among the subsample of all Athens HIV-positive participants.
In Odessa, number of network members recruited by each participant was not found to be related to perceptions of stigma or support or to experiences of stigma or support (Table 3). This was also true among HIV status-based subsamples in Odessa (Table S4). However, among all Odessa participants, fewer reported experiences of support (B = −0.24; p = .003) and perceptions of more stigma among participants’ social networks (B = 1.20; p = .008) predicted significantly higher interviewer-rated enthusiasm for naming network members’ names (Table 3). This pattern of association seemed to be driven primarily driven by HIV-negative participants, as it held true for that subgroup, but not for HIV-positive or recently-infected participants (Table S4).
Also among Odessa participants, a greater number of reported experiences of stigma (B = 1.51; p < .0005) and perceptions of more stigma among participants’ social networks (B = 2.13; p < .0005) were both associated with higher interviewer-rated participant enthusiasm for recruiting network members (Table 3). Also, fewer reported experiences of HIV-related support (B = −1.27; p < .0005), but perceptions of more HIV-related support among participants’ social networks (B = 1.40; p < .0005) predicted higher interviewer-rated participant enthusiasm for recruiting network members. The association between experiences of stigma and participant enthusiasm for recruiting network members again appeared to be driven by HIV-negative participants (Table S4), but the associations between enthusiasm for recruiting network members and both experiences of support and perceptions of both stigma and support all maintained the same patterns among both HIV-positive and HIV-negative participants in Odessa.
Discussion
The TRIP intervention study represents an important part of the growing body of evidence that network-based interventions are an effective method of locating recent (Nikolopoulos et al., 2016; Vasylyeva et al., 2014) and/or undiagnosed (Smyrnov et al., 2018) HIV infections (and possibly also of locating individuals who would not otherwise be likely to present for standard HIV testing mechanisms - e.g., clinics) and linking them to care to improve health outcomes and reduce HIV transmission. However, to date, there has been limited empirical evidence about potential risks and other potential benefits of such interventions to participants. Since network-based interventions involve social processes (specifically, recruiting and being recruited by one’s risk and/or social contacts to an HIV-related study), their potential effects on important HIV-related psychosocial outcomes, including HIV-related stigma and support, should be monitored and assessed empirically.
Conversely, given the great deal of available evidence suggesting that HIV-related stigma can be a barrier to HIV-related services (e.g., Carr et al., 2004; Weiser et al., 2003; Williams & Aber, under review), and predicts HIV-related outcomes (e.g., Williams & Aber, under review) and HIV-related health and risk behaviors (e.g., Rao et al., 2007; Vanable et al., 2006; Wolitski et al., 2009), its potential effects on participants’ ability and willingness to engage in network-based recruitment for HIV-related health services need to be better understood. The efficacy of network-based HIV testing interventions depends upon participants’ recruiting others (and feeling comfortable doing so), and fear of stigma could impede this process to a degree that could be detrimental to the intervention’s potential effects on HIV transmission. On the other hand, social support could facilitate it. Better understanding these relationships could have important implications for learning “best practices” of implementation in contexts with varying levels of HIV-related stigma and/or support.
The present study assessed the extent to which HIV-related stigma and support were experienced by TRIP participants (Question 1), whether such experiences varied significantly by study site, study arm, HIV status, and other important participant characteristics (Question 2), and whether such experiences increased or decreased during the course of the study (Question 3). Results suggest that the TRIP intervention does not present an unduly high risk of experiencing increased HIV-related stigma. First, reports of stigma were low, overall (Question 1). The mean number of types of stigma experienced by any TRIP participant was 0.16 out of a possible 4. Only 11.3% of participants reported any experiences of stigma (and only 16.8% of HIV-positive participants). This is consistent with results from Project Protect (the pilot study preceding TRIP), during which no reports of major stigmatizing events occurred (Vasylyeva et al., 2014). Second, neither PwRI and their networks nor LT positive seeds and their networks (i.e., those who participated in network recruitment) experienced significantly more stigmatizing experiences than did negative control participants (Question 2). This means that recently-infected and longer-term positive participants who recruited their network members to the study (and thereby potentially risked others inferring or assuming they were HIV-positive) were no more likely to experience stigma than were participants who were HIV-negative and did not recruit anyone to the study. In other words, the groups that we would expect to be most at risk for stigma-related harms did not seem to have experienced them.
Experiences of HIV-related stigma did not change significantly between baseline and follow-up for the full TRIP sample, and actually significantly decreased during the course of the study at the Chicago site (Question 3). Perceptions of HIV-related stigma among family and friends also significantly decreased among the full TRIP sample during the course of the study. In addition to the strong unlikelihood that participation in TRIP did any stigma-related harm to participants, these findings suggest a possibility that TRIP may benefit participants. It is plausible, for example, that the social processes involved in recruiting and being recruited by one’s network members for HIV testing facilitate more open discussion about HIV and HIV-related care, and change stigma-related norms within networks (and/or individuals’ perceptions of stigma). Additionally, participants’ perceptions of stigma among people they know may decrease during the course of the study as they become better acquainted with project personnel who care about them. Although the design of the present study does not allow for causal inferences to be drawn, it provides preliminary data supporting the merit of future research assessing whether network-based interventions such as TRIP can actually cause reductions in stigma through such processes.
Our analyses on HIV-related support similarly provide preliminary evidence that TRIP could possibly benefit participants by increasing HIV-related support. First, reports of HIV-related support were high, overall (Question 1). The mean number of types of support experienced by TRIP participants was 0.83 out of a possible 3. Almost 44% of participants (and 56% of HIV-positive participants) reported experiences of HIV-related support. Second, experiences of HIV-related support were significantly higher among both PwRI and their networks, and LTP seeds and their networks (i.e., those who participated in network recruitment) than among negative control participants (Question 2). This means that recently-infected and longer-term positive participants who recruited their network members to the study (and thereby potentially risked others inferring or assuming they were HIV-positive) were more likely to experience HIV-related social support than were participants who were HIV-negative and did not recruit anyone to the study. While this is truly a “common sense” finding in that we would intuitively expect HIV-positive participants to receive more HIV-related support than HIV-negative participants, it at least suggests that participation in TRIP does not likely pose any impediments to such support. This tentative conclusion is greatly strengthened by results from Question 3 which indicate that experiences of HIV-related support significantly increased between baseline and follow-up 1) among recently-HIV-infected TRIP participants across all sites and 2) among all participants at the Odessa site. However, perceptions of HIV-related support by friends and family members were found to significantly decrease among the full TRIP sample and among HIV-positive TRIP participants. Although certainly not indicative of a benefit to participants, it is plausible that this finding is due in part to participant interactions during the course of the TRIP study with highly supportive project and/or medical staff which led participants to re-evaluate (i.e., to more critically evaluate) the degree of support of friends and family members.
Finally, the present study assessed the extent to which HIV-related stigma and support predicted participant engagement in TRIP (Question 4). In Athens, where stigma was highest, perceptions of stigma were found to predict (in a negative direction) number of network members recruited to the study among recently-infected participants, and experiences of stigma were found to (negatively) predict interviewer-rated enthusiasm both for naming and for recruiting network members. Also in Athens, where HIV-related support was least frequent, both perceptions and experiences of HIV-related support were found to predict both types of participant engagement (number of network members recruited and enthusiasm for naming and/or recruiting network members).
Interestingly, in Odessa, where stigma was lowest and support was highest, stigma and support were found to be unrelated to the number of network members each participant recruited to the study. However, both experiences and perceptions of stigma and support in Odessa significantly predicted interviewer-rated enthusiasm for engaging in the components of the intervention (naming and recruiting network members). The pattern of associations among these constructs in Odessa is somewhat complex and counter-intuitive: participants who reported fewer experiences of support, but perceptions of more support among their networks, were rated by interviewers as higher in enthusiasm for engaging in the intervention; participants who reported more experiences of stigma and perceptions of more stigma among their networks were also rated by interviewers as higher in enthusiasm for engaging in the intervention. One possible interpretation for this pattern of associations is based on both participants’ positive interactions with interviewers and on participants’ altruism towards their network members. Participants may experience very positive interactions with the interviewer which are very uncharacteristic of the interactions they often have with service providers and other people with whom they interact. It may then be that the more unlike their previous experiences this particular interaction was (i.e., among those with worse previous experiences), the more intensely they reacted to it, and the more eager participants then were to present their friends and other network members with an opportunity to themselves have such an uncharacteristically positive and supportive experience (i.e., to recruit them to the study). Of course, this interpretation is speculative, and more work must be done by future research to better understand the implications of this somewhat complex pattern of results.
Taken together, these findings from both Athens and Odessa suggest that HIV-related stigma and support may have important implications for implementation of the TRIP intervention, and perhaps for other similar network-based HIV interventions as well. Particularly in settings with high average levels of stigma and low average levels of HIV-related support, individual variation in experiences and perceptions of HIV-stigma may in fact be a barrier to fully engaged participation in network recruitment, and individuals’ experiences and perceptions of greater HIV-related support may help to facilitate intervention engagement. Future research should work to unpack site-level variation in patterns of association, and should test possible strategies and mechanisms to address stigma as a barrier to (and to capitalize on or to possibly even increase available social support as a potential facilitator of) participant engagement in interventions involving network-based participant recruitment (e.g., the use of a stigma-reducing module preceding network-tracing and recruitment), since improving participant engagement could help to optimize intervention efficacy (i.e., to locate more HIV-infected unaware and successfully link them to care) and thereby improve the ability of such interventions to reduce HIV transmission.
Limitations
The present findings cannot be appropriately interpreted without acknowledging this study’s most critical limitations. First, the TRIP study was not an experimental one. Therefore, our findings are purely associational. There are many threats to internal validity which cannot be addressed with our data, and which prevent us from inferring that participation in TRIP did not cause increased stigma and/or did cause social support-related benefits. Future studies should address this limitation directly using experimental designs.
Second, the measures used for stigma and support (both for experiences and for perceptions) were developed by the TRIP team to assess stigma and support related to recent infection. The use of these new measures introduced two important limitations: 1) they were comprised of only a few items (with only binary response options, in the case of experiences of stigma) and some therefore achieved only moderate internal consistency; and 2) although the items asked explicitly about experiences related to recent infection, our experiences using these items during the TRIP study suggested that LTP participants were very likely referring to their experiences related to HIV infection generally (and not to recent infection). Both of these limitations could be reduced in future studies on the degree to which stigma is related to participation in network-based interventions by using more standard, validated measures of perceptions and/or experiences of HIV-related (and not recent HIV-infection-related) stigma. Such measures (e.g., Westbrook & Bauman, 1996; Genberg et al., 2008) are typically comprised of a greater number of items, and are thus able to achieve better reliability than did the measures used in the present study. More importantly, their use would likely improve consistency across participant groups (i.e., across groups of recently-infected and not recently-infected participants) in terms of the range of experiences to which their responses refer. Additionally, our measures of participants’ enthusiasm for naming and recruiting network members were based on interviewer perceptions, and were therefore highly subjective and prone to a high degree of measurement error. Future research should ask participants directly about their enthusiasm (or reticence) for participating, and could also potentially triangulate such participant self-report data with interviewer perceptions.
Third, retention rates between baseline and follow-up were low for the present study – particularly for the Odessa site. We were able to retain participants with characteristics that may make them more “vulnerable” to stigma (e.g., PWID, HIV-positive, non-heterosexual) at higher rates than those without such characteristics (although we did have trouble retaining homeless participants for follow-up, particularly in Odessa), and we also found that those who completed follow-up were no different from those who did not in terms of experienced stigma, and were actually more likely to have perceived higher stigma among their social networks than were people who did not complete follow-up. We therefore conclude that the present findings are not likely biased towards reflecting experiences and perceptions of a “less-relevant” subsample (i.e., of people who experienced less stigma). Our results are nonetheless biased in some unknown direction by participant selection into completion of follow-up interviews. Relatedly, it is possible that our baseline sample was also biased towards including people who had previously experienced less stigma or who were less vulnerable to stigma. It is quite plausible that people with the most acute past experiences of stigma and with the highest levels of fear of stigma would not have agreed to participate in the study at all. This is unfortunately a potentially major limitation of the present study. Although we are hopeful that network members’ recruitment to the study by trusted risk partners would help to encourage such people to participate, we cannot know this.
Fourth, there were some noteworthy differences in implementation between sites which may have impacted results. Specifically, 1) sites varied on the key populations of focus that were most heavily recruited (Athens recruited mostly PWID; Chicago recruited predominately MSM; and Odessa recruited a mix of PWID and sex workers); 2) sites varied dramatically on the degree to which venue-based recruitment was used as compared to network-based recruitment, with Odessa recruiting far more participants from venues than did the other sites; 3) sites varied in terms of community outreach efforts that were done at baseline to distribute educational pamphlets on recent HIV infection and to discourage associated stigma; and 4) sites varied on the degree to which seeds were recruited based on their heavy involvement with other HIV services and programs (e.g., in Odessa, most LTP seeds had long-term relationships with an organization providing them with HIV-related services, which likely contributed to the high levels of support they reported). We have dealt with these differences in part by presenting site-specific findings for each research question, but additional research needs to be done before we can fully understand how such differences should affect our interpretation of findings for each site.
Finally, analyses of the relationships between stigma and support and participant engagement (Question 4) were limited in Odessa by a small sample size of relevant cases (i.e., those who were asked to recruit others to the study). A large proportion of Odessa participants were recruited via venue-based recruiting, and were therefore excluded from these analyses, which focused on direct coupon-based recruitment among network members.
Conclusions
The present findings, though limited in their ability to draw causal inferences, provide preliminary evidence that the TRIP network-based case-finding intervention was safe for participants in all three of its sites (Athens, Odessa, and Chicago) in terms of stigma-related risks. The results of this study - in combination with the fact that constant monitoring by project staff for potential adverse events uncovered no such events, and also with the fact that TRIP’s predecessor, Project Protect, also precipitated no such negative events (Vasylyeva et al., 2014) - strongly suggest that TRIP did no stigma-related harm to participants. Additionally, the present findings provide preliminary support for the notion that TRIP may have some potential benefits to participants in the form of reduced stigma and/or increased social support among some groups of participants at some sites. Such potential benefits, however, must be investigated in future studies which can achieve a higher degree of internal validity.
Taken together with our previous findings that TRIP was effective at locating recent (Nikolopoulos et al., 2016) and undiagnosed HIV infections (Smyrnov et al., 2018; Morgan et al., 2018) and linking them to treatment (Psichogiou et al., under review), the present promising findings serve to strengthen our conclusions that TRIP (and perhaps other similar network-based interventions) has (have) an important place in public health efforts to move towards 90-90-90 goals. Our current finding that stigma and support predict participant engagement in TRIP, at least in some contexts and for some key groups of participants, suggests that there might be ways to improve implementation practices for network-based HIV interventions in varying contexts (e.g., by decreasing levels of stigma and/or increasing levels of support as needed). Future work should strive to better understand how local conditions affect stigma and support, and how implementation of network-based interventions like TRIP should be modified to accommodate such site-level differences, so that intervention efficacy can be optimized and the largest possible impact on HIV transmission and HIV health outcomes can be achieved.
Supplementary Material
Acknowledgments
Research reported in this presentation was supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number DP1 DA034989 Transmission Research Intervention Project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Our NIDA Project Officer, Elizabeth Lambert, has been a great source of wise counsel and advice. We also acknowledge assistance from the hundreds of participants in this project and hope that it has improved their lives and health as well as those of people in their networks and communities.
Funding. This study was funded by the National Institute on Drug Abuse (DP1DA034989; PI Dr. Samuel R. Friedman).
Footnotes
Respondent-driven sampling is an approach that uses participant referrals and participant-driven recruitment with an aim of reaching a representative sample of a hidden population (e.g., a very high-risk group) that is otherwise not easy to sample. Social network-based and risk network-based sampling, on the other hand, aim to recruit all (realistically, as large a proportion as possible of) relevant members of participants’ social network or risk network in order to understand social and/or risk connections and patterns or to perform a public health intervention within them.
Note that analyses of changes between baseline and follow-up for the present study reflect only associations, as data for the TRIP study are not experimental.
Disclosure of Potential Conflicts of Interest. None of the authors have any conflicts of interest to declare. Leslie D. Williams declares that she has no conflict of interest. Ania Korobchuk declares that she has no conflict of interest. Eirini Pavlitina declares that she has no conflict of interest. Georgios K. Nikolopoulos declares that he has no conflict of interest. Britt Skaathun declares that she has no conflict of interest. John Schneider declares that he has no conflict of interest. Evangelia-Georgia Kostaki declares that she has no conflict of interest. Pavlo Smyrnov declares that he has no conflict of interest. Tetyana I. Vasylyeva declares that she has no conflict of interest. Mina Psichogiou declares that she has no conflict of interest. Ethan Morgan declares that he has no conflict of interest. Andria Hadjikou declares that she has no conflict of interest. Martin J. Downing, Jr. declares that he has no conflict of interest. Angelos Hatzakis declares that he has no conflict of interest. Samuel R. Friedman declares that he has no conflict of interest.
Research Involving Human Participants. Institutional review board at National Development and Research Institutes (NDRI) in New York, NY approved all study procedures. Additionally, the institutional review board at the University of Chicago in Chicago, Illinois approved all procedures for the Chicago study site; the Medical Ethics Committee at Gromashevsky Institute of Epidemiology and Infectious Diseases in Ukraine approved all procedures for the Odessa, Ukraine study site; and the institutional review board at the Hellenic Society for the Study of AIDS and Sexually Transmitted Diseases in Athens, Greece approved all study procedures for the Athens, Greece study site.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval: This article does not contain any studies with animals performed by any of the authors.
Informed Consent. Informed consent was obtained for all study participants. All consent and assent forms were reviewed and approved by all institutional review boards in New York, Chicago, Athens, and Odessa, as described above.
References
- Amirkhanian YA, Kelly JA, Kabakchieva E, McAuliffe TL, & Vassileva S (2003). Evaluation of a social network HIV prevention intervention program for young men who have sex with men in Russia and Bulgaria. AIDS Education and Prevention, 15(3), 205. [DOI] [PubMed] [Google Scholar]
- Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C et al. (2007). High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis, 195(7):951–959. DOI: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- Carr RL, & Gramling LF (2004). Stigma: A health barrier for women with HIV/AIDS. Journal of the Association of Nurses in AIDS Care, 15(5), 30–39. [DOI] [PubMed] [Google Scholar]
- Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim AA, Nkengasong JN, Parekh BS, 2012. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One 7, e33328. doi: 10.1371/journal.pone.0033328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Downing MJ, Smyrnov P, Nikolopoulos G, Schneider J, Livak B & Hatzakis A. (2014). Socially-integrated transdisciplinary HIV prevention. AIDS and Behavior, 18(10), 1821–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg BL, Kawichai S, Chingono A, et al. Assessing HIV/AIDS stigma and discrimination in developing countries. AIDS Behav. 2008;12:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Penedo FJ, Antoni MH, Durán RE, McPherson-Baker S, Ironson G, & Schneiderman N. (2004). Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology, 23(4), 413. [DOI] [PubMed] [Google Scholar]
- Hall HI, Holtgrave DR, Maulsby C. (2012). HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS, 26(7):893–896. DOI: 10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- Hickey MD, Salmen CR, Omollo D, Mattah B, Fiorella KJ, Geng EH, & Tessler RA (2015). Implementation and operational research: pulling the network together: quasiexperimental trial of a patient-defined support network intervention for promoting engagement in HIV care and medication adherence on Mfangano Island, Kenya. JAIDS Journal of Acquired Immune Deficiency Syndromes, 69(4), e127–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, DiMarco M, Austin J, Luke W, & DiFonzo K. (2003). Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. Journal of Behavioral Medicine, 26(4), 315–332. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Davey-Rothwell MA, Knowlton AR, Alexander KA, Williams CT, & Boodram B (2013). Social network approaches to recruitment, HIV prevention, medical care, and medication adherence.Journal of acquired immune deficiency syndromes (1999), 63(0 1), S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzel A, Shilaih M, Yang WL, Böni J, Yerly S, Klimkait T et al. (2016). HIV-1 Transmission During Recent Infection and During Treatment Interruptions as Major Drivers of New Infections in the Swiss HIV Cohort Study. Clin Infect Dis, 62(1):115–122. DOI: 10.1093/cid/civ732. [DOI] [PubMed] [Google Scholar]
- Morgan E, Skaathun B, Nikolopoulos GK, Paraskevis D, Williams LD, Smyrnov P, Friedman SR, & Schneider JA. (2018). A network intervention to locate newly HIV infected persons within MSM networks in Chicago. AIDS and Behavior. 10.1007/s10461-018-2202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ncama BP, McInerney PA, Bhengu BR, Corless IB, Wantland DJ, Nicholas PK, & Davis SM. (2008). Social support and medication adherence in HIV disease in KwaZulu-Natal, South Africa. International Journal of Nursing Studies, 45(12), 1757–1763. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos GK, Katsoulidou A, Kantzanou M, Rokka C, Tsiara C, Sypsa V, & Hatzakis A (2017). Evaluation of the limiting antigen avidity EIA (LAg) in people who inject drugs in Greece. Epidemiology & Infection, 145(2), 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos GK, Pavlitina E, Muth SQ, Schneider J, Psichogiou M, Williams LD, Paraskevis D, Sypsa V, Magiorkinis G, Smyrnov P, Korobchuk A, Vasylyeva T, Skaathun B, Malliori M, Kafetzopoulos E, Hatzakis A & Friedman SR. (2016). A network intervention that locates and intervenes with recently HIV-infected persons: The Transmission Reduction Intervention Project (TRIP). Scientific Reports, 6:38100 DOI: 10.1038/srep38100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passin WF, Kim AS, Hutchinson AB, Crepaz N, Herbst JH, Lyles CM, & HIV/AIDS Prevention Research Synthesis Project Team. (2006). A systematic review of HIV partner counseling and referral services: client and provider attitudes, preferences, practices, and experiences. Sexually transmitted diseases, 33(5), 320–328. [DOI] [PubMed] [Google Scholar]
- Power R, Koopman C, Volk J, Israelski DM, Stone L, Chesney MA, & Spiegel D. (2003). Social support, substance use, and denial in relationship to antiretroviral treatment adherence among HIV-infected persons. AIDS Patient Care and STDs, 17(5), 245–252. [DOI] [PubMed] [Google Scholar]
- Psichogiou M, Giallouros G, Pantavou K, Pavlitina E, Papadopoulou M, Williams LD, Paraskevis D, Friedman SR, & Nikolopoulos GK. (under review). Identifying, linking, and treating people who inject drugs and were recently infected with HIV. Manuscript submitted to AIDS Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulerwitz J, Michaelis AP, Lippman SA, Chinaglia M, & Diaz J (2008). HIV-related stigma, service utilization, and status disclosure among truck drivers crossing the Southern borders in Brazil. AIDS care, 20(7), 764–770. [DOI] [PubMed] [Google Scholar]
- Rao D, Kekwaletswe TC, Hosek S, Martinez J, & Rodriguez F (2007). Stigma and social barriers to medication adherence with urban youth living with HIV. AIDS care, 19(1), 28–33. [DOI] [PubMed] [Google Scholar]
- Sherman SG, Sutcliffe C, Srirojn B, Latkin CA, Aramratanna A, & Celentano DD (2009). Evaluation of a peer network intervention trial among young methamphetamine users in Chiang Mai, Thailand. Social Science & Medicine, 68(1), 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyrnov P, Williams LD, Korobchuk A, Sazonova Y, Nikolopoulos G, Skaathun B & Friedman SR. (2018). Risk network approaches to locating undiagnosed HIV cases in Odessa, Ukraine. Journal of the International AIDS Society, 21(1), e25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan JM, & Nyblade L (2013). HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: a review of the evidence. AIDS and Behavior, 17(7), 2528–2539. [DOI] [PubMed] [Google Scholar]
- UNAIDS. (2014). 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS): UNAIDS/JC2684; http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf [Google Scholar]
- Vanable PA, Carey MP, Blair DC, & Littlewood RA (2006). Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS and Behavior, 10(5), 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasylyeva TI, Friedman SR, Smyrnov P, & Bondarenko K (2015). A new approach to prevent HIV transmission: Project Protect intervention for recently infected individuals. AIDS care, 27(2), 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasylyeva TI, Friedman SR, Lourenco J, Gupta S, Hatzakis A, Pybus OG et al. (2016). Reducing HIV infection in people who inject drugs is impossible without targeting recently-infected subjects. AIDS, 30(18): 2885–2890. DOI: 10.1097/QAD.0000000000001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, Makhema J, & Marlink R (2003). Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. JAIDS-HAGERSTOWN MD-, 34(3), 281–288. [DOI] [PubMed] [Google Scholar]
- Westbrook L & Bauman L (1996). Perceived Stigma of HIV/AIDS: Public View. Bronx, NY: Albert Einstein College of Medicine. [Google Scholar]
- Williams LD, Aber JL, & SIZE Research Group. (Under review). HIV/AIDS-related Stigma, Health Service Barriers, and HIV Outcomes in South Africa: Using a Multilevel Framework to Test Empirical Relationships. Manuscript submitted to AIDS and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LD, Korobchuk A, Smyrnov P, Sazonova Y, Nikolopoulos G, Skaathun B, Morgan E, Schneider J, Vasylyeva TI, Duong YT, Chernyavska S, Goncharov V, Kotlik L, & Friedman SR (under review). Social network approaches to locating people recently infected with HIV in Odessa, Ukraine. Manuscript submitted to Journal of the International AIDS Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitski RJ, Pals SL, Kidder DP, Courtenay-Quirk C, & Holtgrave DR (2009). The effects of HIV stigma on health, disclosure of HIV status, and risk behavior of homeless and unstably housed persons living with HIV. AIDS and Behavior, 13(6), 1222–1232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


