TO THE EDITOR:
Platin-based chemotherapeutic agents are associated with hypersensitivity reactions (HSRs), occurring in 12% of patients with gynecologic malignancy receiving carboplatin.1 HSRs occur in 5–16% of patients with gynecologic malignancy receiving cisplatin, and up to 25% in patients with multiple cancer types (including gynecologic) receiving oxaliplatin.2–3 Platin HSRs may lead to use of alternative therapies with potentially worse outcomes particularly for platin responsive-tumors.4 Although skin testing (ST), desensitization and risk stratification protocols for platin HSRs allow patients to safely receive platins, they are not widely used.5
To better understand the safety of platin ST, we conducted a literature review from January 2000 to June 2018 (Table 1) using the following search terms in PubMed: carboplatin ST (69 results), cisplatin ST (95 results), oxaliplatin ST (44 results), and platinum ST (166 results). Other search terms (e.g., carboplatin testing, cisplatin testing, oxaliplatin testing, platin ST) did not yield additional articles. We found 44 studies describing 1,393 patients who received platin ST. Two (0.1%) systemic reactions occurred immediately after ST. One patient with positive carboplatin intradermal testing (concentration not reported) developed symptoms 30 minutes later: diffuse erythroderma and subjective chills, dyspnea, and chest discomfort.6 The authors reported no signs of anaphylaxis. Symptoms resolved with corticosteroids and antihistamines. The patient did not receive further platin agents. The second patient, a 45 year-old woman with metastatic colorectal cancer, developed eyelid swelling, chest erythema, diffuse pruritus, and restlessness 15 minutes after a positive intradermal oxaliplatin test (0.5 mg/mL).7 The patient received intramuscular epinephrine, intravenous dexclorpheniramine and hydrocortisone. Symptoms resolved within minutes. The patient underwent an oxaliplatin 12-step desensitization with premedication (aspirin, montelukast, ranitidine, and dexchlorpheniramine). At the last step, the patient experienced pruritus on the palms and soles. The infusion was stopped, and the patient received dexchlorpheniramine. The infusion was restarted after 30 minutes at the last step, which the patient tolerated. The patient underwent four additional desensitizations without reaction.
TABLE 1.
Published studies with platin skin testing since 2000
| Author (last name) | Year | Drug(s) tested: Ca, Cis, O | Total number of patients |
Systemic reactions |
|---|---|---|---|---|
| Zanotti | 2001 | Ca | 47 | None |
| Meyer | 2002 | O | 8 | Not discussed |
| Porzio | 2002 | Ca, Cis | 1 | None |
| Garufi | 2003 | O | 5 | Not discussed |
| Markman | 2003 | Ca | 126 | “A single individual developed a 3-cm wheal and flare around the injection site that progressed over a few minutes to total body erythroderma. The patient complained of chills, chest discomfort, and mild dyspnea, but there were no other signs of severe anaphylaxis (e.g., wheezing, intense anxiety, hypotension). After treatment with diphenhydramine and corticosteroids, the symptoms completely resolved.” |
| Moreno-Ancillo | 2003 | Ca | 1 | Not discussed |
| Thomas | 2003 | O | 3 | Not discussed |
| Choi | 2004 | Ca | 8 | Not discussed |
| Lee | 2004 | Ca | 5 | Not discussed |
| Confino-Cohen | 2005 | Ca | 23 | Not discussed |
| Lee | 2005 | Ca | 26 | Not discussed |
| Herrero | 2006 | O | 5 | Not discussed |
| McAlpine | 2006 | Ca | 12 | None |
| Edmondson | 2007 | O | 1 | None |
| Leguy-Seguin | 2007 | Ca, Cis, O | 21 | None |
| Castells | 2008 | Ca | 88 | Not discussed |
| Enrique | 2008 | Ca | 2 | Not discussed |
| Pagani | 2008 | O | 4 | None |
| Gomez | 2009 | Ca | 54 | Not discussed |
| Hesterberg | 2009 | Ca | 38 | Not discussed |
| Syrigou | 2009 | Ca | 3 | None |
| Visitsunthorn | 2009 | Ca | 2 | None |
| Gottlieb | 2010 | Ca, O | 11 | None |
| Herrera | 2010 | Ca | 1 | None |
| Gastaminza | 2011 | Ca, O | 2 | None |
| Pagani | 2012 | Ca | 3 | Not discussed |
| Patil | 2012 | Ca | 39 | Not discussed |
| Caiado | 2013 | Ca, O | 12 | Not discussed |
| Cortijo-Cascajares | 2013 | O | 21 | Not discussed |
| Madrigal-Burgaleta | 2013 | Ca, Cis, O | 32 | Not discussed |
| Wong | 2013 | O | 46 | Not discussed |
| Bruchim | 2014 | Ca | 49 | None |
| Martin-Lazaro | 2014 | O | 1 | “Within 15 minutes, the patient began to experience itching of the eyes and nose, palpebral swelling, generalized pruritus (especially on the palms, soles and genitals), chest erythema, and restlessness. She received intramuscular epinephrine, and intravenous dexchlorpheniramine and hydrocortisone, and recovered within minutes. She did not experience any delayed reaction.” |
| Alvarez-Cuesta | 2015 | Ca, Cis, O | 74 | Not discussed |
| D’Amelio | 2015 | Ca, Cis, O | 1 | None |
| Wang | 2015 | Ca, O | 142 | Not discussed |
| Altwerger | 2017 | Ca | 129 | Not discussed |
| Brault | 2017 | Ca, Cis, O | 86 | Not discussed |
| Galvao | 2017 | Ca | 138 | Not discussed |
| Giavina-Bianchi | 2017 | Ca, O | 15 | Not discussed |
| Mawhirt | 2017 | Ca, O | 36 | Not discussed |
| Solomon | 2017 | Cis | 1 | None |
| Capelle | 2018 | Ca, Cis | 22 | Not discussed |
| Perez-Rodriguez | 2018 | Ca, Cis, O | 49 | Not discussed |
Ca, carboplatin; Cis, cisplatin; O, oxaliplatin.
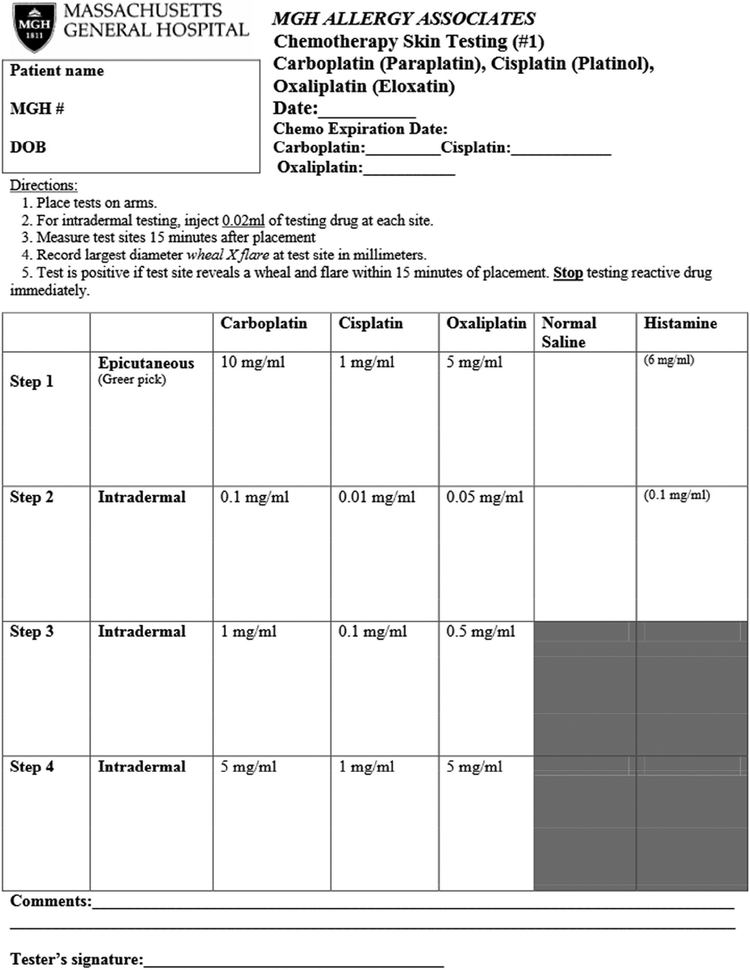
Experience at our own institution supports the safety of platin ST. Our group performed platin ST (Figure 1) on 179 patients from October 2013 to May 2018, and no systemic reactions during ST occurred. Despite this safety profile, there is a theoretical risk of anaphylaxis with any allergy testing procedure. It is important to perform ST in a supervised medical setting with trained staff and emergency and hypersensitivity medications (such as epinephrine, glucagon, corticosteroids, antihistamines, and albuterol) available. Chemotherapy ST is resource-intensive and requires careful planning prior to implementation. Allergy practices need access to a pharmacy that prepares chemotherapy dilutions, and staff trained to handle chemotherapeutic agents safely with appropriate personal protective equipment.
FIGURE 1.
Platin ST Protocol
Platin drug challenge, although not routinely performed in the United States, has been studied in platin HSR evaluations.8,9 In a 2013 study, low-risk patients with platin HSRs and negative platin ST underwent platin challenge; 7 out of 12 (58%) were negative and did not need desensitization.8 Another study concluded that antineoplastic drug challenges can reduce desensitizations (32% of platin challenges were negative).9 However, there were 21 positive platin challenges and one patient had anaphylaxis (hives, hypoxemia, hypotension, dyspnea, and wheezing) and required epinephrine. Platin drug challenges may be harder to spread in the United States because of this high risk of HSR.
Risk stratification and drug desensitization are critical steps in HSR evaluation. Our group developed and published chemotherapy risk stratification algorithms that identify patients that are not allergic (see Figure E1 in the Online Repository). Patients start in the inpatient setting, generally with an intermediate desensitization protocol (see Figure E2 in the Online Repository).5 Protocol progression is determined by undergoing desensitization without reactions and remaining negative on repeat ST. The risk stratification process identifies patients who are not allergic to platin agents, despite a supposed allergic reaction history. The goal is for non-allergic patients to safely return to outpatient treatments, typically at a 50% infusion rate. By reducing the number of inpatient desensitizations, this progression ideally leads to increased patient satisfaction and compliance, as well as cost savings, while ensuring the safety of these patients.
While chemotherapy desensitization is safe and effective, it is also resource-intensive.5
Desensitizations require prolonged infusions, dedicated infusion beds/chairs, pharmacy access to chemotherapy, trained staff, and space, which increase costs. At our institution, we implemented an outpatient infusion chair program in which patients at low risk for HSR (e.g. no or minimal reactions during prior desensitizations) receive their desensitization in an outpatient infusion chair. These desensitizations are treated as outpatient procedures. Chemotherapy orders and dilutions are prepared in advance to ensure a timely start. In a process improvement analysis, we showed that utilizing outpatient chemotherapy desensitization chairs shortened time to starting desensitizations and increased patient satisfaction, while maintaining safety.10 This work suggests that low-risk desensitizations appear safe to conduct in an outpatient setting.
Despite the utility and safety of chemotherapy ST and desensitization, these procedures are infrequently performed. In early 2018, we conducted a national survey of practicing allergy/immunology physicians, via an email through the AAAAI leadership. Our 8-question survey aimed to investigate practice patterns for chemotherapy HSR evaluation. Though few respondents (13/72; 18%) performed chemotherapy ST in their office or performed desensitizations, most respondents (51/71; 72%) expressed interest in learning how to implement these protocols (unpublished data). The major limitation of this survey study was the low response rate (9% of AAAAI membership, consistent with average AAAAI survey response rates). Still, these survey results highlighted respondents’ interest in education about chemotherapy testing.
In summary, chemotherapy ST, risk stratification, and desensitization can be performed safely and improve patient outcomes. A platin HSR should not lead to use of second-line agents, as allergic patients can safely receive chemotherapy through desensitization. Risk stratification protocols can identify non-allergic patients, allowing first-line therapy and returning patients to an outpatient setting for infusions.5 Though many practices may not have every resource needed for desensitization, they can begin the process with ST and/or risk stratification. Those patients that require desensitization can then be referred to desensitization centers. Allergists can safely expand their scope of practice and work closely with oncologists to improve patient care.
Supplementary Material
Clinical Implications:
Hypersensitivity reactions to platin chemotherapy are common and associated with worse patient outcomes when first-line treatment is consequently avoided. Platin chemotherapy skin testing and desensitization are safe, improve patient care, and should be utilized more broadly by allergists.
Acknowledgments
Funding: A.S. Levin is supported by the National Institutes of Health award T32HL116275. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
REFERENCES
- 1.Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. [DOI] [PubMed] [Google Scholar]
- 2.Koren C, Yerushalmi R, Katz A, Malik H, Sulkes A, Fenig E. Hypersensitivity reaction to cisplatin during chemoradiation therapy for gynecologic malignancy. Am J Clin Oncol. 2002;25:625–626. [DOI] [PubMed] [Google Scholar]
- 3.Polyzos A, Tsavaris N, Gogas H, Souglakos J, Vambakas L, Vardakas N, et al. Clinical features of hypersensitivity reactions to oxaliplatin: a 10-year experience. Oncology. 2009;76:36–41. [DOI] [PubMed] [Google Scholar]
- 4.Bruchim I, Jarchowsky-Dolberg O, Fishman A. Advanced (>second) line chemotherapy in the treatment of patients with recurrent epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2013;166:94–98. [DOI] [PubMed] [Google Scholar]
- 5.Wang AL, Patil SU, Long AA, Banerji A. Risk-stratification protocol for carboplatin and oxaliplatin hypersensitivity: repeat skin testing to identify drug allergy. Ann Allergy Asthma Immunol. 2015;115:422–428. [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Zanotti K, Peterson G, Kulp B, Webster K, Belinson J. Expanded experience with an intradermal skin test to predict for the presence or absence of carboplatin hypersensitivity. J Clin Oncol. 2003;21:4611–4614. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Lazaro J, Firvida JL, Berges-Gimeno P. Anaphylaxis after oxaliplatin allergy skin testing. J Investig Allergol Clin Immunol. 2014;24:269–270. [PubMed] [Google Scholar]
- 8.Madrigal-Burgaleta R, Berges-Gimeno MP, Angel-Pereira D, Ferreiro-Monteagudo R, Guillen-Ponce C, Pueyo C, et al. Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy. 2013;68:853–861. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Cuesta E, Madrigal-Burgaleta R, Angel-Pereira D, Ureña-Tavera A, Zamora-Verduga M, Lopez-Gonzalez P, et al. Delving into cornerstones of hypersensitivity to antineoplastic and biological agents: value of diagnostic tools prior to desensitization. Allergy. 2015;70:784–794. [DOI] [PubMed] [Google Scholar]
- 10.Barmettler S, Wolfson A, Slawski B, Jordan J, Blumenthal K, Banerji A. Safe and effective implementation of chemotherapy outpatient desensitizations [abstract]. Ann Allergy Asthma Immunol. 2017;119:S2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.