Abstract
Objective:
To test the feasibility of an exercise augmentation to pharmacotherapy in depressed younger and older adults while exploring neural mechanisms.
Methods:
A randomized, double-blind, controlled clinical trial was conducted in fifteen inactive younger (20–39 years) and older (60–79 years) adults meeting DSM-5 criteria for a major depressive episode (https://clinicaltrials.gov/ct2/show/NCT02407704). Participants were randomized to receive a 12-week regimen of venlafaxine XR or venlafaxine XR plus supervised exercise. Cardiorespiratory fitness was assessed using a submaximal VO2 test and neuroimaging assessments were conducted using a 7-Tesla MR scanner.
Results:
Attrition was 38% and 14% for the medication and exercise groups, respectively. Attendance was 91% for the exercise intervention. Exploratory analyses revealed an association between improvement in fitness and increased cortical thickness in the anterior cingulate cortex.
Conclusion:
Exercise augmentation to pharmacotherapy is feasible for depressed younger and older adults and may have neural benefits in a core brain region implicated in depression.
Keywords: Exercise, Depression, Brain, MRI
Exercise has emerged as an effective non-pharmacological treatment for depression in older and younger adults (1). The neural benefits of exercise overlap with several regional structural brain abnormalities in depression (i.e., prefrontal cortex, anterior cingulate, and hippocampus) (2). Only one study (N=41) has examined structural brain changes associated with exercise in depression and did not find changes in hippocampal volume; however, poor intervention adherence (mean=30%) limits interpretation of these findings (3).
The aim of this pilot trial was to establish the feasibility of conducting an exercise intervention in younger and older adults with major depression receiving antidepressant pharmacotherapy while exploring neural mechanisms. We explored patterns in efficacy of the exercise intervention and examined links between change in cardiorespiratory fitness (CRF) and change in cortical thickness in regions sensitive to depression.
Methods
Participants
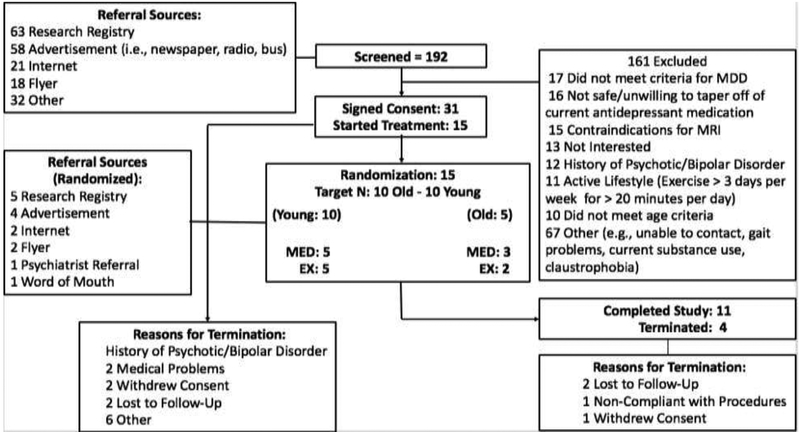
Participants included inactive adults aged 20 to 39 years and 60 to 79 years who met criteria for a current major depressive episode as defined by the Primary Care Evaluation of Mental Disorders (PRIME-MD) (4). Exclusion criteria included contraindications for MRI, a self-reported active lifestyle (i.e., exercise > 3 days per week, >20 minutes per day) , self-reported gait or balance difficulties, safety concerns for engaging in regular moderate- intensity aerobic exercise based on a physical exam conducted by the study nurse practitioner or disapproval by the participant’s primary care provider for older adult participants, uncontrolled hypertension, acute risk for a cardiovascular event (i.e., cardiovascular event within past 12 months), Type 2 diabetes (but later removed from exclusion criteria), substance use problems in the past three months, lifetime diagnosis of bipolar disorder or any psychotic disorder, and clinically significant cognitive impairment (e.g., diagnosis of dementia or Modified Mini-Mental State Exam (3MS score < 84)) (5). Please see figure 1 for a summary of study recruitment and enrollment.
Figure 1.
Summary of Recruitment and Enrollment in Study.
Procedures
Eligible participants who provided informed consent completed mood, cognitive, CRF, physical activity (PA) monitoring, and neuroimaging baseline assessments. Participants were then randomized to the medication only (MED) or medication plus exercise (EX) group. Participants on a different antidepressant medication regimen were first tapered off any previous antidepressant medication over a period of two weeks before being administered the study medication (venlafaxine XR). Study clinicians provided biweekly medication management throughout the study without knowledge of the participants’ group assignment.
Exercise Intervention
Participants randomized to the EX group participated in individualized supervised exercise sessions at the University of Pittsburgh three times per week for 12-weeks. Exercise sessions included warm-up and cool-down periods and participants exercised for one hour, including a gradual ramp-up period. Participants engaged in moderate-intensity aerobic exercise for approximately 45-minutes using a motor-driven treadmill and/or recumbent bike. Moderate-intensity was defined by continuous heart rate monitoring and maintaining a heart range of 60–75% of age-based heart rate based on the Karvonen method (i.e., 220-age). For participants on beta blockers, Rating of Perceived Exertion (RPE) (Borg 6 – 20) was used to measure intensity at a goal of 13 – 15 RPE. After completion of the intervention, all clinical, cognitive, CRF, and neuroimaging assessments were repeated for all participants.
Measures
Screening Measures
Participants scoring >2 on the Physical Activity Readiness Questionnaire (PAR-Q) required PCP approval before participation (6). The Primary Care Evaluation of Mental Disorders (PRIME-MD) was used to confirm diagnosis of major depression, and to exclude other psychiatric diagnoses (i.e., bipolar disorder) (4). Scores <84 on the 3MS was used to exclude participants with possible dementia (5).
Outcome Measures
Depression Severity:
The Montgomery-Asberg Depression Rating Scale (MADRS) was used to assess severity of depressive symptoms (7). A score cutoff of <10 was used to determine remission. Clinicians were instructed to not ask participants if they were in the EX group, and participants were instructed to not divulge this information.
Cardiorespiratory Fitness:
A submaximal VO2 test assessed CRF. Participants underwent testing with a modified Balke protocol (i.e., speed remained constant with 2% grade increments every two minutes). The test was terminated when subject’s heart rate reached 85% of the age-based maximum (220-age), volitional fatigue, or RPE >=15.
Physical Activity:
PA was monitored for one-week pre and post intervention using the Body Media Sensewear armband (Model MF-SW. BodyMedia Inc., Pittsburgh PA). These devices were placed on the left arm and use a triaxial accelerometer to assess amount and intensity of PA.
Neuroimaging Assessments
Twelve participants underwent structural brain MRI scanning on a 7 Tesla (7T) Siemens scanner within one-week prior to initiating and after completing the intervention . T1 weighted images were collected using a 3D Magnetization Prepared Rapid Gradient Echo Imaging (MPRAGE) protocol. Scanning parameters include an echo time (TE) of 2.5 ms, repetition time (TR) of 3,000ms, and field of view (FOV) of 176 × 223 mm. The three remaining participants completed baseline and post-intervention MRI scans on a 3 Tesla scanner. Similar scanning parameters were used for the 3T.
Cortical Thickness
Given the small sample size, Spearman’s rank-order (rho) correlations were used to examine the association between change in CRF and cortical thickness. Vertex-based estimates of regional gray matter thickness were generated using Freesurfer, version 6.0 (Dale, Fischl (8).
RESULTS
Feasibility
We screened 192 adults (see Figure 1). Thirty-one participants (16%) enrolled, of which 15 (48%) were randomized (MED=8; EX=7) and 11 participants (73%) completed the study (MED=5 EX=6). Two participants were lost to follow-up, one participant was non-adherent to study procedures (e.g., nonadherent to medication), and one participant withdrew consent. An initial recruitment challenge was to identify strategies to enroll depressed older adults. Nonetheless, we identified several successful recruitment strategies, including the University of Pittsburgh CTSI research registry (n=5), paper-based or radio advertisements (n=4), flyers (n=2), online advertisements (n=2), referral from a medical provider (n=1), and self-referral through word of mouth (n=1). All participants completing the study completed MRI, biomarker, cognitive, mood, fitness, and PA assessments without difficulty or endorsing burden related to the length of time required to complete study assessments.
Participant Characteristics
Fifteen participants (10 younger adults and 5 older adults with major depression) were randomized to receive venlafaxine XR (MED) or venlafaxine XR and supervised aerobic exercise (EX). Randomization was performed by the study data manager using the permuted block randomization method. Study clinicians were blinded to group assignments. Eleven participants returned for follow-up assessments. Participants completing the study were adherent to the medication regimen, and those in the EX group attended 91% of the sessions on average. PA levels as assessed by accelerometers and self-report did not differ between treatment groups at baseline.
Cardiorespiratory Fitness
Significant changes in fitness were not observed in the exercise group (paired-samples t-test; t=−0.96; df=5; p=0.38). One out of six participants in the exercise group showed a decline in fitness (~2 SD below the mean) while the other five participants showed an increase in fitness. Given the violation of normality in these data, an exploratory analysis was conducted using the non-parametric Related-Samples Wilcoxon Matched-Pair Signed-Rank test with and without excluding the participant showing a decline in fitness. Results indicated the exercise group overall did not show an increase in fitness (df=5, p= 0.25) but an increase in fitness was observed in the exercise group after excluding the sole participant showing a decline in fitness (df=4, p=0.04).
Depressive Symptoms
Participants in both treatment groups showed a significant reduction in depressive symptoms (Mean (SD) percent reduction in MADRS score: MED= −63.85 (35.56); EX= −74.34 (16.37); paired-samples t-test MED: t=8.36; df=4; p=0.001; EX: t=6.46; df=5; p=0.001). There was no group difference in trajectory of decline in depressive symptoms during the intervention while covarying for medication titration (Repeated Measures ANOVA group x time interaction: F= 0.23; df=6; p= 0.97); however, the EX group appeared to have a more efficient trajectory of decline in depressive symptoms (i.e., remitted after a shorter treatment duration relative to the MED group) based on visualization of the data (See Supplemental Data File).
Cortical Thickness
Cross-sectional associations between depression severity and cortical thickness in regions commonly showing structural abnormalities in depression were explored at baseline (i.e., prefrontal, anterior cingulate, hippocampal, and striatal regions). Depression severity was negatively associated with cortical thickness in the right rostral anterior cingulate cortex (ACC) (Spearman’s rho correlation r = −0.75; df=8; p=0.01; r2=0.56), the right medial orbitofrontal cortex (OFC) (Spearman’s rho correlation r= −0.78; df=8; p=0.008, r2=0.61), and right parahippocampal gyrus (PHCG) (Spearman’s rho correlation r = −0.93; df=8; p<0.001, r2=0.86). Change in depressive symptoms was not associated with change in cortical thickness in the OFC, ACC, or PHCG (p > 0.10). However, a pattern was observed such that improvement in CRF was associated with an increase in cortical thickness in the R rostral ACC (Spearman’s rho correlation; r= 0.64, df=8, p=0.04, r2=0.40), a region in which greater depression severity was linked to reduced cortical thickness at baseline. Similar patterns (not statistically significant) were observed for the R medial OFC (Spearman’s rank order correlation; r=0.60, df=8, p=0.06, r2=0.40) (See Supplemental data file for visualization of these results). These results suggest a link between improvement in CRF and an increase in cortical thickness in the rostral ACC, which was sensitive to greater depression severity in this sample and is a core brain region implicated in depression.
DISCUSSION
Our pilot study showed that an exercise intervention as augmentation to pharmacotherapy in depressed younger and older adults was feasible and acceptable, without excessive or unacceptable participant burden. Notable strengths of this pilot study include the double-blind, randomized nature of the study design, exceptional exercise adherence (91% attendance), and an exploration of neuroimaging outcomes. Exploratory analyses suggested improvement in CRF was associated with an increase in cortical thickness in the rostral ACC, a core brain region affected in depression.
Our findings are limited by the small sample size, disproportionate representation of depressed younger relative to older adults, exclusion of middle-aged adults, and group differences in gender and antidepressant medication use prior to the study. Further, technical difficulties related to the 7T MR scanner resulted in a few participants being scanned on a 3T MR scanner, which may have affected our ability to detect changes in regional brain morphology.
Conclusions
This pilot study demonstrated the feasibility of developing an exercise intervention in depressed older and younger adults taking antidepressant medication. Recruitment challenges were overcome, and intervention adherence was exceptional. Further, an association between exercise-related improvement in fitness and an increase in cortical thickness in a key region sensitive to depression is a promising avenue for future large-scale studies to explore.
Supplementary Material
Highlights.
Exercise augmentation to pharmacotherapy is a feasible intervention for treatment of depression in younger and older adults.
Depressed younger and older adults can maintain exceptionally high exercise adherence (i.e., 91% attendance) in the context of a supervised exercise intervention.
Exercise-related changes in cardiorespiratory fitness may have neural benefits for the anterior cingulate cortex, a core region implicated in depression.
Acknowledgements
Funding Acknowledgment: SG was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 2014192810 and is currently funded through the VA MIRECC Advanced Fellowship. KIE was supported by research grants (R01 DK095172, P30 AG024827, P30 MH90333, R01 CA221882, R01 AG053952, R01 CA196762). Partial support also received from the UPMC Endowment in geriatric psychiatry (CFR and HA). MAB was supported by research grants (P50 AG005133, P30 MH090333). JFK was supported by research grants (P30 MH090333).
Sponsor’s Role: Financial support for the development of this paper was exclusively from the Advanced Center in Intervention and Services Research in Late-life Depression Prevention: P30MH090333.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: JFK has received medication supplies from Indivior and Pfizer for Investigator Initiated Trials.
This manuscript was submitted for presentation to the American Association for Geriatric Psychiatry Annual Convention 2019.
References
- 1.PAGAC. Physical Activity Guidelines Advisory Committe Scientific Report, 2018. Washington, D.C: 2018 [Available from: https://health.gov/paguidelines/second-edition/report/pdf/09_F-3_Brain_Health.pdf. [Google Scholar]
- 2.Gujral S, Aizenstein H, Reynolds CF 3rd, Butters MA, Erickson KI. Exercise effects on depression: Possible neural mechanisms. General hospital psychiatry. 2017;49:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krogh J, Rostrup E, Thomsen C, Elfving B, Videbech P, Nordentoft M. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression--a randomized clinical trial. Journal of affective disorders. 2014;165:24–30. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer RL, Kroenke K, Linzer M, Hahn SR, Williams JB, deGruy FV 3rd, et al. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 Study. Jama. 1995;274(19):1511–7. [PubMed] [Google Scholar]
- 5.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987;48(8):314–8. [PubMed] [Google Scholar]
- 6.Thomas S, Reading J, Shephard RJ. Revision of the Physical-Activity Readiness Questionnaire (Par-Q). Can J Sport Sci. 1992;17(4):338–45. [PubMed] [Google Scholar]
- 7.Montgomery S, Asberg M. A new depression scale designed to be sensitive to change. Brit J Psychiat. 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 8.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.