Abstract
BCG unresponsive bladder cancer is an inherently resistant disease state for which the preferred treatment is radical cystectomy. To date, no effective intravesical therapies exist for patients who possess these resistant tumors. For this reason, many research groups are actively investigating/testing novel therapeutic agents to aid in bladder preservation for this patient population. This review article describes our 15-year experience developing and testing IFN-based gene therapy. Gene therapy has become a powerful tool in our fight against cancer and herein; we outline how it has been developed and is currently being tested to treat patients with BCG unresponsive disease.
Keywords: BCG unresponsive bladder cancer, gene therapy, Interferon-α, Instiladrin
Summary
BCG unresponsive bladder cancer represents an advanced state of disease in which no effective therapies exist to aid in bladder preservation. Our work developing and optimizing IFNα-based gene therapy over the past 15 years is now coming to fruition with promising results from phase I & II clinical trials and pending results from a phase III trial. After overcoming the initial barriers associated with gene delivery to the bladder, we have made significant strides forward in developing this novel therapeutic strategy for treatment of this inherently resistant disease.
Background
Bladder cancer is the second most common genitourinary malignancy in the United States with 70,030 new cases and 16,870 deaths in 2017 [1,2]. Approximately 70% of newly diagnosed bladder cancers are non-muscle invasive (NMIBC) for which the current standard initial treatment is transurethral resection followed by intravesical therapy. Radical cystectomy is an option for refractory or persistent tumors [3–5]. The key to successful management of NMIBC is preventing recurrence and progression, which can occur in 70% and 10–20% of patients, respectively [1]. To achieve this goal a variety of intravesical therapies are utilized in the management of this disease, BCG being the optimal choice for intermediate-risk and highrisk patients [4]. Despite the importance of intravesical treatment for NMIBC, only four agents (including BCG) have been approved in the past 60 years [6–8]. Although BCG has stood its ground as a frontline immunotherapy for decades, a significant number of patients with NMIBC eventually progress to a BCG unresponsive state [9]. BCG unresponsive patients pose a significant challenge to those managing NMIBC. Radical cystectomy remains the preferred treatment in these high-risk patients however, being such an invasive and potentially morbid procedure not all patients are willing to accept it and prefer repeated attempts at intravesical therapy despite proven futility in the majority of cases. It is clear that more effective therapies are needed for the treatment of this disease state.
Significant effort has been, and continues to be put forth in developing therapy for BCG unresponsive bladder cancer. This review article describes our 15-year experience developing IFN-based gene therapy to treat this disease. Herein, we describe our experience from the early days of preclinical work to a current phase III clinical trial, and beyond.
Prior to describing our experience with gene therapy development, we must first understand the evolution of the BCG unresponsive definition and current Food and Drug Administration (FDA) expectations for trial design/outcomes in this disease state.
The evolution of the BCG unresponsive definition and current clinical trial expectations
Prior to 2005, no formal definition of BCG unresponsive disease existed. The definition was simply based on the treating physicians interpretation of their patients disease state [10]. In 2005, following a meeting of bladder cancer experts, the definition of “BCG refractory” disease was developed as non-improving or worsening disease at 3 months after initial BCG, or failure to achieve a disease free state by 6 months after initial BCG with either maintenance or re-induction [11]. The current terminology, “BCG unresponsive” disease was coined in 2015 to broaden the definition covering both refractory and relapsing disease [12]. In 2018, the FDA defines BCG unresponsive disease as: “persistent or recurrent CIS alone or with recurrent Ta/T1 disease within 12 months of completion of adequate BCG therapy, or recurrent high-grade Ta/T1 disease within 6 months of completion of adequate BCG therapy, or T1 high-grade disease at the first evaluation following an induction BCG course” [13]. It is of utmost importance that when using this terminology treating physicians ensure that patients have received an “adequate” amount of BCG. The FDA defines adequate BCG therapy as “at least 5/6 doses of initial induction plus at least 2/3 maintenance doses or alternatively at least 5/6 doses of an initial induction plus at least 2/6 doses of an re-induction”[13].
Due to the heterogeneous nature of the disease and strict criteria for defining various disease states the FDA has published guidelines for industry to aid in drug development for BCG unresponsive NMIBC [13]. In this document, the FDA highlights the intricacies of, and differences between BCG unresponsive papillary disease and CIS that is unresponsive to BCG. They set forth what is expected of new therapeutics, both intravesical and systemic treatments. They state the only appropriate trial endpoint is complete response (CR) for patients with CIS, defined as negative cystoscopy and negative cytology. Positive cystoscopy with biopsy proven benign or low-grade NMIBC/negative cytology, or the presence of a positive /suspicious cytology with a negative biopsy are not considered to be a treatment failure. Recurrence is simply defined as anything not meeting the aforementioned criteria [13].
Contemporary research in the management of BCG unresponsive NMIBC
In order to address a significant unmet need for new therapies in NIMBC, many research groups are actively testing novel therapeutic strategies for this disease. Although it has been decades since the last drug approval, many are optimistic that the landscape of NMIBC management is about to change. Siddiqui et al. recently published a review of 18 completed/ongoing clinical trials in BCG unresponsive disease since 2014 [14]. Interestingly, significant variety exists in the treatment mechanisms underlying these therapies ranging from vaccines to enhanced drug delivery systems to gene therapy, which is the focus of this review.
Choosing the correct therapy - Interferon (IFN) as a treatment for NMIBC
Historically, many intravesical agents have been tested in hopes of preventing recurrence and progression in NMIBC; recombinant human interferon-α amongst them (rhIFNα). Interferon is a pleiotropic cytokine that has been shown to be cytotoxic and anti- angiogenic in bladder cancer [15,16]. Early work by our group showed that systemic administration of rhIFNα to bladder tumor bearing mice was associated with decreased angiogenic factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) [17,18].
Over the past several decades rhIFN has been used as a monotherapy and in combination with other intravesical agents [19–22]. Early studies examining the efficacy of rhIFNα monotherapy noted moderate anti-tumor responses in patients with CIS, however its use in T1 disease was found to be inferior when compared to standard intravesical therapy [21,23].
The potential for synergistic anti-tumor activity when rhIFNa is used in combination with BCG has been assessed in several clinical trials, two of which assessed its activity in “BCG failure” patients [19,24]. Although they report relatively high disease-free rates following combination therapy, these results are misleading. A significant number of patients classified as “BCG failures” only received one prior course of BCG, therefore not meeting contemporary definitions of this disease state [24,19]. Furthermore, a recent Cochrane review assessing combination BCG/rhIFNα found no clear benefit for prevention of recurrence or progression when compared to BCG monotherapy [25].
The limitations seen with intravesical rhIFNα therapy are likely a result of short drug exposure time rather than its inherent anti-tumor activity. The limited dwell time that occurs with intravesical therapy does not allow for the development of a significant immunological response. rhIFNa monotherapy does not provide the necessary treatment durability with most patients relapsing within the first year of treatment [26]. To overcome this limitation, we have invested our research efforts into gene therapy in hopes of creating an environment within the bladder in which the urothelium is exposed to IFNα for sustained periods (Figure 1a).
Figure 1a -.
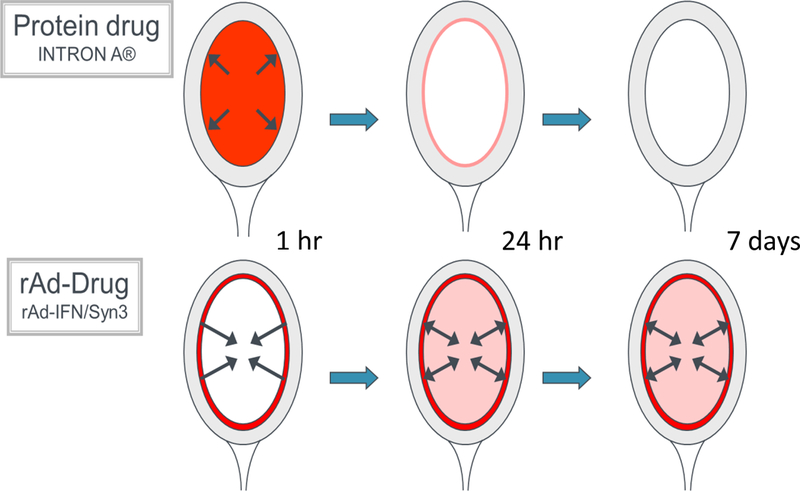
Depiction of sustained urothelial exposure with AdIFNα/Syn3 compared with rhIFNα
Gene therapy concept and definitions
Gene therapy can be defined as the therapeutic delivery of nucleic acid into a host’s cell to treat a disease [27]. Although originally developed to treat genetic diseases, the majority of gene therapy research today focuses on the treatment of cancer [28]. Following the first successful therapeutic gene transfer for melanoma almost 30 years ago, gene therapy has evolved into a strategic tool in our armamentarium against cancer [29,30].
Gene therapy can be divided into two broad categories: therapies targeting the tumor cell and therapies targeting the immune system. Our focus has been the latter, so called ‘immunogene therapy’. Immunogene therapy is a term used when the genetic material delivered elicits and/or modulates the host’s immune response [31]. The fundamental basis of immune therapy involves the host’s immune system mounting an anti-tumor response against foreign tumor cells. Cytokines, such as IFNα, are pleiotropic and possess the ability impede tumor cell growth directly and activate both the innate and adaptive immune response against tumor cells [31]. Ideally, the goal with any antineoplastic agent is to maximize the effect in the target tissue with minimal systemic side effects. Systemic cytokine therapy has been used successfully in several malignancies, however widespread adoption has not occurred secondary to serious adverse events associated with its administration [32,33]. We know from prior work testing intravesically delivered rhIFNα that local cytokine delivery appears to be well tolerated, reassuring us that it will be tolerated as a local therapy via gene transfer [20].
The bladder serves as an ideal organ for gene therapy. It is a defined cavity that provides direct contact between vector and tumor cells at the same time allowing relatively easy access to urine and tissue to monitor effect and perform correlative studies. Despite these favorable conditions for gene transfer, early trials investigating gene therapy in bladder cancer were disappointing with effective gene delivery being the major barrier [34].
Overcoming ‘the barrier’ to gene therapy in bladder cancer - the discovery of Syn3
The bladder urothelium possesses a unique protective layer called the “glycosaminoglycan layer” (GAG). This layer is composed of a thick layer of glycoproteins and proteoglycans covering the surface of urothelial cells [35]. Although the exact function of this layer is unknown, many believe it to serve as a barrier to infection and other harmful urinary constituents. As a result, successful intravesical gene therapy must overcome this natural barrier in order to allow for viral transduction to the underlying urothelium.
In efforts to enhance gene transduction within the bladder, early studies used harsh chemical reagents such as ethanol and acetone to serve as a detergent in hopes of increasing gene delivery [36]. Although they witnessed enhanced transgene expression, the harsh nature of these chemicals would not prove feasible in a clinical setting. In 2001, Connor et al. tested a variety of chemical detergents in vivo in hopes of identifying a less toxic reagent that would enhance β-galactosidase transgene expression [37]. In doing so, they identified a candidate compound called “BigCHAP”. Interestingly, researchers noted significant heterogeneity in the results using different lots of the Big CHAP compound, which led to further bioanalysis. This revealed three impurities in the bioactive version of BigCHAP of which one, impurity no.3, was found to be the active component. Further chemical modification of this impurity led to the develop of ‘Syn3’, a compound proven to enhance transgene delivery and expression [38]. This discovery laid the foundation for further preclinical work assessing the safety, feasibility, and efficacy of gene transfer in bladder cancer.
Preclinical testing of gene therapy for bladder cancer
Preclinical work performed by Iqbal et al. in 2001 showed that a recombinant adenovirus expressing human interferon-α2b was able to produce biologically active protein both in vitro and in vivo. rAdIFNα2b, originally known as “IACB”, was effective in suppressing tumor growth in both primary and metastatic tumor models [39].
At this time, with the recent discovery of the excipient Syn3 and a proven antineoplastic gene therapeutic construct (rAdIFNα), we began assessing the efficacy of these agents as a intravesical treatment strategy for bladder cancer. In 2004, Benedict et al. used an orthotopic model of human bladder cancer in nude mice to test the delivery/efficacy of AdIFNα/Syn3 [40]. In this model, tumor presence within the bladder was confirmed by green fluorescence and treatment was initiated with 1×1011 P/mL AdIFNα/Syn3 instillations into the bladder on two consecutive days. With this model, we were able to demonstrate high urinary IFN levels and marked tumor regression following treatment (Figure 1b/c). Interestingly, the rAdIFNα2b treatment also had cytotoxic effects on cells that were previously shown to be resistant to rhIFNα. Perhaps, one of the most important findings was the durability of IFNα levels following intravesical instillation. Elevated bladder tissue IFNα levels were noted for at least 7 days following AdIFNα/Syn3 compared with rhIFNα, which lasted less than 12 hrs (Figure 1c). This important discovery emphasized the benefit of gene therapy in overcoming the issue of durability with intravesically-administrated rhIFNα [40].
Figure 1b -.

Effect of intravesical treatment with AdIFNα/Syn3 in an orthotopic bladder cancer model (figure used with permission [40])
Figure 1c -.
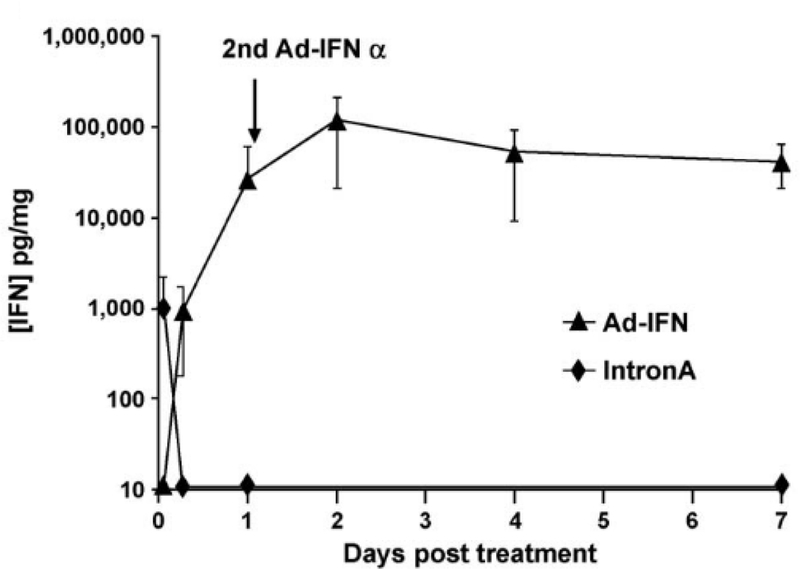
Prolonged IFNα levels are seen with gene therapy (Ad-IFNα) compared to recombinant IFNα (IntronA) following intravesical treatment (figure used with permission [40])
From our preclinical work, at least three mechanisms mediating the anti-tumor effects of IFNa based gene therapy have emerged. In 2002, Izawa et al. demonstrated that inhibition of tumorgenicity by AdIFNβ was related to inhibition of angiogenesis factors and neovascularization; confirming the antiangiogenic effects of IFN observed in our early work [41]. Other preclinical work has demonstrated that rhIFNα induced TRAIL leads to cell death via an IRF-1 dependent mechanism in human bladder cancer cells [15]. TRAIL-mediated cell death appears a likely factor in the clinical setting as well with elevated TRAIL levels noted in patients with detectable urinary IFNα following transduction [42]. A third, less understood mechanism involves soluble bystander factor(s) that are secreted by both AdlFNa infected tumor cells and normal urothelial cells. Our hypothesis is that this bystander effect is selectively cytotoxic to tumor cells, but not normal urothelial cells [43,44]. We recognize that invading immune response cells would likely be past the transduction window by the vector (24hrs) and expressed IFN protein is unlikely to be directly transferred to such cells via the ‘ bystander effect’. However, whether there are downstream immune mechanisms contributing to AdlFNa’s bystander effects on the tumor microenvironment remains unknown. Our prior preclinical data were generated in human xenografts in nude mice [40]. We are now conducting mechanistic and correlative studies using spontaneous and syngeneic mouse models that may provide insight into the immune mechanisms underlying AdlFNα’s anti-tumor activity.
From bench to bedside: Preparation for phase I testing
In a study evaluating the dosing regimens for this novel agent, Connor et al. examined the pharmacodynamics and efficacy of re-dosing rAdIFNα2b in immunocompetent rats [45]. High and sustained levels of IFNα were found in the urine and bladder tissue following intravesical therapy. Minimal levels were detected in systemic circulation. In this re-dosing experiment we demonstrated that longer intervals between doses allowed for a dampened immune response, therefore facilitating increased duration and magnitude of adenoviral gene expression [45].
Prior to initiating a phase I clinical study several unanswered questions needed to be addressed. Are two consecutive doses necessary? What is the ideal dose? Does efficacy correlate with urinary IFN levels? To address these questions we examined single dosing and three different concentrations of AdIFNα2b/Syn3 in an orthotropic bladder cancer model [46]. In doing so, we found that the lowest dose was relatively ineffective at inhibiting bladder tumor growth when compared to the higher doses. Furthermore, we found that a single instillation was as effective as two consecutive instillations providing the rationale for single instillation only in human studies. Another important finding was that tumor response correlated with sustained elevation of IFNα levels in the urine. The use of this correlation in our human trials would allow for quantification of gene transfer. Table 1a summarizes select preclinical studies from the development of AdIFNα/Syn3.
Table 1a –
Select preclinical studies in the development of AdIFNα/Syn3
| Author | Year | Main findings |
|---|---|---|
| Iqbal et al. 2001 (39) | 2001 | rAdIFNα2b produced biologically active protein both in vitro and in vivo rAdIFNα2b was effective in suppressing tumor growth |
| Yamashita et al. (38) | 2002 | Syn 3 described as a novel excipient to enhance transgene expression within the bladder |
| Benedict et al. (40) | 2004 | rAdIFNα2b/Syn3 causes marked tumor regression in an orthotopic bladder cancer model |
| Tao et al. (46) | 2006 | Single instillation (vs. two) of rAdIFNα2b/Syn3 causes tumor regression Tumor response correlates with urinary IFNα levels |
A previous concern with using adenoviral vectors in gene therapy was the feasibility of large-scale production to meet potential market demand [47]. Until recently, this shortcoming hindered the clinical application of this novel therapeutic technique. Fortunately, we were able to overcome this obstacle by developing technology to aid in large-scale adenoviral production, in turn allowing us to perform the clinical studies detailed below. Adenoviral vectors provide several other unique advantages as well. Adenovirus provides high transduction efficiency in humans but lacks the associated DNA machinery to integrate the transgene and hence does not carry the integrational mutagenic risks of random retroviral and lentiviral vectors. The resultant short-term expression of the interferon α−2b protein peaks within 48hrs and declines thereafter over the next 14 days. Adenoviral mediated short-term expression trigger’s interferons pleiotropic effects whilst, minimizes the possibility of interferon conditioning and induction of PDL1 response mechanisms in the tumor [Plote, 2018 under review], Induction of such checkpoints is undesirable as it negates downstream immune mediated anti-tumor effects.
Phase I clinical study - rAdlFNα2b/Syn3
A phase I clinical study of “SCH721015/SCH209702” (rAdIFNα2b/Syn3) was initiated in April 2011 [48]. The primary endpoint was safety with secondary endpoints being effective gene transfer and preliminary evidence of clinical activity at 3 months. Seventeen patients with disease recurrence following 2 cycles of BCG (defined as 6-week induction followed by a 3-week maintenance or a second induction) were enrolled in the study. Following a single treatment dose, safety was evaluated for 12 weeks and efficacy of gene transfer assessed by measuring urinary IFNα levels. Preliminary drug efficacy was assessed at 3 months.
Overall, the therapy was well tolerated with no dose limiting toxicities. Lower urinary tract symptoms (LUTS) mainly in the form of urgency were noted in 88% of patients, but were well managed with anticholinergics. Effective gene transfer was noted in all patients except those receiving the lowest dose. Detectable levels of IFNa were noted in patient’s urine for up to 10 days. Of the 14 patients treated with effective dosage and confirmed gene transfer (ie. detectable urinary IFNα), 6 (43%) experienced a CR at 3 months with 2 remaining disease-free at 29 and 39 months, respectively [48].
Phase II clinical study - Instiladrin®
The encouraging results from the Phase I trial led to the initiation of a multi-center Phase II study governed by the Society of Urologic Oncology Clinical Trials Consortium (SUO- CTC). In this single-arm trial, 40 BCG unresponsive patients at 13 centers were randomized to two dosages of AdIFNα/Syn3 every 3 months for 1 year while CR was maintained. The primary endpoint was defined as 25% freedom from high-grade recurrence (biopsy mandated) at 12 months [49]. Evidence of effective gene transfer was found in all trial patients with detectable levels of urinary IFNα2b at 24 hours. The primary outcome was similar between the two dosage groups with 7 (33%) and 7 (37%) achieving 12-month RFS in the low and high dose groups, respectively, although the median time to recurrence seemed to favor the high dose. In the 14 patients with 12-month RFS, durable responses were seen with no documented bladder recurrence before 21 months and some lasting beyond 36 months. Importantly, AdIFNα/Syn3 appears to act on both papillary and CIS BCG unresponsive lesions. Subset analyses demonstrated 50% and 30% 12-month RFS for papillary and CIS lesions, respectively [49]. This represents a significant gain in the management of BCG unresponsive disease, particularly for those with CIS whose only approved option is Valrubicin with a 12-month RFS of just 10% [50].
Similar to the phase I study the most common adverse events were LUTS. Importantly, no patients discontinued therapy due to toxicity, and AE’s were similar in both the high and low dose groups. Serum IFNα2b levels were extremely low and there was no measurable IFNα2b DNA, both indicators of systemic biosafety. Table 1b summarizes published clinical studies using Instiladrin.
Table 1b–
Published clinical studies of AdIFNα/Syn3 (Instiladrin)
| Author | Year | Phase | Primary and Secondary endpoints |
Main findings |
|---|---|---|---|---|
| Dinney CP. et al. (48) | 2013 | I | 1° - safety 2° - assessment of gene transfer and early efficacy |
rAdIFNα2b/Syn3 was well tolerated with no DLTsb Gene expression was confirmed with dose dependent urinary IFNα levels 43% experienced a CRc at 3 months |
| Shore N. et al. (49) | 2017 | II | 1° - 25% freedom from HGa recurrence at 12 months (biopsy mandated) | rAdIFNα2b/Syn3 was well tolerated Effective gene transfer was found in all patients 35% of patients were free of recurrence at 2 months |
= high-grade
= dose-limiting toxicities
= complete response
Phase III multi-institutional study - Instiladrin®
A multi-institutional phase III registration trial of Instiladrin was initiated in late 2016. This trial population consisted of high-grade BCG unresponsive patients of whom at least 100 must have CIS. The primary endpoint is twofold: percent of CIS patients with CR at 3 months and secondly, the durability of CR. Secondary endpoints include percent RFS at 3,6,9 and 12 months for Ta/T1 lesions without CIS. In this trial, intravesical treatment with Instiladrin was administered every 3 months for 1 year while CR was maintained and those who remained a CR at 12 months are eligible for 3 years of maintenance treatment. Accrual for this trial completed in Q2 2018 and we keenly await the results.
Current and future work optimizing IFN-based gene therapy for bladder cancer
Although results thus far are very encouraging, much work lies ahead to better understand and optimize this novel therapeutic strategy. As we enter the world of personalized medicine, it is crucial that with this new therapy that we optimize patient selection. Our current work is focused on identifying biomarkers that will predict sensitivity or resistance to IFNα- based gene therapy. Candidate markers such as various cytokines, miRNA’s, intrinsic molecular subtypes, and immune biomarkers are all being investigated from the Phase I and II trials correlative data.
As detailed above, our prior preclinical work was performed in human xenografts and therefore not suitable to assess the immune activity of AdIFNα/Syn3 therapy. To address this, we have tested IFN based therapy in syngeneic immunocompetent mouse models assessing immune cell infiltrate and modulation of the immune checkpoint marker PDL-1 (Plote 2018 under review). We found both to be markedly increased following rhIFN treatment, providing rationale to test combination therapy with immune checkpoint blockade. Using the same syngeneic mouse tumor model we tested combination therapy with poly:IC (a TLR3 agonist which acts as a synthetic analogue of IFN) and anti-PDL1 and demonstrated significant improvement in survival with combination therapy (Plote, 2018 under review). A phase I/II clinical trial to test the combination of intravesical Instiladrin with systemic anti-PD1 therapy is currently being developed.
In addition to the work above, we are testing novel vectors for the delivery of IFNα in NMIBC. Lentivirus (LV) is another potential vector for intravesical delivery of IFN α, which unlike adenovirus, can integrate into the host genome allowing for continual production and sustained levels of IFNα. Our preliminary work using this vector has shown that LV-IFNα effectively upregulates IFNα target genes including PDL-1, is cytotoxic to murine bladder cancer cell lines in-vitro, and improves the survival of BBN tumor-bearing mice. Therefore, LV appears to be a promising vector for intravesical gene delivery and warrants further research.
Other gene therapy approaches in NMIBC
In addition to our research group, others are working diligently to move this field forward by way of novel therapeutic targets and innovative techniques. Recently, Packiam et al. published interim results of their gene therapeutic construct, CG0070 oncolytic vector [51]. In this model, an oncolytic adenovirus targets Rb-negative bladder cancer cells for destruction via direct tumor lysis and/or immune mediated killing [51]. They assessed the oncolytic activity of CG0070 in a cohort of BCG unresponsive patients. With this being an interim analysis, only 6- month CR rates were available and found to be 47% in patients with BCG unresponsive NIMBC. Although intriguing, these results are limited by small sample size, short follow-up and a rigorous dosing schedule. The lack of Rb-status is also a significant limitation to this study, as this is the integral to the proposed mechanism of action [51].
Acknowledgments
Funding: The University of Texas MD Anderson SPORE in Genitourinary Cancer P50CA091846, NIH/NCI under P30CA016672
Footnotes
Research involving human participants and/or animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
Informed consent was obtained from all individual participants of clinical studies included in this review article.
Conflicts of Interest
Nigel Parker - leadership, consulting, and research funding - FKD Therapies Oy Seppo Yla-Herttulla - consultant for FKD Therapies Oy Colin Dinney - consultant on advisory board for FKD Therapies Oy All remaining authors declare no conflicts of interest.
References
- 1.Kaufman DS, Shipley WU, Feldman AS (2009) Bladder cancer. Lancet (London, England) 374 (9685):239–249. doi: 10.1016/s0140-6736(09)60491-8 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA: a cancer journal for clinicians 67 (1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M, van Rhijn BW, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R (2017) EAU Guidelines on Non¬Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. European urology 71 (3):447–461. doi: 10.1016/j.eururo.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD, Skinner EC, Smith ND, McKiernan JM (2016) Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. The Journal of urology 196 (4):1021–1029. doi: 10.1016/j.juro.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 5.Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, Downs TM, Efstathiou JA, Flaig TW, Friedlander T, Greenberg RE, Guru KA, Hahn N, Herr HW, Hoimes C, Inman BA, Jimbo M, Kader AK, Lele SM, Meeks JJ, Michalski J, Montgomery JS, Pagliaro LC, Pal SK, Patterson A, Plimack ER, Pohar KS, Porter MP, Preston MA, Sexton WJ, Siefker-Radtke AO, Sonpavde G, Tward J, Wile G, Dwyer MA, Gurski LA (2017) Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN 15 (10):1240–1267. doi: 10.6004/jnccn.2017.0156 [DOI] [PubMed] [Google Scholar]
- 6.Jarow J, Maher VE, Tang S, Ibrahim A, Kim G, Sridhara R, Pazdur R (2015) Development of Systemic and Topical Drugs to Treat Non-muscle Invasive Bladder Cancer. Bladder cancer (Amsterdam, Netherlands) 1 (2):133–136. doi: 10.3233/blc-150016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prout GR Jr., Koontz WW Jr., Coombs LJ, Hawkins IR, Friedell GH (1983) Long-term fate of 90 patients with superficial bladder cancer randomly assigned to receive or not to receive thiotepa. The Journal of urology 130 (4):677–680 [DOI] [PubMed] [Google Scholar]
- 8.Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M (2000) Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. The Journal of urology 163 (3):761–767 [PubMed] [Google Scholar]
- 9.Kamat AM, Colombel M, Sundi D, Lamm D, Boehle A, Brausi M, Buckley R, Persad R, Palou J, Soloway M, Witjes JA (2017) BCG-unresponsive non-muscle-invasive bladder cancer: recommendations from the IBCG. Nature reviews Urology 14 (4):244–255. doi: 10.1038/nrurol.2017.16 [DOI] [PubMed] [Google Scholar]
- 10.Steinberg RL, Thomas LJ, Mott SL, O’Donnell MA (2016) Bacillus Calmette-Guerin (BCG) Treatment Failures with Non-Muscle Invasive Bladder Cancer: A Data-Driven Definition for BCG Unresponsive Disease. Bladder cancer (Amsterdam, Netherlands) 2 (2):215–224. doi: 10.3233/blc-150039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieder AM, Brausi M, Lamm D, O’Donnell M, Tomita K, Woo H, Jewett MA (2005) Management of stage T1 tumors of the bladder: International Consensus Panel. Urology 66 (6 Suppl 1):108–125. doi: 10.1016/j.urology.2005.08.066 [DOI] [PubMed] [Google Scholar]
- 12.Lerner SP, Dinney C, Kamat A, Bivalacqua TJ, Nielsen M, O’Donnell M, Schoenberg MP, Steinberg G (2015) Clarification of Bladder Cancer Disease States Following Treatment of Patients with Intravesical BCG. Bladder cancer (Amsterdam, Netherlands) 1 (1):29–30. doi: 10.3233/blc-159002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Document FG (2018) BCG Unresponsive Non-muscle Invasive Bladder Cancer - Developing drugs and biologics for treatment - a guideline for industry. [Google Scholar]
- 14.Siddiqui MR, Grant C, Sanford T, Agarwal PK (2017) Current clinical trials in non-muscle invasive bladder cancer. Urologic oncology 35 (8):516–527. doi: 10.1016/j.urolonc.2017.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papageorgiou A, Dinney CP, McConkey DJ (2007) Interferon-alpha induces TRAIL expression and cell death via an IRF-1-dependent mechanism in human bladder cancer cells. Cancer biology & therapy 6 (6):872–879 [DOI] [PubMed] [Google Scholar]
- 16.von Marschall Z, Scholz A, Cramer T, Schafer G, Schirner M, Oberg K, Wiedenmann B, Hocker M, Rosewicz S (2003) Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. Journal of the National Cancer Institute 95 (6):437–448 [DOI] [PubMed] [Google Scholar]
- 17.Dinney CP, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, Fidler IJ (1998) Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer research 58 (4):808–814 [PubMed] [Google Scholar]
- 18.Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ (1999) Interferon-alpha-mediated down- regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clinical cancer research : an official journal of the American Association for Cancer Research 5 (10):2726–2734 [PubMed] [Google Scholar]
- 19.Joudi FN, Smith BJ, O’Donnell MA (2006) Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urologic oncology 24 (4):344–348. doi: 10.1016/j.urolonc.2005.11.026 [DOI] [PubMed] [Google Scholar]
- 20.Lamm D, Brausi M, O’Donnell MA, Witjes JA (2014) Interferon alfa in the treatment paradigm for non-muscle-invasive bladder cancer. Urologic oncology 32 (1):35.e21–30. doi: 10.1016/j.urolonc.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Malmstrom PU (2002) A randomized comparative dose-ranging study of interferon-alpha and mitomycin-C as an internal control in primary or recurrent superficial transitional cell carcinoma of the bladder. BJU international 89 (7):681–686 [DOI] [PubMed] [Google Scholar]
- 22.Rajala P, Kaasinen E, Raitanen M, Liukkonen T, Rintala E (2002) Perioperative single dose instillation of epirubicin or interferon-alpha after transurethral resection for the prophylaxis of primary superficial bladder cancer recurrence: a prospective randomized multicenter study--FinnBladder III long-term results. The Journal of urology 168 (3):981–985. doi: 10.1097/01.ju.0000026417.33622.7d [DOI] [PubMed] [Google Scholar]
- 23.Torti FM, Shortliffe LD, Williams RD, Pitts WC, Kempson RL, Ross JC, Palmer J, Meyers F, Ferrari M, Hannigan J, et al. (1988) Alpha-interferon in superficial bladder cancer: a Northern California Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 6 (3):476–483. doi: 10.1200/jco.1988.6.3.476 [DOI] [PubMed] [Google Scholar]
- 24.Correa AF, Theisen K, Ferroni M, Maranchie JK, Hrebinko R, Davies BJ, Gingrich JR (2015) The Role of Interferon in the Management of BCG Refractory Nonmuscle Invasive Bladder Cancer. Advances in urology 2015:656918. doi: 10.1155/2015/656918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd AR, Shepherd E, Brook NR (2017) Intravesical Bacillus Calmette-Guerin with interferon-alpha versus intravesical Bacillus Calmette-Guerin for treating non-muscle-invasive bladder cancer. The Cochrane database of systematic reviews 3:Cd012112. doi: 10.1002/14651858.CD012112.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belldegrun AS, Franklin JR, O’Donnell MA, Gomella LG, Klein E, Neri R, Nseyo UO, Ratliff TL, Williams RD (1998) Superficial bladder cancer: the role of interferon-alpha. The Journal of urology 159 (6):1793–1801 [DOI] [PubMed] [Google Scholar]
- 27.Kaji EH, Leiden JM (2001) Gene and stem cell therapies. Jama 285 (5):545–550 [DOI] [PubMed] [Google Scholar]
- 28.Walther W, Schlag PM (2013) Current status of gene therapy for cancer. Current opinion in oncology 25 (6):659–664. doi: 10.1097/cco.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL, et al. (1990) Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. The New England journal of medicine 323 (9):570–578. doi: 10.1056/nejm199008303230904 [DOI] [PubMed] [Google Scholar]
- 30.Wirth T, Parker N, Yla-Herttuala S (2013) History of gene therapy. Gene 525 (2):162–169. doi: 10.1016/j.gene.2013.03.137 [DOI] [PubMed] [Google Scholar]
- 31.Wysocki PJ, Karczewska-Dzionk A, Mackiewicz-Wysocka M, Mackiewicz A (2004) Human cancer gene therapy with cytokine gene-modified cells. Expert opinion on biological therapy 4 (10):1595–1607. doi: 10.1517/14712598.4.10.1595 [DOI] [PubMed] [Google Scholar]
- 32.Baldo BA (2014) Side effects of cytokines approved for therapy. Drug safety 37 (11):921–943. doi: 10.1007/s40264-014-0226-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonasch E, Haluska FG (2001) Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. The oncologist 6 (1):34–55 [DOI] [PubMed] [Google Scholar]
- 34.Bass C, Cabrera G, Elgavish A, Robert B, Siegal GP, Anderson SC, Maneval DC, Curiel DT (1995) Recombinant adenovirus-mediated gene transfer to genitourinary epithelium in vitro and in vivo. Cancer gene therapy 2 (2):97–104 [PubMed] [Google Scholar]
- 35.Klingler CH (2016) Glycosaminoglycans: how much do we know about their role in the bladder? Urologia 83 Suppl 1:11–14. doi: 10.5301/uro.5000184 [DOI] [PubMed] [Google Scholar]
- 36.Engler H, Anderson SC, Machemer TR, Philopena JM, Connor RJ, Wen SF, Maneval DC (1999) Ethanol improves adenovirus-mediated gene transfer and expression to the bladder epithelium of rodents. Urology 53 (5):1049–1053 [DOI] [PubMed] [Google Scholar]
- 37.Connor RJ, Engler H, Machemer T, Philopena JM, Horn MT, Sutjipto S, Maneval DC, Youngster S, Chan TM, Bausch J, McAuliffe JP, Hindsgaul O, Nagabhushan TL (2001) Identification of polyamides that enhance adenovirus-mediated gene expression in the urothelium. Gene therapy 8 (1):41–48. doi: 10.1038/sj.gt.3301348 [DOI] [PubMed] [Google Scholar]
- 38.Yamashita M, Rosser CJ, Zhou JH, Zhang XQ, Connor RJ, Engler H, Maneval DC, Karashima T, Czerniak BA, Dinney CP, Benedict WF (2002) Syn3 provides high levels of intravesical adenoviral-mediated gene transfer for gene therapy of genetically altered urothelium and superficial bladder cancer. Cancer gene therapy 9 (8):687–691. doi: 10.1038/sj.cgt.7700488 [DOI] [PubMed] [Google Scholar]
- 39.Iqbal Ahmed CM, Johnson DE, Demers GW, Engler H, Howe JA, Wills KN, Wen SF, Shinoda J, Beltran J, Nodelman M, Machemer T, Maneval DC, Nagabhushan TL, Sugarman BJ (2001) Interferon alpha2b gene delivery using adenoviral vector causes inhibition of tumor growth in xenograft models from a variety of cancers. Cancer gene therapy 8 (10):788–795. doi: 10.1038/sj.cgt.7700364 [DOI] [PubMed] [Google Scholar]
- 40.Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, McConkey DJ, Papageorgiou A, Munsell M, Philopena J, Engler H, Demers W, Maneval DC, Dinney CP, Connor RJ (2004) Intravesical Ad-IFNalpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Molecular therapy : the journal of the American Society of Gene Therapy 10 (3):525–532. doi: 10.1016/j.ymthe.2004.05.027 [DOI] [PubMed] [Google Scholar]
- 41.Izawa JI, Sweeney P, Perrotte P, Kedar D, Dong Z, Slaton JW, Karashima T, Inoue K, Benedict WF, Dinney CP (2002) Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. Clinical cancer research : an official journal of the American Association for Cancer Research 8 (4):1258–1270 [PubMed] [Google Scholar]
- 42.Benedict WF, Fisher M, Zhang XQ, Yang Z, Munsell MF, Dinney CN (2014) Use of monitoring levels of soluble forms of cytokeratin 18 in the urine of patients with superficial bladder cancer following intravesical Ad-IFNalpha/Syn3 treatment in a phase l study. Cancer gene therapy 21 (3):91–94. doi: 10.1038/cgt.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Dong L, Chapman E, Benedict WF (2008) Conditioned medium from Ad-IFN-alpha-infected bladder cancer and normal urothelial cells is cytotoxic to cancer cells but not normal cells: further evidence for a strong bystander effect. Cancer gene therapy 15 (12):817–822. doi: 10.1038/cgt.2008.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Yang Z, Dong L, Papageorgiou A, McConkey DJ, Benedict WF (2007) Adenoviral-mediated interferon alpha overcomes resistance to the interferon protein in various cancer types and has marked bystander effects. Cancer gene therapy 14 (3):241–250. doi: 10.1038/sj.cgt.7701011 [DOI] [PubMed] [Google Scholar]
- 45.Connor RJ, Anderson JM, Machemer T, Maneval DC, Engler H (2005) Sustained intravesical interferon protein exposure is achieved using an adenoviral-mediated gene delivery system: a study in rats evaluating dosing regimens. Urology 66 (1):224–229. doi: 10.1016/j.urology.2005.02.015 [DOI] [PubMed] [Google Scholar]
- 46.Tao Z, Connor RJ, Ashoori F, Dinney CP, Munsell M, Philopena JA, Benedict WF (2006) Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: implications for clinical investigation. Cancer gene therapy 13 (2):125–130. doi: 10.1038/sj.cgt.7700865 [DOI] [PubMed] [Google Scholar]
- 47.Vellinga J, Smith JP, Lipiec A, Majhen D, Lemckert A, van Ooij M, Ives P, Yallop C, Custers J, Havenga M (2014) Challenges in manufacturing adenoviral vectors for global vaccine product deployment. Human gene therapy 25 (4):318–327. doi: 10.1089/hum.2014.007 [DOI] [PubMed] [Google Scholar]
- 48.Dinney CP, Fisher MB, Navai N, O’Donnell MA, Cutler D, Abraham A, Young S, Hutchins B, Caceres M, Kishnani N, Sode G, Cullen C, Zhang G, Grossman HB, Kamat AM, Gonzales M, Kincaid M, Ainslie N, Maneval DC, Wszolek MF, Benedict WF (2013) Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for Bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. The Journal of urology 190 (3):850–856. doi: 10.1016/j.juro.2013.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, Gomella LG, Kamat AM, Lotan Y, Svatek RS, Bivalacqua TJ, Grubb RL 3rd, Krupski TL, Lerner SP, Woods ME, Inman BA, Milowsky MI, Boyd A, Treasure FP, Gregory G, Sawutz DG, Yla-Herttuala S, Parker NR, Dinney CPN (2017) Intravesical rAd- IFNalpha/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non¬Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 35 (30):3410–3416. doi: 10.1200/jco.2017.72.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinney CP, Greenberg RE, Steinberg GD (2013) Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urologic oncology 31 (8):1635–1642. doi: 10.1016/j.urolonc.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 51.Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL 3rd, Clark W, Kroeger M, Dumbadze I, Chamie K, Kader AK, Curran D, Gutheil J, Kuan A, Yeung AW, Steinberg GD (2017) An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urologic oncology. doi: 10.1016/j.urolonc.2017.07.005 [DOI] [PubMed] [Google Scholar]


