Abstract
Endocrine disrupting chemicals (EDCs) in the environment are considered to be a contributing factor to the decline in the sperm quality. With growing evidence of the harmful effects of EDCs on the male reproductive system, we tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture adversely affects reproductive outcomes and androgen synthesis. In this study, an environmentally relevant composition of phthalates (15% DiNP, 21% DEHP, 36% DEP, 15% DBP, 8% DiBP, and 5% BBzP) that were detected in urine samples of pregnant women in Illinois, United States, was used. Pregnant CD-1 mice (F0) were orally dosed with a vehicle or the phthalate mixtures (20 µg/kg/day, 200 µg/kg/day, 200 mg/kg/day, or 500 mg/kg/day) from gestational day 10.5 to the day of birth. Then, the indices of the reproductive function of the F1 males born to these dams were assessed. Those male mice prenatally exposed to the phthalate mixture had smaller gonads, prostates and seminal vesicles, especially in the 20 µg/kg/day and 500 mg/kg/day phthalate mixture groups, compared to the controls. Importantly, at the age of 12 months, those prenatally exposed mice had significantly lower serum testosterone concentrations accompanied by the decreased mRNA expression of testicular steroidogenic genes (StAR, Cyp11, and Cyp17) and impaired spermatogenesis. Taken together, this study found that prenatal exposure to environmentally relevant doses of a phthalate mixture caused a life-long impact on the reproduction in male mice.
Keywords: Phthalates, Endocrine disruptor, steroidogenesis, Fertility, Testes
1. Introduction
Infertility is a growing concern with a 44% decrease in sperm counts between 1960 and 2002 in the United States (Hamilton and Ventura, 2006). Recent studies indicate that endocrine disrupting chemicals (EDCs) in the consumer products may contribute to the decline of sperm counts (Knez, 2013) due to their antiandrogenic activity (Swan et al., 2005; Main et al., 2006).
Phthalates are environmental toxicants that are widely used in plastics, toys, cosmetics, food containers, and other consumer products (Dai et al., 2015). Their ubiquitous presence allows humans to be exposed to phthalates through various routes such as dermal contact, oral ingestion, and inhalation (Kavlock et al., 2006). The most commonly used phthalates are di(2-ethylhexyl) phthalate (DEHP), di-n-butyl phthalate (DBP), diethyl phthalate (DEP), diisobutyl phthalate (DiBP), butyl benzyl phthalate (BBzP), diisononyl phthalate (DiNP), and diisodecyl phthalate (DiDP) (Johansson et al., 2016). Phthalates as DEHP, DEP, DBP, BBzP, and DiBP have been detected in urine samples taken from pregnant mothers (Silva et al., 2004).. Importantly, phthalates are detected in human amniotic fluids, suggesting that a developing fetus may be exposed to phthalates in utero (Silva et al., 2004). When rodents were prenatally exposed to DBP or BBzP, they developed phthalate syndrome which is characterized by testicular dysgenesis and low testosterone production accompanied by de-masculinization (Foster, 2006). Indeed, we showed that prenatal exposure to DEHP induces premature reproductive senescence in male mice, exhibiting reproductive senescence at a much earlier age than controls (Barakat et al., 2017).
While the mechanism of anti-androgenic action of phthalates is not fully discovered, recent studies have shown that DBP gives its anti-androgenic effects by suppressing the production of testosterone via altering the expression of key steroidogenic genes in the testis (Barlow and Foster, 2003; Shultz et al., 2001). Androgens that are synthesized in the Leydig cells of testes are important for male fertility, spermatogenesis, and the development of the male reproductive organs (Wang et al., 2009). The effect of prenatal exposure to phthalates not only cause reproductive abnormalities in its own generation, but the impact is transmitted to next generations via epigenetic modification (Doyle et al., 2013).
In females, prenatal exposure to a physiologically relevant level of mixture of phthalates such as DEHP, DBP, DEP, and BBzP was shown to induces uterine malformation, disrupt estrus cyclicity, and ultimately impact fertility (Zhou et al., 2017; Johansson et al., 2016). However, no studies yet have assessed the effects of the exposure to phthalate mixture on the male reproductive system (Barakat et al., 2017; Barlow and Foster, 2003; Foster, 2006; Shultz et al., 2001; Swan et al., 2005). Humans are constantly exposed to a mixture of many phthalates. Therefore, investigation using an environmentally relevant mixture of phthalates is necessary. In this study, we formulated a phthalate mixture based on the amounts of phthalates measured in urine samples of pregnant women and used it to test the hypothesis that prenatal exposure to a phthalate mixture adversely affects reproductive outcomes and androgen synthesis in the male mice.
2. Materials & Methods
2.1. Chemicals
Phthalates (>98% purity) were purchased from Sigma-Aldrich (St. Louis, MO). The phthalate mixture was made of 15% DiNP, 21% DEHP, 36% DEP, 15% DBP, 8% DiBP, and 5% BBzP. The percentages were in proportion to the levels of phthalate metabolites measured in urine samples of pregnant women (Yazdy et al., 2018). These doses of individual phthalates are relatively lower than those used for rodent study in literature and align within the range of the estimated daily intakes of humans (Heudorf et al., 2007).
2.2. Animal dosing and regimen
CD-1 male and female mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were housed in the animal care facility at the University of Illinois at Urbana-Champaign under 12-hour light/dark cycles and allowed to acclimate to the facility for at least two weeks before use. The female dams were mated with male breeders at two months of age. Successful mating was confirmed by the presence of a vaginal sperm plug. The pregnant female mice were dosed with 20 µg/kg/day, 200 µg/kg/day, 200 mg/kg/day, or 500 mg/kg/day of the phthalate mixture in tocopherol-stripped corn oil (MP Bio Medicals, OH) from gestational day 10.5 to the day of birth. Doses of the phthalate mixture were chosen to cover a wide environmentally relevant range and to include some of the doses of individual phthalates that were shown to adversely affect reproductive health (Manikkam et al., 2013; Rattan et al., 2017; Zhou et al., 2017). The exposure window was chosen because it is a critical period for gonadal development in the mouse. The mice were dosed orally using a pipet tip into the mouth. All procedures involving animal care, euthanasia, and tissue collection were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign.
2.3. Measurement of body weight, gonadal weight, and anogenital distance
Body weight and gonadal weight of the male offspring were measured during the entire experimental period at postnatal day (PND) 21, 60, and at 12 months of age. Weights of liver, prostate and seminal vesicle were measured at 12 months of age. Anogenital distance (AGD) was determined at PND 21, and 2 and 12 months of ages by measuring the distance from the urethral opening to the cranial opening of the anus using a caliper.
2.4. Measurement of serum testosterone
Peripheral blood was collected at 2 and 12 months of age by cardiac puncture. The blood was centrifuged at 2000 xg, and then serum was collected and stored at −20° C until further analyses. The concentrations of serum testosterone were measured by using ELISA kits (DRG Diagnostic, Springfield, NJ) with a reportable range of 0.06–25 ng/ml.
2.5. Fertility test
Each male mouse was housed with a proven breeder female for two weeks. The fertility rate (number of male that produce litter/total number of males x 100), litter size (number of pups per litter), and sex ratio (numbers of female/numbers of male pups) were recorded.
2.6. Semen analysis
Male mice were euthanized by CO2 asphyxiation followed by cervical dislocation. The cauda of the epididymis was excised at 12 months of age and minced with fine scissors in a warm phosphate buffered saline. The sperm suspension was incubated at 37°C for 10 minutes to allow spermatozoa to swim out of the minced epididymis. Sperm motility was then analyzed by a computer-assisted sperm analyzer (CASA; Sperm Vision II, Minitube of America, Vernon, WI) by examining 10 microscopic fields. Sperm motility was measured by the percentage of progressive motile sperms, percent of local motile sperms, and percentage of immotile sperms. For total sperm counts, two aliquots of semen samples were collected from each mouse and diluted 1:200 in formalin for immobilization. Sperm concentration was measured using a hemocytometer, and the average number of sperms was reported as million sperms/ml.
2.7. Tissue collection and testicular histopathology
The testis and epididymis were collected at 12 months of age, then fixed in Bouin’s solution (Ricca chemical Co., Arlington, TX) for 24 hours and then transferred to 70% ethanol. The tissues were embedded in paraffin, sectioned at 7 µm thickness, stained with hematoxylin and eosin, and examined using light microscopy (Olympus BX 51, Tokyo, Japan).
2.8. Steroidogenic gene expression analysis
Testes were collected at 12 months of age and snap frozen for quantitative real-time polymerase chain reaction (qPCR) analysis. Total RNA was extracted using TrizolVR solution (Ambion, Carlsbad, CA) and then purified with a RNeasy Kit (Qiagen, Valencia, CA). Concentration and quality of total RNA was analyzed using a Nanodrop (Thermo Scientific, Waltham, MA) and stored at –80°C until use. Complementary DNA was generated by M-MLV Reverse Transcriptase (Thermo Scientific). The mRNA expression levels of steroidogenic acute regulatory protein (Star), cytochrome P450 cholesterol side-chain cleavage (Cyp11a1), 3β-hydroxysteroid dehydrogenase 1 (Hsd3b1), 17β-hydroxysteroid dehydrogenase 1 (Hsd17b1), and cytochrome P450 aromatase (Cyp17a1) were measured by real-time PCR. The primers are listed in Table 1. Ribosomal protein L19 (RPL19) was used as the internal control and data from each gene was normalized to the corresponding value of L19 to calculate relative fold changes, which were used for statistical analysis.
Table 1.
Primer sequences used for RT-PCR.
| Target gene | Forward primer (5’−3’) | Reverse primer (5’−3’) | Fragment size (bp) |
|---|---|---|---|
| Star | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG | 262 bp |
| Cyp11a1 | AGATCCCTTCCCCTGGTGACAATG | CGCATGAGAAGAGTATCGACGCATC | 192 bp |
| Hsd3b1 | CAGGAGAAAGAACTGCAGGAGGTC | GCACACTTGCTTGAACACAGGC | 280 bp |
| Hsd17b1 | ACTGTGCCAGCAAGTTTGCG | AAGCGGTTCGTGGAGAAGTAG | 310 bp |
| Cyp17a1 | CCAGGACCCAAGTGTGTTCT | CCTGATACGAAGCACTTCTCG | 250 bp |
| Rpl19 | CCTGAAGGTCAAAGG GAAT | GTCTGCCTTCAGCTTGTG GA | 73 bp |
2.9. Statistical Analysis
The data was analyzed using the statistical software package SPSS. The comparison was between control and treated groups and the same age point, and the statistical sampling unit was litter. Multiple comparisons between normally distributed continuous experimental groups were analyzed by the one-way analysis of variance (ANOVA) as a parametric test followed by the Dunnett (two-sided) post hoc test. Multiple comparisons between non-normally distributed experimental groups were analyzed by Kruskal-Wallis as a nonparametric test. Fertility data was compared using Fisher’s exact test for each treatment group against the control group. The numbers of animals used for statistical analyses were 6–8 mice. The data is expressed as mean ± SEM. Statistical significance was assigned as P ≤ 0.05 and marked as (*), whereas statistical tendency was set as P ≤ 0.09 and marked as (^).
3. Results and Discussion
Previous studies showed the impacts of prenatal exposure to different phthalates on the male reproductive system (Barakat et al., 2017; Barlow and Foster, 2003; Foster, 2006; Shultz et al., 2001; Swan et al., 2005). In this study, we tested the hypothesis that prenatal exposure to an environmentally relevant mixture of phthalates adversely affects male reproduction. Pregnant female mice were dosed with 20 µg/kg/day, 200 µg/kg/day, 200 mg/kg/day, or 500 mg/kg/day of the six-phthalate mixture (15% DiNP, 21% DEHP, 36% DEP, 15% DBP, 8% DiBP, and 5% BBzP) from gestational day 10.5 to the day of birth. These doses of each individual phthalates are relatively low as reported in the rodent literature and align within the range of the estimated daily intakes of humans (Heudorf et al., 2007). The reproductive indices of the F1 males born to these dams were assessed by the age of 12 months.
3.1. Effects of prenatal exposure to phthalate mixture on body weights and anogenital distance
Body weights were measured to assess a potential impact on their general health and the anogenital distance (AGD) as a proxy of prenatal androgen synthesis (Eisenberg & Lipshultz, 2015). Mice prenatally exposed to phthalate mixture did not exhibit a difference in their body or liver weights compared to control group when measured at PND 21 and 60 (Table 2). At 12 months of age, however, all the phthalate mixture group showed a trend of gaining body weights, 200 µg/kg/day dose group being most impacted (P=0.04) (Table 2). Previous studies have reported similar impacts of a single phthalate exposure as DEHP on body weight (Hao et al. 2013; Kavlock et al., 2006). Interestingly, no observable difference of AGD among the groups. These results differ from previous studies that assessed the impact by the exposure to individual phthalate such as BBzP, DBP, or DEHP, in which neonatal exposure to each individual phthalate shortened the AGD (Barakat et al., 2018; Chen et al., 2015; Gray et al., 2000; Swan et al., 2005). The reason for differences between our current mixture study versus previous studies using single phthalates is not currently known. It is likely due to the difference in the doses of individual phthalates and/or dosing periods between this and previous studies (Chen et al., 2015; Gray et al., 2000).
Table 2.
Effect of prenatal exposure to phthalate mixture on body weight, organs weight, and anogenital distance.
| Treatment | PND 21 | PND 60 | 12 Months |
|---|---|---|---|
| Body weight (g) | |||
| Control | 16.59 ± 1.91 | 39.47 ± 5.3 | 51.01 ± 3.24 |
| 20 µg/kg/day | 15.36 ± 1.47 | 37.61 ± 3.45 | 54.40 ± 7.22 |
| 200 µg/kg/day | 15.82 ± 1.29 | 40.20 ± 5.50 | 61.81 ± 0.28* |
| 200 mg/kg/day | 15.15 ± 1.91 | 40.52 ±3.94 | 56.21 ± 7.37 |
| 500 mg/kg/day | 16.59 ± 1.12 | 41.37 ± 4.59 | 58.30 ± 3.76 |
| AGD (mm) | |||
| Control | 10.66 ± 0.89 | 18.11 ± 1.10 | 22.13 ± 0.71 |
| 20 µg/kg/day | 10.58 ± 0.87 | 18.83 ± 1.17 | 21.51 ± 0.95 |
| 200 µg/kg/day | 10.25 ± 0.62 | 19.12 ± 1.26 | 22.12 ± 0.01 |
| 200 mg/kg/day | 10.14 ± 1.17 | 19.16 ± 0.98 | 20.75 ± 0.94 |
| 500 mg/kg/day | 10.37 ± 0.71 | 18.51 ± 0.55 | 21.33 ± 0.88 |
| Liver weight (g) | |||
| Control | - | - | 2.86 ± 0.17 |
| 20 g/kg/day | - | - | 2.72 ± 0.35 |
| 200 g/kg/day | - | - | 3.67 ± 0.49 |
| 200 mg/kg/day | - | - | 3.11 ± 0.48 |
| 500 mg/kg/day | - | - | 3.11 ± 0.33 |
, significantly different compared to control group (P ≤ 0.05)
3.2. Prenatal exposure to phthalate mixture decreases prostate, seminal vesicle, and gonadal weights
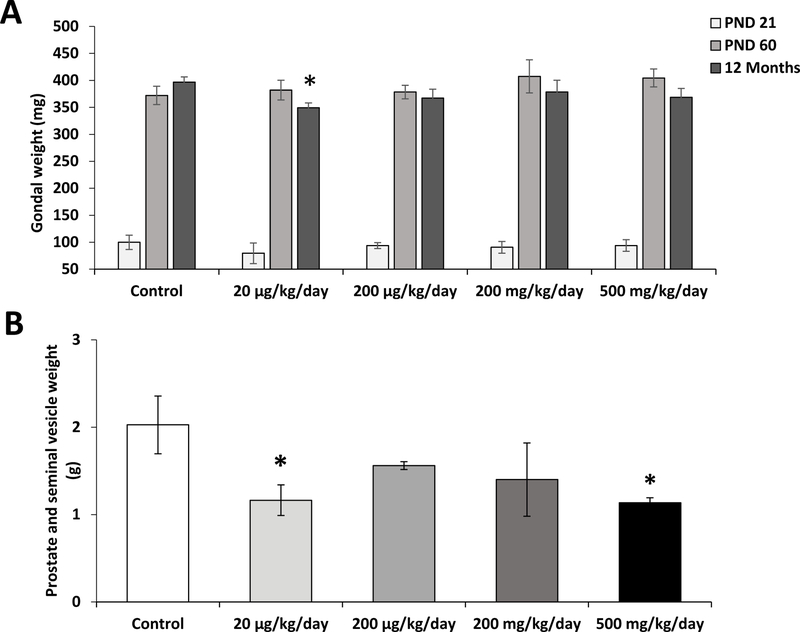
To determine the effect of prenatal exposure to phthalate mixture on gonadal development, the gonadal weights were measured at PND 21 and 60, and 12 months of ages (Figure 1A). Prenatal exposure to phthalate mixture did not affect gonadal weights when measured at PND 21 and 60. However, at 12 months of age, the gonadal weight of the 20 µg/kg/day dose group was significantly decreased than those of controls (P=0.03). At the same age, prostate and seminal vesicles of 20 µg/kg/day and 500 mg/kg/day dose groups were lighter than those of the control (P=0.04 and P=0.03, respectively) (Figure 1B). These results are consistent with previous studies that showed an exposure to individual phthalates, such as DEHP, DBP, or BBzP, altered gonadal weights (Do et al., 2012; Zhou et al., 2010; Barakat et al., 2017; Gray et al., 2001; Swan et al., 2005). In agreement with the previous report that in utero and lactational exposure to 750 mg/kg DEHP was reported to negatively affect the weight of prostate and seminal vesicles (Moore et al., 2001). The weight decrease of the gonads, seminal vesicle and the prostate is an indicator of low testosterone production or activity (Wang et al., 2009). Indeed, we found that the prenatally exposed mice had significantly lower serum testosterone levels and lower steroidogenic gene expression in their gonads than those of controls.
Figure 1.
Effects prenatal exposure to phthalate mixture on the weights of gonads, prostate and seminal vesicles.
3.3. Prenatal exposure to phthalate mixture impacts litter size and sex ratio
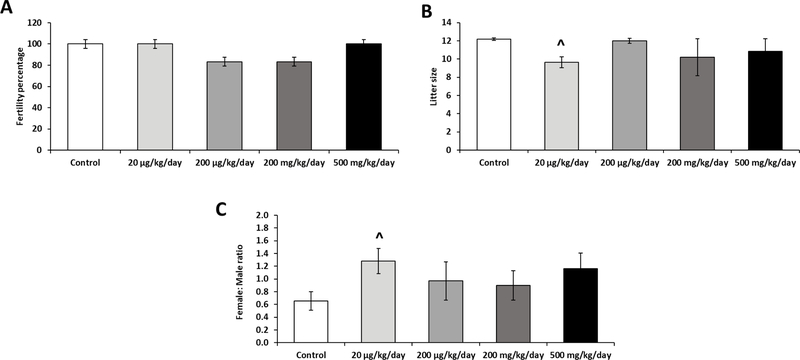
To determine the effects of prenatal exposure to a phthalate mixture on fertility, 4-month old male mice were mated with proven females. Rate of fertile males, their litter size, and the sex ratio of their offspring were measured (Figure 2). There were no significant differences between the control and treatment groups in fertility rate (Figure 2A). Litter size was recorded to assess fecundity and the 20 µg/kg/day phthalate mixture group showed a decrease in litter size (P= 0.06) compared to control (Figure 2B). Female to male ratio of the litter size was also recorded to determine if the phthalate mixture influenced sex ratios in the litter (Figure 2C). The 20 µg/kg/day phthalate mixture group showed an increase in female to male ratio when compared to the control group (P= 0.07). Taken together, these results revealed that prenatal phthalate mixture exposure did not exhibit a major reproductive disability by the age of 12 months. However, the possibility that their reproductive dysfunction would be manifested later in their life should not be excluded as we previously observed that male mice that were prenatally exposed to DEHP showed a severe reproductive dysfunction only after they reached the age of 15 months (Barakat et al., 2017). The fertility impact seen at 20 µg/kg/day dose group is consistent with previous studies that reported the non-monotonic dose-response effects of phthalates exposure (Barakat et al., 2017; Dai et al., 2015; Do et al., 2012).
Figure 2.
Effects of prenatal exposure to phthalate mixture on fertility outcomes at 4 months of age.
3.4. Prenatal exposure to phthalate mixture decreases serum testosterone in adults
At PND 60, serum testosterone levels of the prenatally phthalate mixture exposed mice were not different from controls (Figure 3A). However, when measured at the age of 12 months, the 20 µg/kg/day and 500 mg/kg/day phthalate mixture groups had significantly lower serum testosterone levels (P=0.02 and P=0.01, respectively) compared to the control (Figure 3B). This results are consistent with the previous study that showed prenatal exposure to DEHP or DBP lead to premature decline on testosterone level and reproductive health on male mice (Barakat et al., 2017; Gray et al., 2001; Shultz et al., 2001) and the smaller testicular, seminal vesicle and prostate sizes of the prenatally exposed mice observed in this study (Figure 1). The observed no impact on the testosterone levels when measured at PND 60, but the significantly lower testosterone levels at the age of 12 months may indicate that the prenatal exposure to phthalate may alter the development of progenitor cells of adult Leydig cells but not fetal Leydig cells (Martinez-Arguelles & Papadopoulos, 2015). In the testis, adult Leydig cell population arises from peritubular progenitor cells at puberty and maintains testosterone synthesis throughout life (Griswold and Behringer, 2009). Because male mice used in this study were not directly exposed to phthalates mixture at the advent of puberty but during fetal development, our results indicate that prenatal exposure might impact progenitor cells of adult Leydig cells.
Figure 3.
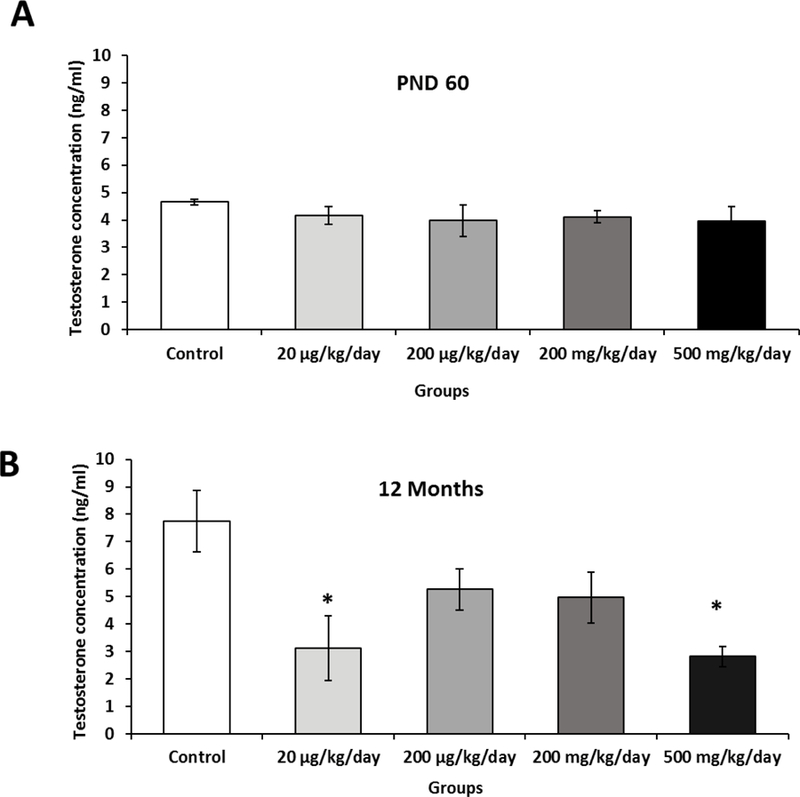
Effects of prenatal exposure to phthalate mixture on serum testosterone levels.
3.5. Prenatal exposure to phthalate mixture disrupts testicular steroidogenesis
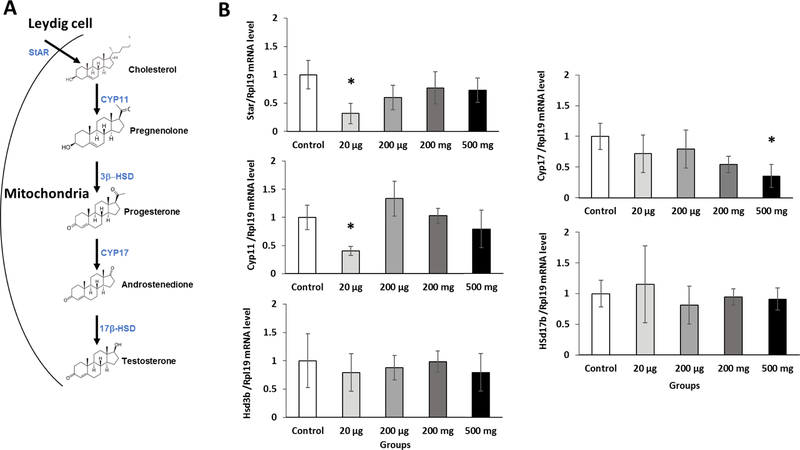
The lower testosterone level in the serum prompted us to determine if the testosterone synthesis pathway was affected by the prenatal exposure to the phthalate mixture. As serum testosterone levels are predominantly regulated by testicular testosterone synthesis (Wang et al., 2009) (Figure 4A), the mRNA expression levels of genes that are involved in testosterone synthesis were measured by qPCR. Prenatal exposure to phthalate mixture tended to decrease the expression of StAR mRNA, the 20 µg/kg/day group being most impacted (P=0.01) compared to control group (Figure 4B). The StAR is responsible for cholesterol transport into the inner mitochondria (Figure 4A) and catalyzes a rate-limiting step in testosterone synthesis in the testis (Clewell et al., 2010). Its down-regulation is associated with reduced cholesterol uptake, leading to decreased testosterone synthesis (Rone et al., 2009). The 20 µg/kg/day phthalate mixture group had a significantly lower Cyp11a1 mRNA expression than controls (P=0.04). Cyp11a1 is critical in converting cholesterol to pregnenolone, which is then converted to progesterone by Hsd3b1 (Sekaran and Jagadeesan, 2015). A previous study showed that prenatal exposure to 100 mg/kg DEHP down-regulated Cyp11a1 mRNA expression (Sekaran and Jagadeesan, 2015). The mRNA expression Hsd3b1 and Hsd17b1 was not altered in the prenatally exposed mice. Lastly, the expression of Cyp17a1 gene was significantly down-regulated in the 500 mg/kg/day phthalate mixture group (Figure 4B). Previous findings showed that prenatal exposure to 500 mg/kg/day DBP significantly decreased the expression of Cyp11a1, StAR, and Cyp17a1 (Shultz et al., 2001) and that prenatal exposure to 250 mg/kg/day DEHP significantly decreased the expression of Cyp17a1 and Hsd3b1 (Ungewitter et al., 2017). Taken together, these results suggest that the low serum testosterone levels seen in the phthalate mixture exposure group may be primarily due to adversely affected testicular steroidogenesis.
Figure 4.
Prenatal exposure to phthalate mixture effects on the expression of steroidogenic genes.
3.6. Prenatal exposure of phthalate mixture leads to histopathological abnormalities in testes and epididymis
To determine if prenatal exposure to the phthalate mixture affected gonadal function, testes and epididymis were collected at 12 months of age and microscopically examined. Testicular samples were cross-sectioned and stained with hematoxylin and eosin. The control mice exhibited active spermatogenesis in the seminiferous tubules (Figure 5A) and had densely populated sperms in the epididymis (Figure 6A). However, the testes of phthalate mixture mice showed hypospermatogenesis, degenerative changes in the seminiferous tubules, germ cell degeneration, fewer developing spermatids, and abnormal residual bodies in the lumen of seminiferous tubules (Figure 5B–E). Furthermore, the abnormal residual bodies were found in the lumen of seminiferous tubules, which is an indication of testosterone depletion and failure of spermiation (Saito et al., 2000). The epididymis of the phthalate mixture groups had a cribriform appearance of vacuoles in the epithelial lining and sloughed germ cells the in the lumen (Figure 6B–E). Quantitative histological analysis found that the 20 µg/kg/day and 500 mg/kg/day phthalate mixture groups had the higher percentage of the pathological abnormalities exhibiting as hypospermatogenesis, sloughed germ cell, and epididymal vacuoles (Table 3). These results are consistent with the reduced sperm quality and testosterone concentration seen in these two groups (Figure 3B, 7A). Our results are similar to previous individual phthalates studies showed that prenatal exposure to DEHP at 500 mg/kg/day induced testicular germ cell disorganization and impaired spermatogenesis (Doyle et al., 2013). Prenatal DBP exposure at doses of 50, 100 and 500 mg/kg lead to degeneration of seminiferous tubules, focal interstitial cell hyperplasia and adenoma at 500 mg/kg, but not at 100 mg/kg (Mylchreest et al., 2000). Testosterone is an essential hormone for spermatogenesis and for the maintenance of the blood-testis barrier, meiosis of germ cells, Sertoli cell-spermatid adhesion, and sperm release (Smith and Walker, 2014). Therefore, pathological abnormalities, including hypospermatogenesis and sloughed germ cells caused by exposure to phthalate mixture are considered to be a direct effect of reduced testosterone synthesis in the Leydig cells.
Figure 5.
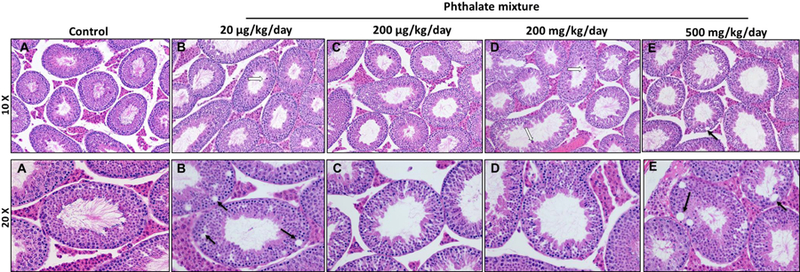
Prenatal exposure to phthalate mixture effects on the testis.
Figure 6.
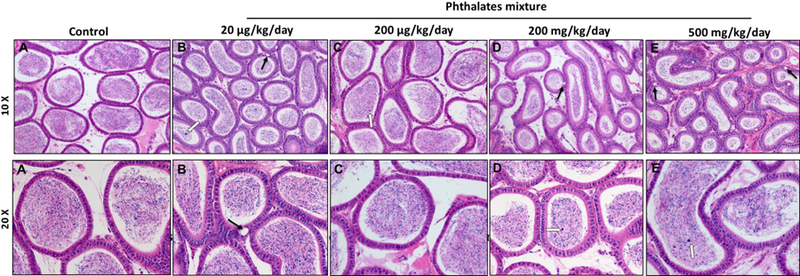
Prenatal exposure to phthalate mixture effects on the epididymis.
Table 3.
Histopathological impact of prenatal exposure to phthalate mixture.
| Control | 20ug/kg/day | 200ug/kg/day | 200mg/kg/day | 500mg/kg/day | |
|---|---|---|---|---|---|
| Testis | |||||
| -Hypospermatogenesis | 0% (0/4) | 75% (3/4) | 66% (2/3) | 75% (3/4) | 66% (2/3) |
| -Germ cell degeneration | 0% (0/4) | 100% (4/4) | 66% (2/3) | 50% (2/4) | 100% (3/3) |
| -Failure of spermiation. | 0% (0/4) | 50% (2/4) | 33% (1/3) | 50% (2/4) | 66% (2/3) |
| -Abnormal residual bodies | 0% (0/4) | 50% (2/4) | 66% (2/3) | 50% (2/4) | 33% (1/3) |
| Epididymis | |||||
| -Epididymal vacuoles | 0% (0/4) | 100% (4/4) | 33% (1/3) | 75% (3/4) | 33% (1/3) |
| -Germ cell in lumen of epididymis | 0% (0/4) | 75% (3/4) | 0% (0/3) | 50% (2/4) | 66% (2/3) |
, the number of mice showing each abnormality per treatment group was divided by the total mice per treatment group to calculate a percentage of affected mice for each abnormality (affected mice/total number of mice)
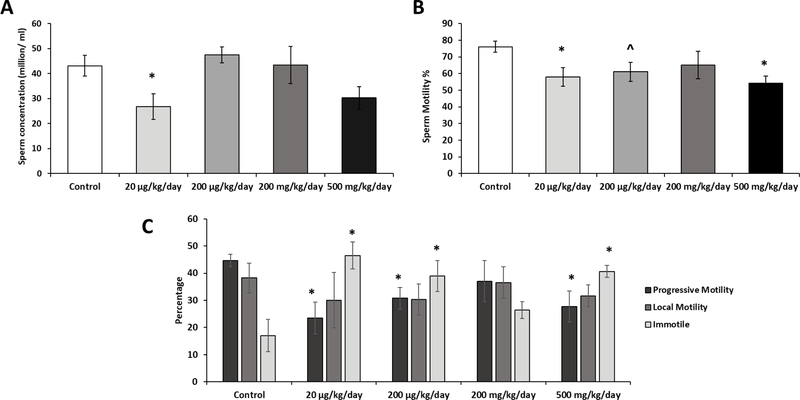
Figure 7.
Prenatal exposure to phthalate mixture effects on epididymal sperm quality.
3.7. Prenatal exposure of phthalate mixture adversely impacts sperm quantity and quality
To determine if sperm quality was affected by prenatal exposure to the phthalate mixture, sperm concentration and sperm motility were accessed by CASA at 12 months of age. Sperm concentration was significantly lower in the 20 µg/kg/day phthalate mixture group (P=0.05) compared to the control, whereas the other treatment groups showed no significant difference (Figure 7A). Because spermatogenesis is testosterone-dependent process (Smith and Walker, 2014), the decreased sperm counts in the prenatally exposed mice were expected. Interestingly, phthalate mixture treated groups exhibited a reduction in the percentage of motile sperms with significantly lower percentages in the 20 µg/kg/day and 500 mg/kg/day phthalate mixture groups (P=0.02 and P=0.03, respectively) compared to the control (Figure 7B). The progressive motile sperms percentage was significantly decreased in the 20 µg/kg/day, 200 µg/kg/day, and 500 mg/kg/day phthalate mixture groups (P=0.03, P=0.06, and P=0.04, respectively) (Figure 7C), and increased numbers of immotile sperms were seen in the 20 µg/kg/day, 200 µg/kg/day, and 500 mg/kg/day exposure groups (P=0.01, P=0.06, and P=0.02, respectively) compared to the control (Figure 7C). Sperm motility is influenced by various factors such as spermatogenesis itself, epididymal secretion and contents of seminal plasma (Pereira et al., 2017). Since the development and function of these reproductive organs are regulated by testosterone (Griswold and Behringer, 2009), it is likely that decreased testosterone by the phthalate exposure may be in part responsible for the altered sperm motility.
Our findings are consistent with previous reports of decreased epididymal sperm concentration following single exposures of DEHP, BBP, and DINP. Previous studies have shown that individual phthalate exposures, such as DEHP (500 mg/kg/day) or DBP (2000 mg/kg.bw), lead to reduction of sperm concentration and motility, and increase the incidences of urogenital malformations (Dobrzyńska et al., 2011; Doyle et al., 2013; Gray et al., 2000; Wang et al., 2004; Gray et al., 2000; Moore et al., 2001).
Conclusion
Taken together, these results show that prenatal exposure to a mixture of phthalates that are comparable to human exposure levels disrupts testicular steroidogenesis, sperm quality, and fertility in the adulthood male mice. Importantly, this study found that among the doses tested in this study, the lowest dose group (20μg/kg/day) gave the severst impact on the reproduction of the tested animals, displaying a non-monotonic dose response, against the general norm that a lower dose of a toxicant would be safer than a higher dose. Overall, this study found that prenatal exposure to environmentally relevant doses of a phthalate mixture caused a life-long impact on the reproduction in male mice.
Highlights:
Prenatal exposure to phthalate mixture adversely affects androgen synthesis accompanied by the decreased mRNA expression of testicular steroidogenic genes (StAR, Cyp11, and Cyp17).
Prenatally exposed mice had significantly lower sperm concentration and impaired spermatogenesis.
Prenatal exposure to environmentally relevant doses of a phthalate mixture caused a life-long impact on the reproduction in male mice.
Acknowledgments
Funding
This work was supported by National Institute of Environmental Health Sciences grant (P01-ES022848) and Environmental Protection Agency grant (RD-83459301), Egyptian Higher Ministry of Education Scholarship (JS-3041), Summer Undergraduate Research Experience in Toxicology (SURE Tox, R25 ES025059).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest here.
Reference
- Barakat R, Lin P-CP, Rattan S, Brehm E, Canisso IF, Abosalum ME, Flaws JA, Hess R, Ko C, 2017. Prenatal exposure to DEHP induces premature reproductive senescence in male mice. Toxicol. Sci 1–13. 10.1093/toxsci/kfw248 [DOI] [PMC free article] [PubMed]
- Barakat R, Park CJ, Perez PA, Chiu K, Ko C, 2018. Female Antiestrogens, in: Reference Module in Biomedical Sciences Elsevier; 10.1016/B978-0-12-801238-3.64414-8 [DOI] [Google Scholar]
- Barlow NJ, Foster PMD, 2003. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol. Pathol 31, 397–410. 10.1080/01926230390202335 [DOI] [PubMed] [Google Scholar]
- Chen J, Wu S, Wen S, Shen L, Peng J, Yan C, Cao X, Zhou Y, Long C, Lin T, He D, Hua Y, Wei G, Wu CJ, Wen S, Shen S, Peng L, Yan J, 2015. The Mechanism of Environmental Endocrine Disruptors (DEHP) Induces Epigenetic Transgenerational Inheritance of Cryptorchidism 10.1371/journal.pone.0126403 [DOI] [PMC free article] [PubMed]
- Clewell RA, Campbell JL, Ross SM, Gaido KW, Clewell HJ, Andersen ME, 2010. Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol. Vitr 24, 327–334. 10.1016/J.TIV.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Dai Y, Yang Y, Xu X, Hu Y, 2015. Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Horm. Behav 71, 41–48. 10.1016/j.yhbeh.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Do PR, Stahlhut R. w., Ponzi D, Saal FS vom, Taylor JA, 2012. Non-monotonic dose effects of in utero exposure to di(2- ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses Rylee 34, 614–621. 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyńska MM, Tyrkiel EJ, Pachocki KA, 2011. Developmental toxicity in mice following paternal exposure to Di-N-butyl-phthalate (DBP). Biomed. Environ. Sci 24, 569–78. 10.3967/0895-3988.2011.05.017 [DOI] [PubMed] [Google Scholar]
- Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH, 2013. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod 88, 112 10.1095/biolreprod.112.106104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH, 2013. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod 88, 112 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, & Lipshultz LI, 2015. Anogenital distance as a measure of human male fertility. Journal of Assisted Reproduction and Genetics, 32(3), 479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Miyawaki E, 2002. Effects on development of the reproductive system in male offspring of rats given butyl benzyl phthalate during late pregnancy. Reprod. Toxicol 16, 71–6. [DOI] [PubMed] [Google Scholar]
- Foster PMD, 2006. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl 29, 140-7–5. 10.1111/j.1365-2605.2005.00563.x [DOI] [PubMed] [Google Scholar]
- Gray LE, Laskey J, Ostby J, 2006. Chronic Di-n-butyl Phthalate Exposure in Rats Reduces Fertility and Alters Ovarian Function During Pregnancy in Female Long Evans Hooded Rats. Toxicol. Sci 93, 189–195. 10.1093/toxsci/kfl035 [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Price M, Veeramachaneni DNR, Parks L, 2000. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci 58, 350–365. 10.1093/toxsci/58.2.350 [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L, 2001. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update 7, 248–264. 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Griswold SL, Behringer RR., 2009. Fetal Leydig cell origin and development. Sex Dev 3:1–15. doi: 10.1159/000200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BE, Ventura SJ, 2006. Fertility and abortion rates in the United States, 1960–2002. Int. J. Androl 29, 34–45. 10.1111/j.1365-2605.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- Hao C, Cheng X, Guo J, Xia H, & Ma X, 2013. Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Frontiers in Bioscience, 5(12), 725–733. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J, 2007. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 210, 623–634. 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Johansson KL,H, Rosenskjold Jacobsen P, Hass U, Svingen T, Marie Vinggaard A, Krag Isling L, Axelstad M, Christiansen S, Boberg J, 2016. Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging. Reprod. Toxicol 61, 186–194. 10.1016/j.reprotox.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, Williams P, 2006. NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reproductive Toxicology, 22(3), 291–399. [DOI] [PubMed] [Google Scholar]
- Knez J, 2013. Endocrine-disrupting chemicals and male reproductive health. Reprod. Biomed. Online 26, 440–448. 10.1016/J.RBMO.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen JH, Andersson AM, Toppari J, Skakkebaek NE, 2006. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect 114, 270–276. 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK, 2013. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS One 8 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, & Papadopoulos V, 2015. Mechanisms mediating environmental chemical-induced endocrine disruption in the adrenal gland. Frontiers in Endocrinology, 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RW, Rudy TA, Lin TM, Ko K, Peterson RE, 2001. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer di(2-ethylhexyl) phthalate. Environ. Health Perspect 109, 229–237. 10.1289/ehp.01109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, Foster PM, 2000. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol. Sci 55, 143–51. [DOI] [PubMed] [Google Scholar]
- Pereira R, Rosália SA, Barros A, Sousa M. Major regulatory mechanisms involved in sperm motility. Asian J Androl 2017;19:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Brehm E, Gao L, Niermann S, Flaws JA, 2017. Prenatal exposure to di(2- ethylhexyl) phthalate (DEHP) disrupts ovarian function in a transgenerational manner in female mice. Biol. Reprod 10.1093/biolre/iox154. [DOI] [PMC free article] [PubMed]
- Rehman S, Usman Z, Rehman S, AlDraihem M, Rehman N, Rehman I, Ahmad G, 2018. Endocrine disrupting chemicals and impact on male reproductive health. Transl. Androl. Urol 7, 490–503. 10.21037/tau.2018.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V, 2009. Cholesterol transport in steroid biosynthesis: Role of protein–protein interactions and implications in disease states. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1791, 646–658. 10.1016/j.bbalip.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, O’Donnell L, Mclachlan RI, Robertson DM, 2000. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 141, 2779–2785. 10.1210/en.141.8.2779. [DOI] [PubMed] [Google Scholar]
- Sekaran S, Jagadeesan A, 2015. In utero exposure to phthalate downregulates critical genes in Leydig cells of F1 male progeny. J. Cell. Biochem 116, 1466–1477. 10.1002/jcb.25108. [DOI] [PubMed] [Google Scholar]
- Shultz VD, Phillips S, Sar M, Foster PM, Gaido KW, 2001. Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol. Sci 64, 233–42. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy J. a, Herbert a R., Preau JL, Needham LL, Calafat a M., 2004. Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol 72, 1226–1231. 10.1007/s00128-004-0374-4 [DOI] [PubMed] [Google Scholar]
- Smith LB, Walker WH., 2014. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Kim S, Koschorreck J, Kho Y, Choi K, 2016. Alteration of sex hormone levels and steroidogenic pathway by several low molecular weight phthalates and their metabolites in male zebrafish (Danio rerio) and/or human adrenal cell (H295R) line. J. Hazard. Mater 320, 45–54. 10.1016/J.JHAZMAT.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL, Drobnis EZ, Carter BS, Kelly D, Simmons TM, Wang C, Lumbreras L, Villanueva S, Diaz-Romero M, Lomeli MB, Otero-Salazar E, Hobel C, Brock B, Kwong C, Muehlen A, Sparks A, Wolf A, Whitham J, Hatterman-Zogg M, Maifeld M, 2005. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect 113, 1056–1061. 10.1289/ehp.8100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewitter E, Rotgers E, Bantukul T, Kawakami Y, Kissling GE, Yao HH-C, 2017. From the Cover: Teratogenic Effects of in Utero Exposure to Di-(2-Ethylhexyl)-Phthalate (DEHP) in B6:129S4 Mice. Toxicol. Sci 157, 8–19. 10.1093/toxsci/kfx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R-S, Yeh S, Tzeng C-R, Chang C, 2009. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev 30, 119–32. 10.1210/er.2008-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Song L, Chen J, He J, Liu R, Zhu Z, Wang X, 2004. [Effects of di-butyl phthalate on sperm motility and oxidative stress in rats]. Zhonghua Nan Ke Xue 10, 253–6. [PubMed] [Google Scholar]
- Yazdy MM, Coull BA, Gardiner JC, Aguiar A, Calafat AM, Xiaoyun Ye X, Schantz SL, Korrick SA, 2018. A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy. J. Expo. Sci. Environ. Epidemiol 28, 448–460. 10.1038/s41370-018-0050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Gao L, Flaws JA, 2017. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol. Appl. Pharmacol 318, 49–57. 10.1016/j.taap.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Wang H, Zhang J, Gao X, Zhao W, Zheng Y, 2010. Di-n-Butyl Phthalate (DBP) Exposure Induces Oxidative Damage in Testes of Adult Rats. Syst. Biol. Reprod. Med 56, 413–419. 10.3109/19396368.2010.509902 [DOI] [PubMed] [Google Scholar]