Abstract
Tendons and ligaments play essential roles in connecting muscle and bone and stabilizing the connections between bones. The damage to tendons and ligaments caused by aging, injury, and arthritis induces the dysfunction of the musculoskeletal system and reduces the quality of life. Current therapy for damaged tendons and ligaments depends on self-repair; however, it is difficult to reconstruct normal tissue. Regeneration therapy for tendons and ligaments has not been achieved, partly because the mechanism, cell biology, and pathophysiology of tendon and ligament development remain unclear. This review summarizes the role of the transcription factor, Mohawk, which controls tendon and ligament cell differentiation, in the maintenance of cell homeostasis, as well as its function in disease and the possibility of new therapeutic approaches.
Keywords: Tendon and ligament, regeneration, transcription factor
Introduction
Currently, musculoskeletal disorders are a major problem that affects healthy life(1). Within the musculoskeletal system, tendons and ligaments serve as connective tissue. Tendons connect muscles and bones and play an important role in transmitting force, and ligaments connect bone to bone and regulate mobility and stability. Tendons and ligaments function in various parts of the body. For example, the annulus fibrosus connects the vertebral bodies, maintaining stabilization and allowing flexible movement of the spine(2). The periodontal ligament (PDL) connects the teeth with the alveolar bone and acts not only as a stabilizer but also as a sensory receptor for the masticatory system(3). Damage to and degeneration of these tissues causes disorders and diseases associated with pain and disability, but the current therapy that relies on self-repair is not sufficient for the reacquisition of mechanical strength(4). A better understanding of the molecular mechanism of the development and homeostasis of tendons and ligaments is required for the conception of more advanced therapy for these tissues. While research in this area is progressing, it is not as advanced as that in other aspects of the musculoskeletal system.
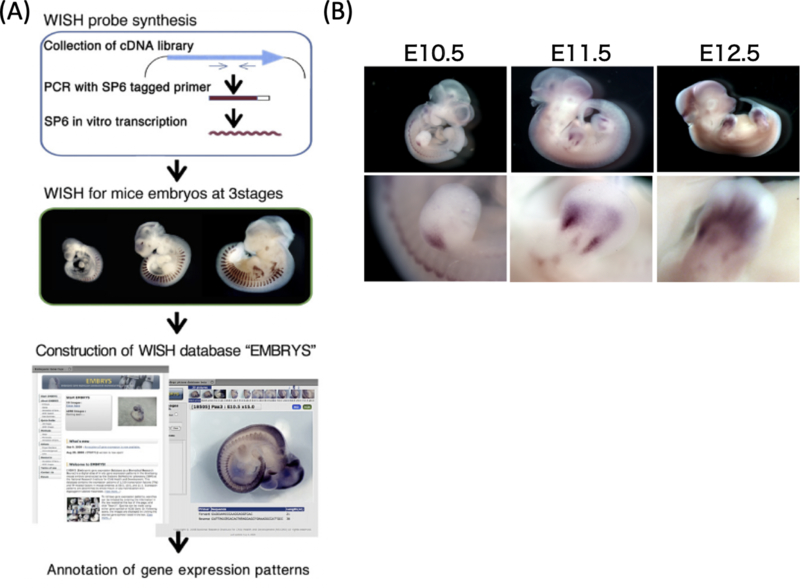
To identify tissue-specific transcription factors present during embryogenesis and to clarify the gene network involved in tissue development, we previously generated a whole-mount in situ hybridization database, EMBRYS that details the expression of ~1,500 transcription factors and cofactors during embryogenesis(5). Using this system, we found that the homeobox protein Mohawk (Mkx) is specifically expressed in tendon- and ligament-related tissues6) (Figure 1). Based on these findings, we generated Mkx-knockout mice and rats, analyzed the function of this transcription factor under physiological conditions, and obtained several interesting results(6)–(12). In this review, we report the mechanism underlying tendon and ligament development and homeostasis, with special attention to Mkx.
Figure 1. Development and regeneration research based on EMBRYS.
(A) The scheme of WISH database creation. (B) WISH image of Mkx in EMBRYS.
Structure of tendons/ligaments and limitation of the self-repair system
The smallest unit of tendon and ligament tissue is the collagen molecule. Initially, collagen molecules organize into triple helical polypeptide chains, and these chains form cross-links with each other. These structures are called collagen fibrils and they provide mechanical strength. A collagen fiber is an aggregate of collagen fibrils wrapped with endotenon, and a fiber bundle is an aggregate of collagen fibers. Aggregates of fiber bundles are wrapped with epitenon, and these are named tendons(13), (14) (Figure 2).
Figure 2. Schematic drawing of tendon and ligament tissue.
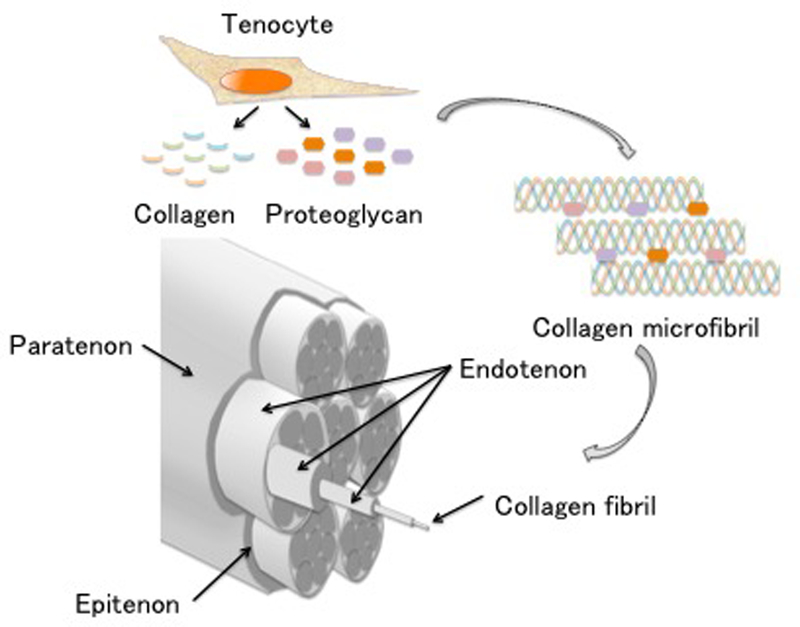
Tenocytes synthesize collagens and proteoglycans. Collagen is organized in triple helical polypeptide chains, and collagen fibrils are formed from collagen microfibrils. Collagen fibrils are connected and wrapped with endotenon. A fiber bundle is an aggregate of collagen fibers. Aggregates of these fiber bundles are wrapped with epitenon and paratenon, and these are named tendon and ligament.
Collagen fibrils are mainly composed of type I collagen, and endotenon and epitenon are composed of type III collagen. Other collagen types that make up tendons include types IV, V, and VI15). Blood vessels, lymphatic vessels, and nerve tissues are present in endotenon and epitenon, and they play an essential role in the maintenance of tendon and ligament cells(16). Other extracellular matrix components include proteoglycans Decorin and Tenascin C, which are important for collagen fiber polymerization, and Aggrecan, which plays a role in reducing friction when collagen fibrils glide(16).
In the previous study, the repair system of tendon and ligament is reported that is divided three phases(17)–(22): an inflammatory phase, a cell proliferative phase, and a remodeling phase. The inflammatory phase occurs immediately after injury to several days thereafter. Blood vessels present in the tendon sheath cause hematoma, which contains fibrin, platelets, various growth factors, and cytokines. These attract neutrophils and macrophages to the injured site, and angiogenic factors are also released at this time. The cell proliferation phase begins from day 3 and lasts for several weeks after injury. This phase is classified into two categories: exogenous repair and endogenous repair. In exogenous repair, fibroblasts migrate to the injured site via macrophages because of lactic acid produced by ischemia tissue(23). Fibroblasts produce irregular extracellular matrix mainly composed of type III collagen, which forms mechanically weak and non-gliding tissue. In endogenous repair, tenocytes migrate from the inner and outer tendon sheath and form a structure imitating collagen fibrils(24). Both repair systems work at the injured site, but exogenous repair works slightly earlier than endogenous repair. In addition, the balance between the two repair systems depends on the distance between the stump ends of the injured tendons, the injury range, and post-therapy. The remodeling phase occurs 6 to 8 weeks after injury, and type III collagen is slowly replaced with type I collagen. Type I collagen is rearranged in the axial direction of the tendon, and its mechanical strength increases (Figure 3).
Figure 3. Process of tendon and ligament healing.
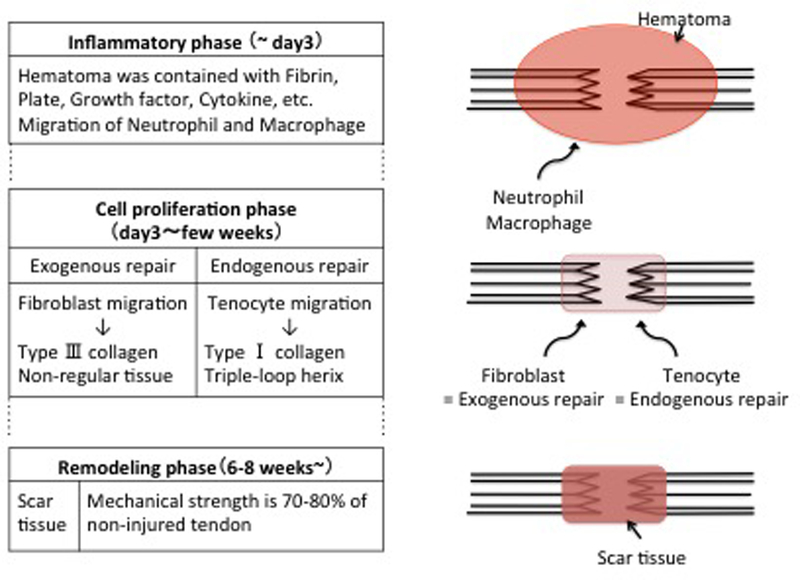
The process is divided into three phases: inflammatory phase, cell proliferation phase, and remodeling phase. Tissues repaired by natural healing have insufficient mechanical strength when compared with uninjured tendon and ligament.
However, the strength of the repaired tendon is only 70–80% that of uninjured tendon even if the repair system works sufficiently well, and currently it is difficult to mechanically complete repair(4). Many studies have attempted to increase the regeneration potential using transforming growth factor β (TGFβ), basic fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), insulin-like growth factor 1 and 2, plateletderived growth factor, etc., which have been implicated in tendon and ligament repair. However, there is no report stating that the strength of the repaired tissue attained the same level as that of uninjured tendon and ligament(25)–(29). Therefore, to promote regeneration therapy, studies on the mechanism of tendon and ligament development have drawn attention.
Current knowledge of tendon development
A transcription factor is a protein that binds the sequences of DNA and controls the rate of transcription of multiple genes. Approximately 1800 genes in the human genome encode transcription factors. Transcription factors play an essential role in determining cell fate and development. Therefore, it is necessary to know what transcription factors are required during tendon and ligament development.
Scleraxis (Scx) is involved in the early stage of tendon development(30), (31). Analysis of Scx expression in wild-type mouse embryo confirmed expression in tendon progenitor cells at the E9.5, early developmental stage(30), (31). The knockout of Scx causes the development of hypoplastic tendon tissue in the entire body of the mouse. The induction of tendon progenitor cells was intact in Scx−/− mice, but tendon formation was disrupted(32). As a result, Scx is considered an important early marker in tendon development. In addition, the expression of Scx was observed between the sclerotome and the myotome in the embryonic somite. This area is named the syndetome and is the origin of the axial tendons(33). The discovery of Scx accelerated the research on tendon development, and there have been reports on the relationship between Scx and growth factors. FGFs induce the expression of Scx in the syndetome and are involved in the differentiation of and interaction between muscle and tendon(34). The induction of bone morphogenetic protein BMP12 (growth/differentiation factor, GDF 5), which belongs to the TGFβ superfamily, in mesenchymal stem cells (MSCs) resulted in increased expression of Scx and type I collagen. BMP12 plays an important role in tendon development via the TGFβ/Smad pathway(35). In addition, the expression of Scx was maintained through the TGFβ/Smad 2/3 pathway by adding mechanical stress to tenocytes, and this mechanical stress is important in the maintenance of tendon homeostasis(36). Tenomodulin (Tnmd), which inhibits VEGF-induced angiogenesis(37), is strongly expressed in mature tenocytes and it is useful as a late marker of tendon development. Furthermore, Scx induces the expression of Tnmd(38). In Tnmd-knockout mice, the cell proliferative capacity of tenocytes is low, and the collagen fiber bundles in tendons exhibit a non-uniform morphology(39). TGFβ can induce the expression of Scx and Tnmd. Since TGFβ is expressed only in muscles and chondrocytes at the differentiation stage, it is suggested that interactions between muscle, tendon, and cartilage are important during the differentiation stage(40). Furthermore, to evaluate the function of Scx about tendon healing, is it is reported that Scx-induced MSCs are increased the expression of Col1a1, Dcn, and Tnmd and are lost multipotency(8),(41),(42). In addition, several reports indicate Scx positive progenitor cells exist in wound site within tendon healing(43),(44). These reports have indicated that Scx has an important role not only in the tendon development but also tendon regeneration. However, it is known that the expression of Scx is substantially decreased after birth(45). Therefore, it is not only Scx that regulates the differentiation of stems cells into tenocytes, but also other factors that are involved in the homeostasis of post-natal tendons may be critical.
Curiously enough, many reports are focused the relationship between Scx and tendon, but not focused about ligament. Recently it is reported that although Scx is expressed both tendon and ligament in developmental stage, in Scx−/− mice , disrupted phenotype of ligament is not seen in neonatal stage and hypoplastic phenotype of ligament is seen in postnatal stage(32),(46). The closer analysis of this difference is also interesting.
Egr1 is also an important transcription factor in tendon and ligament development. Egr1 is first detected at 12.5 of mice in the syndetome(47). Egr1−/− mice showed a reduction in the number of collagen fibrils in embryonic tendons and exhibited reduced expression of tendon- and ligament-related genes involved Scx(47). Moreover, the overexpression of Egr1 in MSCs induced the expression of tendon- and ligament-related genes(48). It is reported that Egr1 regulate the expression of Scx through TGFβsignal pathway(48), but Scx occurs in earlierstage than Egr1 in development(30),(31),(47), so Egr1 has the function to maintain the expression of Scx.
The function of Mkx in the development of tendons and ligaments
Mkx is the sole member of a newly characterized class within the three amino acid loop extension (TALE) superclass of atypical homeobox genes encoding 353 amino acids. In mouse embryos, the expression of Mkx is observed on E12.5 of mice in the syndetome(49).
There are two major phenotypes of Mkx−/− mice and rats. The first is hypoplastic tendons and ligaments in the body at birth and the second is the degeneration of tendons and ligaments after birth. For example, the Achilles tendon, the biggest tendon in the body, is hypoplastic at birth in Mkx−/− mice and rats, and it was observed that the diameter of collagen fibrils in the tendon was smaller in the Mkx−/− mice than in wild-type animals(6), (10). Furthermore, bone tissue is newly formed within the Achilles tendon three weeks after birth(10) (Figure 4). In the intervertebral discs, Mkx is expressed mainly in the outer annulus fibrosus (OAF), the fibrous tissue that connects the vertebral bodies. In Mkx−/− mice, the OAF is hypoplastic compared to that of wild-type mice at birth. The diameter of collagen fibrils in the OAF is also smaller in the Mkx−/− mice than in the wildtype animals. In addition, the intervertebral discs of Mkx−/− mice gradually degenerate with age causing degenerative spondylosis(11) (Figure 4). These findings suggest that the OAF is important for maintaining the structure of the intervertebral disc and that Mkx plays an essential role in this process.
Figure 4. Venus expression in Venus knock-in mice and representative phenotype in Mkx-knockout mice.
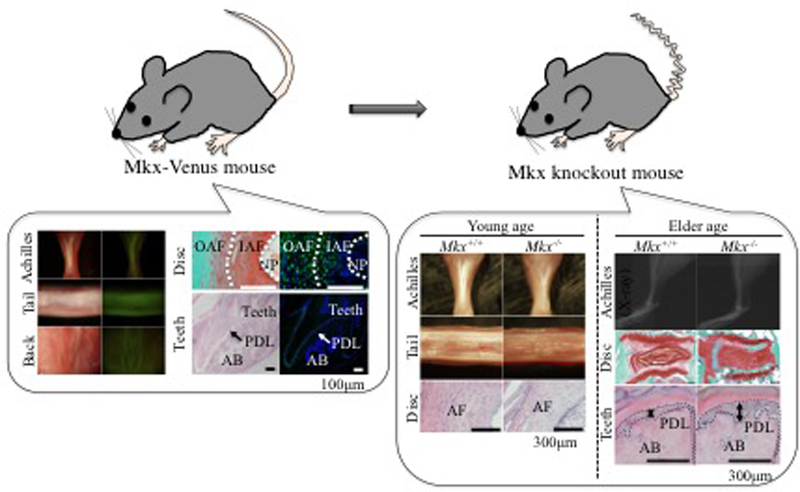
The figures on the left show Achilles tendon, tail tendon, back tendon, annulus fibrosus, and periodontal ligament in Venus knock-in (= Mkx+/−) mice. Venus expression is identified in tendons and ligaments. The figures on the right are comparisons between Mkx+/+ and Mkx−/− mice. The major phenotype at young age is hypoplastic tendons and ligaments. The major phenotype in adults is chondrogenic or osteogenic changes in tendons and ligaments. These images are modified from references 6, 11, and 12.
The common point about these tissues is that they are all fibrous tissues formed mainly of type I collagen. When Mkx is knocked out, the expression of type I collagen is reduced, the tissues become hypoplastic, and the structure gradually degenerates. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis revealed the down-regulation of tendon- and ligament-related genes, including Col1a1, Col1a2, and genes in the SLRP family in the OAF cells of Mkx−/− mice compared with those of wild-type mice(10), (11). Electron microscopic observations showed that the collagen fibrils were significantly narrower in Mkx−/− mice than in wild-type mice(6), (10), (11). These findings suggest that Mkx has an important function in type I collagen tissue formation in tendons and ligaments.
The phenotypes of the Scx−/− and Mkx−/− mice are different. In Scx−/− mice, the formation of tendons was disrupted, but in Mkx−/− mice, the tendons were formed but were hypoplastic, and the number of tenocytes did not decrease(6), (32). These differences suggest that Scx is essential in the early stage of tendon development and Mkx plays a critical role in tendon maturation.
So, is there a direct or indirect relationship between Mkx and Egr1? Egr1−/− mice did not reduced expression of Mkx(47). Moreover, the overexpression of Egr1 in MSCs did not induce the expression of Mkx(48). On the other hand, the overexpression of Mkx in MSCs did not affect the expression of Egr1(11). These results suggest that Mkx and Egr1 affect tendon and ligament development through different mechanisms. Furthermore, neither Mkx nor Egr1 knockout mice show any complete loss of tendon or ligament tissue. These results indicate that some parts are linked or that unknown factors may exist in tendon and ligament development. Current knowledge of the tendon transcription factor network in the development of tendon in embryogenesis are shown (Figure 5)
Figure 5. Current summary of key transcription factor in tendon development.
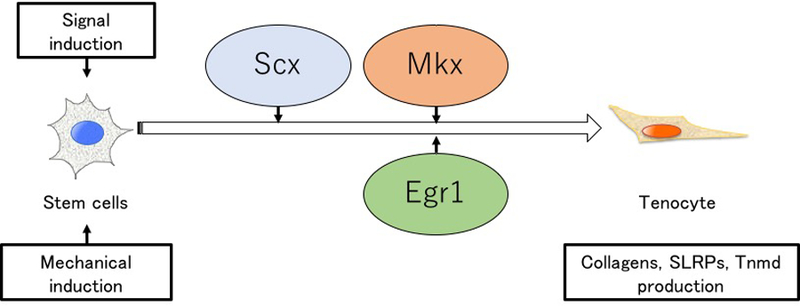
The expression of Scx, Mkx, and Egr1 are induced by signaling cues and mechanical cues. Scx is first expressed, and Mkx and Egr1 are subsequently expressed. These factors activate the expression of tendon and ligament related genes.
The function of Mkx in the adult tendons and ligaments
Mkx expression is maintained in murine and human tendon and ligament cells. In human anterior cruciate ligament (ACL) cells, the expression of Mkx gradually decreases during aging or OA change progression7). Moreover, Mkx knockout in human ACL and OAF cells resulted in a decrease in the expression of tendonrelated genes(7),(11). These findings suggest that Mkx has the potential to maintain tendon and ligament cell productivity in tissues at the adult stage.
Chondrogenic metaplasia is a representative degenerative change in the ACL. As mentioned above, heterotopic ossification is seen in the Achilles tendon of Mkx−/− rats(10). Mkx is also expressed and is functional in the PDL. Although no significant changes were observed in the PDL of young Mkx−/− mice when compared with that of wild-type animals, the PDL of aged Mkx−/− mice underwent osteogenic changes(12). Moreover, when the tenocytes in the non-ossified patellar tendons of Mkx−/− rats were extracted and induced to differentiate in vitro, the tenocytes progressed toward both cartilage and bone differentiation more readily than those from wild-type animals(10). In contrast, MSCs overexpressing Mkx resisted chondrogenic, osteogenic, and adipogenic differentiation(11). These findings suggest that Mkx has the potential to maintain tendon and ligament cell homeostasis at the adult stage.
The function of Mkx in the mechanobiology of tendon and ligament cells
Proper mechanical stimulation is essential for collagen production(50), and mechanical signals promote MSC differentiation into tenocytes(51). Mkx is also responsive to mechanical stimulation. Wild-type mice exhibited an increase in collagen fiber diameter and density in response to physical treadmill exercise, whereas in Mkx−/− mice, tendons failed to respond to the same mechanical stimulation. This suggests that Mkx has a role in the response to mechanical stimulation(9). Furthermore, general transcription factor II-I repeat domain-containing protein 1 (Gtf2ird1) was found to play an important role in translating mechanical stress to Mkx expression9). Gtf2ird1 is localized in the cytoplasm of unstressed tenocytes and is translocated into the nucleus upon mechanical stretching to activate Mkx expression, thereby acting as a mechanosensor(9).
The potential of regeneration therapy using stem cells inducing Mkx
One of the main approaches in tissue regenerative therapy is cell transplantation using stem cells. It was reported that undifferentiated tendon stem/progenitor cells (TSPCs) as well as the stem cell niche are present in tendon tissue(52). However, the number of TSPCs is very low in adult tissues and their proliferative capacity is limited. Although many trial has done for TSPC to increase viability or proliferation using compounds, growth factor, and mechanical stimulation(53)–(55), it is presently difficult to achieve regenerative therapy by using cell therapy directly with TSPCs. To overcome the above problems, it is necessary to establish a system for the in vitro differentiation of tendon cells and to discover tendon-specific master transcription factors. The identified master transcription factor can also be used as a new differentiation marker of the tenocyte lineage, and it can be expected to lead to the elucidation of the differentiation signal cascade.
In order to analyze the characteristics of Mkx as a key transcription factor, we prepared the Mkxoverexpressing C3H10T1/2 cell line using a retrovirus. At first, the steady-state expression of Mkx in C3H10T1/2 changed the cell morphology to a spindle shape similar to tendon cells. Then, gene expression analysis by qRT-PCR was performed. Several genes that are important in the formation of type I collagen tissue, such as the tenogenetic markers Scx, Col1a1, and TNC, were strongly expressed when compared with the control group(11). These results suggested that due to the expression of the tendon-specific transcription factor Mkx, C3H10T1/2 differentiated into the tenocyte lineage and expressed the components of extracellular matrix in tendon tissue. Further, it was confirmed that when these cells were transplanted into mice with defects in OAF, type I collagen tissue of a size equal to those in normal OAF tissue was observed(11). Moreover, to study the physical property of the newly formed tissue, this transplant model was subjected to disc degeneration. Therefore, it was confirmed that this newly formed tissue had the same physical properties as those of healthy tissue(11). Although the lamellar structure of OAF was not fully reconstituted, the OAF tissues regenerated by transplanted Mkx-overexpressing stem cells had sufficient physical biomechanical property. These results show that the transcription factor Mkx plays an important role in inducing the differentiation of MSCs to tendon and ligament cells and that these induced cells have potential application in regenerative therapy of tendon and ligament tissue. In the future, it is necessary to establish a method for regulating the orientation of collagen fibers synthesized by the transplanted cells (Figure 6).
Figure 6. Future plan for tendon and ligament regeneration or reconstruction therapy.
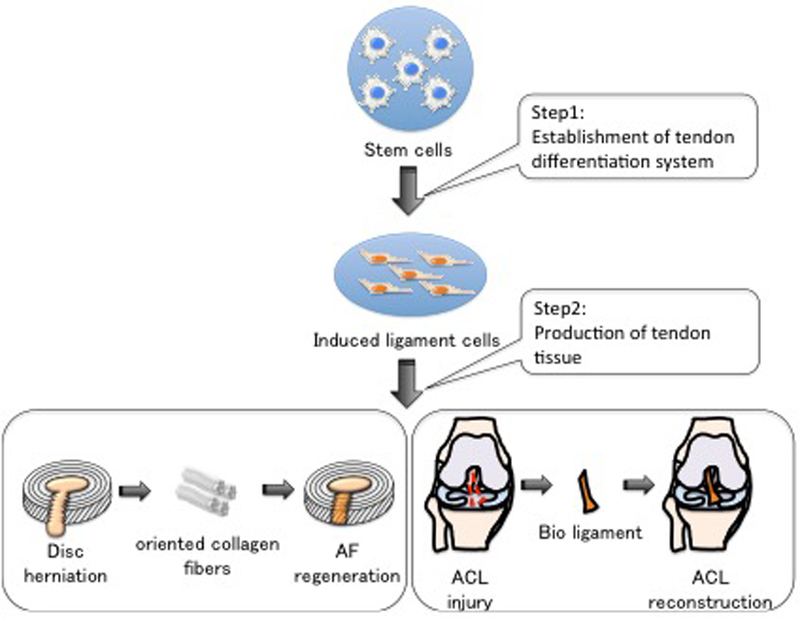
We hope that further analysis of the intergenic network will make it possible to establish a differentiation system for tendon cells (Step 1), and to produce tendon tissue-like organoids (Step 2).
Conclusion and future direction
We performed comprehensive gene expression analysis using a whole-mount in situ hybridization database, EMBRYS that details the expression of transcription factors and cofactors during embryogenesis in the mouse. As a result, we discovered the new master transcription factor Mkx in tendons and ligaments. Further, we succeeded in analyzing its function under physiological conditions and provided insight for the improvement of tendon and ligament regeneration therapy. Based on these findings, we are carrying out further analysis of the intergenic network in order to establish a differentiation system for tendon cells independent of viral vectors, and to produce tendon tissue-like organoids. We will continue to pursue the advancement of regenerative therapy through integrative research on the musculoskeletal system.
Acknowledgement
We thank Naoki Koda and all other members of the Asahara lab. This research is supported by AMEDCREST from AMED (JP15gm0410001, JP17gm0810008), JSPS KAKENHI [Grant Number: 26113008, 15H02560, 15K15544], and grants from the National Institutes of Health [AR050631, AR065379]
Footnotes
Conflict of interest
None.
References
- 1.Horton R GBD 2010: understanding disease, injury, and risk. Lancet 2012;15;380(9859):2053–2054. [DOI] [PubMed] [Google Scholar]
- 2.Bron JL, Helder MN, Meisel HJ, Van Royen BJ, Smit TH Repair, regenerative and supportive therapies of the annulus fibrosus: Achievements and challenges. Eur. Spine J 2009;18(3):301–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beertsen W, McCulloch CAG, Sodek J The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 1997;13:20–40. [DOI] [PubMed] [Google Scholar]
- 4.Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med 1992. ;11:533–78. [PubMed] [Google Scholar]
- 5.Yokoyama S, Ito Y, Ueno-Kudoh H, Shimizu H, Uchibe K, Albini S et al. A systems approach reveals that the myogenesis genome network is regulated by the transcriptional repressor RP58. Dev. Cell 2009;17: 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci 2010;107:10538–10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakahara H, Hasegawa A, Otabe K, Ayabe F, Matsukawa T, Onizuka N, et al. Transcription factor Mohawk and the pathogenesis of human anterior cruciate ligament degradation. Arthritis Rheum 2013;65:2081–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, et al. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J. Orthop. Res 2015;33:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayama T, Mori M, Ito Y, Matsushima T, Nakamichi R, Suzuki H, et al. Gtf2ird1-dependent Mohawk (Mkx) expression regulates mechanosensing properties of tendon. Mol. Cell. Biol 2016;36:1297–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H, Ito Y, Shinohara M, Yamashita S, Ichinose S, Kishida A, et al. Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc. Natl. Acad. Sci 2016;113:7840–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamichi R, Ito Y, Inui M, Onizuka N, Kayama T, Kataoka K,et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat Commun 2016;7:12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koda N, Sato T, Shinohara M, Ichinose S, Ito Y, Nakamichi R, et al. The transcription factor mohawk homeobox regulates homeostasis of the periodontal ligament. Development 2017;144,313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dykyj D, Jules KT. The clinical anatomy of tendons. J Am Podiatr Med Assoc 1991;81:358–365. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn K The structure of collagen. Essays Biochem 1969;5:59–87. [PubMed] [Google Scholar]
- 15.Kannus P Structure of the tendon connective tissue. Scandinavian journal of medicine & science in sports 2000;10:312–320. [DOI] [PubMed] [Google Scholar]
- 16.Ochiai N, Matsui T, Miyaji N, Merklin RJ, Hunter JM. Vascular anatomy of flexor tendons. I. Vincular system and blood supply of the profundus tendon in the digital sheath. J Hand Surg 1979;4:321–330. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand KA, Frank CB. Scar formation and ligament healing. Can J Surg 1998;41(6):425–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med 2003;33(5):381–94. [DOI] [PubMed] [Google Scholar]
- 19.Thaker H, Sharma AK. Engaging stem cells for customized tendon regeneration. Stem Cells Int 2012; 309187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today 2013. ;99(3):203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng 2012; 14:47–71 [DOI] [PubMed] [Google Scholar]
- 22.Frank C, Schachar N, Dittrich D. Natural history of healing in the repaired medial collateral ligament. J Clin Periodontol 1982;9(6):428–40. [DOI] [PubMed] [Google Scholar]
- 23.Klein MB, Pham H, Yalamanchi N, and Chang J. Flexor tendon wound healing in vitro the effect of lactate on tendon cell proliferation and collagen production. J Hand Surg Am 22001;26:847–854 [DOI] [PubMed] [Google Scholar]
- 24.Graham MF, Becker H, Cohen IK, Merritt W, and Diegelmann RF. Intrinsic tendon fibroplasia: documentation by in vitro studies. J Orthop Res 1983;1:251–256 [DOI] [PubMed] [Google Scholar]
- 25.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg Am 2002;27(4): 615–620 [DOI] [PubMed] [Google Scholar]
- 26.Tsuzaki M, Brigman BE, Yamamoto J, Lawrence WT, Simmons JG, Mohapatra NK, et al. Insulin-like growth factor-I is expressed by avian flexor tendon cells. J Orthop Res 2000; July;18(4):546–556 [DOI] [PubMed] [Google Scholar]
- 27.Wang XT, Liu PY, Tang JB. Tendon healing in vitro: genetic modification of tenocytes with exogenous PDGF gene and promotion of collagen gene expression. J Hand Surg Am 2004;29(5):884–890. [DOI] [PubMed] [Google Scholar]
- 28.Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lineaweaver WC. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. J Hand Surg Am 1998;23(6):1052–1058. [DOI] [PubMed] [Google Scholar]
- 29.Petersen W, Unterhauser F, Pufe T, Zantop T, Sudkamp NP, Weiler A. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed during the remodeling of free tendon grafts in sheep. Arch Orthop Trauma Surg 2003;123:168 –174. [DOI] [PubMed] [Google Scholar]
- 30.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, et al. Scleraxis: a basic helix-loophelix protein that prefigures skeletal formation during mouse embryogenesis. Development 1995;121(4):1099–1110 [DOI] [PubMed] [Google Scholar]
- 31.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001;128(19):3855–3866. [DOI] [PubMed] [Google Scholar]
- 32.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007;134(14):2697–2708. [DOI] [PubMed] [Google Scholar]
- 33.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell 2003;113(2):235–48. [DOI] [PubMed] [Google Scholar]
- 34.Brent AE, Schweitzer R and Tabin CJ A somitic compartment of tendon progenitors. Cell 2003;113(2):235–248. [DOI] [PubMed] [Google Scholar]
- 35.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng 2005;100(4):418–22. [DOI] [PubMed] [Google Scholar]
- 36.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, et al. Conversion of Mechanical Force into TGF-β-Mediated Biochemical Signals. Current Biol 2011;21(11);933–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oshima Y, Sato K, Tashiro F, Miyazaki J, Nishida K, et al. Anti-angiogenic action of the C-terminal domain of tenomodulin that shares homology with chondromodulin-I. J Cell Sci 2004;117(Pt 13):2731–44. [DOI] [PubMed] [Google Scholar]
- 38.Shukunami C, Takimoto A, Oro M, Hiraki Y Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol 2006;298(1):234–247. [DOI] [PubMed] [Google Scholar]
- 39.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 2005;25(2): 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N,Schweitzer R Recruitment and maintenance of tendon progenitors by TGFc signaling are essential for tendon formation. Development 2009;136(8):1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alberton P, Popov C, Prägert M, Kohler J, Shukunami C, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev 2012;21(6):846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Zhang C, Zhu S, Lu P, Zhu T, Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells 2015;33(2):443–55. [DOI] [PubMed] [Google Scholar]
- 43.Tokunaga T, Shukunami C, Okamoto N, Taniwaki T, Oka K, et al. FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin-Positive Tenocytes in a Rat Rotator Cuff Healing Model. Am J Sports Med 2015;43(10):2411–22. [DOI] [PubMed] [Google Scholar]
- 44.Sakabe T, Sakai K, Maeda T, Sunaga A, Furuta N, et al. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J Biol Chem 2018. [DOI] [PMC free article] [PubMed]
- 45.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 2010;137(17):2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimoto Y, Takimoto A, Watanabe H, Hiraki Y, Kondoh G, Scleraxis is required for maturation of tissue domains for proper integration of the musculoskeletal system. Sci Rep 2017;7:45010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin MA, Havis E, et al. EGR1 and EGR2 involvement in vertebrate tendon differentiation. J Biol Chem 2011;286(7):5855–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin MA, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest 2013;123(8):3564–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson DM, Arredondo J, Hahn K, Valente G, Martin JF, Wilson-Rawls J, et al. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn 2006;235(3):792–801. [DOI] [PubMed] [Google Scholar]
- 50.Yang G, Crawford RC,Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech 2004;37(10):1543–1550. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Kahn CJ, Chen HQ, Tran N, Wang X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol Int 2008;32(3):344–352. [DOI] [PubMed] [Google Scholar]
- 52.Bi Yanming, Ehirchiou Driss, Kilts Tina M, Inkson Colette A, Embree Mildred C, Sonoyama Wataru, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med 2007;13(10):1219–1227 [DOI] [PubMed] [Google Scholar]
- 53.Gallorini M, Berardi AC, Berardocco M, Gissi C, Maffulli N, et al. Hyaluronic acid increases tendon derived cell viability and proliferation in vitro: comparative study of two different hyaluronic acid preparations by molecular weight. Muscles Ligaments Tendons J 2017. September 18;7(2):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown JP, Galassi TV, Stoppato M, Schiele NR, Kuo CK. Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Res Ther 2015. May 9;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.di Giacomo V, Berardocco M, Gallorini M, Oliva F, Colosimo A, et al. Combined supplementation of ascorbic acid and thyroid hormone T3 affects tenocyte proliferation. The effect of ascorbic acid in the production of nitric oxide. Muscles Ligaments Tendons J 2017. May 10;7(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]