Abstract
Nonalcoholic fatty liver disease (NAFLD) is a spectrum comprised of isolated steatosis, nonalcoholic steatohepatitis (NASH), advanced fibrosis, and cirrhosis. The majority of NAFLD subjects do not have NASH and do not carry a significant risk for liver-related adverse outcomes (cirrhosis and mortality). Globally, the prevalence of NAFLD is approximately 25%. In Asia, a gradient of high to low prevalence rates is noted from urban to rural areas. Given the prevalence of NAFLD, the clinical and economic burden of NAFLD and NASH can be substantial. With increasing recognition of NASH as an important liver disease, the diagnosis of NASH still requires a liver biopsy that is suboptimal. Although liver biopsy is the most accurate modality to diagnose and stage the severity of NASH, this method suffers from being invasive, costly, associated with potential complications, and plagued with interobserver variability of individual pathological features. A number of noninvasive modalities to diagnose NASH and stage liver fibrosis are being developed. These modalities include predictive models (NAFLD fibrosis score) and serum biomarkers such as enhanced liver fibrosis (ELF). Other tests are based on radiological techniques, such as transient elastography (TE) or magnetic resonance elastography (MRE), which are used to estimate liver stiffness as a potential surrogate of hepatic fibrosis. Although a dynamic field of research, most of these diagnostic modalities have area under the curve ranging between 0.76 and 0.90%, with MRE having the best predictive performance. In summary, developing safe and easily accessible noninvasive modalities to accurately diagnose and monitor NASH and associated fibrosis is of utmost importance in clinical practice and clinical research. These tests are not only important to risk stratify subjects at the greatest risk for progressive liver disease, but also to serve as appropriate surrogate endpoints for therapeutic clinical trials of NASH.
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease (CLD), worldwide.(1) Based on a recent meta-analysis, 25% of the general global adult population are potentially affected by NAFLD.(1) Although NAFLD was initially reported to be more prevalent among Hispanics, it is now increasingly reported from all regions of the world.(1) Furthermore, the prevalence of NAFLD in children is also high and estimated at approximately 10%.(2–4)
Data on the incidence of NAFLD are quite sparse. Of the available data, the estimated incidence of NAFLD is reported to be between 28 and 52 per 1,000 person-years.(1) However, these incidence rates are probably underestimations, given the rising incidence of obesity and diabetes, two of the primary associated risk factors for NAFLD.
The long-term outcome of the spectrum of NAFLD has been described in observational studies. In fact, data suggest that most patients with NAFLD die primarily of cardiovascular complications.(4–6) Nevertheless, there are subgroups of NAFLD patients, primarily those with hi tological nonalcoholic steatohepatitis (NASH) or significant hepatic fibrosis (HF), who are at risk for developing advanced liver disease, hepatocellular carcinoma (HCC), excess liver mortality, or become candidates for liver transplantation.(1,7–12) In fact, more recent studies have suggested that stage of fibrosis, independent of any other histological feature, predicts mortality in NAFLD
In addition to the clinical burden, NASH places significant burden on patient-reported outcomes (PROs) and the economy.(13–18) A recent Decision Analytic Markov Model estimated tremendous economic burden of NAFLD and nonalcoholic steatohepatitis (NASH) to the United States and European economies.(19) These data provide strong evidence that the progressive form of NAFLD or NASH, especially those with significant fibrosis, poses tremendous clinical, PRO, and economic burden to individuals and society.(14–20)
This article is the summary of data presented at a recent American Association for the Study of Liver Diseases (AASLD) Emerging Trend Conference on NASH on the state of diagnostic modalities for NASH and fibrosis.
The Initial Steps in the Diagnosis of NAFLD
Before the initiation of exhaustive diagnostic tests for NAFLD, it is important for health care providers to exclude other etiologies for steatosis and coexisting common causes of CLD. Specifically, excessive alcohol consumption and other exogenous factors (e.g., steatogenic medications) must be excluded.(1,3,21) Furthermore, in patients with persistently high serum ferritin, and increased iron saturation, especially in the context of homozygote or heterozygote C282Y HFE mutation, iron overload must be considered. Although lowtiter autoimmune markers can be frequently observed in patients with NAFLD, autoimmune liver disease should be ruled out in patients with high serum titers of autoantibodies in association with other features such as high serum globulins.(1,3,21)
Once other causes of steatosis have been ruled out, NAFLD should be considered. In this context, presence of commonly associated comorbidities, such as obesity, dyslipidemia, insulin resistance or diabetes, hypothyroidism, polycystic ovary syndrome, and sleep apnea, should be determined.(3,21)
Role of Histopathology in the Clinical Research and Management of Patients With NAFLD
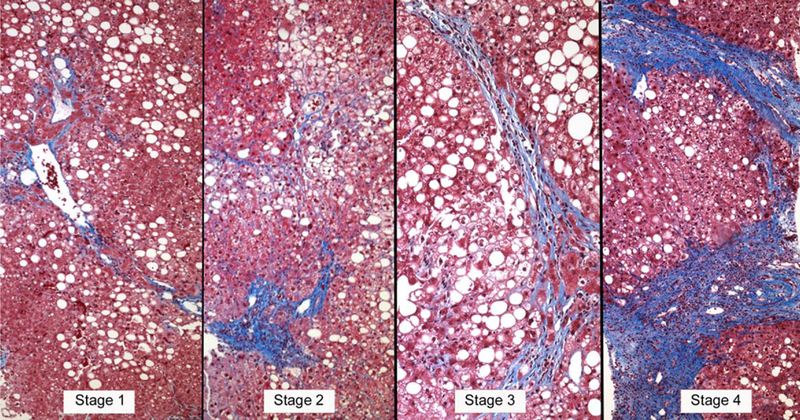
The liver biopsy is the definitive technique for the diagnosis and classification of NAFLD, in which the role of histopathology is to establish a diagnosis, exclude other causes of liver disease, characterize the liver lesions, and correlate the lesions with potential clinical outcomes in the context of the natural history of the disease.(7,23–30) Historically, the terminology and concepts of the histopathological features of NAFLD were derived from alcoholic liver disease. Thus, alcoholic fatty liver (AFL) is analogous to simple steatosis (nonalcoholic fatty liver; NAFL), alcoholic hepatitis to NASH, and alcoholic cirrhosis to the cirrhotic stage of NASH.(23) Although there may be differences in degree, the features are sufficiently similar to preclude an etiological diagnosis based on histology alone. Therefore, the characteristic histopathological features that are investigated when diagnosing alcoholic or non-alcoholic fatty liver disease include: (1) fat: hepatocellular triglyceride accumulation; (2) hepatocellular injury in the centrilobular location that is most severe in the acinar zone; (3) cytoskeletal damage shown as hepatocellular ballooning with or without Mallory-Denk bodies; (4) parenchymal inflammation where lymphocytes and macrophages predominate, though neutrophils may be present in severe cases; and (5) perisinusoidal fibrosis observed as collagen deposition in the space of Disse (Fig. 1).
FIG. 1.
Stages of fibrosis in NAFLD according to the NASH CRN staging system (Masson trichrome stains). Collagen is blue with this method. Stage 1 (left): centrilobular perisinusoidal fibrosis. Blue stained collagen fibers outline the sinusoids surrounding the central vein in the center of the field. Stage 2 (left middle): centrilobular perisinusoidal fibrosis and periportal fibrosis. Delicate collagen fibers are deposited around the sinusoids in the upper part of the field while denser collagen expands the portal tract in the lower part of the field. Stage 3 (right middle): bridging fibrosis. A vascularized septum of fibrous tissue transects the hepatic parenchyma. Stage 4 (right): cirrhosis. Nodules of hepatocytes are surrounded by variable-size fibrous septa.
As an aid to help characterize these lesions and allow for statistical analysis in clinical trials, the pathologists of the National Institutes of Health’s NASH Committee (NIH NASH CRN) devised a grading system called the NAFLD Activity Score (NAS)(29) (Table 1). After studying the interobserver and intraobserver variability of a variety of histological features, the features with the greatest reproducibility (severity of steatosis, hepatocellular ballooning, and lobular inflammation) were chosen to formulate the NAS score. The NAS system then assigns a numerical grade to each feature such that the severity of steatosis is graded from zero to three (0–3), hepatocellular ballooning is graded from zero to two (0–2), and lobular inflammation is graded from zero to three (0–3). The NAS score is the unweighted sum of these three numbers with a range from zero to eight (0–8). Improvement in histological severity is accompanied by a decrease in the NAS.(29) More recently, a refinement of this scoring system, called the SAF Score, has been proposed. Although SAF appears to be a very similar system, the SAF Score separates degree of steatosis (S) from grade of necroinflammatory activity (A) and so may provide greater granularity in terms of disease activity and help distinguish steatosis from NASH.(30)
TABLE 1.
Histological Features of NASH CRN NAS and Fibrosis Staging(27)
| Feature | Definition | Score or code |
|---|---|---|
| Steatosis grade | Low- to medium-power evaluation of parenchymal involvement by steatosis) | |
| <5% | 0 | |
| 5%−33% | 1 | |
| 33%−66% | 2 | |
| >66% | 3 | |
| Lobular inflammation | Overall assessment of all inflammatory foci per 200 × field | |
| No foci | 0 | |
| <2 foci per 200 field | 1 | |
| 2–4 foci per 200 field | 2 | |
| >4 foci per 200 field | 3 | |
| Ballooning* | ||
| None | 0 | |
| Few (or borderline) balloon cells | 1 | |
| Many cells/prominent ballooning | 2 | |
| NAS | Sum of Steatosis + Lobular Inflammation + Ballooning | 0–8 |
| Fibrosis stage | ||
| None | 0 | |
| Perisinusoidal or periportal | 1 | |
| Mild, zone 3, perisinusoidal | 1A | |
| Moderate, zone 3, perisinusoidal | 1B | |
| Portal/periportal | 1C | |
| Perisinusoidal and portal/periportal | 2 | |
| Bridging fibrosis | 3 | |
| Cirrhosis | 4 |
However, the histological feature with the greatest reproducibility is fibrosis, a feature that is not part of the NAS score because fibrosis is considered a sign of the stage of disease rather than a grade of injury. Fibrosis staging is therefore scored separately. Accordingly, in the NASH CRN system, fibrosis stage 0 = no fibrosis; stage 1 = centrilobular pericellular fibrosis (or periportal fibrosis in children); stage 2 = centrilobular and periportal fibrosis; stage 3 = bridging fibrosis; and stage 4 = cirrhosis(8,22,30) (Fig. 1).
As a result of this histological work done on NAFLD, the natural history of the various lesions associated with NAFLD is gradually being elucidated. For example, fatty liver, alone or with some lobular inflammation but without evidence of cytoskeletal damage (ballooning or Mallory-Denk bodies) or fibrosis, has long been considered nonprogressive liver disease; however, recent follow-up studies have found that a few patients do, in fact, eventually develop fibrosis and even cirrhosis.(30) Another example is NASH with ballooning 6 Mallory-Denk bodies, which has long been thought to be the progressive form of NAFLD; however, recent long-term follow-up studies have found that the single histological feature that predicted mortality was not NASH, but fibrosis in the liver biopsy.(8,22,30) Furthermore, studies of NAFLD patients with paired biopsies found that spontaneous regression of fibrosis may be an important feature in NASH, both in the long term and short term.(22,25)
With these findings in mind, a collaborative effort with NIH NASH CRN was undertaken where two scoring systems were developed to grade the level of liver injury associated with NAFLD (NAS) and stage the level of disease associated with NAFLD Fibrosis Score (NFS). As such, the use of histology in diagnosing NAFLD has allowed a more in-depth understanding of the natural history of the various NAFLD histological lesions, but more information is still needed on the mechanisms of fibrosis progression and development of cirrhosis in patients with NAFLD.
The Role of Radiological Modalities for Diagnosing, Staging, and Monitoring NASH
Although liver biopsy is the gold standard to diagnose NASH and assess the stage of fibrosis in patients with NAFLD, its many limitations (cost, sampling error, complications leading to morbidity, and, though rare, death) prohibit its routine use.(11,25,26) However, when seeing a patient for the first time with suspected NAFLD, a clinician would like to know the following: (1) whether the patient has NAFLD; (2) whether the patient is likely to have underlying NASH; (3) whether the patient has any fibrosis; and (4) whether the patient has advanced fibrosis. As a result, there is an urgent need for an accurate noninvasive diagnostic modality for the diagnosis and staging of NASH.(31)
In the context of NAFLD, the first diagnostic challenge is to accurately show the presence of fat in the liver. Currently, 20%−33% is considered a reliable level at which steatosis is detected by conventional means; however, the presence of fat greater than 5%−10% is considered abnormal.(22,31–36)
Fat is thought to have its own chemical signature, which can be detected directly by magnetic resonance spectroscopy (MRS). When performed properly, MRS quantifies the proton density fat fraction (PDFF), a standardized measure of liver tissue (triglyceride).(34) However, the limitations of MRS include: limited availability, need for expertise in protocol prescription, data collection, and spectral analysis is required. Furthermore, MRS is not available on routine scanners. Therefore, now, magnetic resonance imaging (MRI) based methods have been developed using MRI-PDFF to quantify liver fat without needing spectroscopy coils using routinely available clinical MRI scanners.(33–40) MRI-PDFF addresses confounding factors and is not affected by scanner field strength, patient factors (age, sex, body mass index [BMI], and etiology of liver disease), and concomitant liver abnormalities such as iron overload or necroinflammation.(34,41–43)
In contrast, fibrosis has no molecular signature that can be detected by current imaging techniques so all imaging tests for fibrosis attempt to detect fibrosis indirectly using proposed biomarkers, which include: stiffness, diffusion, perfusion, metabolites, and image texture. However, the leading biomarker is liver “stiffness” (or “elasticity”) and its family of related parameters.(34) The rationale for using stiffness or elasticity is that the collagen deposition associated with fibrosis imparts parenchymal rigidity, which, on imaging tests, is considered as assessing stiffness or “elastography.”(34–43)
The most accurate noninvasive methods to assess the stiffness of the liver and to dichotomize the patient into advanced versus nonadvanced fibrosis include transient elastography (TE), magnetic resonance elastography (MRE), and emerging techniques such as shear wave elastography and acoustic radial force imaging(38,44–46) (Fig. 2).
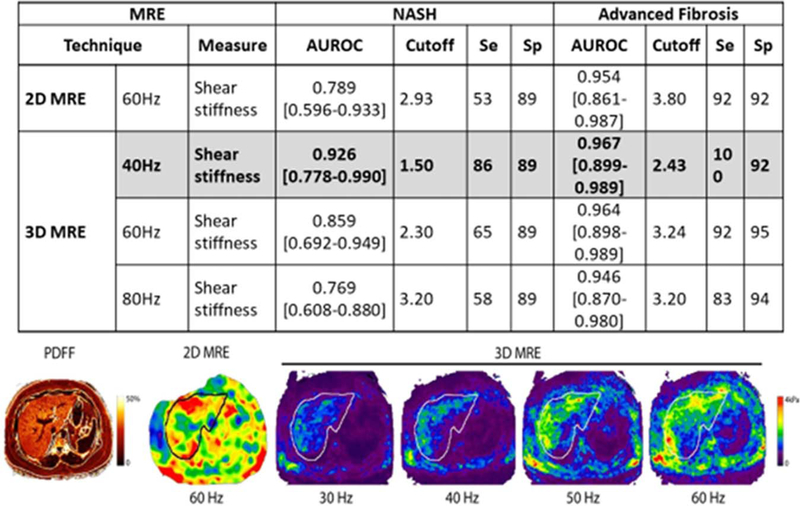
FIG. 2.
Novel 3D MRE and diagnosis of NASH and AF.
TE has been shown to have an area under the curve (AUROC) of 0.83 for advanced fibrosis (AF) when compared to blood tests. Overall, TE has a 90% negative predictive value (NPV), a sensitivity of 88%, a positive predictive value (PPV) of <65% and places the fewest patients (43.6%) in what is considered “the gray zone” compared to blood tests. Furthermore, TE may also have prognostic ability. Because TE has diagnostic accuracies of 80.8%, it is thus able to categorize patients into subgroups found to be have different prognoses.(47)
Using a stiffness cutoff of 3.63 kilopascals, MRE has a sensitivity of 0.86 (95% confidence interval [CI], 0.65–0.97), a specificity of 0.91 (95% CI, 0.83–0.96), a PPV of 0.68 (95% CI, 0.48–0.84), and a NPV of 0.97 (95% CI, 0.91–0.99) with an AUROC of 0.924 for diagnosing AF.(39) In addition, the use of three-dimensional (3D) MRE has shown at 40 Hz and a stiffness cutoff of 2.43, to have an AUROC of 0.962 for diagnosing AF.(46) In fact, in a recent study comparing acoustic radiation force impulse–based versus MRE-based NAFLD fibrosis assessment, MRE was significantly better.(45) Furthermore, MRE was significantly better than TE when diagnosing cirrhosis.(48) There are several caveats that must be addressed before using MRE. These caveats include: the cost of the procedure, patient size, claustrophobia, or presence of metal implants.(46,48)
Although TE or other ultrasound-based tests are more accessible and easier to use, they are limited when used in patients with obesity, ascites, or acute inflammation.(34) However, even though 2D and 3D MRE are able to overcome all these issues except for iron overload or acute inflammation, MRE is limited by restricted accessibility at many centers, especially worldwide, and the required expertise needed to obtain adequate results in the setting of 3D MRE. Currently, accurate imaging is a trade-off between specificity, accessibility, and ease of use such that as specificity goes up, accessibility and ease of use go down. It is also reasonable to use ultrasound-based tests in nonobese individuals and consider using MRE in individuals with obesity, especially morbid obesity.(34,38,45) Further research is needed to quantify the exact trade-off that occurs when one imaging technique is traded off for the other.
Noninvasive Biomarkers in NASH
In addition to the noninvasive tests based on the imaging modalities, there is an attempt to define noninvasive biomarkers using predictive models or serum biomarkers. These noninvasive markers include those that are based on alanine aminotransferase (ALT) levels, those that include components of metabolic syndrome (MetS), measuring circulating keratin18 fragment levels as well as tests based on soluble markers such as FibroMeter, microRNA (miRNA) panels, and lipidomic panels.(49–61)
Using these noninvasive tests to diagnose for NASH, current studies have found that the frequency of NASH in individuals with normal ALT (<35 U/L) was 11% whereas the frequency was 29% in those with elevated ALT ( 35 U/L); and if the ALT was 2 times the upper limit of normal (>70 U/L), predicting NASH was found to have a 50% sensitivity and 61% specificity for NASH.(49) Another study found that individuals with NAFLD can have normal ALT levels as the disease progresses.(62)
Feldstein et al. in their seminal work, found that circulating levels of cytokeratin-18 (CK-18) fragments were predictors of NASH in patients with NAFLD.(53) Since the release of their observations, there has been intense investigations that have unequivocally found that increased circulating levels of CK-18 fragments are associated with NASH.(54,55) However, at the same time, there are a number of issues with this diagnostic method (lack of a commercially available clinical test, poor reproducibility with limited sensitivity/specificity at the individual patient level,(51) and a lack of a clear cut-off point), which limit its clinical utility at the present time.(62)
Others have looked into combining these different measures to diagnose NASH. Investigators have examined the efficacy of a panel of eicasanoids in the detection of NASH using a lipidomics-based approach and demonstrated excellent diagnostic accuracy ranging between 0.9 and 1.0.(63) These data need to be confirmed in larger, multicenter studies.
The NASH test combines demographic characteristics (age, sex, and BMI), serum parameters (aminotransferases and lipids), and alpha-2 macroglobulin, apolipoprotein A1, and haptoglobin. The NASH test sensitivity is 33% with a specificity of 94%, indicating that it has a good NPV for NASH of 81%.(54) Another combined test, the NASH Diagnostics Panel, which uses the presence of CK-18 fragments, adiponectin, and resistin, initially performed well, but, in a larger study, was not found to be as effective.(55) The NAFLD Diagnostic Panel used CK-18 fragments in combination with the presence of type 2 diabetes mellitus, triglycerides, and sex, but it did not perform any better than the NASH Diagnostics Panel.(56)
On the other hand, the OXNASH score, which uses aspartate aminotransferase (AST), age, BMI, and a ratio of 13-hydroxy-octadecadienoic acid to linoleic acid, correlates with histological features of NASH and provides AUROCs of 0.730 (95% CI, 0.637, 0.823) for inflammation; followed by ballooning of 0.723 (95% CI, 0.630, 0.816), steatosis 0.705 (95% CI, 0.570, 0.840); and fibrosis 0.673 (95% CI, 0.577, 0.770).(57,58) The Hepascore, derived from age, sex, bilirubin, gamma glutamytransferase, hyaluronic acid (HA), alpha-2 macroglobulin, when compared to a simple noninvasive score (BARD Index [BMI, AST, ALT, diabetes]), performed reasonably well in identifying fibrosis F2-F4 with AUROCs of 0.73 to 0.91 with more accuracy noted for patients with a fibrotic stage of 4.(47,59) The FibroMeter NAFLD (age, weight, fasting glucose, AST, ALT, ferritin, and platelet count) performs reasonably well in identifying mild-to-moderate fibrosis. The PPV for correctly identifying F = 1 was 84.9% for the FibroMeter NAFLD with a NPV of 66.7% and a diagnostic accuracy of 80%. The PPV for F = 3 was found to be 74.5% with a NPV of 86.2 and a diagnostic accuracy of 82.1%.(64) Several prognostic scores (Palekar score, Shimada index, Nice model, and Gholam’s model) have also been developed, with all performing some-what similarly with AUROC ranging from 0.76 to 0.90.(60,61)
MetS is another commonly used index to identify individuals with NAFLD at risk for NASH. Several studies have found a significant relationship between the increasing number of MetS components and the likelihood of NASH in patients with NASH.(65–67) Yet, what has not been explored is the combination of MetS, levels of ALT, and age to predict NASH in NAFLD, leaving another area for future development.
With these continuing challenges in correctly diagnosing NASH, the NAFLD scientific community needs to re-evaluate the need for predicting NASH in patients with NAFLD. Instead, it may best to focus on NASH with stage ≥2 fibrosis given that it is the subphenotype that is primarily targeted in phase 2B and 3 clinical trials.
Serum Fibrosis Markers in NASH
Given that stage of fibrosis is the most important predictor of outcome,(7,8,10,68) much effort has focused on determining presence of fibrosis.(7) In this context, NAFLD biomarkers can target domains that can be defined as the following: (1) diagnostic markers, reflecting current stage of fibrosis; (2) prognostic markers, stratifying individuals by fibrosis progression risk, discriminating fast versus slow progressors, and/or predicting long-term outcomes and hard endpoints; and (3) monitoring markers that may be used to track disease progression or treatment response. Such biomarkers should be at one of four qualification levels: (1) exploration (early-phase experimental biomarkers); (2) demonstration (“probable valid” biomarkers); (3) characterization (“known valid” biomarkers); and (4) surrogacy (registerable “surrogate endpoint”).(69–82) Although there has been some progress in biomarker development for detection of AF, existing biomarkers are generally at the first two qualification levels and need further independent validation.
Serological markers for the evaluation of liver fibrosis (LF) may be divided into “indirect” markers (that reflect alterations in hepatic function, but not collagen turnover, e.g., ALT, AST, and platelet levels) and “direct” markers (that directly measure aspects of extracellular matrix (ECM) deposition and/or turnover).(69,71) Furthermore, novel kinetic biomarkers using deuterated water-based approaches are emerging to assess dynamic changes in the fractional synthesis rate of collagen turnover in the liver in patients with NAFLD.(83)
Indirect Markers and “Simple Panels”
Significant hepatic fibrosis can lead to hepatocellular dysfunction and portal hypertension, which are reflected by changes in standard biochemical and hematological parameters. These tests, alone or combined as “simple panels,” are potentially attractive clinical tools given that they are inexpensive and many indices are already routinely measured in patients with liver disease.(70) The results of a head-to-head comparison in a large cohort with biopsy-proven NAFLD patients have been published. In general, simple panels have a relatively robust NPV, so they can reliably exclude AF, but have poor PPV (ranging from 27% to 79%).(71) Using such tests may help to mitigate the health care burden such a large “at-risk” population places on resources by not allowing those considered to have “low-risk” scores to undergo further investigation. As LF progresses toward AF/cirrhosis, serum ALT levels tend to fall whereas AST levels remain stable or increase, leading to an increase in the AST/ALT ratio. This phenomenon is exploited as a component of many of the simple panels. The NFS is calculated using six routinely measured parameters found to be independently associated with AF on multivariate analysis.(62) By applying a low cutoff (<–1.455), AF can be excluded with high accuracy (NPV 93%) whereas a high cut-off threshold (>0.676) offers accurate detection of AF (PPV 90%).(62) Use of this score has been suggested to reduce the need for liver biopsy by 75%. In this context, NFS can accurately exclude AF in NAFLD and is clinically useful.
The Fibrosis-4 (FIB-4) score is one of the best performing simple noninvasive tests for AF in NAFLD. A score of <1.3 has a 90% NPV for stage 3–4 fibrosis, whereas a score of >2.67 has an 80% PPV with only one quarter of the cohort being unclassified 1.3 or above 2.67.(73) Other studies have also found that the FIB-4 score narrowly outperforms other simple noninvasive tests in predicting AF.(71) The specificity for AF using the FIB-4 and NFS declines with age, becoming unacceptably low in patients aged >65 years; however, age-adjusted lower cutoffs (NFS <0.12 and FIB-4 <2) have been derived that maintain the high NPV and so help to exclude AF in those aged ≥65 years.(74) It is also important to note that these scores may not be helpful in the younger age group and perform poorly with relatively low AUROC. In this context, the exact cut-off threshold and validity of these noninvasive tests in the clinical setting require further external validation before their full clinical use can be recommended.(75–79)
Direct Markers: Collagen Turnover
When the severity of ongoing liver injury exceeds that of hepatic regeneration, hepatocytes are replaced by an ECM composed of collagens (I, III, and IV), fibronectin, undulin, elastin, laminin, hyaluronan, and proteoglycans 3. Candidate biomarkers derived from these processes are appealing targets and are currently an area of active investigation.(75,76)
One such biomarker is HA. HA production is increased when collagen synthesis is accelerated, so this is a marker of increased ECM production. Similarly, LF results in the deposition of collagen and release of propeptides, predominantly Pro-Collagen III (PIIINP). The terminal peptide of PIIINP correlates with the NAS, and its constituent components (P < 0.001), where a threshold of 6.6 ng/mL provides an NPV for AF of 95%−97% and 100% for cirrhosis.(75) The Enhanced Liver Fibrosis (ELF) test is a commercial panel of markers focusing on matrix turnover comprised of tissue inhibitors: matrix metalloproteinase 1 (tissue inhibitor of matrix metalloproteinase 1), HA, and aminoterminal peptide of pro-collagen III (P3NP).(76,80) When compared with the NFS, this test performed only marginally better for severe fibrosis (AUROC 0.93 vs. 0.89) and moderate fibrosis (AUROC 0.90 vs. 0.86), but combining the two modalities enhanced performance (AUROC 0.98 for severe fibrosis and 0.93 for moderate fibrosis).(81) FibroTest is a commercial panel, with a reported AUROC of 0.75–0.86 for F2-F4 and 0.81–0.92 for F3-F4.(81) Other commercial assays currently in development with promising preliminary results detect pathologically modified proteins generated by specific proteases such as the specific collagen fragments of Pro-C3 and Pro-C6 using proprietary Protein Finger-print enzyme-linked immunosorbent assay assays.(82)
Other Promising Experimental Markers: Genetics/Epigenetics, Metabolomics, and Lipidomics
Interpatient variation in NAFLD progression risk is, at least in part, determined by genetic modifiers that influence individual response to environmental (diet, lifestyle) factors.(22) Mounting evidence indicates that epigenetic factors, such as differential DNA methylation and circulating cell-free DNA methylation signatures in plasma, may potentially stratify patients with NAFLD into mild versus severe fibrosis.(83,84) miRNA is another genetic marker that appears to be relatively stable and can be detected in plasma following release from injured tissue and may serve as another disease biomarker.(85–87) These modalities remain highly experimental, however, and require further validation.
Summary
Over the last 40 years, NAFLD has evolved from an unrecognized entity to a heterogeneous collection of overlapping liver diseases with a common phenotype of hepatic steatosis. Although NAFLD is quite common, affecting approximately 25% of the world’s adult population, it is increasingly clear that subjects with NASH and especially those with significant fibrosis are at the greatest risks for excess mortality and adverse clinical outcomes as well as impairment of PRO and significant economic burden.
Despite the growing recognition of this important burden, there are significant challenges to accurately and noninvasively diagnose the progressive form of NAFLD. Although liver biopsy is considered the current imperfect “gold” standard for diagnosing NASH and staging fibrosis, it is an invasive procedure with some variability in assessment of the key features of NASH.
Therefore, a number of serum markers, radiographical modalities, and noninvasive predictive algorithms have been or are currently undergoing investigation. To date, most of these modalities suffer from suboptimal performance. However, MRI-PDFF seems to be the most accurate modality for detecting hepatic fat whereas MRE seems to be the most accurate test for staging liver disease. Thus, a combination of MRI-PDFF and MRE may provide a relatively accurate method to risk stratify subjects with NAFLD. But availability and cost of these modalities presents a major challenge to most clinical practices.
As a result of the limitations of the noninvasive tests and radiographical imaging, the regulatory authorities (United States Food and Drug Administration (FDA) and European Medicines Agency (EMA)] now require histological endpoints for approval of drugs and diagnostic modalities. As such, until noninvasive measures are perfected and robustly validated, the diagnosis of NAFLD may continue to be underestimated and the development of therapeutic options may be hindered.
Acknowledgments:
The authors of this manuscript participated as faculty in the Emerging Trend for NAFLD conference sponsored by AASLD in March of 2017. None of the authors have any conflicts related to this article. The authors acknowledge and thank Linda Henry, Ph.D., who assisted with manuscript editing.
Potential conflict of interest: Dr. Younossi consults for Bristol-Myers Squibb, Novo Nordisk, Novartis, Intercept, Gilead, Allergan, and GlaxoSmithKline. He advises for Bistol Myers Squib and Novartis. Dr. Charlton consults for and received grants from Gilead, Intercept, NGM, Genfit, and Novartis. He received grants from Conatus. Dr. Abdelmalek received grants from Promethus and Metabolon. Dr. Kowdley consults for, advises for, is on the speakers’ bureau for, and received grants from Gilead and Intercept. He advises for and received grants from Allergan. He consults for Verlyx. He advises for Conatus. He received grants from Galectin, Immuron, NGM, Prometheus, and Zydus. Dr. Goodman consults for and received grants from Allergan. He consults for Pfizer. He received grants from Gilead, Galectin, Conatus, Exalenz, Intercept, Sanofi, Novartis, and Bristol-Myers Squibb. Dr. Ratziu consults for and received grants from Intercept. He consults for Galmed, Genfit, Boehringer Ingelhim, Pfizer, and Allergan. He received grants from Gilead. Dr. Anstee consults for, is on the speakers’ bureau for, and received grants from Allergan/Tobira and Genfit. He consults for and is on the speakers’ bureau for Abbott. He consults for and received grants from Eli Lilly, Intercept, Novartis, and Pfizer. He consults for Acuitas, E3Bio, Galmed, Gilead, Grunthal, Imperial Innoations, Inventiva, Janssen, Kenes, MedImmune, NewGene, and Raptor. He is on the speakers’ bureau for Clinical Care Options. He received grants from AbbVie, Antaros, AstraZeneca, Boehringer Ingelheim, Ellegaard Gottigen, Exalenz, GlaxoSmithKline, Vertex, iXscient, Nordic Bioscience, Novo Nordisk, One Way Liver Genomics, Perspectum, Sanofi-Aventis, SomaLogic, and Takeda. Dr. Harrison consults for and owns stock in Cirius and Madrigal. He consults for Cymetrix, Novartis, Perspectum, Intercept, Novo Nordisk, Capulus, CiVi, NGM, CLDF, Genfit, Echosens, High Index, and Prometheus. He advises for Gilead. He is on the speakers’ bureau for Alexion. Dr. Tetri consults for and advises Nimbus, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, Enanta, Novartis, Galmed, Zafgen, Receptos, Pfizer, Allergan, MedImmue/AstraZeneca, ConSynance, Tobira, Karos, Afimmune, NuSirt, Arrowhead, Reset, Intercept, Gilead, Abide, and Cymabay. Dr. Friedman consults for, has research contracts with, and owns stock in Scholar Rock. He consults for and has research contracts with Bristol-Myers Squibb, Enanta, and Zafgen. He consults for and owns stock in Exalenz, Blade, DeuteRx, Galectin, Genfit, Glympse, Jecure, Lifemax, and Northern Biologics. He consults for Abide, Affimune, Allegan, Angion, Arubutus, Arrowheard, Avaliv, Axcella, Boehringer Ingelheim, Can-Fite, ChemomAb, Contravir, CymaBay, Five Prime, Fortress, Gemphire, Glycotest, Inception, Lexicon, Metacrine, Metagenix, Morphic Rock, Nitto, Novartis, Ocera, Perspectum, Pfizer, Revive, RiverVest, Roche/Genentech, Sandhill, Second Genome, Surrozen, Takeda, Teva, Third Rock, Tokai, Viking, Vivace, VL-45, X-Tuit, and Zydus. He has research contracts with AbbVie and 3V Bio. He owns stock in Akarna, BirdRock, Conatus, Intercept, Nimbus, and Tobira. Dr. Mathurin consults for and is on the speakers’ bureau for Gilead. He consults for Sanofi and Verlyx. Dr. Marchesini advises for and received grants from Gilead and Eli Lilly. He received grants from Novo Nordisk, Genfit, and Janssen. Dr. Negro consults for and received grants from Gilead and AbbVie. He consults for Merck. Dr. Rinella consults for Intercept, Gilead, Immuron, NGM, Nusirt, Enanta, and Novartis. Dr. Caldwell consults for Shionogi and Gencia. He received grants from Gilead, Genfit, Galmed, NGM, Immuron, Conatus, Vital Therapy, Target, and TaiwanJ. Dr. Chalansani consults for and received grants from Lilly. He consults for AbbVie, Nusirt, Tobira/Allergan, Affimune, Axovant, Immuron, Ardelyx, and Madrigal. He received grants from Gilead, Galectin, and Cumberland. Dr. Sanyal consults for and received grants from Gilead, Malinckrodt, and Salix. He consults for and is employed by Sanyal Bio. He consults for Pfizer, Nimbus, Nitto Denko, Hemoshear, and Lilly. He received grants from Conatus, Novartis, Galectin, Bristol-Myers Squibb, Merck, and Sequana. He received royalties from Elsevier and Uptodate. He owns stock in Akarna, GenFit, and NewCo.
Abbreviations:
- 3D
three-dimensional
- AASLD
American Association for the Study of Liver Diseases
- AF
advanced fibrosis
- AFL
alcoholic fatty liver
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUROC
area under the curve
- BMI
body mass index
- CI
confidence interval
- CK-18
cytokeratin-18
- CLD
chronic liver disease
- ECM
extracellular matrix
- ELF
enhanced liver fibrosis
- EMA
European Medicines Agency
- FDA
United States Food and Drug Administration
- FIB-4
Fibrosis-4
- HA
hyaluronic acid
- HCC
hepatocellular carcinoma
- HF
hepatic fibrosis
- LF
liver fibrosis
- MetS
metabolic syndrome
- miRNA
microRNA
- MRE
magnetic resonance elastography
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
non-alcoholic steatohepatitis
- NIH NASH CRN
National Institutes of Health Nonalcoholic Steatohepatitis Clinical Research Network
- NFS
NAFLD Fibrosis Score
- NPV
negative predictive value
- PDDF
proton density fat fraction
- PIIINP
Pro-Collagen III
- PPV
positive predictive value
- PROs
patient-reported outcomes
- SAF
semiquantitative scoring of steatosis (S) activity (A) and fibrosis (F)
- TE
transient elastography
REFERENCES
- 1).Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. HEPATOLOGY 2016,64:73–84. [DOI] [PubMed] [Google Scholar]
- 2).Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–1393. [DOI] [PubMed] [Google Scholar]
- 3).Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis 2009;13:511–531. [DOI] [PubMed] [Google Scholar]
- 4).Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 2013;230:258–267. [DOI] [PubMed] [Google Scholar]
- 5).Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, et al. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J Hepatol 2015;63:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Lonardo A, Ballestri S, Guaraldi G, Nascimbeni F, Romagnoli D, Zona S, et al. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease—evidence from three different disease models: NAFLD, HCV and HIV. World J Gastroenterol 2016;22:9674–9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. HEPATOLOGY 2011. 53:1874–1882. [DOI] [PubMed] [Google Scholar]
- 8).Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. HEPATOLOGY 2013; 57:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. HEPATOLOGY 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 12).Younossi ZM, Stepanova M, Rafiq N, Henry L, Loomba R, Makhlouf H, Goodman Z. Nonalcoholic Steatofibrosis Independently Predicts Mortality in Nonalcoholic Fatty Liver Disease. Hepatol Commun 2017;1:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148: 547–555. [DOI] [PubMed] [Google Scholar]
- 14).Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017;152:1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Sayiner M, Stepanova M, Pham H, Noor B, Walters M, Younossi ZM. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol 2016;3:e000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Younossi ZM, Stepanova M, Henry L, Racila A, Lam B, Pham HT, Hunt S. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int 2017;37:1209–1218. [DOI] [PubMed] [Google Scholar]
- 17).Sayiner M, Otgonsuren M, Cable R, Younossi I, Afendy M, Golabi P, et al. Variables Associated With Inpatient and Outpatient Resource Utilization Among Medicare Beneficiaries With Nonalcoholic Fatty Liver Disease With or Without Cirrhosis. J Clin Gastroenterol 2017;51:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol 2015;49:222–227. [DOI] [PubMed] [Google Scholar]
- 19).Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. HEPATOLOGY 2016;64:1577–1586 [DOI] [PubMed] [Google Scholar]
- 20).Younossi Z What Is the Ethical Responsibility of a Provider When Prescribing the New Direct-Acting Antiviral Agents to Patients With Hepatitis C Infection? Clin Liver Dis 2015;6:117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Rafiq N, Younossi ZM. Nonalcoholic fatty liver disease: a practical approach to evaluation and management. Clin Liver Dis 2009;13:249–266. [DOI] [PubMed] [Google Scholar]
- 22).Anstee QM, Seth D, Day CP. Genetic Factors That Affect Risk of Alcoholic and Nonalcoholic Fatty Liver Disease. Gastroenterology 2016;150:1728–1744.e7. [DOI] [PubMed] [Google Scholar]
- 23).Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis. Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 198055:434–438. [PubMed] [Google Scholar]
- 24).Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis 1986;8:283–298. [PubMed] [Google Scholar]
- 25).Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, Rybicki L. Nonalcoholic fatty liver disease; Assessment of variability in pathologic interpretations. Mod Pathol 1998;11:560–565. [PubMed] [Google Scholar]
- 26).Younossi ZM. Long-term outcomes of Nonalcoholic fatty liver disease: From nonalcoholic steatohepatitis to nonalcoholic steatofibrosis. Clin Gastroenterol Hepatol 2017;15:1144–1147. [DOI] [PubMed] [Google Scholar]
- 27).Stumptner C, Fuchsbichler A, Heid H, Zatloukal K, Denk H. Mallory body—a disease-associated type of sequestrosome. HEPATOLOGY 2002;35:1053–1062. [DOI] [PubMed] [Google Scholar]
- 28).Telli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver. HEPATOLOGY 1995;22: 1714–1719. [PubMed] [Google Scholar]
- 29).Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. HEPATOLOGY 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 30).Kleiner DE, Makhlouf HR. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children. Clin Liver Dis 2016;20:293–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Jayakumar J, Harrison SA, Loomba R. Noninvasive markers of fibrosis and inflammation in nonalcoholic steatohepatitis. Curr Hepatol Rep 2016;15:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Idilman IS, Keskin O, Elhan AH, Idilman R, Karcaaltincaba M. Impact of sequential proton density fat fraction for quantification of hepatic steatosis in nonalcoholic fatty liver disease. Scand J Gastroenterol 2014;49:617–624. [DOI] [PubMed] [Google Scholar]
- 33).Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol 2013;10:666–675. [DOI] [PubMed] [Google Scholar]
- 34).Dulai PS, Sirlin CB, Loomba R. MRI and MRE for noninvasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016;65:1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:6821–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 37).Tapper EB, Challies T, Nasser I, Afdhal NH, Lai M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients With Nonalcoholic Fatty Liver Disease. Am J Gastroenterol 2016;111:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017;152:598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Chen J, Yin M, Talwalkar JA, Oudry J, Glaser KJ, Smyrk TC, et al. Diagnostic Performance of MR Elastography and Vibration-controlled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology 2017;283:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Hu HH, Krishna S. Nayak KS, and Michael I. Goran MI. Assessment of Abdominal Adipose Tissue and Organ Fat Content by Magnetic Resonance Imaging. Obes Rev 2011;12:e504–e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, et al. ; San Diego Integrated NAFLD Research Consortium (SINC). Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. HEPATOLOGY 2012;56:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. HEPATOLOGY 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. ; San Diego Integrated NAFLD Research Consortium (SINC). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). HEPATOLOGY 2015;61:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. HEPATOLOGY 2016;63:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Loomba R, Cui J, Wolfson T, Haufe W, Hooker J, Szeverenyi N, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol 2016;111:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol 2016;65:570–578. [DOI] [PubMed] [Google Scholar]
- 48).Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016;150:626–637.e7. [DOI] [PubMed] [Google Scholar]
- 49).Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int 2013;33:1398–1405. [DOI] [PubMed] [Google Scholar]
- 50).Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. HEPATOLOGY 2012;55:2005–2023. [DOI] [PubMed] [Google Scholar]
- 51).Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 2014;60:167–174. [DOI] [PubMed] [Google Scholar]
- 52).Baranova A, Younossi ZM. The future is around the corner: Noninvasive diagnosis of progressive nonalcoholic steatohepatitis. HEPATOLOGY 2008;47:373–375. [DOI] [PubMed] [Google Scholar]
- 53).Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. HEPATOLOGY 2009;50:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Anty R, Iannelli A, Patouraux S, Bonnafous S, Lavallard VJ, Senni-Buratti M, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther 2010;32:1315–1322. [DOI] [PubMed] [Google Scholar]
- 55).Yilmaz Y, Ulukaya E, Dolar E. A biomarker biopsy for the diagnosis of NASH: promises from CK-18 fragments. Obes Surg 2008;18:1507–1508. [DOI] [PubMed] [Google Scholar]
- 56).Younossi ZM, Page S, Rafiq N, Stepanova M, Hossain N, Afendy A, et al. A biomarker panel for nonalcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg 2011;21: 431–439. [DOI] [PubMed] [Google Scholar]
- 57).Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res 2010;51:3046–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Alkhouri N, Berk M, Yerian L, Lopez R, Chung YM, Zhang R, et al. OxNASH score correlates with histologic features and severity of nonalcoholic fatty liver disease. Dig Dis Sci 2014;59: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Adams LA, George J, Bugianesi E, Rossi E, De Boer WB, van der Poorten D, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2011;26:1536–1543. [DOI] [PubMed] [Google Scholar]
- 60).Palekar NA, Naus R, Larson SP, Ward J, Harrison SA. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int 2006;26:151–156. [DOI] [PubMed] [Google Scholar]
- 61).Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, et al. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol 2007;102:1931–1938. [DOI] [PubMed] [Google Scholar]
- 62).Rinella ME, Loomba R, Caldwell SH, Kowdley K, Charlton M, Tetri B, Harrison SA. Controversies in the Diagnosis and Management of NAFLD and NASH. Gastroenterol Hepatol 2014; 10:219–227. [PMC free article] [PubMed] [Google Scholar]
- 63).Loomba R, Quehenberger O, Armando A, Dennis EA. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res 2015;56:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Siddiqui MS, Patidar KR, Boyett S, Luketic VA, Puri P, Sanyal AJ. Performance of non-invasive models of fibrosis in predicting mild to moderate fibrosis in patients with non-alcoholic fatty liver disease. Liver Int 2016;36:572–579. [DOI] [PubMed] [Google Scholar]
- 65).Barr J, Caballeria J, Martinez-Arranz I, Dom´ınguez-D´ıez A, Alonso C, Muntané J, et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res 2012;11:2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism 2013;62:352–360. [DOI] [PubMed] [Google Scholar]
- 67).Wainwright P, Byrne CD. Bidirectional Relationships and Disconnects between NAFLD and Features of the Metabolic Syndrome. Int J Mol Sci 2016;17:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. HEPATOLOGY 2015;61:1547–1554. [DOI] [PubMed] [Google Scholar]
- 69).Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol 2008;5:95–106. [DOI] [PubMed] [Google Scholar]
- 70).Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol 2013;66:1033–1045. [DOI] [PubMed] [Google Scholar]
- 71).McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–1269. [DOI] [PubMed] [Google Scholar]
- 72).Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. HEPATOLOGY 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 73).Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol 2017;112:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Tanwar S, Trembling PM, Guha IN, Parkes J, Kaye P, Burt AD, et al. Validation of terminal peptide of procollagen III for the detection and assessment of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease. HEPATOLOGY 2013; 57:103–111. [DOI] [PubMed] [Google Scholar]
- 76).Karsdal MA, Henriksen K, Nielsen MJ, Byrjalsen I, Leeming DJ, Gardner S, et al. Fibrogenesis assessed by serological type III collagen formation identifies patients with progressive liver fibrosis and responders to a potential antifibrotic therapy. Am J Physiol Gastrointest Liver Physiol 2016;311:G1009–G1017. [DOI] [PubMed] [Google Scholar]
- 77).Estep JM, Birerdinc A, Younossi Z. Non-invasive diagnostic tests for non-alcoholic fatty liver disease. Curr Mol Med 2010; 10:166–172. Review. [DOI] [PubMed] [Google Scholar]
- 78).Armstrong MJ, Schmidt-Martin D, Rowe IA, Newsome PN. Caution in Using Non-Invasive Scoring Systems in NAFLD Beyond Highly Selected Study Populations. Am J Gastroenterol 2017;112:653–654. [DOI] [PubMed] [Google Scholar]
- 79).Doycheva I, Shaker M, Allende D, Lopez R, Watt KD, Alkhouri N. Low Utility of Noninvasive Fibrosis Scores in Young Adults with Nonalcoholic Fatty Liver Disease. Am J Gastroenterol 2017;112:652–653. [DOI] [PubMed] [Google Scholar]
- 80).Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:1704–1713. [DOI] [PubMed] [Google Scholar]
- 81).Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. HEPATOLOGY 2008;47:455–460. [DOI] [PubMed] [Google Scholar]
- 82).Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Decaris ML, Li KW, Emson CL, Gatmaitan M, Liu S, Wang Y, et al. Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. HEPATOLOGY 2017;65:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Hardy T, Zeybel M, Day CP, Dipper C, Masson S, McPherson S, et al. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut 2017;66:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Pirola CJ, Fernandez Gianotti T, Castano GO, Mallardi P, San Martino J, Mora Gonzalez Lopez, et al. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum noncoding RNAs to liver histology and disease pathogenesis. Gut 2015;64:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Tan Y, Ge G, Pan T, Wen D, Chen L, Yu X, et al. A pilot study of serum microRNAs panel as potential biomarkers for diagnosis of nonalcoholic fatty liver disease. PLoS One 2014;9: e105192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Zarrinpar A, Gupta S, Maurya MR, Subramaniam S, Loomba R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut 2016;65:1546–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]